Abstract
Interferon-α (IFN-α) produced at high levels by human plasmacytoid dendritic cells (pDCs) can specifically regulate B-cell activation to Toll-like receptor (TLR) 7/8 stimulation. To explore the influence of IFN-α and pDCs on B-cell functions in vivo, studies in non-human primates that closely resemble humans in terms of TLR expression on different subsets of immune cells are valuable. Here, we performed a side-by side comparison of the response pattern between human and rhesus macaque B cells and pDCs in vitro to well-defined TLR ligands and tested whether IFN-α enhanced B-cell function comparably. We found that both human and rhesus B cells proliferated while pDCs from both species produced high levels of IFN-α in response to ligands targeting TLR7/8 and TLR9. Both human and rhesus B-cell proliferation to TLR7/8 ligand and CpG class C was significantly increased in the presence of IFN-α. Although both human and rhesus B cells produced IgM upon stimulation, only human B cells acquired high expression of CD27 associated with plasmablast formation. Instead, rhesus B-cell differentiation and IgM levels correlated to down-regulation of CD20. These data suggest that the response pattern of human and rhesus B cells and pDCs to TLR7/8 and TLR9 is similar, although some differences in the cell surface phenotype of the differentiating cells exist. A more thorough understanding of potential similarities and differences between human and rhesus cells and their response to potential vaccine components will provide important information for translating non-human primate studies into human trials.
Keywords: adjuvants, B cells, CpGDNA, dendritic cells, plasmacytoid, Type I interferon/toll-like receptors
Introduction
Human plasmacytoid dendritic cells (pDCs) via their high secretion of type I interferon (IFN), have a unique capacity to enhance B-cell activation in response to specific toll-like receptor (TLR) ligand stimulation.1–4 Using in vitro culture systems, pDCs were shown to both synergize with and substitute for CD4 T-cell help during TLR-mediated stimulation of human B cells into IgM-producing cells.3,5 In addition, mouse models revealed that direct type I IFN-mediated B-cell activation significantly augments the quality and magnitude of anti-viral humoral responses.6,7 Also, IFN-α induced by virus infection,8 or administered together with soluble protein antigen, increases antigen-specific antibody responses.9 Given their unique capacity to produce high levels of type I IFN, it has been suggested that pDCs play an important role in regulating the development of humoral immune responses during infection and in response to some types of vaccines. As human candidate vaccines are often evaluated in non-human primates and synthetic TLR ligands are under consideration as components of vaccine adjuvants,10–12 we sought to directly compare the responsiveness of pDCs and B cells to selected TLR ligands. The TLRs represent a group of pattern recognition molecules expressed on distinct immune cells for sensing infections.13 Although incompletely documented, non-human primates appear to possess subpopulations of dendritic cells (DCs) and B cells that are similar to those present in humans.14,15 Non-human primates are therefore valuable for studies aimed at investigating immune responses induced by human pathogens and vaccine components aimed for human use.16,17 Several reports indicate that TLR ligands show potency as vaccine adjuvants when tested in rhesus macaques18–20 or in human clinical trials.21–23
Subsets of human DCs and B cells express distinct repertoires of TLRs and they respond to TLR stimulation accordingly.2,24,25 Unlike rodents, rhesus macaques express a similar repertoire of TLRs on immune cells such as DCs and B cells as humans.26 Some differences between the human and rhesus macaque immune systems have been reported.17 An improved understanding about similarities and disparities between human and non-human primate immune functions is therefore important and would provide valuable information for translating non-human primate studies for the design of clinical trials aimed at testing new vaccine and treatment strategies.
In this study, we performed a side-by side comparison of the phenotypes of human and rhesus DCs and B cells and we examined their responsiveness to well-defined ligands targeting TLR3, 7/8, and 9. We further asked if IFN-α comparably enhanced B-cell functions such as proliferation and differentiation into antibody-producing cells as observed in culture systems of human cells. We found similar responses in human and rhesus primary cell cultures to TLR ligand stimulation in terms of B-cell proliferation and induction of IFN-α production by pDCs. In both species, B-cell proliferation to the TLR7/8 ligand (-L) and CpG class C showed a significant increase in the presence of IFN-α. Some phenotypic differences between human and rhesus B cells were observed as the cells differentiated into antibody-producing cells, although in both species TLR stimulation promoted maturation of B cells into IgM-producing cells and this effect was enhanced in the presence of IFN-α.
Materials and methods
Animals
Untreated and healthy rhesus macaques of Chinese origin, 5–6 years old, were housed in the Astrid Fagraeus laboratory at the Swedish Institute for Infectious Disease Control. Housing and care procedures were in compliance with the provisions and general guidelines of the Swedish Animal Welfare Agency. All procedures were approved by the Local Ethical Committee on Animal Experiments. The animals were housed in pairs in 4-m3 cages and enriched daily. All blood samplings were performed under sedation with ketamine at 10 mg/kg (100 mg/ml Ketaminol; Intervet, Sollentuna, Sweden). All animals were confirmed negative for simian immunodeficiency virus, simian T-cell lymphotropic virus, and simian retrovirus type D.
Isolation of B cells
Rhesus macaque peripheral blood mononuclear cells (PBMCs) were separated from EDTA blood by Ficoll-Paque Plus (GE Healthcare Biosciences AB, Uppsala, Sweden) as described previously.27,28 The cells were used freshly for experiments or frozen in fetal calf serum (Sigma-Aldrich, Schelldorf, Germany) and 10% DMSO (Sigma-Aldrich), and stored at – 150°. Frozen PBMCs were thawed and rested overnight in medium at 37°. Cell viability was > 90%. Rhesus B cells were isolated by magnetic bead separation using CD20 microbeads on an AutoMacs (Miltenyi Biotec, Bergisch Gladbach, Germany). Human PBMCs were obtained from healthy blood donors by collection of buffy coats. Human B cells were isolated from buffy coats by magnetic bead separation on an AutoMacs using CD19 microbeads (Miltenyi Biotec) as described previously.2,29 The purity was > 98% and > 85% for sorted human and rhesus B cells, respectively, as determined by staining for CD20 (clone 2H7), CD27 (clone M-T271), CD3 (clone SP34) and CD14 (clone TUK4) (all BD Pharmingen, San Jose, CA) (Fig. 1a). Propidium iodide staining (Sigma-Aldrich) was used to monitor cell viability.
Figure 1.
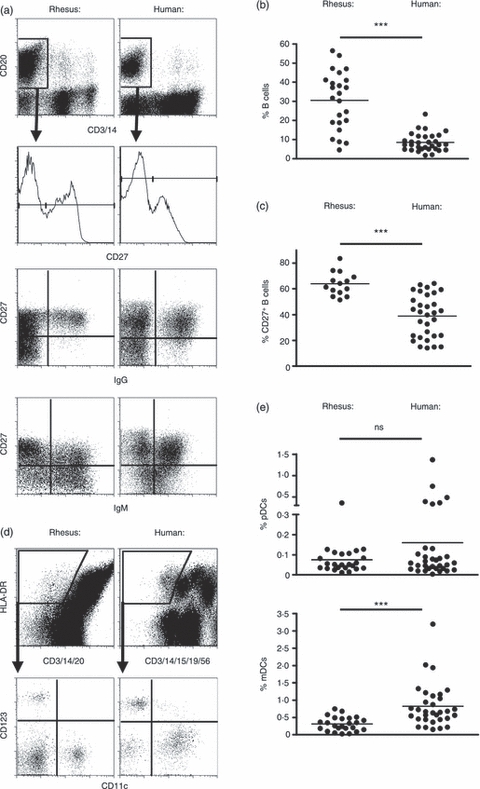
B cell and dendritic cell (DC) subsets can be identified using the same markers in humans and rhesus macaques. (a) B cells were characterized based on their expression of CD20. Memory and naive B cells were further distinguished by their expression of levels of CD27 (high for memory B cells, low for naive B cells) in both human and rhesus macaques. Graphs show individual data of the percentages of B cells out of total peripheral blood mononuclear cells (PBMCs) (b) and percentages of CD27+ memory B cells out of total B cells (c). (d) DCs in both human and rhesus macaques were identified using exclusion of lineage markers, positive expression of MHC class II (HLA-DR) and CD123 and CD11c expression for plasmacytoid DCs (pDCs) and myeloid DCs (mDCs), respectively. Flow cytometry plots of representative stainings are shown. (e) Graph shows data obtained from individual donors/animals on the percentages of pDCs and MDCs out of total PBMCs.
Characterization of rhesus and human B-cell and DC subtypes
To determine the percentage of myeloid DCs (mDCs) and pDCs of the total PBMCs, rhesus PBMCs were stained with HLA-DR (clone L243), CD11c (clone S-HCL-3), CD123 (clone 7G3) (all BD Pharmingen) and the lineage markers CD3 (clone SP34), CD14 (clone TUK4) and CD20 (clone 2H7). Human PBMCs were stained with the same antibody for HLA-DR, CD11c and CD123 and for the lineage markers CD3 (clone SK7), CD14 (clone TUK4), CD15 (clone MMA), CD19 (clone 4G7) and CD56 (clone NCAM16.2), (all BD Pharmingen). After 20 min, the cells were washed and resuspended in PBS containing 1% paraformaldehyde. The cells were analysed by flow cytometry (FACSCalibur, BD Biosciences) and data were evaluated using FlowJo software (Treestar Inc., San Carlos, CA). The mDCs and pDCs were identified as described.15 The phenotype of naive and memory B cells was characterized as described3,27,30 using staining for CD27 (clone M-T271), IgG (clone G18-145) and IgM (clone G20-127) (all BD Pharmingen).
Cell stimulation
For stimulation of cells, the following TLR ligands were used; TLR3: the dsRNA complex polyinosinic : polycytidylic acid (poly(I : C), Sigma-Aldrich); TLR7/8: the imidazoquinoline compound (3M-012)31 referred to as TLR7/8-L (3M Pharmaceuticals, St. Paul, MN); TLR9: CpG ODN 2336 (CpG A), CpG ODN 10103 (CpG B); and CpG ODN 2395 (CpG C) (Coley Pharmaceutical Group, Ottawa, Canada).32 The contaminating endotoxin levels were ≤ 0·0125 ng/ml in all TLR ligands as measured using a Limulus amoebocyte lysate assay. Rhesus or human PBMCs were cultured at 1 × 106 to 2 × 106 cells/ml in 96-well plates or in polystyrene round-bottom tubes in complete medium (RPMI-1640 containing 10% fetal calf serum, 2 mm l-glutamine, 100 U/ml penicillin, 100 μm streptomycin (all from Sigma-Aldrich) and 1% HEPES (Gibco, Invitrogen, Carlsbad, CA). The cultures were analysed for proliferation by carboxyfluorescein diacetate N-succinimidyl ester (CFSE) dilution and thymidine incorporation after 5–6 days. Where indicated, human cells were stimulated in the presence of human IFN-α (1000 U/ml; PBL Biomedical Laboratories, Piscataway, NJ) and rhesus cells with universal type I IFN (1000 U/ml; PBL Biomedical Laboratories). To support viability in the rhesus B-cell cultures, IL-2 (100 ng/ml, PeproTech, Rocky Hill, NJ) and B-cell activation factor of the tumour necrosis factor family (BAFF; 100 ng/ml, PeproTech) were added to the rhesus cultures in the experiments where differentiation and antibody production were measured.
B-cell proliferation assays
Human and rhesus PBMCs were labelled with 0·25 μm CFSE (Molecular Probes, Eugene, OR) for 7 min at 37° and thoroughly washed with complete medium as described elsewhere.2,3 Using the conditions described above 2 × 106 cells/ml were cultured at 37° in polystyrene round-bottom tubes in complete medium. TLR ligands were used at 1 μg/ml (Poly I:C and TLR7/8-L) and 5 μg/ml (CpG classes), optimal concentrations of each ligand that caused peak B-cell activation. Proliferation was measured by flow cytometry and data were analysed using FlowJo software. Live cells were gated on by exclusion of propidium iodide staining. B cells were gated based on expression of CD20 and CD19 for rhesus and human B cells, respectively, and lack of CD3 and CD14. Alternatively, proliferation was measured by thymidine incorporation where PBMCs or B cells were cultured in 96-well plates and pulsed with [3H]thymidine (1 μCi/well, Amersham Bioscience, GE Healthcare Biosciences AB, Uppsala, Sweden) for 16 hr after 4 days of culture. The level of incorporation of [3H]thymidine was measured by a 1450 MicroBeta PLUS counter (Wallac, PerkinElmer Sverige AB, Upplands Väsby, Sweden) and expressed as counts per minute (c.p.m.).
Measurement of IFN-α production
Human or rhesus PBMCs at 6 × 106 cells/ml were exposed to the TLR7/8-L (1 μg/ml) or CpG ODN class C (5 μg/ml) for 1 hr at 37° in polystyrene round-bottom tubes, followed by an additional 10 hr in the presence of the secretion inhibitor Brefeldin A (10 μg/ml; Sigma-Aldrich) and then stained as described previously.33,34 Briefly, the cells were fixed and permeabilized for 15 min using a BD Cytofix/Cytoperm kit (BD Pharmingen). The cells were then washed twice and stained with antibodies specific for IFN-α (clone MMHA-11, PBL Biomedical Laboratories), CD3, CD14, CD20, CD123, HLA-DR (antibodies as described above). The cells were analysed by flow cytometry. In addition, IFN-α levels in the supernatants of cells exposed for 24 hr to the TLR ligands were measured by ELISA (Mabtech, Stockholm, Sweden) performed according to the manufacturer's instructions.
Analysis of B-cell differentiation and antibody production
Phenotypic differentiation of B cells was assessed for up to 6 days of culture by flow cytometry using antibodies against CD20, CD27, IgG and IgM (all BD Pharmingen). Expression of IgG and IgM was assessed by intracellular staining using the BD Cytofix/Cytoperm kit before staining. The presence of IgM and IgG in the supernatants from B cells harvested after 6 days of the indicated culture conditions was detected by ELISA. Briefly, 96-well Nunc Maxisorp microtitre plates (Nunc A/S, Roskilde, Denmark) were coated with 1 μg/ml purified goat anti-human IgM (Jackson ImmunoResearch, West Grove, PA). After washing with PBS containing 0·05% Tween and blocking with PBS supplemented with 2% milk, standards and supernatants of the cultured cells at different dilutions were added to the plates and incubated for 2 hr at 37°. The plates were then washed and incubated with biotin-conjugated isotype-specific secondary antibodies for IgM (Biosource) followed by washing and incubation with streptavidin-horseradish peroxidase (Mabtech). The reaction was developed using o-phenylenediamine dihydrochloride (OPD) in hydrogen peroxide/buffer (SIGMAFAST OPD, Sigma) as a soluble substrate for the detection of peroxidase activity. Substrate reactions were terminated with 2·5 m H2SO4, and the optical density (OD) was read at 490 nm.
Statistical analyses
Statistical analyses were performed using paired or unpaired Student's t-test, Wilcoxon's paired t-test or Mann–Whitney U-test with GraphPad Prism software (*P < 0·05, **P < 0·01, ***P < 0·001, NS = not significant).
Results
Human and rhesus macaque B-cell and DC subsets are identified using similar markers
In our comparison of rhesus macaque and human B-cell and pDC activation, we first assessed the levels of B cell, pDC and mDC subsets in the blood. PBMCs were isolated from healthy blood donors and rhesus macaques, stained and analysed by flow cytometry. As we and others have reported previously, CD20 was used to identify rhesus B cells in place of the classical marker CD19 for human B cells.35,36 Rhesus and human B cells were therefore identified based on expression of CD20 and the absence of CD3 and CD14 expression (Fig. 1a top row). In rhesus macaques, higher percentages of CD20+ B cells of the total PBMC population (mean ± SD 28·3 ± 7·3%) were detected compared with in human PBMCs (8·6 ± 4·7%) (P < 0·0001; Fig. 1b). When the percentages of CD19+ B cells were assessed in the human samples, the levels of CD20+ B cells were still higher in rhesus (data not shown). The CD20+ B-cell population was further characterized based on the level of CD27 expression to distinguish CD27+ memory and CD27− naive B cells. CD27 is a commonly used marker for human memory B cells2,37 but was recently also shown to identify rhesus memory B cells.30 The proportion of memory CD27+ B cells (of total B cells) was higher in the rhesus B cells (63·95 ± 9·06%) compared with human cells (38·87 ± 16·84%) (P < 0·0001) (Fig. 1c). To further detail the memory and naive B cells, we evaluated the expression of surface IgG and IgM. As expected for B cells with a memory phenotype, IgG+ B cells were almost exclusively observed in the CD27+ population. In contrast, IgM+ cells were found both in the CD27+ and CD27− B cell populations. This pattern was similar for rhesus and human B cells.
For staining of pDCs and mDCs, expression of MHC class II (HLA-DR) and the lack of a number of lineage markers were used to identify total DCs as described15 (Fig. 1d). Some of the lineage markers used for rhesus macaque cells differed from those used for human cells. CD20 replaced CD19 for staining of rhesus B cells as mentioned above. CD56 was excluded from the rhesus staining panel because it is expressed both on rhesus natural killer cells and on subpopulations of monocytes and mDCs.14,15,38,39 The total DC population was further subdivided into mDCs and pDCs based on their expression of CD11c and CD123, respectively (Fig. 1d). For these stainings, the same clones of antibodies worked well for both human and rhesus DCs. We found no significant difference in the percentage of rhesus pDCs (0·07 ± 0·06%) and human pDCs (0·16 ± 0·28%) of total PBMCs (P = 0·145) (Fig. 1e). In contrast, the percentage of rhesus mDCs (0·31 ± 0·19%) was lower than of human mDCs (0·83 ± 0·63%) (P = 0·0003). These levels are comparable to values reported in previous studies.14,15,26,40,41
Rhesus and human B-cell proliferation in response to TLR ligands
We next compared the proliferative response of rhesus and human B cells to selected TLR ligands (TLR3, 7/8, 9 ligands) in vitro. We first analysed the proliferation of B cells in total PBMC cultures induced by the three distinct classes of CpG ODN (A, B and C), the imidazoquinoline compound 3M-0012 referred to as TLR7/8-L binding both TLR7 and TLR8, and polyI:C binding TLR3. Proliferation was measured using thymidine incorporation at day 5 of culture. Both human and rhesus B cells express TLR8 and TLR9 but not TLR3 and TLR7.26,42 According to this expression pattern, we observed that all the CpG classes and TLR7/8-L induced significant proliferation compared with unstimulated cultures in both the rhesus and human culture systems (Fig. 2a,b). In contrast, poly I:C did not induce proliferation. CpG class B and C as well as TLR7/8-L induced the strongest proliferation both in rhesus and human cultures. However, while CpG C was significantly more potent in its ability to induce proliferation in rhesus cultures than the other ligands, CpG B was superior in the human cultures, consistent with previous reports.2,43 The proliferative response was also examined using CFSE dilution allowing us to determine the identity of the proliferating cells (Fig. 2a,b, histograms). The vast majority of cells that divided within the PBMCs were found to be CD20+ and CD19+ B cells in the rhesus and human cultures, respectively (data not shown), indicating that mainly B cells proliferated in response to these TLR ligands. In general, rhesus B cells showed lower proliferative capacity compared with human B cells, as found by both detection methods. Human B cells also exhibited somewhat higher spontaneous proliferation in the unstimulated cultures. Taken together, we concluded that rhesus macaque and human B cells proliferated in response to the same TLR ligands, with only CpG B and CpG C displaying a difference in rank order.
Figure 2.
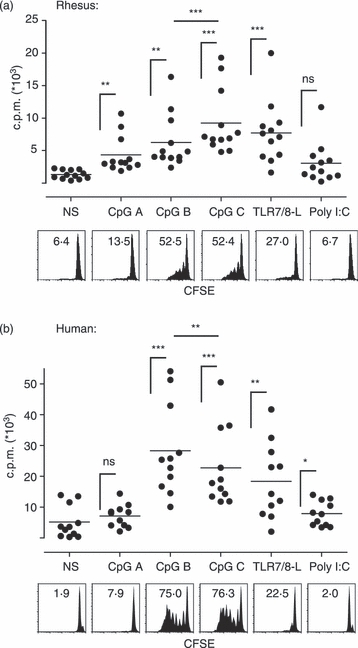
Human and rhesus B cells proliferate in response to Toll-like receptor 7/8 (TLR7/8) and TLR9 but not TLR3 ligation. Total rhesus (a) and human (b) peripheral blood mononuclear cells (PBMCs) were cultured in the presence of indicated TLR ligands. Proliferation was measured by thymidine incorporation after 5 days (top panels). Values are indicated in counts per minute (c.p.m.). The proliferation was also analysed using carboxyfluorescein succinimidyl ester (CFSE) dilution after 6 days (lower panels) gating on B cells lacking CD3 and CD14 expression and expressing some degree of CD20 or CD19 in the rhesus and human cultures, respectively. Numbers indicate percentages of proliferating B cells.
IFN-α produced by TLR7/8-L and CpG C stimulation of rhesus and human pDCs
Type I IFNs (IFN-α/IFN-β) can be produced in response to microbial products by most cell types through distinct innate signalling pathways, but are made in extraordinarily large amounts by pDCs. Human pDCs secrete high levels of IFN-α in response to TLR7/8-L and CpG class A and C while other cells show no or low detectable amounts of IFN-α.2,3,25,32 Because pDCs are rare cells in the immune system, direct isolation to study these cells in detail requires large volumes of blood. To compare IFN-α secretion in rhesus and human pDCs we therefore used the staining panel presented above for identification of these cells out of total PBMCs. As the objective of the present study was to compare pDC-mediated enhancement of B-cell responses, we only compared the IFN-α production with the ligands that also induce B-cell proliferation, i.e. CpG C and TLR7/8-L here. Hence, PBMCs were stimulated for 12 hr with CpG C or TLR7/8-L, intracellularly stained for IFN-α production in CD123+ pDCs and analysed by flow cytometry. In both rhesus and human cultures, IFN-α-secreting pDCs were detected in response to CpG C and TLR7/8-L. Markedly higher frequencies of producing cells were observed in response to TLR7/8-L (Fig. 3a). No IFN-α expression was detected by flow cytometric intracellular staining in any other cell population than CD123+ pDCs (data not shown). We previously reported that a large proportion of human pDCs display a rapid IFN-α secretion on a per cell basis after TLR7/8-L stimulation and that other stimuli such as virus exposure exhibit delayed kinetics where the IFN-α levels accumulate over time.34 Although virus exposure may be different from stimulation with single TLR ligands, we observed a similar phenomenon where the supernatants from parallel rhesus and human cultures harvested at 24 hr and analysed by ELISA showed that the levels of IFN-α induced by CpG C exceeded the levels found by TLR7/8-L (Fig. 3b). This effect was more pronounced in the human cultures (P = 0·001) than in the rhesus cultures (P = 0·556). When comparing the absolute IFN-α levels between human and rhesus cultures, CpG C was shown to induce higher levels in the human cultures whereas TLR7/8-L induced higher levels in the rhesus cultures (Fig. 3c). Since the detection reagents used in both methods are reported to be cross-reactive between rhesus and human IFN-α, we concluded from these data that although human and rhesus pDCs produce IFN-α in response to both TLR7/8-L and CpG C, the levels and kinetics appear to differ.
Figure 3.
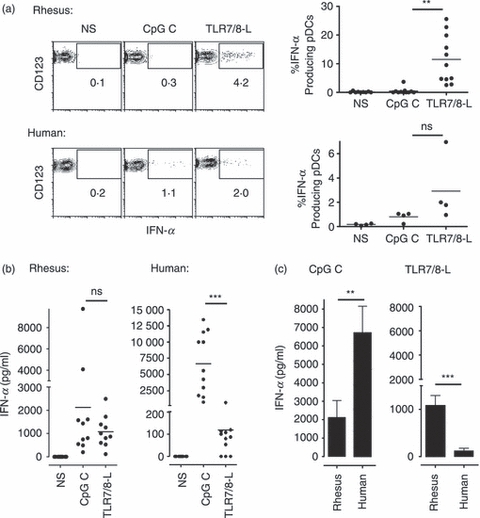
Human and rhesus plasmacytoid dendritic cells (pDCs) produce interferon-α (IFN-α) in response to Toll-like receptor 7/8 (TLR7/8) and TLR9 ligation. Total human and rhesus peripheral blood mononuclear cells (PBMCs) were stimulated with CpG C or TLR7/8-L. (a) IFN-α expression in CD123+ pDCs was measured after 11 hr by intracellular staining and flow cytometry. One representative donor is shown. Data for separate individuals are shown to the right. (b) IFN-α levels in similar cultures were measured by ELISA after 24 hr of stimulation (n = 10 rhesus and n = 11 human). (c) IFN-α levels in response to CpG C and TLR7/8-L were compared between rhesus and human cultures using a Mann–Whitney U-test. Data represents mean ± SEM.
Human and rhesus B-cell proliferation to TLR7/8-L is enhanced by IFN-α
Emerging data indicate that pDCs via production of IFN-α play an important role in shaping the humoral immune response induced by virus infections or vaccination. Human B-cell proliferation and differentiation into antibody-producing plasmablasts in response to TLR7/8 ligation were shown to be significantly augmented by IFN-α produced by pDCs.1,2,4 The increased B-cell responsiveness to TLR7/8 ligation may at least in part be the result of the up-regulation of the cognate receptor TLR7/8 and downstream molecules in the TLR7/8 signalling pathway, such as MyD88 and interferon regulatory factor 7 (IRF-7) in IFN-α-stimulated B cells.1,44 To examine whether IFN-α exerts a comparable effect on rhesus B-cell responses under conditions of TLR7/8 stimulation, rhesus and human B cells were sorted based on their expression of CD20 and CD19, respectively, and stimulated with TLR7/8-L or CpG C in the presence or absence of exogenous IFN-α. The optimal dose of IFN-α for enhancing B-cell proliferation was first determined on human B cells (Fig. 4a) and used for subsequent experiments (1000 U/ml). This is in the same range as the concentration of IFN-α found in TLR-stimulated PBMC cultures. We found that although there was a considerably lower level of proliferation of rhesus B cells than human B cells, there was a clear augmentative effect of IFN-α in both cases (Fig. 4b). As found for human B cells, IFN-α enhanced rhesus B-cell proliferation the strongest in response to TLR7/8-L although there was also a significant effect in response to CpG C. Our data therefore suggest that the presence of IFN-α significantly enhances rhesus B-cell proliferation in response to TLR7/8-L similarly as previously reported for human B cells.
Figure 4.
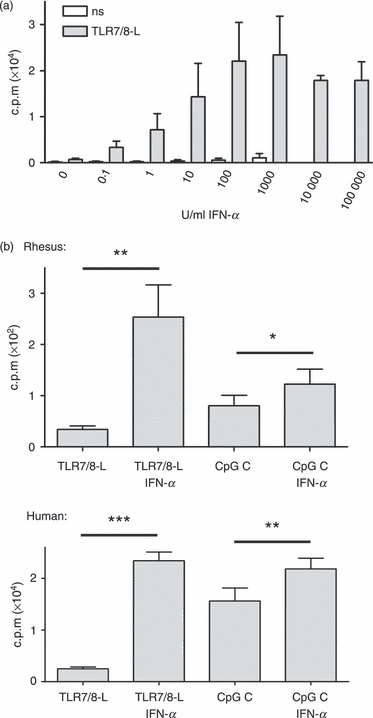
Interferon-α (IFN-α) enhances B-cell proliferation to Toll-like receptor 7/8 ligand (TLR7/8-L) and CpG C both in human and rhesus cultures. (a) Increasing concentrations of IFN-α were added to sorted human B cells with or without TLR7/8-L stimulation. Proliferation was measured by thymidine incorporation after 5 days (n = 5). (b) Sorted human and rhesus B cells were stimulated with TLR7/8-L or CpG C in presence or absence of IFN-α for 5 days and proliferation was analysed (n = 11). Data represent mean ± SEM.
Differentiation markers are different for human and rhesus macaque B cells
We next investigated whether the IFN-α-mediated increased B-cell proliferation led to an increased differentiation into antibody-secreting cells. We and others have previously found that human B-cell differentiation into antibody-producing cells can be defined by up-regulation of CD27 to a distinct CD27high population and that the number of CD27high B cells in the culture strongly correlates with the level of antibody production.2,3 Although it was recently shown that CD27 expression identifies B cells of the memory phenotype in rhesus macaques,30 the presence of CD27high B cells and their potential link to antibody-producing cells were not previously investigated in the rhesus system. To compare the phenotypic differentiation of human versus rhesus B cells, we stimulated B cells with TLR7/8-L and CpG C in the presence or absence of IFN-α and analysed the cells for a series of markers. As expected in the human cultures, a distinct population of CD27high B cells was observed in response to CpG C treatment but not in response to TLR7/8-L alone (Fig. 5a). However, when the B cells were treated with IFN-α together with TLR7/8-L a significant fraction of the B cells differentiated into CD27high cells. In contrast, no CD27high B-cell population was observed in the rhesus cultures in response to any of the stimulation conditions (Fig. 5b). Another indicator of human B-cell differentiation is the loss of CD20 expression together with the up-regulation of CD38.45 In the human cultures, we found that there was a slight up-regulation of CD20 when analysing the total CD20 expression in the culture, which depended on the potency of the stimuli. In contrast, in the human CD27high B-cell population the expression of CD20 was generally lower than in the rest of the B cells. In the rhesus B cells, a more pronounced and consistent down-regulation of CD20 was observed as a consequence of B-cell activation (Fig. 5b). We have earlier found that up-regulation of CD38 occurs simultaneously with CD27high expression on differentiated human B cells.2,3 This remains to be elucidated for rhesus B-cell activation and would require evaluation of cross-reactivity of antibody clones. Here, we instead focused on the up-regulation of CD27 and down-regulation of CD20 on human and rhesus B cells, respectively, and found that there was a significant increase of the percentage of IgM-expressing cells along with stimulation (Fig. 6a,b). In cultures from both species, addition of IFN-α to TLR7/8-L stimulation led to a twofold to threefold increase in the number of IgM-expressing cells compared with the numbers induced by TLR7/8-L alone (Fig. 6a,b). The number of IgG-expressing cells did not increase in a similar way, which may be because the stimulation conditions used here favoured IgM memory cell activation as previously reported.5,46 In contrast to IgM, the frequencies of IgG-expressing B cells did not correlate with B-cell activation in either of the species. There was a strong correlation between the percentages of IgM+ and CD27high cells in the human B-cell cultures (P < 0·0001) and the percentage of IgM+ and CD20low cells in the rhesus cultures (P = 0·0050) (Fig. 6c,d). Therefore, while identification of CD27high cells is a hallmark for differentiation of human B cells into antibody-producing cells, this does not determine differentiation of rhesus B cells. In contrast, down-regulation of CD20 and up-regulation of IgM were shown to be useful for rhesus B-cell differentiation. Importantly, although there were disparities in the differentiation markers between human and rhesus plasmablasts, B-cell differentiation in response to TLR7/8-L stimulation was significantly enhanced by IFN-α in both human and rhesus B-cell cultures.
Figure 5.
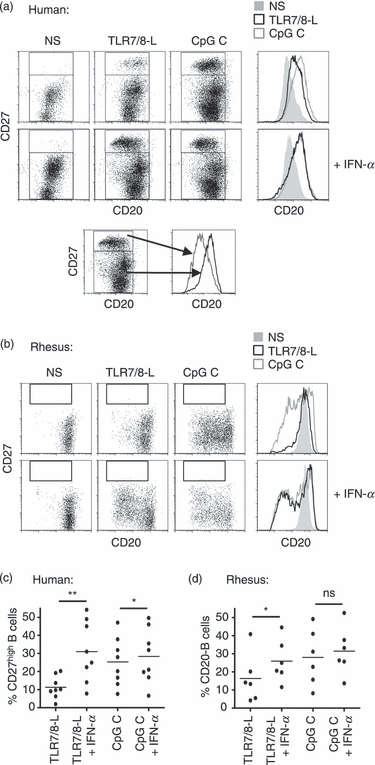
Discrepancy of human and rhesus B-cell differentiation markers. (a) Sorted B cells were stimulated with Toll-like receptor 7/8 ligand (TLR7/8-L) or CpG C in the presence or absence of interferon-α (IFN-α) for 5 days. Differentiation to plasma cells was measured by flow cytometry using high expression of CD27 in human cells. CD20 expression is also depicted in the histograms and below, CD20 histograms are shown from CD27high versus the rest of the B cells. (b) The same staining was applied for rhesus cells. Graphs show data obtained from different individuals of (c) CD27high human B cells or (d) rhesus B cells with low expression of CD20.
Figure 6.
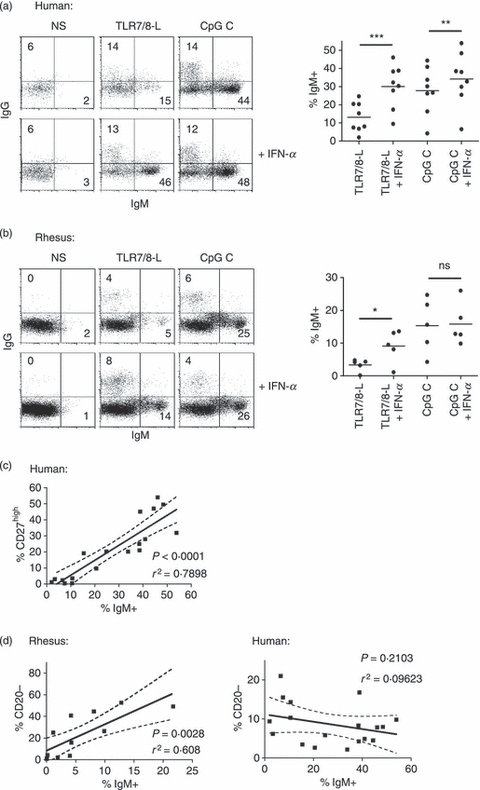
IgM expression in human and rhesus B cells as a measure of differentiation. (a, b) Differentiation to plasma cells was measured using IgG and IgM stainings in both human and rhesus macaque cells, respectively. Compiled data of percentage of IgM-expressing B cells in human and rhesus are shown to the right. (c) Percentage of CD27high cells are compared with the percentage IgM in human B cells. (d) Percentage CD20− cells are compared with the percentage IgM in rhesus as well as human B cells. The correlation analysis shows compiled data from all the different stimulation conditions used.
IFN-α enhances TLR7/8-L induced antibody production in human and rhesus B cells
To investigate if the human and rhesus B cells defined as plasmablasts in the phenotypic analysis described above were antibody-producing cells, we measured IgM secretion in the culture supernatants. CpG C stimulation induced high levels of IgM in both human and rhesus cultures. The levels produced upon stimulation with TLR7/8-L were lower; however, they were increased in the presence of IFN-α (Fig. 7a,b). For both rhesus and human B-cell cultures, we found strong correlations between the percentages of IgM+ B cells in the culture and the levels of secreted IgM (P < 0·0001) (Fig. 7c). In addition, this was confirmed by strong correlations of the levels of secreted IgM in the human and rhesus B-cell cultures and the percentage of CD27high human B cells and CD20low rhesus B cells, respectively (P < 0·0001) (Fig. 7d). Hence, determining B-cell differentiation based on the IgM markers as well as CD27high and CD20low stainings in human and rhesus B cells, respectively, can be translated to levels of antibody-producing cells. In summary, we conclude from these data that TLR stimulation and specifically pDC-produced IFN-α has the ability to modulate both human and rhesus B-cell responses in terms of proliferation and differentiation into antibody-producing cells.
Figure 7.
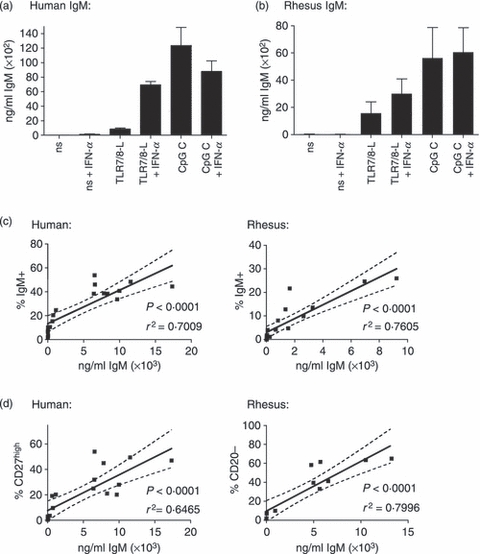
The respective B-cell differentiation pattern in human and rhesus B cells correlate to IgM production. IgM levels were measured by ELISA in supernatants of B cells that were stimulated with Toll-like receptor 7/8 ligand (TLR7/8-L) or CpG C in the presence or absence of interferon-α (IFN-α) for 5 days. Levels of IgM secretion by stimulated human B cells (a) as well as rhesus B cells (b) are shown (n = 3 for human, n = 5 for rhesus). Data represents mean ± SEM. (c) Percentages of IgM+ B cells found by flow cytometry strongly correlate with the IgM levels measured by ELISA in both human and rhesus cultures. (d) Percentage of CD27high B cells in human and CD20low B cells in rhesus, respectively, correlate strongly to IgM levels found in the culture. The correlation analysis includes data points from all the different stimulation conditions.
Discussion
The development and quality of the humoral immune response is to a large extent influenced by the immunological environment of the responding B cell. An expanding body of literature indicates that IFN-α contributes to shaping the adaptive immune responses47,48 and that direct type I IFN-mediated B-cell activation significantly affects the quality and magnitude of the antiviral humoral responses.6–9 We and others previously reported that human pDCs, via their secretion of IFN-α, enhance B-cell responses induced by TLR ligation and/or T helper cell stimulation in vitro.1–4 Compared with mDCs, pDCs have shown less efficiency in presenting antigens to naive T cells and induce cellular immune responses.25,34 However, an increased understanding of the contribution of pDCs in shaping B cell responses is needed, especially with regard to vaccine-induced responses as antibodies are known to provide the protective effect of most successful vaccines. To this end, central questions concern whether pDCs should be specifically targeted and activated by vaccine components. In the last decade, the clinical utility of TLR ligands as vaccine adjuvants and immune stimulatory therapies has evolved as an intensive area of investigation.10,12 Selected TLR ligands are under evaluation for their adjuvant effect both in non-human primate studies18–20 and in human trials21–23 with promising results. As rhesus macaques to a large extent express similar repertoires of TLRs on immune cells as humans do,26 they represent an indispensible in vivo model for testing of TLR ligands. In this study, we found that proliferation of human and rhesus B cells was induced by ligands targeting TLR7/8 and 9 but not TLR3. The different CpG classes, all binding TLR9, are well characterized on human cells in vitro2,32 and to some extent in vitro and in vivo in rhesus macaques.11,40,43,49 We found that CpG B was superior to CpG C at inducing proliferation in human B cells and this effect was inverted for rhesus B cells, which is consistent with previous reports.2,43 CpG B was originally identified to be a particularly potent stimulus of human B cells.50,51 There may be differences in CpG recognition mechanisms among primates making CpG C more efficient in the rhesus system. CpG A, in contrast, induces high amounts of type I IFN from pDCs2,32,40 because of its palindromic CpG phosphodiester sequences with phosphorothioate G-rich ends. The phosphorothioate CpG C with a stimulatory CpG and a palindromic sequence at the 5′ or 3′ end combines the effects of CpG A and CpG B32,52 and may exhibit fewer species-specific features. Regardless of stimuli, a higher level of proliferation was observed for human B cells compared with rhesus B cells by TLR ligand stimulation. This could relate to differences in age, gender and environmental exposures between the human blood donors and the rhesus animals,17 but may also be because the cell culture conditions were originally optimized for human cells. Rhesus and human pDCs in PBMC cultures responded to stimulation by CpG C and TLR7/8-L by production of large amounts of IFN-α at levels comparable to previous reports.26,32,52 This is consistent with the constitutively high expression of IRF-7 reported in both human and rhesus pDCs.41 When IFN-α was measured by ELISA after 24 hr of stimulation, the IFN-α levels in both human and rhesus cultures were higher in response to CpG C compared with TLR7/8-ligand. This may at least in part be because of the higher stability of CpG C, leading to more persistent stimulation. TLR7/8-ligand was shown to be most efficient as an adjuvant when administered in a conjugated form.19,53 Further, we found that IFN-α effectively enhanced B-cell function to TLR7/8 ligation both in human and rhesus B cells. This enhancing effect included proliferation, phenotypic differentiation and induction of IgM secretion. It is therefore plausible that both the human and rhesus immune system have similar regulatory mechanisms for how B-cell responses evolve to virus infections or other conditions engaging TLR7/8 signalling. However, there were marked differences between human and rhesus B cells with regard to alterations of cell surface markers during differentiation. The distinct CD27high populations observed in human B-cell cultures associated with plasmablast formation2,3,45,54 was absent from rhesus B-cell cultures under conditions when both human and rhesus B cells produced increased levels of IgM. Instead, rhesus B cells showed a distinct down-regulation of CD20, which correlated with the levels of IgM production. Hence, CD20 down-regulation may be a useful marker for monitoring rhesus B-cell differentiation. One cannot rule out that the lack of a CD27high plasmablast population in rhesus B-cell cultures reflects a functional difference between the two species. CD27 and its ligand CD70, which is expressed on activated CD4+ T cells, B cells and DCs, play a critical role in T-cell-dependent B-cell responses. CD27 activation was shown to induce antibody production after an initial phase of cellular expansion that involves CD40 : CD40 ligand interactions.55,56 B cells with up-regulated CD27 expression therefore probably possess an increased ability to receive signals via this receptor. The impact of CD27 signalling in B cells on antibody production may therefore be greater in humans compared with in rhesus macaques. CD20 is expressed on almost all B cells and can be targeted by the mAb rituximab. Although this antibody is used for several applications including immunotherapy, knowledge about the biology of CD20 is relatively limited. CD20 has no known natural ligand and CD20 knockout mice display an almost normal phenotype.57 CD20 has been shown to be resident in lipid raft domains of the plasma membrane and may function as a calcium channel following ligation of the B-cell receptor.57 The more pronounced down-regulation of CD20 in activated rhesus B cells may have implications in experimental settings or evaluation of treatment strategies that use antibodies to CD20 for selective depletion of B cells.
The type of adjuvant to be chosen for a certain vaccine depends on the nature of the antigen and the type of immune response required for optimal protection. CpG has been used successfully in clinical trials as an adjuvant to the Engerix-B hepatitis B virus vaccine and an influenza vaccine.21–23 In addition, CpG successfully increased the response to therapeutic vaccination in HIV-infected patients58 and is therefore of interest as an adjuvant for immune-suppressed individuals.10 The use of ligands targeting TLR7/8 may be promising for situations where mDCs and pDCs as well as B cells would be advantageous to directly activate to enhance immune responses including cross-presentation and/or antibody production. Both TLR7/8-L and CpG C have been shown, when administered to rhesus macaques together with an HIV Gag protein, to significantly increase Gag-specific T helper type 1 (Th1) and antibody responses.19,20
The adjuvant effect of several TLR-ligands has been shown to be type I IFN dependent. For example complete Freund's adjuvant and IC31, adjuvants that both include signalling via TLR9, lost their adjuvant effect in mice lacking the IFN-α/β receptor.59,60 Also Poly I:C, when used with a protein-based vaccine in a mouse model, required systemic type I IFN production for its adjuvant activity. Of note, IFN-α production to Poly I:C was TLR-independent and mediated to a large extent by non-haematopoietic stromal cells.61 Therefore, for future adjuvant development, the contribution of both haematopoietic and non-haematopoietic cells needs to be considered in terms of type I IFN production. Although direct IFN signalling on DCs was shown to be central to induce adjuvant effects,60,61 in certain circumstances, adjuvant effects mediated by type I IFN require direct signalling on B cells and T cells.9 Different pathogens may require different types of immune responses to cause protection and so the adjuvant may be chosen accordingly to shape the desired responses.62 The currently most used adjuvant is alum, which functions mainly by induction of humoral responses. Several new vaccines in development are also likely to require effective Th1 immunity to induce protection. Ligation of TLR3, TLR4, TLR7/8 and TLR9 generally elicits Th1 cell responses.62 Therefore, the respective TLR-ligands are promising for use in adjuvant formulations. Considering the potent enhancing effect of IFN-α in our B-cell cultures upon stimulation with TLR7/8-ligand, a combination of TLR7/8-ligand with Poly I:C, which induces systemic IFN-α levels, may be promising. Poly I:C has also been shown to be an effective adjuvant for the induction of potent antibody and Th1 immunity in protein-based vaccines in rhesus macaques63,64 although IFN-α production was not reported in these studies. Further experiments involving studies in rhesus macaques will be required to find optimal adjuvant formulations able to specifically shape protective immune responses to a given pathogen.
In conclusion, the findings reported here contribute to our knowledge about rhesus macaque B-cell responses and support the relevance of using non-human primates for modelling TLR-administration to people. These data will hopefully inform future vaccine design and development of adjuvant strategies.
Acknowledgments
This work was supported by grants from Vetenskapsradet, the Swedish International Development Agency (Sida), the International AIDS Vaccine Initiative (IAVI), the Swedish Governmental Agency for Innovation Systems (Vinnova) and the Swedish Society of Medicine. We are grateful for the assistance of the veterinarians Drs Mats Spångberg and Helene Fredlund, and to the personnel at the Astrid Fagraeus Laboratory at the Swedish Institute for Infectious Disease Control.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–50. doi: 10.4049/jimmunol.174.7.4043. [erratum appears in J Immunol. 2005 May 1;174(9):5884 Note: Berkeredjian-Ding, Isabelle Beatrice [corrected to Bekeredjian-Ding, Isabelle Beatrice]] [DOI] [PubMed] [Google Scholar]
- 2.Douagi I, Gujer C, Sundling C, Adams WC, Smed-Sorensen A, Seder RA, Karlsson Hedestam GB, Lore K. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J Immunol. 2009;182:1991–201. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- 3.Gujer C, Sandgren KJ, Douagi I, et al. IFN-α produced by human plasmacytoid dendritic cells enhances T cell-dependent naive B cell differentiation. J Leukoc Biol. 2011;89:811–21. doi: 10.1189/jlb.0810460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 5.Poeck H, Wagner M, Battiany J, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 6.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–51. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 7.Fink K, Lang KS, Manjarrez-Orduno N, et al. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur J Immunol. 2006;36:2094–105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- 8.Hidmark AS, Nordstrom EK, Dosenovic P, Forsell MN, Liljestrom P, Karlsson Hedestam GB. Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling. J Virol. 2006;80:7100–10. doi: 10.1128/JVI.02579-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–8. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 10.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–96. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann G, Marschner A, Viveros PR, et al. CpG oligonucleotides induce strong humoral but only weak CD4+ T cell responses to protein antigens in rhesus macaques in vivo. Vaccine. 2005;23:3310–7. doi: 10.1016/j.vaccine.2005.01.077. [DOI] [PubMed] [Google Scholar]
- 12.Lore K, Karlsson Hedestam GB. Novel adjuvants for B cell immune responses. Curr Opin HIV AIDS. 2009;4:441–6. doi: 10.1097/COH.0b013e32832da082. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 14.Coates PT, Barratt-Boyes SM, Zhang L, et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–21. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 15.Lore K. Isolation and immunophenotyping of human and rhesus macaque dendritic cells. Methods Cell Biol. 2004;75:623–42. doi: 10.1016/s0091-679x(04)75026-8. [DOI] [PubMed] [Google Scholar]
- 16.Morgan C, Marthas M, Miller C, et al. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 2008;5:e173. doi: 10.1371/journal.pmed.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shedlock DJ, Silvestri G, Weiner DB. Monkeying around with HIV vaccines: using rhesus macaques to define ‘gatekeepers’ for clinical trials. Nat Rev Immunol. 2009;9:717–28. doi: 10.1038/nri2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002;168:1659–63. doi: 10.4049/jimmunol.168.4.1659. [DOI] [PubMed] [Google Scholar]
- 19.Wille-Reece U, Flynn BJ, Lore K, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA. 2005;102:15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wille-Reece U, Flynn BJ, Lore K, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 22.Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22:3136–43. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 23.Halperin SA, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden JJ. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21:2461–7. doi: 10.1016/s0264-410x(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 24.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Lore K, Betts MR, Brenchley JM, et al. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171:4320–8. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 26.Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol. 2008;125:18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Sundling C, Forsell MN, O'Dell S, et al. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J Exp Med. 2010;207:2003–17. doi: 10.1084/jem.20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundling C, O'Dell S, Douagi I, et al. Immunization with wild-type or CD4-binding-defective HIV-1 Env trimers reduces viremia equivalently following heterologous challenge with simian-human immunodeficiency virus. J Virol. 2010;84:9086–95. doi: 10.1128/JVI.01015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams WC, Bond E, Havenga MJ, Holterman L, Goudsmit J, Karlsson Hedestam GB, Koup RA, Lore K. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J Gen Virol. 2009;90(Pt 7):1600–10. doi: 10.1099/vir.0.008342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhrt D, Faith S, Hattemer A, Leone A, Sodora D, Picker L, Borghesi L, Cole KS. Naive and memory B cells in the rhesus macaque can be differentiated by surface expression of CD27 and have differential responses to CD40 ligation. J Immunol Methods. 2011;362:166–76. doi: 10.1016/j.jim.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–68. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer J, Weeratna R, Payette P, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–62. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 33.Lore K, Andersson J. Detection of cytokine- and chemokine-expressing cells at the single cell level. Methods Mol Biol. 2004;249:201–18. doi: 10.1385/1-59259-667-3:201. [DOI] [PubMed] [Google Scholar]
- 34.Lore K, Adams WC, Havenga MJ, Precopio ML, Holterman L, Goudsmit J, Koup RA. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–9. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lore K, Seggewiss R, Guenaga FJ, et al. In vitro culture during retroviral transduction improves thymic repopulation and output after total body irradiation and autologous peripheral blood progenitor cell transplantation in rhesus macaques. Stem Cells. 2006;24:1539–48. doi: 10.1634/stemcells.2005-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–9. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 37.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–6. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 38.Carter DL, Shieh TM, Blosser RL, Chadwick KR, Margolick JB, Hildreth JE, Clements JE, Zink MC. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37:41–50. [PubMed] [Google Scholar]
- 39.Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–14. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abel K, Wang Y, Fritts L, Sanchez E, Chung E, Fitzgerald-Bocarsly P, Krieg AM, Miller CJ. Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells. Clin Diagn Lab Immunol. 2005;12:606–21. doi: 10.1128/CDLI.12.5.606-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung E, Amrute SB, Abel K, Gupta G, Wang Y, Miller CJ, Fitzgerald-Bocarsly P. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin Diagn Lab Immunol. 2005;12:426–35. doi: 10.1128/CDLI.12.3.426-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 43.Teleshova N, Kenney J, Williams V, et al. CpG-C ISS-ODN activation of blood-derived B cells from healthy and chronic immunodeficiency virus-infected macaques. J Leukoc Biol. 2006;79:257–67. doi: 10.1189/jlb.0205084. [DOI] [PubMed] [Google Scholar]
- 44.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 45.Huggins J, Pellegrin T, Felgar RE, et al. CpG DNA activation and plasma-cell differentiation of CD27-naive human B cells. Blood. 2007;109:1611–9. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 47.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–26. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tough DF. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk Lymphoma. 2004;45:257–64. doi: 10.1080/1042819031000149368. [DOI] [PubMed] [Google Scholar]
- 49.Teleshova N, Kenney J, Jones J, et al. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-gamma-secreting simian immunodeficiency virus-specific T cells. J Immunol. 2004;173:1647–57. doi: 10.4049/jimmunol.173.3.1647. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 51.Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164:1617–24. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 52.Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, Rothenfusser S, Endres S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633–41. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 53.Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol. 2005;174:7676–83. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 54.Avery DT, Ellyard JI, Mackay F, Corcoran LM, Hodgkin PD, Tangye SG. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J Immunol. 2005;174:4034–42. doi: 10.4049/jimmunol.174.7.4034. [DOI] [PubMed] [Google Scholar]
- 55.Jacquot S. CD27/CD70 interactions regulate T dependent B cell differentiation. Immunol Res. 2000;21:23–30. doi: 10.1385/IR:21:1:23. [DOI] [PubMed] [Google Scholar]
- 56.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159:2652–7. [PubMed] [Google Scholar]
- 57.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–74. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 58.Sogaard OS, Lohse N, Harboe ZB, Offersen R, Bukh AR, Davis HL, Schonheyder HC, Ostergaard L. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis. 2010;51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 59.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 60.Prchal M, Pilz A, Simma O, Lingnau K, von Gabain A, Strobl B, Muller M, Decker T. Type I interferons as mediators of immune adjuvants for T- and B cell-dependent acquired immunity. Vaccine. 2009;27(Suppl 6):G17–20. doi: 10.1016/j.vaccine.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stahl-Hennig C, Eisenblatter M, Jasny E, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tewari K, Flynn BJ, Boscardin SB, et al. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and alphaDEC-CSP in non-human primates. Vaccine. 2010;28:7256–66. doi: 10.1016/j.vaccine.2010.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]


