Abstract
Classic models suggest maternal tolerance is dependent on regulation of fetal antigen-specific T cell responses. We hypothesize that factors unique to a particular fetal antigen-specific T cell, rather than the state of pregnancy per se, are important determinants of T cell fate during pregnancy. To investigate the fate of fetal antigen-specific CD4 T cells in the systemic circulation, we examined spleen cells in a CD4 T cell receptor transgenic mouse specific for the male antigen H-Y. We observed a transient decrease in CD4+ Vβ6+ cell numbers and, due to transient internalization of CD4, an increase in CD4− Vβ6+ T cells. Antigen-specific in vitro responsiveness was not depressed by pregnancy. These data suggest that pregnancy supports fluidity in this particular CD4 T cell pool that may, in turn, help to meet competing requirements of maternal immune responsiveness and fetal tolerance.
Keywords: CD4, lymphoid tissues, mouse, pregnancy, proliferation, T cell receptor
Introduction
According to classic models the maternal immune system should respond to the fetus because it expresses non-self proteins, and therefore mechanisms must exist to limit or suppress maternal immune function. Recently there has been less emphasis on global suppression, and more focus on the specific regulation of immune responses towards fetal antigen. In particular, recent studies of maternal T cells have identified possible regulatory mechanisms that may be activated by or enhanced during pregnancy.1–4 However, there exists evidence that the maternal immune system can and does respond to fetal antigen,5 although there may be decreased immunologically relevant contact with fetal antigen6,7 and to pathogens existing within the placenta.8 This apparent inconsistency extends to specific T cell subsets. Some studies suggest that fetal antigen-specific CD8 T cells in the maternal circulation can be primed by pregnancy in both mice5 and humans.9 Other studies suggest that these cells are deleted3 or rendered tolerant.10 We hypothesize that a broad range of responses to fetal antigen is possible, which are driven by several factors including the level or form of the inciting fetal antigen, the strength of signal through the relevant T cell receptor (TCR) and the presence of growth or co-stimulatory factors. The presence of maternal antibody to major histocompatibility complex (MHC) suggests that CD4 T cells can participate in anti-fetal responses,11 and this has recently been extended to a very small number of studies using T cell receptor transgenic mice against model antigens expressed on seminal fluid12or fetal tissue.7 However, we are unaware of any studies examining the fine regulation of fetal antigen-specific CD4 T cells during pregnancy. In this study we have examined spleen CD4 T cells of both normal mice and a unique CD4 T cell receptor transgenic mouse13 specific for the male antigen H-Y, which is expressed as early as the blastocyst stage of development.14,15 We found that during pregnancy, these transgenic CD4 T cells become activated and proliferate in response to specific antigen and also transiently modulate the expression of the CD4 co-receptor. These data suggest that during pregnancy this particular T cell pool is dynamic and further suggests a mechanism of fine regulation that helps to maintain the coexistence between fetal tolerance and systemic maternal T cell responsiveness.
Materials and methods
Mice and breeding
CD4 anti-H-Y C57BL/10 RAG-2 KO transgenic mice13 [Marilyn, Vβ6 from Polly Matzinger and Olivier Lantz, National Institutes of Health (NIH), and maintained as an independent strain by E.B.] and normal, non-transgenic C57BL/6 mice were housed under specific pathogen-free and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved housing conditions. The animal use protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Emory University and the University of Vermont. Timed mating was performed as described previously.5 Three days prior to mating, single female mice were transferred into vacant male cages to stimulate oestrous cycling. For timed pregnancies male mice were introduced in the evening, and the next morning female mice were checked visually for the presence of a copulation plug, which was denoted day 0 of pregnancy. Male mice were then removed and plugged female mice were housed in groups of three or four throughout gestation. Mice were euthanized by carbon dioxide treatment followed by cervical dislocation on days 8, 10, 12, 14, 16 and 18 of pregnancy. Multiparous mice, having at least two litters, were also studied.
Isolation of lymphoid cells
Single-cell suspensions were generated from spleen using ‘Ghost special’ medium [Iscove's modified Dulbecco's medium (IMDM), without bicarbonate, with 1% fetal bovine serum (FBS) both from Gibco, Carlsbad, CA] and nylon mesh.
Flow cytometry
Cells obtained from all tissues were counted carefully. Approximately 100 000 or 1 million cells from each of the tissues were placed in individual wells of a 96-well plate or into flow cytometry tubes. Cells were incubated in a 1:50 dilution of 2·4G2 (BD Biosciences, San Jose, CA) in order to block non-specific antibody staining due to Fc receptors. Specific antibodies to the molecules mentioned below were then added. After 30 min, cells were washed in phosphate-buffered saline (PBS) 0·1% bovine serum albumin (BSA) and then analysed by flow cytometry. Antibodies used under saturating conditions in these studies included CD4 (clone GK1·5) conjugated to fluorescein isothiocyanate (FITC), allophycocyanrin (APC) (BD Pharmingen, San Diego, CA) or phycoerythrin (PE)-Texas Red (Invitrogen, Carlsbad, CA); Vβ6 PE (RR4–7, BD), pan TCR-β APC (clone H57, BD), CD69 (H1.SF3 BD), CD44 (IM7 BD), natural killer (NK)1·1 (PK 136, BD), CD25 (7D4, BD), Thy1·2APC (53-2·1, BD), CD8 PE (53-6·7, BD), Ter119 biotin (BD) plus avidin PE cy5 (BD), GR-1 PE (RB6 8C5, BD) or CD11b FITC (RM2801; Caltag Invitrogen, Grand Island, NY).
The samples were then run on an LSR II (BD). Analysis of flow cytometric data was performed using flowjo software (TreeStar, Ashland, OR).
Fluorescence activated cell sorting (FACS) and reverse transcription–polymerase chain reaction (RT–PCR) of sorted cells
To isolate CD4+ Vβ6+ and CD4− Vβ6+ cells, spleens from day 12 pregnant mice and unmated controls were harvested under sterile conditions and single-cell suspensions were generated in IMDM medium containing 10% FBS (Invitrogen Corporation, Carlsbad, CA). After blocking non-specific binding by treatment with 0·5 μm FγIII/II receptor (2·4G2) (BD Biosciences), cells were stained with antibodies to CD4 and Vβ6 for 30 min. After washing with PBS-0·1% BSA, 30 million cells were re-suspended in IMDM medium without serum. The 100 μm tip of the BD FACSAria cell sorter (BD Biosciences) was used for sorting. Nucleated cells were selected by forward- and side-scatter gating, and aggregates were eliminated by doublet discrimination. Double-positive (CD4+ Vβ6+) and single-positive (CD4− Vβ6+) cells were isolated at > 90% purity. Total RNA was extracted from at least 50 000 cells using Trizol reagent (Invitrogen), as per the manufacturer's guidelines. Samples were quantified by ultraviolet (UV) absorbance at 260 nm on a NanoDrop spectrophotometer (ThermoScientific, Wilmington, DE) and RNA integrity was tested using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA).
The iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) was used to synthesize cDNA from 250 ng of RNA template using a mix of random hexamers and oligo-dTs. From each sample, cDNA was used to amplify the following target genes: Cd4 (forward 5′-AAG GGG CAT GGG AGA AAG GAT-3′, reverse 5′-CGG ACT GAA GGT CAC TTT GAA CAC-3′); Cd247 (forward 5′-CCAGGGAAGCAGAAGATGAAGTG-3′, reverse 5′-GGCTGTGATGATGACTCCGTAGAT-3′); Runx1 (forward 5′-CGGAGCGGTAGAGGCAAGAG-3′, reverse 5′-TACTGGTAGGACTGGTCATAGG-3′) and also the housekeeping gene tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (Ywhaz, forward 5′-GC AACGATGTACTTGCTCTTTTGG-3′, reverse 5′-GTCCACAATTCCTTTCTTGTCA TC-3′). Each reaction used 1 μl of cDNA, 150 nm of the forward and reverse primers and 12·5 μl of Power Sybrgreen Master Mix (Applied Biosystems, Carlsbad, CA) in a 25 μl reaction. The reactions were performed on an ABI Prism 7000 (Applied Biosystems) using an initial denaturation of 10 min at 95°, 40 cycles of 15 seconds at 95° and 60 seconds at 60°, followed by a melt curve analysis to ensure that only the correct product was amplified.
Standard curves were generated for all the target genes as well as the housekeeping genes using a single sample, which was serially diluted over the working range of the assay. The relative quantities of each sample were determined using these standard curves. Relative target mRNA values were normalized by dividing the target quantity by the geometric mean of the quantities of the housekeeping gene. Each sample was run in triplicate and averaged. Negative water controls were run for each primer set in the real-time PCR reaction. In each primer set at least one primer was designed over an exon–exon junction.
In vitro re-expression studies
From each sorted population, 100–500 000 cells were placed in IMDM with 10% fetal calf serum (FCS), 100 units/ml penicillin, 100 mg/ml streptomycin (BioWhittaker, Walkersville MD) and 2 mm l-glutamine (BioWhittaker), 0·05 mmβ-mercaptoethanol (Sigma, St Louis, MO) and 0·05 mg/ml Gentamicin (Invitrogen). The cells were washed 36 hr later and stained with antibodies to CD4 and Vβ6. Live cells falling in the lymphocyte by forward- and side-scatter gating were then examined by flow cytometry as above.
Immunofluoresence
Sorted populations of cells were placed on glass slides, air-dried then either stored at −80° or fixed using 4% formaldehyde for 15 min. Slides were washed with PBS and cells were permeabilized with 0·1% Triton X-100 (JT Baker, Phillipsburg, NJ) for 10 min. The slides were washed again and incubated with 10% normal goat serum (Sigma-Aldrich) for 1–3 hr. Further blocking was performed with blocking solutions 1A and 1B (Histomouse™-SP kit Zymed, San Francisco, CA) according to the manufacturer's instructions. Slides were then incubated for 30 min with 1:500 CD4-Texas Red (clone RM4-5; Caltag) or rat IgG2a Texas Red (Caltag) as a control. In other experiments, the slides were blocked only with normal goat serum and then incubated with an antibody to CD3 (1:10 17A2; BD Biosciences or PBS alone as a control) for 1 hr followed by Alexa 488 goat anti-rat (Invitrogen 1:400) for 1 hr. After staining, the slides were mounted with Aqua Mount (Polysciences Inc., Warrington, PA) and examined under an Olympus BX50 Light Microscope (Olympus, Center Valley, PA) with an Optronics MagnaFire digital camera (Goleta, CA).
Proliferation assays
Isolated whole spleen cells from responder pregnant or non-pregnant mice were placed on Histopaque-1077 (Sigma) and the lymphocyte layer was removed and washed in sterile PBS. Cells were examined by flow cytometry and placed in 96-well plates with 200 μl 5% IMDM supplemented with β-mercaptoethanol, l-glutamine, gentamycin, penicillin and streptomycin as above and mitomycin C 50 μg/ml, 1 × 106 cells (Sigma-Aldrich, St Louis, MO). Whole spleen cells from normal C57BL/10 male or female mice were used as stimulators at an effector:stimulator ratio of 1:5. These cultures were incubated for 36 hr, and during the final 12 hr of culture [3H]thymidine was added to a final concentration of 5 μCi/ml. The plates were harvested and incorporation of [3H]thymidine was used as a marker for proliferation. Responder-specific proliferation was calculated as the ratio of proliferation to male versus female cells. Comparisons of response from non-pregnant to pregnant mice were performed after normalization to the percentage of CD4+ Vβ6+ cells in the input culture as determined by flow cytometry.
Statistical considerations
Analysis of cross-sectional data from two or more groups of pregnant and or non-pregnant mice was performed by using the Kruskal–Wallis test followed by Dunn's multiple comparisons test, with significance set at P < 0·05. Isolated analyses used the Mann–Whitney U-test. Data herein are reported as individual values, means with standard error of the mean or as medians with a range, as noted in the figure legends.
Results
CD4 T cells in normal mice
We have observed that the spleen supports the proliferation of several cell types during pregnancy,16 including CD8 T cells in both normal and H-Y-specific CD8 TCR transgenic mice.17 In this examination, we first analysed CD4 T cells in the spleens and uterine draining lymph nodes of normal C57BL/6 females mated to same-strain males. We found that the proportion of CD4 T cells decreases at mid-gestation, due probably to the expansion of non-lymphoid cells expressing an erythroid lineage marker.16 However, the proportion of CD4 T cells in the uterine draining nodes was not altered in normal pregnancy. To characterize further the population of CD4 T cells in these tissues, we examined the expression of the activation markers CD25 and CD44. Figure 1(a) contains representative flow cytometry analysis. In the spleen, the proportion of CD4 T cells expressing CD25 and a low level of CD44 remained stable throughout gestation (Fig. 1b). Pregnancy supported a transient increase in the proportion of CD25-expressing CD4 T cells bearing intermediate levels of CD44. As pregnancy progressed, the proportion of CD4 T cells that were CD25-negative but expressed high levels of CD44 increased in the spleen, which may be consistent with a memory phenotype. By contrast, in the uterine draining nodes, the proportion of CD25-positive CD4 T cells increased only transiently in early–mid-gestation, regardless of CD44 level. This is consistent with previous studies.4 In the uterine draining nodes, the proportion of CD4 T cells expressing high levels of CD44 did not change during pregnancy.
Figure 1.
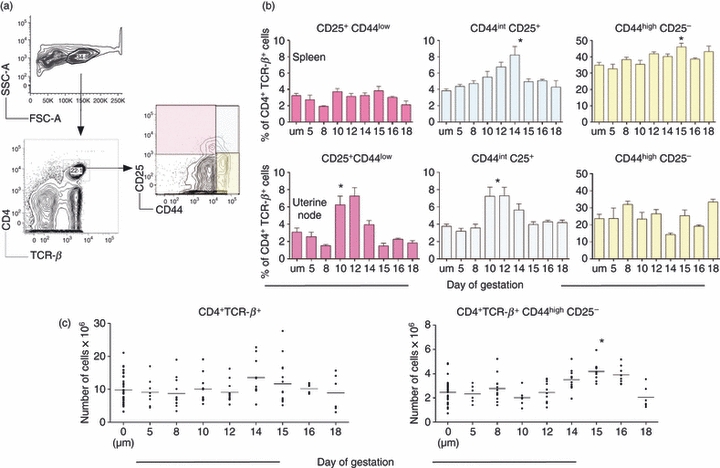
Analysis of CD4 T cells during pregnancy in normal mice. C57BL/6 females were either left naive or mated with same-strain males and euthanized on various days of pregnancy to harvest, analyse and enumerate CD4+ T cells. (a) Example of flow cytometric analysis. Pink quadrant: CD25+ CD44low. Blue quadrant: CD44 intermediate CD25+. Yellow quadrant: CD44high CD25−. (b) Comparison of spleen and uterine draining node CD4+ T cells. Upper panels: spleen. Lower panels: uterine draining nodes. y-Axis: percentage of CD4+ T cell receptor β (TCR-β)+ cells; x-axis: gestational age where ‘um’ denotes unmated mice. Pink bars: percentage of cells falling in pink quadrant by flow cytometry. Blue bars: percentage of cells falling in the blue quadrant. Yellow bars: percentage of cells falling in the yellow quadrant. Each bar represents the mean (± standard error of the mean) of at least five mice. Analysis of the data was performed by Kruskal–Wallis test followed by Dunn's multiple comparisons test. Asterisk: P < 0·05. (c) Enumeration of cells in the spleen. y-Axis: number of cells; x-axis: gestational age; ‘um’ denotes unmated mice. Each symbol represents one mouse. Left: CD4+ TCR-β+ cells. Right: CD4+ TCR+ CD44high CD25− cells. Analysis of the data was performed by Kruskal–Wallis test followed by Dunn's multiple comparisons test. Asterisk: P < 0·05.
The total number of CD4 T cells in the spleens of pregnant animals was not significantly different from those observed in non-pregnant mice (Fig. 1c). However, in late gestation there was a significant but transient increase in the number of activated (CD44high CD25low) CD4 T cells present during the later third of gestation. These data suggested that pregnancy results in the transient activation of a subset of CD4 T cells, while the overall size of the CD4 T cell pool is maintained.
Presence and function of CD4 T cells in H-Y-specific TCR transgenic mice
There are several possible explanations for the observed maintenance of total cell numbers associated with an increase in cells bearing an activated phenotype. These include incomplete activation, rapid activation and proliferation balanced by death, or other subtle regulation of the CD4 T cell pool. To enhance our ability to detect pregnancy-related fluctuations in a particular T cell population, we studied CD4 T cells in a TCR transgenic mouse specific for the male minor histocompatibility antigen H-Y. These mice were bred onto a recombinase 2-deficient background (RAG2KO), so that all the T cells present in these mice are H-Y-specific. H-Y is an antigen expressed by male embryos as early as the blastocyst stage.7 During pregnancy, CD4 T cells in this system would be expected to respond to both non-specific environmental cues (proteins, hormones, etc.) as well as H-Y antigen emanating from male offspring and presented by maternal antigen-presenting cells.
CD4 anti-H-Y TCR transgenic mice were mated and their spleens were harvested on multiple days of gestation and compared to age-matched unmated controls. Spleen T cells were analysed by flow cytometry after incubation with antibodies specific for CD4 and the relevant TCR, Vβ6. The proportion of maternal CD4+ Vβ6+ T cells in the spleen decreased significantly during pregnancy, but this population recovered by 1 week post-partum (Fig. 2). In contrast, the proportion of CD4+ Vβ6+ T cells in the uterine draining nodes was relatively constant throughout gestation.
Figure 2.
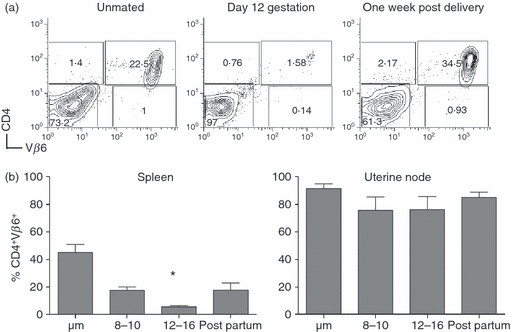
Transient loss of CD4+ Vβ6+ spleen T cells during pregnancy in the spleen of CD4 anti H-Y transgenic mice. (a) Example of flow cytometric analysis. Spleen cells from a never mated (left panel), day 12 pregnant (middle panel) and a post-partum (right panel) mouse were analysed using antibodies to CD4 (y-axis) and Vβ6 (x-axis). (b) Comparison of spleen and uterine draining lymph node. Spleen (left panel, n = 5–8 mice each time-point) and uterine draining lymph nodes (right panel, n = 5–8 mice each time-point) were harvested from pregnant and unmated mice and analysed as in (a). y-Axis: percentage of lymphocytes positive for CD4 and Vβ6; x-axis: time of assay, where ‘um’ refers to unmated mice and ‘post-partum’ refers to mice < 2 weeks from birth of a litter. Asterisk: P < 0·05.
We hypothesized that the proportional decrease in splenic CD4+ Vβ6+ T cells was probably related to an expansion of non-T cells, as was observed in normal mice. Therefore, we examined the number of CD4+ Vβ6+ cells, taking into account the increase in overall cell numbers in the spleen during pregnancy. On days 8–10 of gestation, the number of CD4+ Vβ6+ T cells in the spleen was not statistically different from the number found in unmated mice (Fig. 3a). However, at gestational age 12 the number of T cells dropped significantly and continued to decrease by day 14. By 4–7 days post-delivery, the number of CD4+ Vβ6+ spleen T cells returned to baseline. Moreover, the number of these T cells in the spleens of multiparous females was generally higher than that found in unmated mice.
Figure 3.
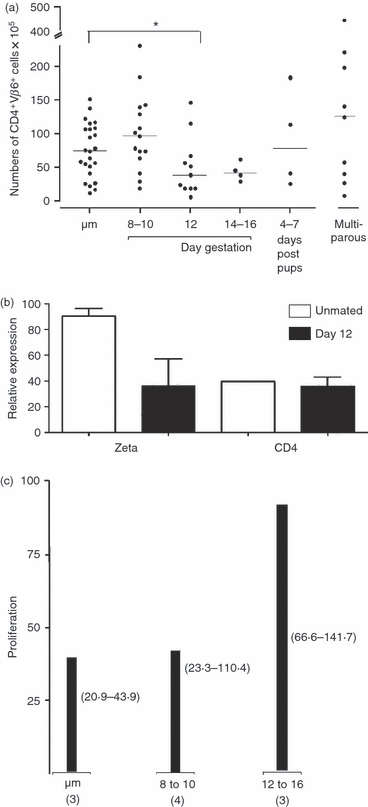
The spleen of CD4 anti-H-Y transgenic mice retains responsiveness despite transient decrease in numbers of CD4+ Vβ6+ spleen T cells during pregnancy. (a) Enumeration of CD4+ Vβ6+ spleen T cells. y-Axis: number of cells; x-axis: status; ‘um’ denotes unmated mice. Each symbol represents one mouse. Asterisk denotes a statistically significant (P < 0·05) difference compared to never-mated mice after application of the Kruskal–Wallis test. (b) Relative expression of T cell receptor zeta and CD4 in CD4+ Vβ6+ CD4 H-Y T cell receptor (TCR) transgenic spleen cells. CD4 H-Y TCR transgenic spleen cells from never mated (white bars, n = 3) and gestational age 12 (black bars, n = 3) were subjected to flow sorting, and the CD4+ Vβ6+ spleen cells (> ∼95% purity) were used to extract RNA which was then used to determine gene expression by quantitative reverse transcription–polymerase chain reaction (qRT–PCR). y-Axis: relative expression; x-axis: gene tested. Comparison of all groups by Kruskal–Wallis test and Dunn's post-test reveals P = 0·24. (c) CD4 H-Y TCR transgenic CD4+ Vβ6+ T cells retain the ability to respond to male cells. y-Axis: specific proliferation of 100 000 CD4 H-Y TCR transgenic spleen cells towards 500 000 male cells divided by the proliferation towards the same number of female spleen cells and by the percent of CD4+ Vβ6+ cells in the input culture as determined by flow cytometry in two experiments. Bars: median values of mice assayed. Numbers in brackets depict the range for the assay; x-axis: gestational age and number of mice assayed.
To investigate the observed changes in the CD4+ Vβ6+ T cell population in the spleen at mid-gestation, we first examined the expression of CD4 and the zeta chain of the TCR complex, both of which have been implicated in T cell signalling. CD4+ Vβ6+ cells were isolated by flow-sorting from spleens of three mid-gestation pregnant mice and three unmated controls, and RNA expression in these cells was analysed by RT–PCR. We observed that CD4+ Vβ6+ T cells from mid-gestation pregnant spleen expressed similar levels of CD4 and the zeta chain compared to cells of the same phenotype isolated from the spleens of unmated controls (Fig. 3b).
In the next experiments, the antigen-specific responsiveness of spleen CD4+ Vβ6+ T cells was determined using an in vitro proliferation assay. Responder spleen cells from pregnant or unmated TCR transgenic females were cultured with stimulator spleen cells from normal C57BL/6 male or female mice (as control). H-Y-specific responsiveness of CD4+ Vβ6+ T cells was determined by calculating the ratio of proliferation against male versus female stimulator cells and normalized to the percentage of CD4+ Vβ6+ T cells input in the culture (Fig. 3c). On days 8–10 of gestation, male-specific proliferation in pregnant samples was similar to unmated mice. On days 12–16 of gestation there was a non-significant trend towards increased H-Y-specific responsiveness in pregnant mice. Using this measure, pregnancy permitted functionality in this specific T cell pool.
Specificity of response in normal mice
The H-Y TCR transgenic mice used in these studies present a unique situation, in that all the T cells they contain are of the same specificity. To examine the behaviour of CD4 anti H-Y cells in a more normal environment, these cells were transferred into normal mice to generate chimeras well before pregnancy (Fig. 4a). Non-transgenic C57BL/6 female mice bearing both the CD45.1 and CD45.2 alleles (‘host’, CD45.2 × CD45.1) were injected with donor cells from CD4 anti-H-Y TCR transgenic mice (CD45.2). These chimeric mice were mated, and on day 12 of gestation the spleens were harvested and compared to naive unmated controls. Examination of the spleen cells present in either the unmated or pregnant mice revealed cells of both host and donor (anti H-Y) origin (Fig. 4b). However, there was a trend towards (P = 0·06) a decrease in the proportion of donor-derived H-Y-specific T cells expressing CD4 compared to non-transgenic T cells (Fig. 4b,c). This suggested that the observed decrease in CD4 expression seen during pregnancy in this population of spleen T cells might be antigen-specific.
Figure 4.
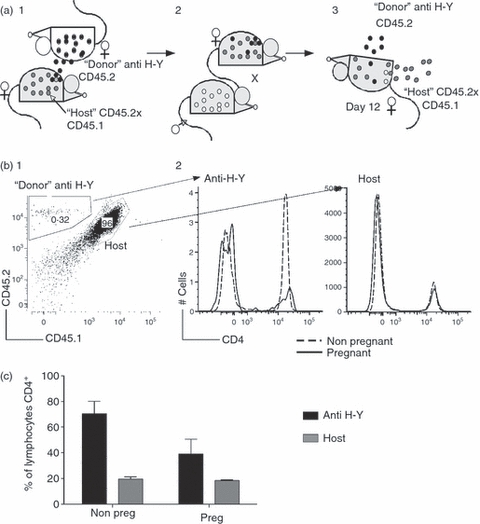
Decrease in the numbers of CD4-expressing cells in the spleen of anti-H-Y T cell receptor (TCR) transgenic mice during pregnancy is antigen-specific. (a) Course of experiment. (1) Normal CD45.2+ CD45.1+ females were injected with CD4 anti-H-Y TCR transgenic RAGKO spleen cells (CD45.2 single-positive, cells presented as black circles) as neonates and allowed to mature to create stable chimeras. (2) Stable chimeras were mated with CD45.1+ males (cells represented as white circles) or left naive. (3) At mid-gestation (day 10), pregnant mice were euthanized and spleens were analysed for the percentage of host (CD45.2+ CD45.1+, grey) or donor (anti H-Y, CD45.2 single-positive, black) lymphocytes expressing CD4. (b) Example of flow cytometry. Spleen cells falling in the lymphocyte gate (not shown) were first analysed for the presence of donor or host markers. These cells were analysed in turn for the surface expression of CD4. (1) Example of spleen from a pregnant chimeric mouse with CD4 H-Y TCR transgenic cells present (∼0·3%) and host cells (96%). A very small proportion of cells bearing only the CD45.1 marker may be of fetal origin. (2) Comparison of CD4 expression in chimeric CD4 H-Y TCR transgenic or host cells. Dotted lines: histograms from a never mated, non-pregnant mouse. Solid lines: histograms from a pregnant mouse. (c) Behaviour of chimeric CD4 anti-H-Y TCR transgenic cells in normal mice. Data are representative of three pregnant and three non-pregnant chimeras. Black bars: anti-H-Y T cells. Grey bars: host T cells. y-Axis: percentage of cell type (donor or host) lymphocytes that are CD4+; x-axis: pregnancy status. Data analysis was performed using two-way analysis of variance (anova), with P = 0·06.
Down-regulation of CD4 in response to antigen or pregnancy in H-Y-specific TCR transgenic mice
The observed decrease in the proportion of transgenic CD4 T cells may reflect a transition in this population from CD4+ Vβ6+ to CD4− Vβ6−. To investigate this possibility, spleen cells from pregnant and unmated transgenic mice were examined for the presence of possible intermediates. First, to determine if exposure to male antigen decreased the surface expression of CD4 in unmated H-Y-specific transgenic mice, these mice were injected with 3 million male cells and 10 days later their spleens examined by flow cytometry. Compared to uninjected mice, mice that received male cells had an increased number of CD4− Vβ6+ cells in the spleen (Fig. 5a). Although the number of male cells injected into unmated mice in these experiments is probably higher than the number found in the circulation of pregnant animals, previous studies have suggested (for examples, see 5,18) maternal immunity is influenced by circulating male fetal cells. To determine if pregnancy confers a similar change, H-Y TCR transgenic mice were mated and euthanized on days 8–10 of gestation and their spleens were analysed for the presence of CD4− Vβ6+ cells. The number of these cells in the spleen was higher in pregnant transgenic mice compared with non-pregnant controls (Fig. 5b). To determine if the appearance of CD4− Vβ6+ T cells was due to a decreased expression of CD4, the RNA expression of the zeta chain of the TCR and CD4 was analysed by RT–PCR. The relative expression of the TCR zeta chain (Fig. 5c, top) was similar in CD4+ Vβ6+ (three pooled samples) and CD4− Vβ6+ (three pooled samples) cells isolated by flow cytometry. There was a non-significant trend towards decreased expression of CD4 in Vβ6+ cells that do not express CD4 on the cell surface (Fig. 5c, middle). However, this did not correlate with increased expression of Runx1 (Fig. 5c, lower), a transcription factor thought to participate in transcriptional down-regulation of CD4.19,20
Figure 5.
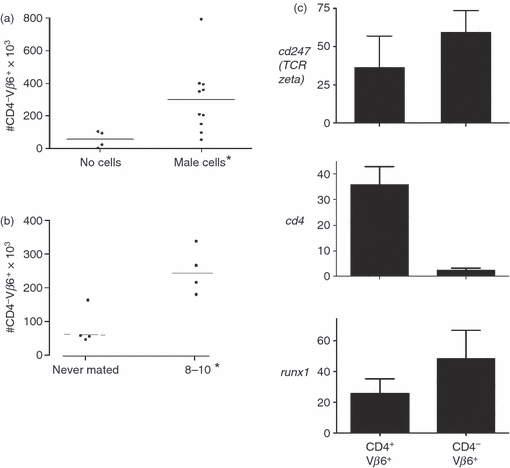
Increase in the number of CD4− Vβ6+ cells in the spleen of pregnant H-Y T cell receptor (TCR) transgenic mice. (a) CD4− Vβ6+ cells in the spleen of non-pregnant mice injected with male cells. Never-mated female H-Y TCR transgenic mice were injected with male cells or not given cells. Ten days later, the mice were euthanized and their spleens harvested and analysed by flow cytometry. y-Axis: numbers CD4− Vβ6+ of cells; x-axis: treatment. Asterisk: P < 0·05. (b) CD4− Vβ6+ cells in the spleen of pregnant and non-pregnant mice. y-Axis: numbers of cells; x-axis: pregnancy status. Asterisk: P < 0·05. (c) Analysis of spleen CD4+ Vβ6+ (double-positive) and CD4− Vβ6+ spleen cells in pregnant mice. Female CD4 anti-H-Y TCR transgenic mice were mated and on day 12 of gestation they were euthanized and the spleens were harvested and subjected to flow sorting as in Fig. 2c. RNA was extracted from three pools each of flow-purified CD4+ Vβ6+ (double-positive) and CD4− Vβ6+ and this was used to determine the relative expression of Cd247 (top panel) Cd4 (middle panel, P = 0·057) and Runx1 (bottom panel). y-Axis: gene examined; x-axis: cell type.
One potential mechanism underlying the decreased surface expression of CD4 may be transient internalization of this molecule into the cytoplasm. To investigate this possibility, spleen cells from pregnant transgenic mice were flow-sorted based on their surface expression of CD4 and Vβ6, and analysed for intracellular CD4 protein by immunohistochemistry. CD4 protein was found to be present in both CD4+ Vβ6+ and CD4− Vβ6+ cells (Fig. 6a). To examine this phenomenon further, CD4+ Vβ6+ and CD4− Vβ6+ cells were placed into culture and after 36 hr CD4 expression was re-examined by flow cytometry. Cells that were initially found to be surface-negative for CD4 re-expressed CD4 after in vitro culture, while cells positive for CD4 retained surface expression (Fig. 6b).
Figure 6.
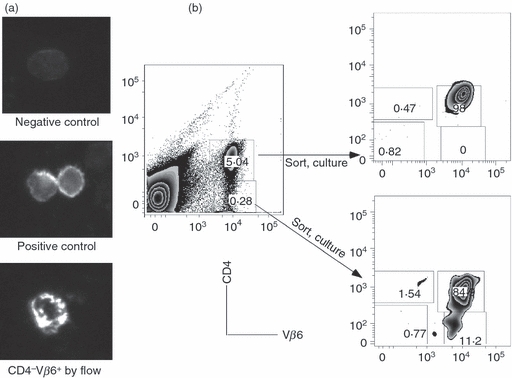
CD4 down-regulation in the spleen cells of CD4 anti-H-Y T cell receptor (TCR) transgenic mice during pregnancy is transient. (a) Intracellular expression of CD4 on CD4 H-Y TCR transgenic spleen cells. CD4 H-Y TCR transgenic spleen cells that were identified as CD4− Vβ6+ or CD4+ Vβ6+ by flow cytometry were placed onto glass slides and analysed by microscopy to determine expression of CD4. Top: Representative cell on negative control slide. Middle: representative CD4+ Vβ6+ cell by flow cytometry. Bottom: representative CD4− Vβ6+ cells by flow cytometry. (b) Re-expression of CD4 in CD4 H-Y TCR transgenic spleen cells in pregnant mice. Left: initial sorting profile. Spleen cells from pooled CD4 H-Y TCR transgenic mice were stained with antibodies to CD4 and Vβ6 and sorted into subpools based on expression of the two markers. Each subpool was incubated in culture medium and then re-examined for expression of CD4 and Vβ6. Right: post-culture analysis of sorted cells. Top right: CD4+ Vβ6+ cells by initial flow cytometry. Bottom right: CD4− Vβ6+ cells by initial flow cytometry.
Discussion
These studies focused on the regulation maternal CD4 T cells in the spleen. We observed a transient increase in the expression of activation markers on CD4 T cells in normal mice during pregnancy. Moreover, we observed a transient decrease in spleen H-Y-specific transgenic CD4 T cells during mid-gestation. These transgenic T cells retained the capacity to proliferate in response to specific antigen in vitro, which agrees with findings in another CD4 TCR transgenic mouse, whose cells proliferate in response to maternally presented fetal antigen at mid-gestation.7 In contrast, expression of antigen in seminal fluid did not elicit early specific proliferation in the spleen CD4 T cell pool.12
We observed an expansion of CD4− Vβ6+ cells in the spleens of H-Y-specific transgenic mice both after injection of male cells and in early pregnancy, and these CD4 down-modulated cells expressed RNA for both CD4 and the TCR. This led us to hypothesize that the observed mid-gestational decrease in the number of H-Y-specific CD4 T cells was related to antigen-induced activation and a transient decrease in the surface expression of the co-receptor CD4 or possibly the TCR, as seen in other systems.10 In these studies we also observed that CD4− Vβ6+ cells express intracellular CD4 (or TCR, data not shown) and can up-regulate rapidly, possibly through rapid protein synthesis or through redistribution to the cell surface.
Down-modulation of CD4 or the TCR is an outcome of activation and a potential mechanism of T cell regulation21,22 in non-pregnant mice. Activation-induced CD4 down-regulation is enhanced by glucocorticoids and can be inhibited by oxidative stress.23 Viruses can down-modulate CD4 expression in human T cells through enhanced endocytosis and intracellular retention of nascent molecules as a method of immune evasion.24 Finally, down-modulation of CD4 in human T cells has been postulated to occur via the actions of TGF-β and support the success of human pregnancy.25
In CD8 T cells, down-regulation of CD8 has also been proposed as a mechanism of peripheral ‘tolerance’26 or of ‘detuning’27 in this T cell population. Such regulation may decrease the avidity of the TCR–MHC+peptide interaction and in turn decrease the sensitivity to activation. This process is regulated by antigen dose28 and may also be related to other factors, such as the cytokine milieu. Such down-regulation is also reversible. In CD8 T cells specific for Listeria monocytogenes,29 a transient decrease in CD8 expression occurred 7 days after infection followed by increased expression after 14 days with the presumed development of a memory cell pool. This time-frame is similar to what we observed in H-Y-specific CD4 T cells in pregnancy. Moreover, while multiple doses of a low-avidity ligand may lead to co-receptor down-regulation and decreased responsiveness in T cells, interaction with a high-avidity ligand may overcome this.27 Although receptor or co-receptor down-regulation may represent a potential mechanism for maternal tolerance in mice10 and in humans,30 our studies suggest that this is a transient phenomenon of pregnancy that does not necessarily inhibit the ability to respond to fetal antigen, at least in culture.
Multiple studies of TCR transgenic mice during pregnancy have generated three common findings. First, the selective loss in utero of fetuses expressing the antigen of interest has not been observed in any transgenic model despite the artificially high frequency of T cells specific for fetal antigens. Secondly, each model shows a variable degree of phenotypic changes in the T cell pool during pregnancy, suggesting that these changes are fetal antigen-specific.3,7,10,31–33 Thirdly, in these models, considerable heterogeneity has been observed in maternal fetal antigen-specific T cells. Even within a given fetal antigen-specific T cell pool, only a fraction of cells studied were tolerant, activated or phenotypically changed, despite what could be a large load of relevant antigen. This variability on a per T cell basis may be due to the local availability of the appropriate antigen-presenting cells.6,7,34 Thus, pregnancy per se does not compel fetal antigen-reactive T cells towards either an up- or down-regulatory pathway.
Pregnancy generates anti-H-Y cytotoxic T lymphocyte (CTL) responses in mice5,35 and humans.18 However, large numbers of gestations (> 8) decrease the ability to reject a male skin graft.36 Because the H-Y response is dependent on CD4 help,37 it is possible that these findings represent the outcome of subtle changes in the CD4 T cell pool that occur over many pregnancies. These changes may be related to altered sensitivity to fetal antigen, migration, life span or the expansion of populations of cells with a regulatory phenotype.2,38–40 In normal mice we observed a transient increase in a population of CD25+ CD4+ CD44 intermediate cells (Fig. 1b) and, moreover, in normal mice there exist data suggestive of the role of ‘regulatory’ T cells in maternal tolerance in general and specifically with regard to H-Y has been suggested.38,41
Although Foxp3 expression can be detected by quantitative RT–PCR (qRT–PCR) (data not shown) in the population of CD4+ Vβ6+ T cells present on day 12 of gestation in the spleen of CD4 H-Y-specific TCR transgenic mice, the robust ability to proliferate in response to male antigen in this pool argues against a preferential expansion of regulatory T cells. Moreover, the CD4 down-regulation described here is unlikely to be related to the actions of a distinct regulatory subset of the CD4+ Vβ6+ T cells remaining in the spleen at this gestational age.42 Thus, down-regulation of CD4 may be a subtle and unique mode of regulation during pregnancy in these mice.
Similar to other models,27,28 we found that CD4 down-regulation can be produced in non-pregnant mice by administration of antigen. A critical question, therefore, is whether the observation of expansion of CD4 down-regulated T cells in pregnant H-Y-specific TCR transgenic mice represents a finding that is unique to pregnancy or the normal functioning of the particular T cell used in our transgenic mice. We found that exogenous delivery of male antigen to non-pregnant H-Y-specific transgenic mice generates a finding similar to that found in early pregnancy. Because it is likely that the amount of antigen (3 million cells) we injected into our non-pregnant mice is higher than that present in pregnant mice on days 8–10 of gestation, we could speculate that pregnancy represents a state of higher and not lower reactivity to antigen. A possible interpretation, therefore, is that these data suggest that pregnancy is a state of enhanced subtle down-regulation during which overall responsiveness is preserved, and a potentially higher but modifiable threshold is set for fetal antigen-specific responses. This would be consistent with our interpretation of the data suggesting that maternal antigen-presenting cells can drive anti-fetal responses, but through inhibited trafficking are limited in their ability to elicit such responses.6,7 In the latter case, the modifiable threshold has to do with regulation of the antigen-presenting cell pool, which exhibits tissue specific and pregnancy-related plasticity in phenotype.16,43,44
An alternative interpretation of these data is that pregnancy is a way to visualize a process that is exactly the same in the non-pregnant state. In this vein, we might speculate that pregnancy does not change the T cell's activation requirements, including reaching an appropriate antigen-presenting cell (dose of antigen, form of antigen), experiencing sufficient strength of signalling through the TCR or receiving co-stimulation. Further, we predict that maternal T cells, just as those in non-pregnant mice, undergo activation by antigen and, in the ‘wrong’ immunological context, down-regulate or die. Thus, maternal tolerance may not be critically dependent on T cell disregulation.45 Nevertheless, it is apparent that the problem of successful pregnancy is solved by complex, overlapping and as yet not fully understood elements that will require increasingly sophisticated models for examination.
Acknowledgments
We thank Juanita Onyekwuluje and Manjula Santhanakrishnan for caring support and technical assistance, Karen Oppenheimer for help with expression studies and Colette Charland of the Flow Cytometry Facility at the Vermont Center for Immunology and Infectious Disease for assistance. We also thank Jon Boyson and Valance Washington for helpful discussions and Karen Fortner for critical review of the manuscript. We finally thank those scientists who understand the continued importance of animal models in the generation of information important to the study of human health.
Disclosures
NIHR01 HD43185 (EAB, PB), T32 AI055402 (MS), P20 RR021905 (the Vermont Center for Immunology and Infectious Disease); the National Science Foundation: NSF 9985780 (Minority Scientist Award to EAB) and the Department of Obstetrics, Gynecology and Reproductive Sciences, University of Vermont, College of Medicine.
References
- 1.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 2.Zenclussen AC, Gerlof K, Zenclussen ML, et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal–maternal interface. Eur J Immunol. 2006;36:82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]
- 3.Vacchio MS, Hodes RJ. Fetal expression of Fas ligand is necessary and sufficient for induction of CD8 T cell tolerance to the fetal antigen H-Y during pregnancy. J Immunol. 2005;174:4657–61. doi: 10.4049/jimmunol.174.8.4657. [DOI] [PubMed] [Google Scholar]
- 4.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 5.Bonney EA, Matzinger P. The maternal immune system's interaction with circulating fetal cells. J Immunol. 1997;158:40–7. [PubMed] [Google Scholar]
- 6.Collins MK, Tay C-S, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–73. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantin CM, Masopust D, Gourley T, Grayson J, Strickland OL, Ahmed R, Bonney EA. Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J Immunol. 2007;179:4383–9. doi: 10.4049/jimmunol.179.7.4383. [DOI] [PubMed] [Google Scholar]
- 9.Cohn M, Epstein R. T-cell inhibition of humoral responsiveness. II. Theory on the role of restrictive recognition in immune regulation. Cell Immunol. 1978;39:125–53. doi: 10.1016/0008-8749(78)90089-8. [DOI] [PubMed] [Google Scholar]
- 10.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T Cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 11.Scott JR, Branch DW. Immunologic disorders in pregnancy. In: Scott JR, DiSaia PJ, Hammond CB, Spellacy WN, editors. Danforth's Obstetrics and Gynecology. 7th edn. Philadelphia, PA: JB Lippincott Company; 1994. pp. 393–426. [Google Scholar]
- 12.Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182:8080–93. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- 13.Lantz O, Grandjean I, Matzinger P, Di Santo P. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–8. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 14.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination, and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 15.Scott DM, Ehrmann JE, Ellis PS, Bishop CE, Agulnik AI, Simpson E, Mitchell MJ. Indetification of a mouse male-specific transplantation antigen, H-Y. Nature. 1997;376:695–8. doi: 10.1038/376695a0. [DOI] [PubMed] [Google Scholar]
- 16.Norton MT, Fortner KA, Bizargity P, Bonney EA. Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol Reprod. 2009;81:457–64. doi: 10.1095/biolreprod.109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norton MT, Fortner KA, Oppenheimer K, Bonney EA. Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology. 2010;131:426–37. doi: 10.1111/j.1365-2567.2010.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James E, Chai J-G, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102:388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 19.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–82. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Zhang F, Kurosu T, Peterlin BM. Runx1 binds positive transcription elongation factor b and represses transcriptional elongation by RNA polymerase II: possible mechanism of CD4 silencing. Mol Cell Biol. 2005;25:10675–83. doi: 10.1128/MCB.25.24.10675-10683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weyand CM, Goronzy J, Fathman CG. Modulation of CD4 by antigenic activation. J Immunol. 1987;138:1351–4. [PubMed] [Google Scholar]
- 22.Hoxie JA, Matthews DM, Callahan KJ, Cassel DL, Cooper RA. Transient modulation and internalization of T4 antigen induced by phorbol esters. J Immunol. 1986;137:1194–201. [PubMed] [Google Scholar]
- 23.Nakamura K, Sasada T, Sono H, Yodoi J. Inhibition of protein kinase C-mediated CD4 down-regulation by oxidative stress in T lymphocytes. J Immunol. 1996;157:5339–49. [PubMed] [Google Scholar]
- 24.Rose JJ, Janvier K, Chandrasekhar S, Sekaly RP, Bonifacino JS, Venkatesan S. CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J Biol Chem. 2005;280:7413–26. doi: 10.1074/jbc.M409420200. [DOI] [PubMed] [Google Scholar]
- 25.Ouellette MJ, Dubois CM, Bergeron D, Roy R, Lambert RD. TGF beta 2 in rabbit blastocoelic fluid regulates CD4 membrane expression: possible role in the success of gestation. Am J Reprod Immunol. 1997;37:125–36. doi: 10.1111/j.1600-0897.1997.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 26.Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- 27.Maile R, Siler CA, Kerry SE, Midkiff KE, Collins EJ, Frelinger JA. Peripheral ‘CD8 tuning’ dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174:619–27. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 28.Ferber T, Schonrich G, Schenkel J, Mellor AL, Hammerling GJ, Arnold B. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 1994;263:674–6. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. J Exp Med. 2007;204:2667–77. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam GK, Whitecar PW, Orton S, Boggess KA, Taylor DD. Differential expression of TcR-CD3 zeta as evidence for altered immunoregulation in preeclamptic versus normotensive women. Am J Obstet Gynecol. 2003;189:843–7. doi: 10.1067/s0002-9378(03)00815-9. [DOI] [PubMed] [Google Scholar]
- 31.Vacchio MS, Ashwell JD. Thymus derived glucocorticoids regulate antigen-specific positive selection. J Exp Med. 1997;185:2033–8. doi: 10.1084/jem.185.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vacchio MS, Hodes RJ. CD28 costimulation is required for in vivo induction of peripheral tolerance in CD8 T cells. J Exp Med. 2003;197:19–26. doi: 10.1084/jem.20021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou M, Mellor AL. Expanded cohorts of maternal CD8+ T cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol. 1998;40:47–62. doi: 10.1016/s0165-0378(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 34.Seavey MM, Mosmann TR. Paternal antigen-bearing cells transferred during insemination do not stimulate anti-paternal CD8+ T cells: role of estradiol in locally inhibiting CD8+ T cell responses. J Immunol. 2006;177:7567–78. doi: 10.4049/jimmunol.177.11.7567. [DOI] [PubMed] [Google Scholar]
- 35.Lengerova A, Vojtiskova M. Postpartum reactivity of female mice to male specific antigens. Folia Biol. 1962;8:21–6. [Google Scholar]
- 36.Breyere EJ, Barrett MK. Prolonged survival of skin homografts in parous female mice. J Natl Cancer Inst. 1960;25:1405–10. [Google Scholar]
- 37.Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992;176:553–64. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. 2010;107:9299–304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mjosberg J, Berg G, Ernerudh J, Ekerfelt C. CD4+ CD25+ regulatory T cells in human pregnancy: development of a Treg–MLC–ELISPOT suppression assay and indications of paternal specific Tregs. Immunology. 2007;120:456–66. doi: 10.1111/j.1365-2567.2006.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+ ‘effector’ regulatory T cells in the gravid uterus. Proc Natl Acad Sci USA. 2007;104:594–9. doi: 10.1073/pnas.0604268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumacher A, Wafula PO, Bertoja AZ, et al. Mechanisms of action of regulatory T cells specific for paternal antigens during pregnancy. Obstet Gynecol. 2007;110:1137–45. doi: 10.1097/01.AOG.0000284625.10175.31. [DOI] [PubMed] [Google Scholar]
- 42.Sukiennicki TL, Fowell DJ. Distinct molecular program imposed on CD4+ T cell targets by CD4+ CD25+ regulatory T cells. J Immunol. 2006;177:6952–61. doi: 10.4049/jimmunol.177.10.6952. [DOI] [PubMed] [Google Scholar]
- 43.Bizargity P, Bonney EA. Dendritic cells: a family portrait at mid-gestation. Immunology. 2009;126:565–78. doi: 10.1111/j.1365-2567.2008.02918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod. 2004;70:1018–23. doi: 10.1095/biolreprod.103.022640. [DOI] [PubMed] [Google Scholar]
- 45.Bonney EA. Preeclampsia: a view through the danger model. J Reprod Immunol. 2007;2:68–74. doi: 10.1016/j.jri.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


