Abstract
Progesterone is the female sex hormone necessary for the maintenance of pregnancy, and is known to modulate macrophage activation. However, studies have concentrated exclusively on the ability of progesterone to negatively regulate the innate and classical pathways of activation, associated with nitric oxide (NO) and interleukin (IL)-12 production. Our aim was to examine the ability of progesterone to modulate alternative macrophage activation. Bone marrow cells were isolated and differentiated from male BALB/c mice, exposed to varying concentrations of progesterone and stimulated with lipopolysaccharide (LPS) (innate activation), IL-4 (alternative activation) or LPS in combination with IL-4. Our present study demonstrates that progesterone not only down-regulates inducible nitric oxide synthase 2 (iNOS) activity in macrophages but also arginase activity, in a dose-dependent manner, independent of the stimuli, whether it is induced by LPS (innate activation), IL-4 (alternative activation) or LPS in combination with IL-4. The ability of progesterone to down-modulate IL-4-induced cell surface expression of the mannose receptor further suggested a negative regulation of alternative macrophage activation by this hormone. Analysis of mRNA expression, by quantitative reverse transcription–polymerase chain reaction (qRT–PCR), of genes associated with innate and alternative macrophage activation revealed that progesterone down-regulated LPS-induced macrophage nos2, argI and p40 (IL-12/IL-23) expression and IL-4-induced argI, mrc-1 and fizz1 expression. However, progesterone up-regulated IL-4-induced macrophage expression of ym1, while dectin-1 expression remained unaltered. Following treatment of macrophages with LPS and IL-4 in combination a similar pattern was observed, with the exception that progesterone up-regulated macrophage expression of fizz1 as well as ym1 and did not modify mrc-1 expression. Our data demonstrate for the first time that a hormone has the ability to regulate selectively the expression of different genes associated with alternative macrophage activation.
Keywords: alternative activation, interleukin-4, macrophages, progesterone
Introduction
Macrophages have been described as having a spectrum of activation states,1 and the plasticity and versatility of macrophages is demonstrated by their ability to adapt their functions as the cytokine environment to which they are exposed changes.2 Nevertheless, it is generally agreed that macrophage activation can be generally described as innate, classical or alternative.3
The innate activation of macrophages is the early response to a microbial stimulus [generally through Toll-like receptor (TLR) ligation] and leads to the production of a range of proinflammatory cytokines and mediators such as tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-12 and nitric oxide (NO). By comparison, classical activation involves the priming of macrophages with interferon (IFN)-γ prior to a second TNF-α-inducing signal such as lipopolysacchride (LPS).4 Classical activation of macrophages (M1 macrophages) is associated with driving the type-1 immune mechanisms, necessary in the defence against a range of pathogens such as Toxoplasma gondii5 and Listeria monocytogenes.6 Alternative activation was first described by Stein et al. (1992)7 and describes the macrophage phenotype (M2 macrophages) induced upon stimulation with T helper type 2 (Th2)-associated cytokines, such as IL-4 and IL-13. Such activation has been associated with wound-healing-type responses through its ability to induce activity of the arginase enzyme.8–10 However, arginase is now known to be also induced following innate stimulation, but inhibited by IFN-γ.11 Many markers of murine alternative activation have now been defined that are not induced by innate stimulation and include the mannose receptor (MR, encoded for by mrc-1),7 dectin-1,12 fizz1 (found in inflammatory zone 1/RELM-α)13 and ym1.3,14,15
MR and dectin-1 are macrophage receptors associated with innate immunity. However, these receptors have also been described as markers of alternative macrophage activation, preferentially up-regulated by exposure to IL-4,7,12,16 and are involved in the innate recognition of fungal and microbial pathogens. In mice, the ym1 gene encodes a 45 000 molecular weight (MW) single-chain polypeptide that is a member of the chitinase family, thought to interact with components of the extracellular matrix.17 Fizz1 is a 9400 MV resistin-like secreted protein that was first identified in an experimentally induced asthma model, and was shown to be able to inhibit nerve growth factor.18 Fizz1 and ym1 have been identified as good markers of alternative activation, with their expression up-regulated by IL-4 and antagonized by IFN-γ/LPS.3,11,15
Macrophages are present within the female reproductive tract and are found in high numbers at the maternal–fetal interface,19 where they may play an important role in the clearance of apoptotic cells.20 The Th2 dominance of the microenvironment surrounding the maternal–fetal interface,21 thought to be established by the constitutive secretion of IL-3, IL-4, IL-5 and IL-10 by cells of the fetal–placental unit during all three trimesters of pregnancy, would be expected to lead to the alternative activation of local macrophages. Indeed, decidual macrophages in human pregnancy exhibit a suppressive phenotype22–24 as well as characteristics associated with alternatively activated macrophages, such as increased expression of the mannose receptor25,26 and stabilin-1 (MS-1), which has been suggested to be involved in the induction of anti-inflammatory pathways.27
Progesterone, the female sex hormone essential for the maintenance of pregnancy, has been demonstrated previously to down-regulate the inflammatory processes associated with innate and classical immunity, and in particular nitric oxide synthase 2 (NOS2) activity.28–30 However, the effect of progesterone on alternative macrophage activation and associated arginase activity,3,31–34 which uses the same substrate (l-arginine) as NOS2, has yet to be investigated. Taking this information into consideration, we hypothesized that progesterone would promote the differentiation of macrophages towards an alternatively activated phenotype, and undertook studies to address this.
Contrary to our hypothesis, our studies demonstrate the ability of progesterone to not only down-modulate NOS2, but also for the first time down-modulate arginase enzyme activity in murine macrophages in a dose-dependent manner. In addition, we present the novel finding that progesterone selectively reduces transcription of a number of genes associated with alternative macrophage activation such as mrc1, but increases transcription of others such as ym1. Consequently, these results demonstrate plasticity in alternative macrophage activation under the influence of progesterone with potential significant consequences for pregnancy.
Materials and methods
Culture of bone marrow-derived (BMD) macrophages
BMD macrophages were prepared from male mice in order to prevent cells being influenced by the cyclical hormone environment present in female mice. Bone marrow cells were flushed from the femurs of 8-week-old male BALB/c mice (maintained at the University of Strathclyde), as described previously.30 The BMD precursor cells were grown for 8 days in Petri dishes containing Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL, Paisley, UK) that had been supplemented with 30% (v/v) l-cell conditioned medium, 20% (v/v) heat-inactivated fetal calf serum (FCS; Sigma-Aldrich, St. Louis, MO), 2 mm l-glutamine (Cambrex BioScience, Veniers, Belgium), 100 U/ml penicillin (Cambrex BioScience) and 100 μg/ml streptomycin (Cambrex BioScience). l-cell conditioned medium provides a source of macrophage colony-stimulating factor (M-CSF) and granulocyte–macrophage (GM)-CSF and was derived from the supernatants of confluent L929 cells.
Treatment of cells
Cells were then harvested and plated out at 106 cell/well in 24-well plates in RPMI-1640 medium (Gibco-BRL) supplemented with 10% (v/v) heat inactivated FCS (Sigma-Aldrich), 2 mm l-glutamine (Cambrex BioScience), 100 U/ml penicillin (Cambrex BioScience) and 100 μg/ml streptomycin (Cambrex BioScience). Progesterone (Sigma, Poole, UK) was dissolved in chloroform (Sigma) at a concentration of 50 mg/ml (160 mm). Dilutions were performed in RPMI-1640 complete medium. Solvent vehicle controls using chloroform was prepared by the same method. Solutions were sterilized using a 0·2 μm MilltexTM filter (Millipore Corporation, Billerca, MA). Cells were stimulated with mouse recombinant IL-4 (100 U/ml; BD PharmingenTM, San Diego, CA) or LPS from Escherichia coli 055:B5 (200 ng/ml; Sigma) for a defined period of time. Cells were then harvested for quantitative real-time polymerase chain reaction (PCR) analysis or for use in arginase assays. Supernatants were collected for analysis of nitrite levels.
Preparation of cDNA
Cells were harvested in 1 ml of Trizol™ reagent (Invitrogen, Paisley, UK) and total RNA isolated according to the manufacturer's instructions. Two μg of total RNA and 300 ng random primers (Promega, Madison, WI) were incubated for 5 min at 65°. The mixture was then incubated for 10 min at room temperature before the addition of 2 μl of AffinityScript™ reverse transcriptase (RT) buffer (Stratagene, Agilent Technologies, Santa Clara, CA), 10 mm dithiothreitol (DTT), 4 mm dNTP mix (Promega) and 1 μl of AffinityScript™ multiple RT (Stratagene). Samples were then incubated for 10 min at 25°, 1 hr at 50° and finally 15 min at 70°.
Quantitative reverse transcription real-time PCR (qRT–PCR) analysis
qRT–PCR experiments were performed and analysed using the Stratagene Mx3000p system. Each reaction contained 10 μl of SYBR® Green JumpStartTM Taq ReadyMixTM (Sigma), 25 pmol of both forward and reverse primers, 8 μl of molecular grade water (Sigma) and 1 μl of cDNA template. Primer nucleotide sequences and associated optimal annealing temperatures are shown in Table 1. Reactions were performed under the following conditions: one cycle at 95° for 10 min and 40 cycles of 1 min at 95°, 1 min at the appropriate annealing temperature and 1 min at 72°. A dissociation curve was generated to ensure amplification of a specific product. This was achieved by subjecting samples to 1 min at 95°, 30 seconds at 55° and 30 seconds at 95°. Relative gene mRNA expression levels were calculated by using an included standard curve for each individual gene and normalized to the housekeeping gene. The maximum gene expression for each experiment was assigned as 100% and all other treatments calculated in comparison to this, similar to previous studies.11 Data are presented as the mean expression ± standard error (SE) for n = 3.
Table 1.
Oligonucleotide sequences of the primers used for analysis of gene expression by real-time polymerase chain reaction (PCR)11
| Primer name | Sequence (5′ to 3′) | Optimal annealing temperature (°) |
|---|---|---|
| argI | Forward TGACATCAACACTCCCCTGACAAC | 61 |
| Reverse GCCTTTTCTTCCTTCCCAGCAG | ||
| dectin-1 | Forward GGAATCCTGTGCTTTGTGGTAGTAG | 64 |
| Reverse GGAAGGCAAGACTGAGAAAAACCTC | ||
| fizz1 | Forward ACCTTTCCTGAGATTCTGCCCC | 64 |
| Reverse CAGTGGTCCAGTCAACGAGTAAGC | ||
| p40(IL-12/IL-23) | Forward CCTGGTTTGCCATCGTTTTG | 62 |
| Reverse TCAGAGTCTCGCCTCCTTTGTG | ||
| mrc-1 | Forward TCTTTTACGAGAAGTTGGGGTCAG | 64 |
| Reverse ATCATTCCGTTCACCAGAGGG | ||
| nos2 | Forward GGTCTTTGACGCTCGGAACTGTAG | 64 |
| Reverse CACAACTGGGTGAACTCCAAGGTG | ||
| Tbp | Forward AACAGCAGCAGCAACAACAGCAGG | 64 |
| Reverse TGATAGGGGTCATAGGAGTCATTGG | ||
| ym1 | Forward GGCTACACTGGAGAAAATAGTCCCC | 64 |
| Reverse CCAACCCACTCATTACCCTGATAG |
Arginase assays
Murine BMD macrophage arginase activity was determined using an assay based on a reaction with α-isonitrosopropiophenon, as described previously.35 Arginase activity was calculated using an internal standard curve generated from known quantities of urea. One unit (U) of arginase activity was defined as the enzyme activity that catalyses the production of 1 μmol urea/min.
Greiss assays for determination of NO production
Quantification of nitrite accumulation, using a method similar to that described previously,36 was used as a measure of NOS2 activity. Greiss reagent, consisting of equal volumes of 2% sulphanilamide in 5% H3PO4 and 0·2% naphylene diamine HCl in water, was added as a volume of 50 μl to a well along with 50 μl of cell supernatant. After incubation for 10 min at room temperature in darkness, absorbance was read at 540 nm. Nitrite production was determined by comparison to a standard curve generated using NaNO2.
Flow cytometry to analyse surface MR expression
After incubation, cells were washed in fluorescence activated cell sorter (FACS) buffer [0·375 g ethylenediamine tetraacetic acid (EDTA), 2·5 g bovine serum albumin (BSA), 500 ml of 1 × concentration phosphate-buffered saline (PBS)] and resuspended in Fc block [purified anti-mouse CD16/CD32 (FcγIII/IIreceptor) IgG2b from BD Pharmingen™]. Samples were incubated at 4° for 20 min. Cells were stained with the pan macrophage marker F4/80 [phycoerythrin (PE)-conjugated rat anti-mouse F4/80 IgG2a (eBioscience, San Diego, CA)] as described previously37 and rat anti-mouse mannose receptor (MR) immunoglobulin G2a (IgG2a) (MR5D3). This primary antibody was developed and kindly provided by Professor Siamon Gordon (University of Oxford).38 Samples were incubated at 4° in the dark for 20 min before washing and incubation with secondary antibody [fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG2a] (STAR113F; AbDSerotec, Kidlington, UK). Cells were centrifuged for 5 min at 200 g, supernatant removed and pellet resuspended in 300 μl of FACS flow (BD Biosciences, San Diego, CA). Isotype controls were set up to gate for background staining using the appropriate isotype-matched antibody [rat IgG2a negative control (MCA1212) from AbDSerotec]. The FACSCanto™ flow cytometer from BD Biosciences was used to perform fluorescence analysis. Compensation controls were set up using CaliBRITE Beads from BD Biosciences. F4/80+ cells were gated on dot-plots and data analysed to show mean fluorescence intensity of these cells using facsdiva software (BD Biosciences).
Statistical analysis
All values are mean ± standard error (SE) of three separate experiments. Statistically significant of differences were calculated using the Mann–Whitney U-test with GraphPad Prism® version 5.0 software. P < 0·05 was accepted as significant.
Results
Progesterone down-regulates arginase activity and nitrite production in a dose-dependent manner
Bone marrow-derived macrophages were treated with a range of concentrations of progesterone and then stimulated with IL-4, LPS or a combination of both, and arginase and nitrite activity measured (Fig. 1). As shown in a previous study from our laboratory,11 IL-4 stimulation induced a small but significant increase in arginase activity (Fig. 1a). Treatment of cells with > 15·625 μm progesterone prior to IL-4 stimulation significantly reduced arginase activity (P < 0·05). Nitrite levels within culture supernatants were undetectable (data not shown). LPS induced a twofold rise in macrophage arginase activity (Fig. 1b), which was significantly (P < 0·05) reduced by the presence of 15·625 μm progesterone. Addition of 31·25 μm progesterone resulted in a further small, but significant, reduction in macrophage arginase activity. Treatment of BMD macrophages with both IL-4 and LPS for 48 hr resulted in a significant increase in arginase activity (Fig. 1c) to a much greater extent than with either stimulant alone, as described previously.11 IL-4 + LPS-induced arginase activity was reduced in a dose-dependent manner by preincubation with progesterone. Culture supernatants from experiments reported in Fig. 1b,c were also analysed for nitrite (Fig. 1d,e). Progesterone induced a significant dose-dependent reduction in macrophage nitrite production induced by either LPS (Fig. 1d) or IL-4 + LPS (Fig. 1e). Arginase activity and nitrite production in stimulated cells were not altered significantly by treatment with the hormone solvent chloroform (data not shown): data were comparable to cells treated with medium alone. The observed effects of the hormone progesterone, or its solvent chloroform, were not due to reductions in cell viability, as determined by measurements of mitochondrial activity by alamarBlue™ (data not shown).
Figure 1.

Progesterone down-modulates arginase activity and nitrite production by bone marrow-derived (BMD) macrophages. Arginase activity (a, b, c) and nitric oxide (NO) production (d, e) in progesterone-treated BMD macrophages that have been subsequently stimulated with interleukin (IL)-4 (100 U/ml) for 48 hr (a), lipopolysaccharide (LPS) (200 ng/ml) for 48 hr (b, d) or both IL-4 (100 U/ml) and LPS (200 ng/ml) for 48 hr (c, e). Open bars represent samples that were treated with progesterone at the stated concentration only. Closed bars represent those samples that were preincubated with progesterone for 16 hr prior to the addition of IL-4, LPS or IL-4 + LPS. Solvent vehicle (0·01–0·04% chloroform) did not result in any significant differences from the medium control (data not shown). Results show mean ± standard error (SE) of n = 3 and are representative of three separate experiments. *P < 0·05 in comparison with stimulated control.
Progesterone down-regulates argI and nos2 gene expression in BMD macrophages
BMD macrophages were treated with progesterone and stimulated with IL-4, LPS or both IL-4 and LPS and real-time PCR carried out to analyse any changes in argI gene expression after 6 or 24 hr of stimulation (Fig. 2). In unstimulated cells, argI and nos2 mRNA levels did not change over the 24-hr period, and both progesterone and chloroform had no significant effect on these levels. IL-4 stimulation induced an increase in macrophage argI expression after 6 hr (Fig. 2a), which was maintained at 24 hr (Fig. 2b). By comparison, LPS induced the maximum levels of argI expression only after 24 hr of stimulation (Fig. 2c,d). Stimulation with both IL-4 and LPS induced arg I expression after 6 hr, but to a lesser extent than with IL-4 alone (Fig. 2e). Progesterone significantly reduced expression of argI mRNA transcripts in IL-4, LPS and IL-4 and LPS-treated macrophages at the 24-hr time point (Fig. 2b,d,f).
Figure 2.
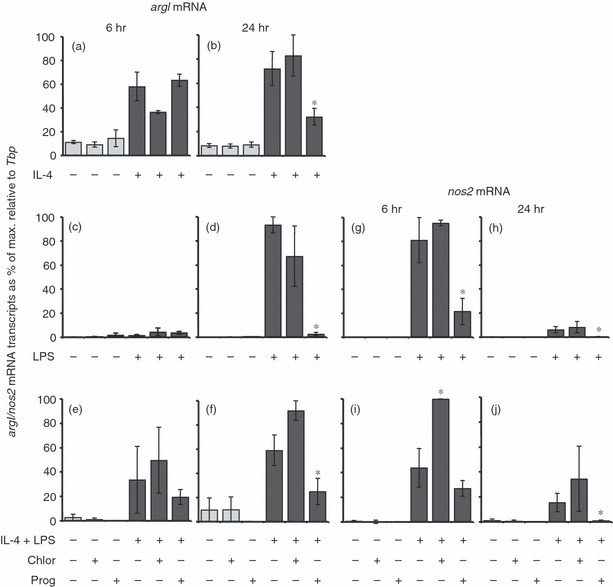
The effect of progesterone on argI and nos2 mRNA expression in bone marrow-derived (BMD) macrophages. Cells were left untreated, incubated with 0·04% chloroform (solvent control) or 62·5 μm progesterone for 16 hr prior to the addition of 100 U/ml interleukin 4 (IL-4) (a, b), 200 ng/ml lipopolysaccharide (LPS) (c, d, g, h) or 100 U/ml IL-4 and 200 ng/ml LPS (e, f, i, j) for 6 hr (a, c, e, g, i) or 24 hr (b, d, f, h, j). Control cultures were not treated with IL-4 and/or LPS. Expression levels of argI and nos2 mRNA transcripts were normalized to the housekeeping gene Tbp for each experimental run. The maximum gene expression for each run was designated 100% and all experiments calculated in comparison to this. Data are represented as the mean expression ± standard error (SE) of n = 3. *P < 0·05 in comparison with stimulated controls not treated with hormone or solvent for the same length of time.
IL-4 does not induce nos2 or p40 (IL-23/IL-23) mRNA transcripts, and treatment with progesterone (or the solvent chloroform) did not modulate the expression of these genes (data not shown). LPS-induced nos2 expression peaked at 6 hr (Fig. 2g) and was significantly reduced by 24 hr (Fig. 2h). Progesterone significantly reduced LPS-induced macrophage nos2 expression at both 6 and 24 hr. Progesterone had no effect on the nos2 mRNA levels after 6 hr of treatment with both IL-4 and LPS (Fig. 2i). However, the solvent control induced a significant increase. By the 24-hr time-point progesterone had down-regulated nos2 gene expression and chloroform had no effect (Fig. 2j).
Progesterone down-regulates mrc-1 and p40 gene expression
Studies in our laboratory have shown that arginase is induced by both innate (LPS) and alternative (IL-4) stimulation in a time-dependent manner.11 Consequently, we examined the ability of progesterone to modulate mrc-1- and p40-specific markers of alternative and innate immunity, respectively. Progesterone caused a reduction in the expression of the alternative macrophage activation-associated marker mrc-1 after 24 hr of stimulation with IL-4 (Fig. 3b). After stimulation with both IL-4 and LPS for 6 hr, levels of mrc-1 (Fig. 3c) mRNA transcripts were significantly increased. These levels were maintained after 24 hr (Fig. 3d). Progesterone had no effect on the expression of IL-4–LPS-induced mrc-1 at either time-point.
Figure 3.
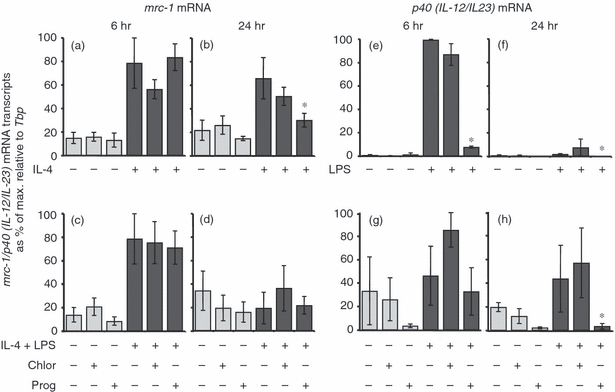
The effect of progesterone on mrc-1 and p40 [interleukin 12 (IL-12)/IL-23] mRNA expression in bone marrow-derived (BMD) macrophages. Cells were left untreated, incubated with 0·04% chloroform (solvent control) or 62·5 μm progesterone for 16 hr prior to the addition of 100 U/ml IL-4 (a, b), 200 ng/ml lipopolysaccharide (LPS) (e, f) or 100 U/ml IL-4 and 200 ng/ml LPS (c, d, g, h) for 6 hr (a, c, e, g) or 24 hr (b, d, f, h). Control cultures were not treated with IL-4, LPS or LPS and IL-4. Expression levels of mrc-1 and p40 (IL-12/IL-23) mRNA transcripts were normalized to the housekeeping gene Tbp for each experimental run. The maximum gene expression for each run was designated 100% and all experiments calculated in comparison to this. Data are represented as the mean expression ± standard error (SE) of n = 3. *P < 0·05 in comparison with stimulated controls not treated with hormone or solvent for the same length of time.
Progesterone and chloroform (used as a solvent) had no effect on the expression of p40 (IL-12/IL-23) mRNA transcripts in unstimulated cells (Fig. 3e–h). After 6 hr of LPS stimulation, p40 (IL-12/IL-23) transcripts were significantly up-regulated (Fig. 3e), but reduced by the 24-hr time-point (Fig. 3f). Progesterone reduced the ability of p40 (IL-12/IL-23) to be induced after 6 hr of LPS exposure, and this was reduced further by the 24-hr time-point. By comparison, progesterone had no effect on p40 (IL-12/IL23) transcripts in IL-4 and LPS-stimulated cells after 6 hr of treatment (Fig. 3g); however, by 24 hr of treatment with progesterone, transcript levels were significantly reduced (Fig. 3h).
Progesterone can modulate surface expression of the mannose receptor
Flow cytometry was carried out to determine the ability of progesterone to modulate the surface expression of the MR (Fig. 4). IL-4 was used to induce the alternative macrophage activation state and induced a significant increase in surface MR expression. Progesterone also induced an increase in surface MR expression in unstimulated cells. However, progesterone significantly reduced IL-4 induced MR expression to levels that were comparable with the unstimulated controls and in agreement with reduced mrc-1 mRNA expression (Fig. 3b).
Figure 4.

Influence of progesterone on interleukin 4 (IL-4) induced expression of mannose receptor (MR) in bone marrow-derived (BMD) macrophages. Cells were preincubated with medium (open bar), 62·5 μm progesterone (black bar), 0·04% chloroform (grey bar) for 16 hr followed by a 48-hr stimulation with IL-4 (100 U/ml). Unstimulated cells received medium. Results are the mean fluorescence intensity ± standard error (SE) of n = 3 samples and are representative of at least two experimental runs. *P < 0·05 compared with medium controls (open bar).
Progesterone differentially modulates other markers of alternative macrophage activation
Progesterone had no effect on IL-4-induced dectin-1 expression at either the 6-hr (Fig. 5a) or 24-hr (Fig. 5b) time-points. After stimulation with both IL-4 and LPS for 6 hr, dectin-1 (Fig. 5c) mRNA transcripts were significantly increased. These levels were maintained after 24 hr (Fig. 5d). Progesterone had no effect on the expression of this gene at either time-point.
Figure 5.
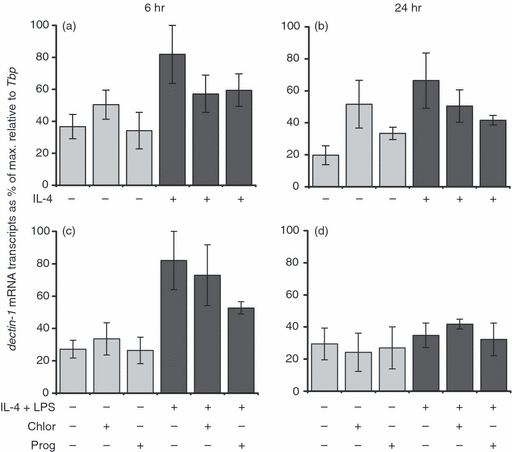
The effect of progesterone on dectin-1 mRNA expression in bone marrow-derived (BMD) macrophages. Cells were left untreated, incubated with 0·04% chloroform (solvent control) or 62·5 μm progesterone for 16 hr prior to the addition of 100 U/ml interleukin 4 (IL-4) (a, b) or 100 U/ml IL-4 and 200 ng/ml lipopolysaccharide (LPS) (c, d) for 6 hr (a, c) or 24 hr (b, d). Control cultures were not treated with IL-4 or LPS and IL-4. Expression levels of dectin-1 mRNA transcripts were normalized to the housekeeping gene Tbp for each experimental run. The maximum gene expression for each run was designated 100% and all experiments calculated in comparison to this. Data are represented as the mean expression ± standard error (SE) of n = 3. *P < 0·05 in comparison with stimulated controls not treated with hormone or solvent for the same length of time.
Macrophage fizz1 (Fig. 6) and ym1 (Fig. 7) mRNA transcripts were also analysed as markers of alternative macrophage activation. While progesterone alone had no effect on background levels of fizz1 mRNA at either time-point (Fig. 6a,b), a significant increase in ym1 mRNA levels after 6 hr and 24 hr was observed (Fig. 7a,b). While preincubation of the cells with progesterone significantly reduced the expression of fizz1 induced after 24 hr of treatment with IL-4 (Fig. 6b) progesterone significantly enhanced macrophage fizz1 mRNA expression induced by stimulation with both IL-4 and LPS for 24 hr (Fig. 6d). Progesterone significantly enhanced early (6 hr) but not late (24 hr) expression of ym1 mRNA transcripts after stimulation with IL-4 (Fig. 7a,b) or with both IL-4 and LPS (Fig. 7c,d).
Figure 6.

The effect of progesterone on fizz1 mRNA expression in bone marrow-derived (BMD) macrophages. Cells were left untreated, incubated with 0·04% chloroform (solvent control) or 62·5 μm progesterone for 16 hr prior to the addition of 100 U/ml interleukin 4 (IL-4) (a, b) or 100 U/ml IL-4 and 200 ng/ml lipopolysaccharide (LPS) (c, d) for 6 hr (a, c) or 24 hr (b, d). Control cultures were not treated with IL-4 or LPS and IL-4. Expression levels of fizz1 mRNA transcripts were normalized to the housekeeping gene Tbp for each experimental run. The maximum gene expression for each run was designated 100% and all experiments calculated in comparison to this. Data are represented as the mean expression ± standard error (SE) of n = 3. *P < 0·05 in comparison with stimulated controls not treated with hormone or solvent for the same length of time.
Figure 7.

The effect of progesterone on ym1 mRNA expression in bone marrow-derived (BMD) macrophages. Cells were left untreated, incubated with 0·04% chloroform (solvent control) or 62·5 μm progesterone for 16 hr prior to the addition of 100 U/ml interleukin 4 (IL-4) (a, b) or 100 U/ml IL-4 and 200 ng/ml lipopolysaccharide (LPS) (c, d) for 6 hr (a, c) or 24 hr (b, d). Control cultures were not treated with IL-4 or LPS and IL-4. Expression levels of ym1 mRNA transcripts were normalised to the housekeeping gene Tbp for each experimental run. The maximum gene expression for each run was designated 100% and all experiments calculated in comparison to this. Data are represented as the mean expression ± standard error (SE) of n = 3. *P < 0·05 in comparison with stimulated controls not treated with hormone or solvent for the same length of time.
Discussion
Macrophages are now recognized as being a versatile and plastic population of cells,1,11,39–42 able to respond to the local microenvironment through altering receptor expression and cytokine production to adapt their function. Despite classification of macrophage phenotypes into distinct categories, such as classically activated (M1), innately activated or alternatively activated (M2), it is now recognized that it is difficult to strictly compartmentalize the different functions of these cells. Our present studies reinforce further this concept of plasticity, with the pregnancy-associated hormone progesterone demonstrated to be able to modulate differentially the so-called defined ‘markers’ of alternatively activated macrophages. Not only does progesterone down-modulate NOS2, but also arginase I enzyme activity in murine macrophages in a dose-dependent manner. In addition, we demonstrate that progesterone can selectively reduce the expression of transcript levels for some genes associated with alternative macrophage activation such as mrc1, but increase expression levels of transcripts for others, such as ym1. The consequences of these progesterone influences for a healthy pregnancy need to be addressed.
Macrophages represent a significant population of immune cells within the non-pregnant and pregnant uterus of both mice and humans.43–53 In addition, macrophages are localized to areas of apoptosis and are implicated in the remodelling of tissue at the maternal–fetal interface.20 The Th2 environment associated with pregnancy would favour the development of macrophages towards the alternatively activated phenotype. Indeed, Cupurdija and colleagues25 have demonstrated that decidual macrophages of early human pregnancy display characteristics associated with the alternative activation phenotype. However, as monocytes are recruited from the circulation into the uterus during the later stages of pregnancy in humans under the apparent influence of trophoblast cells,54 they are programmed towards a decidual phenotype55 that are refractory to IFN-γ influences, but at the same time display characteristics of classically activated, alternatively activated and immunosuppressive/deactivated macrophages. Our data would suggest that progesterone could be playing a significant role in influencing the expression of such a mixture of phenotypes. However, the influence of other pregnancy hormones, such as oestrogen and prolactin, will also be taken into consideration.
Down-regulation of the classical pathway of macrophage activation during pregnancy ensures that proinflammatory cytokines and NO, that would be detrimental to the successful continuation of pregnancy, are not released.56 This is supported by our data and previously published work,30 showing that progesterone acts to down-modulate nitrite production in BMD macrophages as well as down-regulating the expression of nos2 mRNA transcripts and p40 (IL-12/IL-23) mRNA transcripts. To the best of our knowledge, no in vitro studies have considered effects of progesterone on the activity of macrophage arginase, which competes with NOS2 for the common substrate l-arginine. Previously, our group has demonstrated that at the concentrations used, IL-4 is a weaker stimulus of arginase I mRNA expression and activity than stimulation with LPS or a combination of IL-4 and LPS11. Surprisingly, our data demonstrate that progesterone not only suppresses classical activation, but also argI mRNA transcript levels and consequently arginase activity, irrespective of the method of stimulation.
l-arginine metabolism and arginase have been shown to affect the success of pregnancy and this provides a possible evolutionary advantageous reason for progesterone-mediated arginase modulation. It has been shown, for example, that depletion of l-arginine by arginase down-regulates the CD3ζ chain of the T cell receptor (TCR) resulting in the induction of T cell hyporesponsiveness favouring fetal survival.57 Significantly, cervical ripening occurs immediately after a decline in progesterone synthesis which allows the recruitment of immune cells, including macrophages, to promote remodelling.58 During this time, alternatively activated macrophage populations have been found to predominate which would serve to promote post-partum tissue repair via arginase and ym1-associated activities as noted in other tissues.59–63 Our data would indicate that progesterone is one possible candidate for the negative regulation of arginase activity pre-labour.
In addition to elevated arginase I mRNA expression and activity, alternative macrophage activation has been associated with high expression of the pattern recognition receptors MR7 and dectin-112 and mRNA for the ym13,14,15 and fizz113 genes. We have found that in the presence of high levels of progesterone, IL-4 induced mrc-1 and fizz1 expression is reduced, while ym1 mRNA expression is increased further and dectin-1 mRNA expression levels remain unchanged. The ability of progesterone to modulate expression of each of these markers may be related to their individual functions.
MR+ decidual macrophages have been identified at the human maternal–fetal interface26 and microarray analysis of human decidual macrophages showed that mrc-1 was up-regulated in comparison with blood-derived mononuclear cells, along with alternative macrophage activation-associated CC chemokine 1 (AMAC-1) (CCL18),64 a human marker of alternative macrophage activation. In addition, studies using human decidual macrophages have shown that tumour-associated glycoprotein-72 (TAG-72) is a progesterone-dependent molecule, which may be the natural ligand for the MR within the human decidua.26 Ligand binding of the MR leads to an inhibition of Th1 polari zed immune responses65 and so would favour the successful continuation of pregnancy. In the absence of IL-4, progesterone significantly increased the surface expression of the MR on macrophages, and would thus promote the probability of binding of TAG-72 to the MR to inhibit Th1 responses, which are widely viewed as detrimental to pregnancy. By comparison, when stimulated with IL-4, both mrc-1 expression and surface MR expression were reduced by progesterone. Under these circumstances, progesterone may function to regulate the levels of macrophage surface MR in order to limit the extent of Th1 inhibition. In contrast, the expression of dectin-1 remained unaltered in the presence of progesterone, regardless of whether the stimulus was simply IL-4 or IL-4 in combination with LPS. The dectin-1 receptor binds fungal-derived β-glucans66,67 on fungal pathogens, meaning that this aspect of immunity to these pathogens should be unaffected by the presence of high levels of progesterone.
An interesting finding in these studies is the ability of macrophage fizz1 expression to be regulated differentially in the presence of progesterone, dependent upon the type of stimulus; progesterone reduced IL-4 induced fizz1 expression, but increased IL-4+ LPS-induced fizz1 expression; fizz1 is a resistin-like secreted protein also known as Relmα. Despite being classified as a marker of alternative macrophage activation13 and induced by the Th2-associated cytokines IL-4 and IL-13, recent studies employing the use of fizz1-deficient mice (Retnla–/– mice) have demonstrated that this molecule acts to negatively regulate Th2-dependent inflammatory responses.68,69 In the presence of both IL-4 and LPS, which would equate to a type-2 inflammatory response, fizz1 expression by BM macrophages was elevated by progesterone. Generally, Th1 responses are considered detrimental to pregnancy and Th2 responses beneficial (reviewed in 70). However, our data pose the intriguing question as to whether type-2 inflammation (IL-4 plus additional inflammatory signals) could also be damaging through pregnancy. Consequently, progesterone could serve to down-regulate excessive type-2 inflammation via increased macrophage fizz1 production.
Macrophage ym1 mRNA transcripts were up-regulated by progesterone, both in the presence and absence of IL-4. Two major functions have been associated with ym1 activities, namely tissue remodelling and repair,71 and induction of Th2 cytokine production.72 Significantly, both functions would, by general consensus, be important in the maintenance of a healthy pregnancy. Recently, Routely and Ashcroft10 have shown in a murine model of wound repair in the skin that progesterone acts to promote an alternatively activated phenotype, as indicated by increasing levels of ym1 protein expression although not ym1 mRNA expression. Unsurprisingly, therefore, alternative macrophage activation and concomitant ym1 expression are related closely to tissue remodelling and repair during pregnancy in mice.25,59
The ability of progesterone to modulate various aspects of macrophage activity has been well studied (reviewed in 28). However, during the course of the present studies, we have demonstrated for the first time the ability of progesterone to alter expression of markers associated with alternative macrophage activation in vitro. In addition, the novel finding that progesterone regulates differentially markers of alternative macrophage activation, as demonstrated by its ability to down-regulate IL-4-induced argI and mrc-1, but up-regulate ym1 expression, has important implications both for the general study of alternative macrophage activation and for the study of macrophages in pregnancy and remodelling.
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–9. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 3.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 4.Pace JL, Russell SW, Schreiber RD, Altman A, Katz DH. Macrophage activation: priming activity from a T-cell hybridoma is attributable to interferon-gamma. Proc Natl Acad Sci USA. 1983;80:3782–6. doi: 10.1073/pnas.80.12.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson SE, Jr, Remington JS. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974;139:1154–74. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cline MJ. Bactericidal activity of human macrophages: analysis of factors influencing the killing of Listeria monocytogenes. Infect Immun. 1970;2:156–61. doi: 10.1128/iai.2.2.156-161.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witte MB, Barbul A, Schick MA, Vogt N, Becker HD. Upregulation of arginase expression in wound-derived fibroblasts. J Surg Res. 2002;105:35–42. doi: 10.1006/jsre.2002.6443. [DOI] [PubMed] [Google Scholar]
- 9.Witte MB, Barbul A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003;11:419–23. doi: 10.1046/j.1524-475x.2003.11605.x. [DOI] [PubMed] [Google Scholar]
- 10.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009;17:42–50. doi: 10.1111/j.1524-475X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 11.Menzies FM, Henriquez FL, Alexander J, Roberts CW. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol. 2010;160:369–79. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY, Gordon S, Brown GD. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171:4569–73. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- 13.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–80. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 14.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 15.Raes G, Noel W, Beschin A, Brys L, de Baetselier P, Hassanzadeh GH. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev Immunol. 2002;9:151–9. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 17.Jin HM, Copeland NG, Gilbert DJ, Jenkins NA, Kirkpatrick RB, Rosenberg M. Genetic characterization of the murine Ym1 gene and identification of a cluster of highly homologous genes. Genomics. 1998;54:316–22. doi: 10.1006/geno.1998.5593. [DOI] [PubMed] [Google Scholar]
- 18.Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–55. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De M, Wood GW. Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol. 1990;126:417–24. doi: 10.1677/joe.0.1260417. [DOI] [PubMed] [Google Scholar]
- 20.Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol. 2003;1 doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the materna–fetal interface. J Immunol. 1993;151:4562–73. [PubMed] [Google Scholar]
- 22.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol. 2003;50:444–52. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 24.Mizuno M, Aoki K, Kimbara T. Functions of macrophages in human decidual tissue in early pregnancy. Am J Reprod Immunol. 1994;31:180–8. doi: 10.1111/j.1600-0897.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 25.Cupurdija K, Azzola D, Hainz U, et al. Macrophages of human first trimester decidua express markers associated to alternative activation. Am J Reprod Immunol. 2004;51:117–22. doi: 10.1046/j.8755-8920.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Laskarin G, Cupurdija K, Tokmadzic VS, et al. The presence of functional mannose receptor on macrophages at the maternal–fetal interface. Hum Reprod. 2005;20:1057–66. doi: 10.1093/humrep/deh740. [DOI] [PubMed] [Google Scholar]
- 27.Politz O, Gratchev A, McCourt PA, et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002;362:155–64. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59:1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 29.Robert R, Spitzer JA. Effects of female hormones (17beta-estradiol and progesterone) on nitric oxide production by alveolar macrophages in rats. Nitric Oxide. 1997;1:453–62. doi: 10.1006/niox.1997.0157. [DOI] [PubMed] [Google Scholar]
- 30.Jones LA, Anthony JP, Henriquez FL, Lyons RE, Nickdel MB, Carter KC, Alexander J, Roberts CW. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008;125:59–69. doi: 10.1111/j.1365-2567.2008.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3 doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noel W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–33. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, Mantovani A, Sozzani S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J Immunol. 2005;174 doi: 10.4049/jimmunol.174.11.6561. [DOI] [PubMed] [Google Scholar]
- 34.Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Corraliza IM, Soler G, Eichmann K, Modolell M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem Biophys Res Commun. 1995;206:667–73. doi: 10.1006/bbrc.1995.1094. [DOI] [PubMed] [Google Scholar]
- 36.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–80. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–15. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Pomares L, Reid DM, Brown GD, et al. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J Leukoc Biol. 2003;73:604–13. doi: 10.1189/jlb.0902450. [DOI] [PubMed] [Google Scholar]
- 39.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 43.Hunt JS, Manning LS, Mitchell D, Selanders JR, Wood GW. Localization and characterization of macrophages in murine uterus. J Leukoc Biol. 1985;38:255–65. doi: 10.1002/jlb.38.2.255. [DOI] [PubMed] [Google Scholar]
- 44.De M, Choudhuri R, Wood GW. Determination of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from mating through implantation. J Leukoc Biol. 1991;50:252–62. doi: 10.1002/jlb.50.3.252. [DOI] [PubMed] [Google Scholar]
- 45.De M, Wood GW. Analysis of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from implantation through parturition. J Leukoc Biol. 1991;50:381–92. doi: 10.1002/jlb.50.4.381. [DOI] [PubMed] [Google Scholar]
- 46.Stewart IJ, Mitchell BS. The distribution of uterine macrophages in virgin and early pregnant mice. J Anat. 1991;179:183–96. [PMC free article] [PubMed] [Google Scholar]
- 47.Brandon JM. Distribution of macrophages in the mouse uterus from one day to three months after parturition, as defined by the immunohistochemical localization of the macrophage-restricted antigens F4/80 and macrosialin. Anat Rec. 1994;240:233–42. doi: 10.1002/ar.1092400210. [DOI] [PubMed] [Google Scholar]
- 48.Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod. 1997;12:586–90. doi: 10.1093/humrep/12.3.586. [DOI] [PubMed] [Google Scholar]
- 49.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61:879–83. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- 50.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–36. [PubMed] [Google Scholar]
- 51.Mackler AM, Green LM, McMillan PJ, Yellon SM. Distribution and activation of uterine mononuclear phagocytes in peripartum endometrium and myometrium of the mouse. Biol Reprod. 2000;62:1193–200. doi: 10.1095/biolreprod62.5.1193. [DOI] [PubMed] [Google Scholar]
- 52.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–71. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 53.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal–fetal interface. Reprod Sci. 2010;17:209–18. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 54.Fest S, Aldo PB, Abrahams VM, et al. Trophoblast–macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod Immunol. 2007;57:55–66. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 55.McIntire RH, Ganacias KG, Hunt JS. Programming of human monocytes by the uteroplacental environment. Reprod Sci. 2008;15:437–47. doi: 10.1177/1933719107314065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su L, Sun Y, Ma F, Lu P, Huang H, Zhou J. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-kappaB activation and enhancing SOCS1 expression. Immunol Lett. 2009;125:151–5. doi: 10.1016/j.imlet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Kropf P, Baud D, Marshall SE, et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol. 2007;37:935–45. doi: 10.1002/eji.200636542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod. 2009;81:1–6. doi: 10.1095/biolreprod.108.074997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182:2700–7. doi: 10.4049/jimmunol.0803138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106:17475–80. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–96. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McSweeney SJ, Hadoke PW, Kozak AM, Small GR, Khaled H, Walker BR, Gray GA. Improved heart function follows enhanced inflammatory cell recruitment and angiogenesis in 11betaHSD1-deficient mice post-MI. Cardiovasc Res. 2010;88:159–67. doi: 10.1093/cvr/cvq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–77. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 64.Gustafsson C, Mjosberg J, Matussek A, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chieppa M, Bianchi G, Doni A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–60. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 66.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–12. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown J, O'Callaghan CA, Marshall AS, Gilbert RJ, Siebold C, Gordon S, Brown GD, Jones EY. Structure of the fungal beta-glucan-binding immune receptor dectin-1: implications for function. Protein Sci. 2007;16:1042–52. doi: 10.1110/ps.072791207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nair MG, Du Y, Perrigoue JG, et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–52. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF, Jr, Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bansal AS. Joining the immunological dots in recurrent miscarriage. Am J Reprod Immunol. 2010;64:307–15. doi: 10.1111/j.1600-0897.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- 71.Chang NC, Hung SI, Hwa KY, Kato I, Chen JE, Liu CH, Chang AC. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276:17497–506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 72.Cai Y, Kumar RK, Zhou J, Foster PS, Webb DC. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: identification of a novel pathway for regulating allergic inflammation. J Immunol. 2009;182:5393–9. doi: 10.4049/jimmunol.0803874. [DOI] [PubMed] [Google Scholar]


