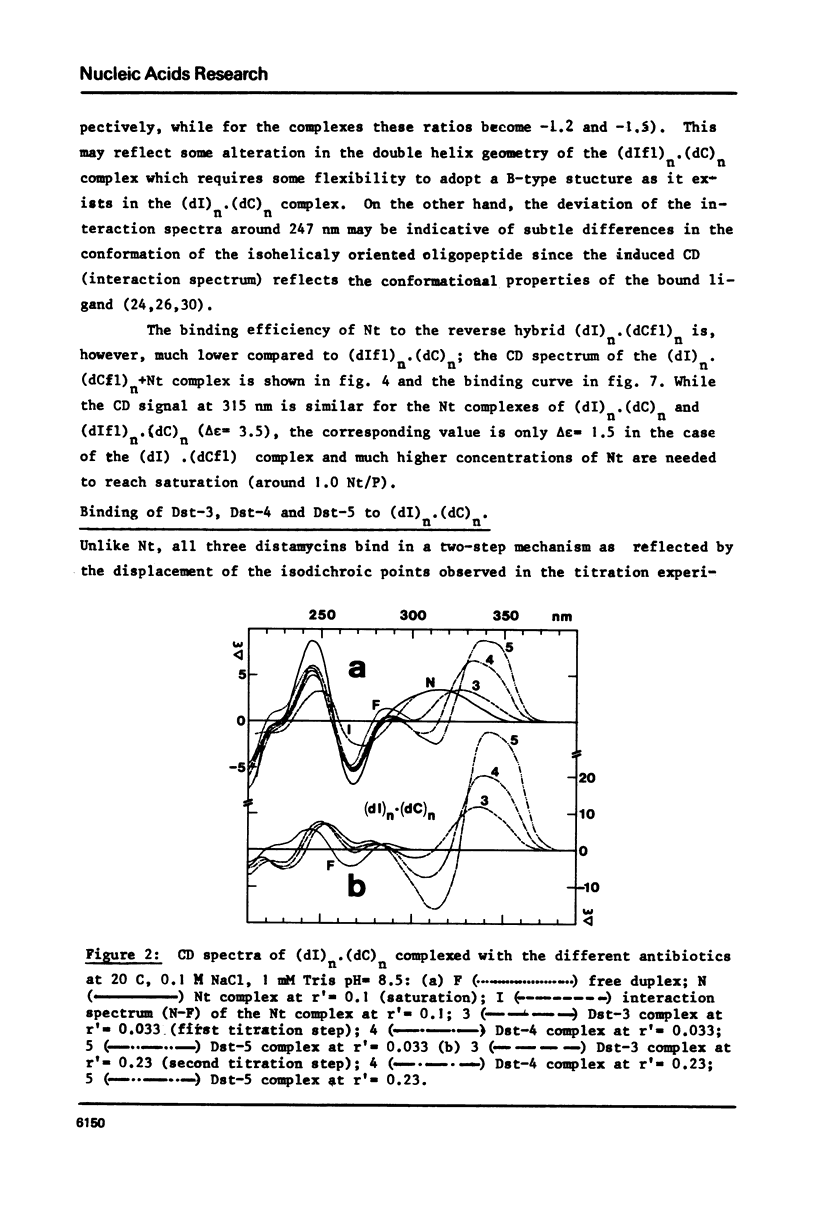
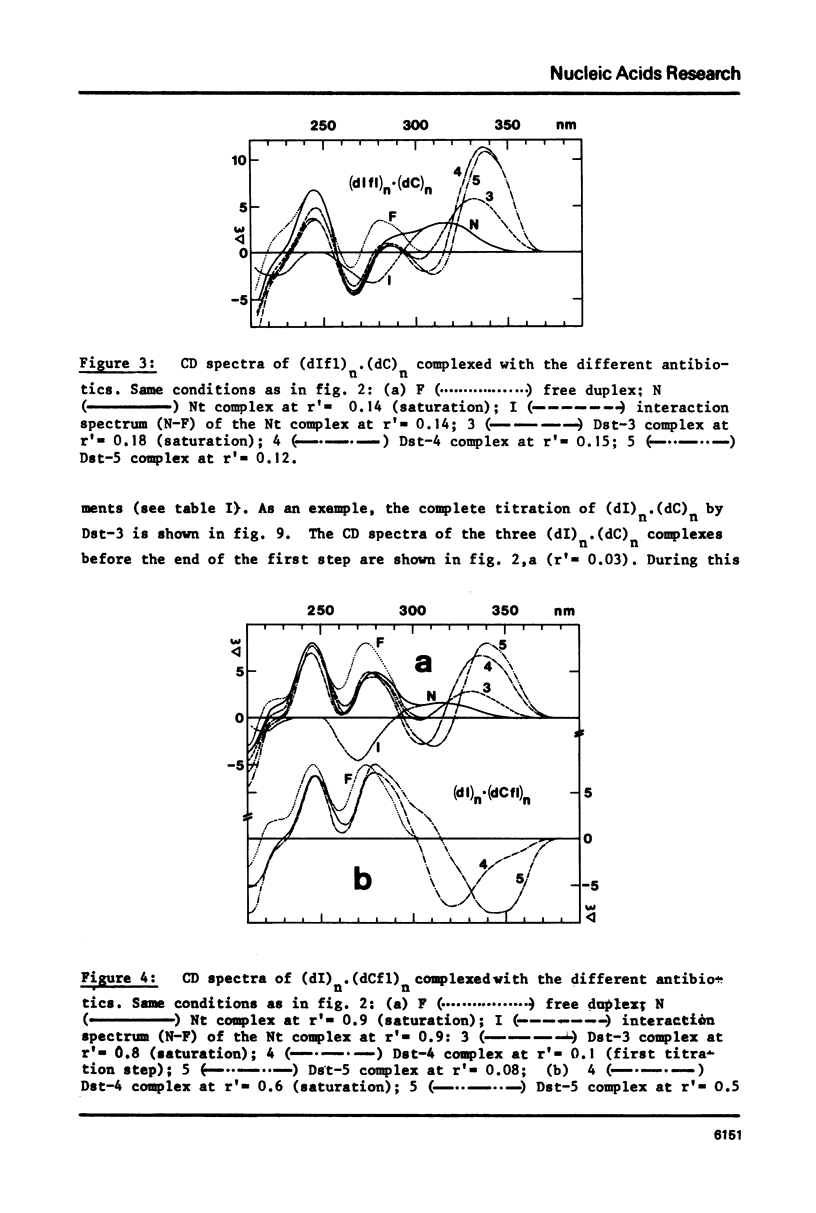
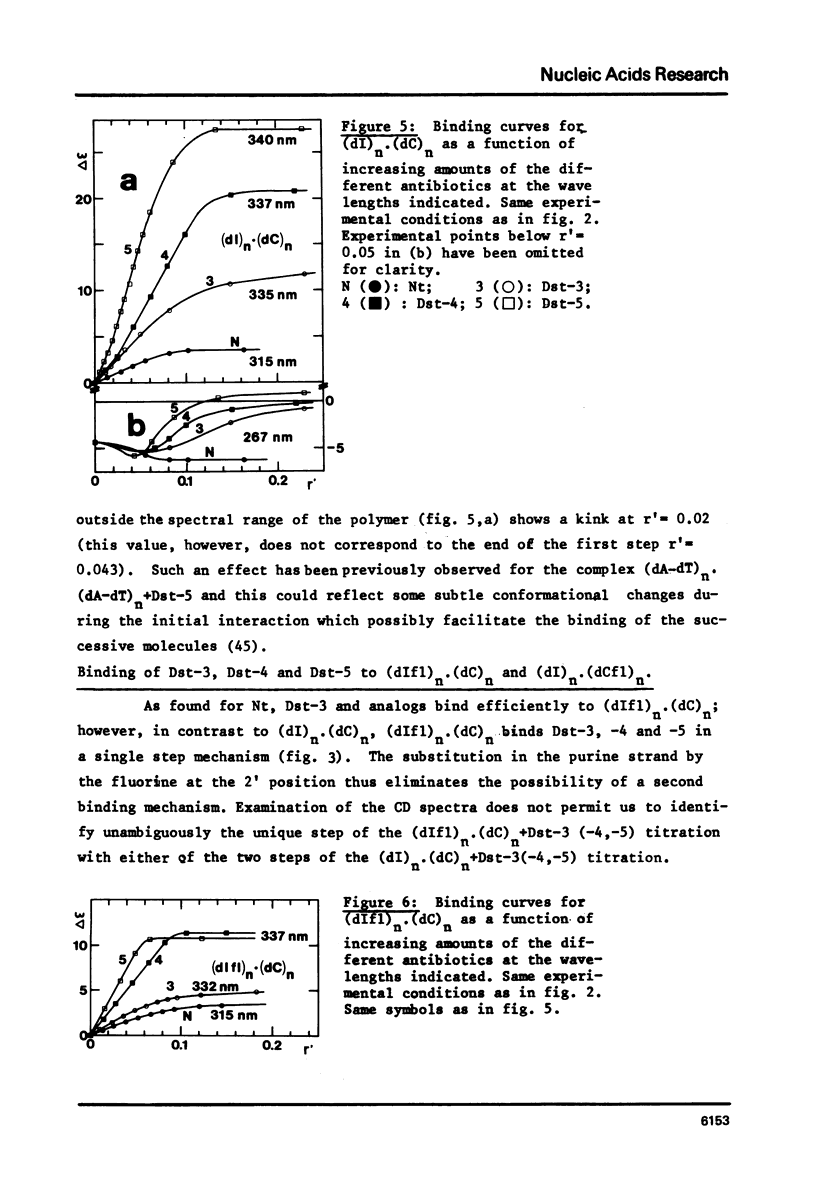
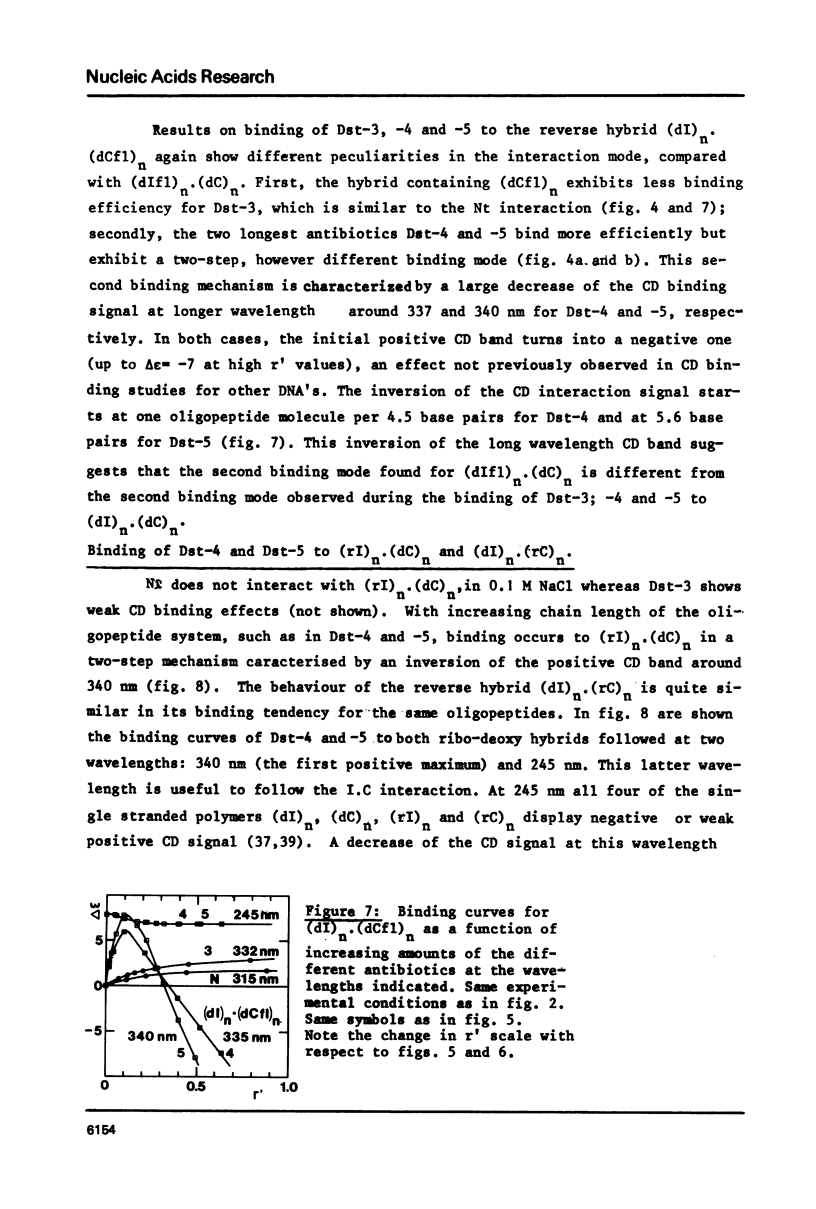
Abstract
Binding of the B-form specific ligands netropsin and distamycin-3, -4 and -5 has been used to monitor the presence and/or the inducibility of a B-type structure in various poly-inosinic.poly-cytidilic double stranded polymers with deoxyribose, ribose or 2'-deoxy-2'-fluororibose as sugar on either strand. The efficiency of binding was followed by circular dichroism and further evaluated by the increase in melting temperature of the complexes. The efficient binding of netropsin and distamycins to the hybrid polymer (dIfl)n. (dC)n demonstrated that the fluorine carrying strand may undergo a A to B-type transition reflecting a change of the 2'-deoxy-2'-fluororibose from the 3'-endo to the 1'-exo or 2'-endo pucker. The less efficient binding of the same ligands to the reverse hybrid (dI)n.(dCfl)n showed that the geometry of the pyrimidine strand is the most critical for the specific interaction. Taking into account the recent findings about the regular hydration in the minor groove of the B-type dodecamer dCGCGAATTCGCG in solid-state, the different binding modes observed between the different polymers and antibiotics are explained by differences in their possibilities of hydration. Binding of netropsin to a double stranded deoxypolymer is interpreted as a local replacement of water molecules by netropsin in the minor groove hydration network which is typical of the B-form.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Arnott S. The geometry of nucleic acids. Prog Biophys Mol Biol. 1970;21:265–319. doi: 10.1016/0079-6107(70)90027-1. [DOI] [PubMed] [Google Scholar]
- Berman H. M., Neidle S., Zimmer C., Thrum H. Netropsin, a DNA-binding oligopeptide structural and binding studies. Biochim Biophys Acta. 1979 Jan 26;561(1):124–131. doi: 10.1016/0005-2787(79)90496-9. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M. J., PATTERSON D. L. PHYSICAL AND CHEMICAL CHARACTERIZATION OF THE ORDERED COMPLEXES FORMED BETWEEN POLYINOSINIC ACID, POLYCYTIDYLIC ACID AND THEIR DEOXYRIBO-ANALOGUES. J Mol Biol. 1965 Jun;12:410–428. doi: 10.1016/s0022-2836(65)80264-9. [DOI] [PubMed] [Google Scholar]
- Conner B. N., Takano T., Tanaka S., Itakura K., Dickerson R. E. The molecular structure of d(ICpCpGpG), a fragment of right-handed double helical A-DNA. Nature. 1982 Jan 28;295(5847):294–299. doi: 10.1038/295294a0. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Kinematic model for B-DNA. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7318–7322. doi: 10.1073/pnas.78.12.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Wing R. M., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T., Kakiuchi N., Ikehara M. Polynucleotides. XLV Synthesis and properties of poly(2'-azido-2'-deoxyinosinic acid). Nucleic Acids Res. 1977 Aug;4(8):2629–2639. doi: 10.1093/nar/4.8.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschlbauer W., Blandin M., Drocourt J. L., Thang M. N. Poly-2'-deoxy-2'-fluoro-cytidylic acid: enzymatic synthesis, spectroscopic characterization and interaction with poly-inosinic acid. Nucleic Acids Res. 1977 Jun;4(6):1933–1943. doi: 10.1093/nar/4.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschlbauer W., Jankowski K. Nucleoside conformation is determined by the electronegativity of the sugar substituent. Nucleic Acids Res. 1980 Mar 25;8(6):1421–1433. doi: 10.1093/nar/8.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. HELIX--RANDOM COIL TRANSITIONS IN DNA HOMOPOLYMER PAIRS. J Mol Biol. 1964 Apr;8:452–469. doi: 10.1016/s0022-2836(64)80003-6. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Janik B., Kotick M. P., Kreiser T. H., Reverman L. F., Sommer R. G., Wilson D. P. Synthesis and properties of poly 2'-fluoro-2'-deoxyuridylic acid. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1153–1160. doi: 10.1016/s0006-291x(72)80095-0. [DOI] [PubMed] [Google Scholar]
- Kakiuchi N., Marck C., Rousseau N., Leng M., De Clerq E., Guschlbauer W. Polynucleotide helix geometry and stability. Spectroscopic, antigenic and interferon-inducing properties of deoxyribose-, ribose-, or 2'-deoxy-2'-fluororibose-containing duplexes of poly(inosinic acid) . poly(cytidylic acid). J Biol Chem. 1982 Feb 25;257(4):1924–1928. [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Luck G., Triebel H., Waring M., Zimmer C. Conformation dependent binding of netropsin and distamycin to DNA and DNA model polymers. Nucleic Acids Res. 1974 Mar;1(3):503–530. doi: 10.1093/nar/1.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck G., Zimmer C., Reinert K. E., Arcamone F. Specific interactions of distamycin A and its analogs with (A-T) rich and (G-C) rich duplex regions of DNA and deoxypolynucleotides. Nucleic Acids Res. 1977 Aug;4(8):2655–2670. doi: 10.1093/nar/4.8.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. C., Wartell R. M., O'Shea D. C. Conformational features of distamycin-DNA and netropsin-DNA complexes by Raman spectroscopy. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5483–5487. doi: 10.1073/pnas.75.11.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizot J. C., Brahms J., Eckstein F. Forces involved ithe conformational stability of nucleic acids. Nature. 1969 May 10;222(5193):559–561. doi: 10.1038/222559a0. [DOI] [PubMed] [Google Scholar]
- Melcher G. The stabilisation of nucleic acid structures. Biophysik. 1970;7(1):29–32. doi: 10.1007/BF01189460. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Netropsin-poly(dA-dT) complex in solution: structure and dynamics of antibiotic-free base pair regions and those centered on bound netropsin. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5207–5211. doi: 10.1073/pnas.74.12.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Shakked Z., Rabinovich D., Cruse W. B., Egert E., Kennard O., Sala G., Salisbury S. A., Viswamitra M. A. Crystalline A-dna: the X-ray analysis of the fragment d(G-G-T-A-T-A-C-C). Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):479–487. doi: 10.1098/rspb.1981.0076. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W., Favre A. Protonated polynucleotide structures. X. Optical properties of poly(I)-poly(C) and its disproportionation complexes. Biochim Biophys Acta. 1972 Jun 22;272(1):22–26. [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. Polynucléotides protonés. VII. Transitions thermiques entre differents complexes de l'acide polyinosinique et de l'acide polycytidylique en milieu acide. Biopolymers. 1969;8(3):361–378. doi: 10.1002/bip.1969.360080307. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Bobst A. M., Waters J. A., Witkop B. Synthesis and characterization of potential interferon inducers. Poly(2'-azido-2'-deoxyuridylic acid). Biochemistry. 1973 Sep 25;12(20):3962–3972. doi: 10.1021/bi00744a028. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Takatsuka Y., Ikehara M., Cheng D. M., Kan L. S., Ts'o P. O. Synthesis and characterization of the dinucleoside monophosphates containing 2'-fluoro-2'-deoxyadenosine. Biochemistry. 1981 May 26;20(11):3056–3062. doi: 10.1021/bi00514a011. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Larson J. E., Wells R. D. Netropsin. A specific probe for A-T regions of duplex deoxyribonucleic acid. J Biol Chem. 1974 Nov 10;249(21):6719–6731. [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]
- Wohlrab F., Haertlé T., Trichtinger T., Guschlbauer W. 2'-Deoxy-2'-fluorouridine-5'-phosphate: an alternative substrate for thymidylate synthetase from Escherichia coli K12. Nucleic Acids Res. 1978 Dec;5(12):4753–4759. doi: 10.1093/nar/5.12.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasedatelev A. S., Gursky G. V., Zimmer C., Thrum H. Binding of netropsin to DNA and synthetic polynucleotides. Mol Biol Rep. 1974 Mar;1(6):337–342. doi: 10.1007/BF00309567. [DOI] [PubMed] [Google Scholar]
- Zimmer C. Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1975;15(0):285–318. doi: 10.1016/s0079-6603(08)60122-1. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Kakiuchi N., Guschlbauer W. Differential stabilization by netropsin of inducible B-like conformations in deoxyribo-, ribo- and 2'-deoxy-2'-fluororibo-adenosine containing duplexes of (dA)n . (dT)n and (dA)n . (dU)na. Nucleic Acids Res. 1982 Mar 11;10(5):1721–1732. doi: 10.1093/nar/10.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Luck G., Thrum H., Pitra C. Binding of analogues of the antibiotics distamycin A and netropsin to native DNA. Effect of chomophore systems and basic residues of the oligopeptides on thermal stability, conformation and template activity of the DNA complexes. Eur J Biochem. 1972 Mar 15;26(1):81–89. doi: 10.1111/j.1432-1033.1972.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Marck C., Schneider C., Guschlbauer W. Influence of nucleotide sequence on dA.dT-specific binding of Netropsin to double stranded DNA. Nucleic Acids Res. 1979 Jun 25;6(8):2831–2837. doi: 10.1093/nar/6.8.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Marck C., Schneider C., Thiele D., Luck G., Guschlbauer W. Magnetic circular dichroism study of the binding of netropsin and distamycin A with DNA. Biochim Biophys Acta. 1980 Apr 30;607(2):232–246. doi: 10.1016/0005-2787(80)90076-3. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Puschendorf B., Grunicke H., Chandra P., Venner H. Influence of netropsin and distamycin A on the secondary structure and template activity of DNA. Eur J Biochem. 1971 Jul 29;21(2):269–278. doi: 10.1111/j.1432-1033.1971.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Reinert K. E., Luck G., Wähnert U., Löber G., Thrum H. Interaction of the oligopeptide antibiotics netropsin and distamycin A with nucleic acids. J Mol Biol. 1971 May 28;58(1):329–348. doi: 10.1016/0022-2836(71)90250-6. [DOI] [PubMed] [Google Scholar]
- Zmudzka B., Bollum F. J., Shugar D. Polydeoxyribouridylic acid and its complexes with polyribo- and deoxyriboadenylic acids. J Mol Biol. 1969 Nov 28;46(1):169–183. doi: 10.1016/0022-2836(69)90064-3. [DOI] [PubMed] [Google Scholar]



