Abstract
Bone morphogenetic protein (BMP) signalling regulates lymphopoiesis in bone marrow and thymus via the interaction of haemato-lymphoid progenitors with the stroma microenvironment. Despite increasing functional evidence for the role of BMP signalling in lymphopoiesis, little is known of the spatial distribution of BMP/BMP antagonists in the thymus and of how BMP signals exert specific functions in developing lymphocytes. We analysed expression of BMP/BMP antagonists in the thymus and bone marrow and determined the topology of BMP/BMP antagonist expression using lacZ reporter mice. Bmp4, Bmp7, Gremlin and Twisted gastrulation (Twsg1) are all expressed in the thymus and expression was clearly different for each gene investigated. Expression was seen both in cortical and medullary regions suggesting that BMP signals regulate all stages of T-cell development. Two genes in particular, Bmp7 and Twsg1, were dynamically expressed in developing T and B lymphocytes. Their conditional ablation in all haematopoietic cells surprisingly did not affect the steady state of B-cell and T-cell development. This indicates that both lymphoid cell-derived BMP7 and TWSG1 are dispensable for normal lymphopoiesis and that bone-marrow stroma-derived TWSG1 is responsible for the lymphoid defects observed in Twsg1 null mice. In summary our data demonstrate a complex network of lymphoid and stroma derived BMP signals involved in the orchestration of lymphopoiesis in both bone marrow and thymus.
Keywords: bone morphogenetic protein, LacZ reporter, lymphopoiesis
Introduction
Both T and B lymphocytes originate from a common lymphoid precursor but their development requires distinct microenvironments. B lymphocytes develop in the bone marrow whereas T cells mature in the thymus. It is well established that reciprocal interactions between developing lymphocytes and stromal cells from the respective organs are key to their successful maturation.1,2 Several growth and differentiation factors have been identified in stromal cells that are instrumental for supporting T-cell and B-cell development.3,4 However, less is known about signals that originate from the developing lymphocytes to reciprocally support stromal cell function.5
Embryonic development and adult homeostasis of most tissues are orchestrated to a large degree by only a few families of conserved signalling factors: the Hedgehog (Hh)-, Notch-, Wnt/β-catenin-, tumour-growth-factor (TGF)/bone morphogenetic protein (BMP)-, and fibroblast growth factor (FGF) families. All these signalling pathways are also important for the development of the lymphoid organs and lymphocytes themselves.6–9
The BMPs, which belong to the TGF-β superfamily, are evolutionary highly conserved molecules and can be separated into two subgroups. BMP2 and BMP4 are orthologues of Drosophila Decapentaplegic (Dpp), whereas BMP5, BMP6, and BMP7 belong to the Glass-bottom-boat (Gbb) subgroup. The name BMP originates from the molecule's ability to induce ectopic bone formation.10 The BMPs are secreted signalling molecules and, contrary to their name, have critical functions in many different biological processes outside bone formation. They are involved in many aspects of embryonic development,11,12 homeostasis and repair of various tissues13 including the haematopoietic system.14,15
The BMPs signal via specific heterodimeric BMP receptors consisting of a type I receptor (Alk1, Alk2, Alk3, Alk6) and a type II receptor (BmpRII, ActRII, ActRIIB) sub-unit. Receptor engagement leads to the phosphorylation of a type I receptor by a type II receptor, which in turn facilitates the activation of either the BMP-specific Smad1/5/8 signalling pathway16 or non-specific signal transduction pathways such as MAPK/PI3K/Akt17. In Smad-dependent signalling, Smad1/5/8 are phosphorylated and translocated together with the co-Smad Smad4 to the nucleus to exert their cellular effects.18
Receptor engagement by BMPs is highly regulated in the extracellular space by secreted BMP antagonists such as Gremlin, Noggin, Chordin and Twisted Gastrulation (TWSG1) that bind BMPs with high affinity and so prevent receptor engagement.19 The binding affinities of the various BMPs to antagonists as well as receptors differ.20 Hence, the various BMPs and BMP antagonists are not simply redundant but are required for the precise spatiotemporal regulation of BMP signalling. By controlling Bmp expression as well as exploiting the different binding affinities to receptors and antagonists,20 a precise spatio-temporal regulation of BMP signals is possible. Because of this complexity, the precise contribution of these different BMP signals to complex biological processes is still poorly understood.
Bone morphogenetic protein signalling plays a prominent role during haematopoiesis: it modulates the developmental programme of human haematopoietic stem cells14,15 and controls the size and number of the haematopoietic stem cell niches.21 Deregulation of Smad molecules affects normal haematopoietic growth and leads to neoplastic haematopoiesis.22
Bone morphogenetic protein signals also regulate thymopoiesis6,23,24 and the development of thymocytes themselves.25–28 Thymocyte development requires reciprocal interactions between thymic stroma and developing thymocytes.29 Similarly, B-cell development requires reciprocal interactions between developing B-lymphocytes and bone marrow stroma.5 Previous studies have shown that thymic stroma-derived BMP2/4 inhibits T-cell development.25–27 Thymocytes can modulate the effect of BMP2/4 by expressing the evolutionarily conserved BMP modifier TWSG1 in a T-cell receptor (TCR) -dependent manner.26,30 Known target genes of Smad-dependent signalling are the inhibitor of differentiation (Id) gene family.31 Id proteins are important regulators of lymphocyte development32 lending further evidence for an important role of BMP signals in coordinating lymphocyte development.
In this study, we have mapped the site of expression for several BMP and BMP antagonists in the mouse thymus, as well as in subsets of developing T and B lymphocytes and examined the effects of conditional ablation of these two molecules in developing lymphocytes.
Materials and methods
Mice
Heterozygote Bmp4+/lacZ,33Bmp7+/lacZ,34gremlin+/lacZ,35 and Twsg1+/lacZ36 reporter mice have been used in this study. Mice carrying conditional allele (Twsg1fl/+)37) were crossed with FlpCre mice to remove the neo-cassette and were subsequently backcrossed to C57BL/6 mice for at least six to eight generations. The F1 progeny homozygous for the floxed allele (Twsg1fl/fl) were crossed with Twsg1wt/kovav::iCre mice to generate mice with a conditional loss of Twsg1 in the haematopoietic cell compartment (Twsg1fl/KOVav::iCre; designated as Twsg1cKO here). Mice carrying the conditional allele (Bmp7fl/+)38 were backcrossed into C57BL/6 mice for at least seven generations. The F1 progeny homozygous for the floxed allele (Bmp7fl/fl) were crossed with Bmp7wt/Δvav::iCre mice to generate mice with a conditional loss of Bmp7 in the haematopoietic cell compartment (Bmp7fl/Δvav::iCre; designated as Bmp7cKO here). Congenic C57BL/6-CD45.1 mice were provided by A. Potocnik (NIMR, London, UK). Mice were maintained at the Biomedical Sciences Research Centre (BSRC) Alexander. Fleming animal facility under specific pathogen-free conditions. Experiments on live animals were approved by the Hellenic Ministry of Rural Development (Directorate of Veterinary Services) and by BSRC Alexander Fleming's Animal Research and Ethics Committee for compliance with Federation of European Laboratory Animal Science Associations’ regulations.
LacZ staining and immunohistochemistry
O.C.T. (Optimal Cutting Temperature; BDH Chemicals, VWR, Vienna, Austria) embedded tissues were ‘snap frozen’ in liquid nitrogen steam; 6–7 μm cryostat sections were placed in gelatin-coated slides, air-dried, fixed in cold gluteraldehyde/formaldehyde or paraformaldehyde/acetone and incubated with 2 mg/ml X-gal (HT Biotechnology, Cambridge, UK) at 37° overnight. Subsequent staining was performed with the following antibodies CD3 (KT3), CD25 (7D4), CD205 (DEC-205) (NLDC) (all Biolegend, San Diego, CA, eBiosciences, San Diego, CA or BD Biosciences, Erembodegem, Belgium), Cytokeratin-8 (CK8) (Troma-1, DSHB, Iowa City, IA). Sections were developed using an appropriate ABC-HRP kit (Vector Laboratories, Burlingame, CA).
Flow cytometry and sorting
For surface antigens, staining was performed using standard procedures. The following antibodies were used CD4 (GK1.5, phycoerythrin-Cy5.5), CD8 [53-6.7, phycoerythrin (PE)], Ly6C (AL-21, FITC), CD31 (390, biotin), B220 (RA3-6B2, PE), CD19 [6D5, allophycocyanin (APC)], CD25 (PC61, Alexa-700), cKIT (2B7, PE-Cy7), FITC), CD45.1 (A20, APC-Cy7), CD45.2 (104, Alexa-700) (all Biolegend, eBioscience, or BD Biosciences), IgM [goat F(ab’)2; Southern Biotech, Birmingham, AL]. For Fluorescein di-β-d-Galactopyranoside (FDG) detection, labelled cells were incubated under hypotonic conditions with 2 mm FDG (Marker Gene Technologies, Eugene, OR) at 37° for 1 min. Aquisition was on FACS CantoII™ using facsdiva™ software (BD Biosciences). Analysis was performed using FlowJo™ (TreeStar, Ashland, OR). Double-negative (DN) thymocytes were sorted as lineage negative (CD4−, CD8−, CD11b−, CD45RB−, CD49b−, TER119−) after depletion using the IMag mouse CD4 lymphocyte enrichment sets (BD Biosciences) followed by FACS sorting on a FACS Aria (BD Biosciences).
Repopulation assay
Fetal liver cells (1 × 106) from 15.5dpc Bmp7Δ/Δ embryos along with fetal liver cells from 15.5dpc Bmp7+/Δ control embryos were injected into the tail-vein of the wild-type irradiated (1038 rads) CD45.1 mice. 7–8 weeks after transfer mice were sacrificed and reconstitution was monitored by flow cytometric analysis of peripheral blood using CD45.1/CD45.2 to gate specifically on fetal liver-derived cells.
Reverse transcription-PCR
RNA was extracted from wild-type thymus, bone marrow, liver and kidney and sorted sub-populations using Trizol (Invitrogen, Life Technologies Europe BV, Bleiswijk, Netherlands) and concentrations were determined by spectrophotometry. For each fraction 2 μg RNA was reverse transcribed into cDNA with superscript II (Invitrogen). Reverse transcription (RT) -PCR was performed in a solution containing 1× reaction buffer, 15mM MgCl2 (both Abgene, UK), 200μM dNTPs (GE Healthcare, UK), 0.5μM of each primer and 0.2 U ThermoPrime Taq polymerase (Abgene, UK). Cycling conditions were as follows: 94° for 5 min, then 35 cycles of 94° for 20 seconds, 60° for 30 seconds and 72° for 90 seconds. For primer sequences see supplemental methods.
Statistical analysis
Student's t-test was used for statistical analysis. Results with a P-value of < 0·05 were considered significant.
Results
Multiple components of the BMP signalling pathway are expressed in primary haematopoietic organs
To be able to address the potential role of BMP signalling for lymphopoeisis we first assessed the expression of various BMP and BMP antagonists in primary lymphoid organs. The RT-PCR analysis showed that Bmp2, Bmp4, Bmp5, Bmp6 and Bmp7 were all expressed in bone marrow whereas Bmp6 expression was not detectable in adult thymocytes. Complementary DNAs from lung and kidney were used as positive controls for the PCR (Fig. 1a). Similarly, expression of several BMP antagonists was found both in thymus and bone marrow, namely Gremlin, Twsg1 and Chordin. Noggin expression was restricted to the bone marrow and was not detected in the thymus (Fig. 1a). The analysis of FACS-purified thymocyte sub-populations by RT-PCR revealed that both Bmp7 and Twsg1 were expressed in all thymocyte subsets investigated, with somewhat stronger expression in the DN fractions. To confirm these findings we also used lacZ reporter mice to assess gene expression in thymocyte subsets by flow cytometry. We detected expression of Twsg1 in DN, double-positive (DP), CD4 and CD8 thymocyte subsets with the relatively highest signal in the DN cell compartment (Fig. 1c). FDG-based expression analysis, however, proved not to be sensitive enough to detect Bmp7 expression in the T-cell compartment (Fig. 1d). As expected, no expression could be detected for Bmp4 and Gremlin (not shown). The expression of BPM/BMP antagonists also in thymocytes suggests that BMP signalling might be actively regulating thymopoiesis.
Figure 1.
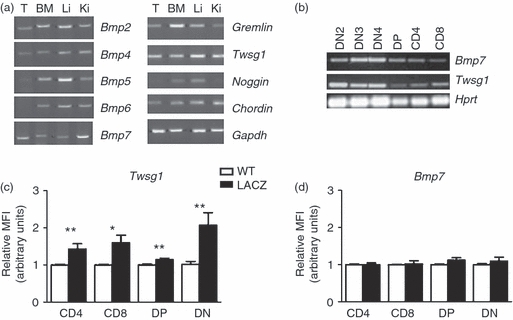
Expression of bone morphogenetic protein (BMP)/BMP antagonists in primary lymphoid organs. (a) reverse transcription-PCR analysis of BMPs, BMP antagonists in the thymus and bone marrow. Lung and kidney served as positive controls. (b) Bmp7 and Twsg1 expression on FACS sorted double-negative (DN) 2–4, double-positive (DP), single-positive (SP) CD4 and SP CD8 thymocyte populations. (c–d) FDG-based quantitative analysis of lacZ gene reporter activity by FACS on thymocyte subpopulations from Twsg1-lacZ (c), Bmp7-lacZ (d) and wild-type (wt) control mice showing significant levels of expression for Twsg1 in the SP CD4, SP CD8, DP and DN compartments in the thymus (n = 9 mice per group), while no significant differences were detected for Bmp7 (n = 5 mice per group). MFI, mean fluorescence intensity, *P < 0·05.
Compartmentalization of BMP/BMP antagonist expression in the adult thymus
To reveal the spatial distribution of the various BMP/BMP antagonists within the adult thymus we analysed lacZ reporter mice for Bmp4, Bmp7, Gremlin and Twsg1. Double staining was performed for LacZ and DEC-205 (CD205) or CK8 to distinguish between thymic cortex and medulla, respectively (Fig. 2). The topology of expression was clearly different for every gene and was typically seen both in the medulla and the cortex. A more systematic analysis of lacZ expression in relation to cell surface markers was performed, which is summarized in Figs 3 and 4.
Figure 2.
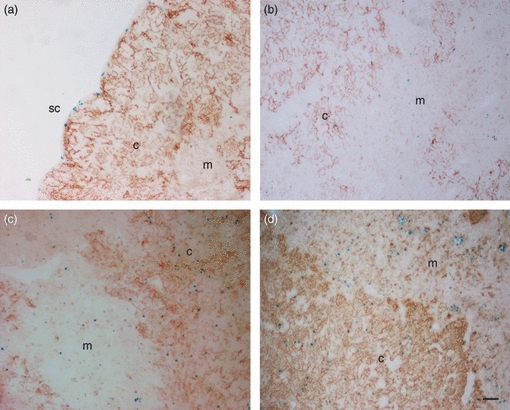
Bone morphogenetic protein (BMP)/BMP antagonist expression in cortex and medulla of the thymus. (a–d) X-gal-based detection of lacZ gene reporter activity for Bmp4 (a), Bmp7 (b), Twsg1 (c), Gremlin (d) (blue) on thymus sections combined with immunohistochemical staining for DEC-205 (CD205, NLDC; brown) to indicate cortical and medullary areas. c, cortex; m, medulla; sc, subcapsular zone (size bar 50 μm).
Figure 3.
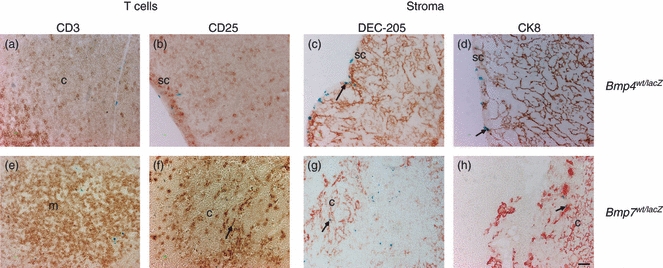
Identity of Bmp4 and Bmp7 expressing cells in the thymus. X-gal-based detection of lacZ gene reporter activity for Bmp4 (a–d) and Bmp7 (e–h) (blue) on thymus sections combined with immunohistochemical staining for for CD3 (a, e), CD25 (b, f), DEC-205 (c, g), cytokeratin8 (CK8) (d, h) (red or brown). c, cortex; m, medulla; sc, subcapsular zone (size bar 50 μm).
Figure 4.
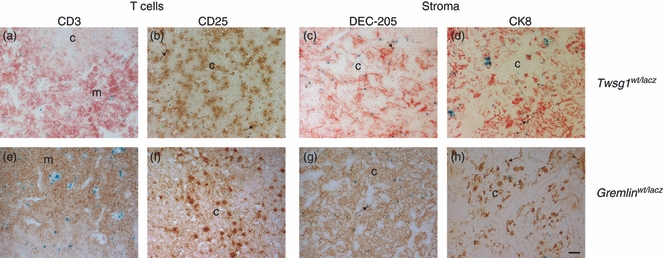
Identity of Twsg1 and Gremlin expressing cells in the thymus. X-gal-based detection of lacZ gene reporter activity for Twsg1 (a–d) and Gremlin (e–h) (blue) on thymus sections combined with immunohistochemical staining for for CD3 (a, e), CD25 (b, f), DEC-205 (c, g), cytokeratin8 (CK8) (d, h) (red or brown). c, cortex; m, medulla; sc, subcapsular zone (size bar 50 μm).
Bmp4 was mainly expressed in the vessels of the cortical region as well as in the sub-capsular region of the thymus (Fig. 3a–d) but not in the medullary area where single-positive CD4 or CD8 T cells reside. Bmp4 does not co-localize with CD25+ thymocytes in the cortical region (Fig. 3a,b) but was observed in dendritic cells and cortical thymic epithelial cells (Fig. 3c,d). Bmp7 was expressed both in the cortex and in the medulla. Expression was seen in some CD25+ cells (Fig. 3f) but not in CD3+ T cells (Fig. 3e). Furthermore, some dendritic cells and some CK8+ cortical thymic epithelial cells expressed Bmp7 (Fig. 3g,h). Twsg1 is abundantly expressed in both cortex and medulla of the adult thymus. It is seen in a few CD3+ cells in the medulla (Fig. 4a) as well as in CD25+ progenitor cells in the cortex (Fig. 4b). Dendritic cells and cortical thymic epithelial cells (Fig. 4c–d) also express Twsg1. Gremlin is also expressed in the cortex and medulla of the adult thymus. Expression was observed in dendritic cells and a few cortical thymic epithelial cells, but not in T cells or their progenitors (Fig. 4e–f).
BMP/BMP antagonist expression in the bone marrow
To complement the expression data in thymocytes we performed flow cytometry-based lacZ-expression analysis also on isolated bone marrow cells. We used Ly6C and CD31 to distinguish blast-like cells, lymphoid cells, myeloid cells, erythrocytes, granulocytes and monocytes.39 Bone marrow cells from Bmp7-lacZ mice showed weak FDG staining in a subset of lymphoid cells (Fig. 5b). No significant expression of Bmp4 was observed (Fig. 5a). Twsg1 expression was detected in nearly all subsets with highest expression in lymphoid cells, monocytes and granulocytes (Fig. 5c). Gremlin expression was detected in erythroid cells, granulocytes and monocytes. Expression in cells of lymphoid origin varied and was not significant (Fig. 5d). Hence, the expression of various BMP/BMP antagonists in different haematopoietic cell subsets strongly suggests that BMP signalling plays an active role in haematopoiesis.
Figure 5.
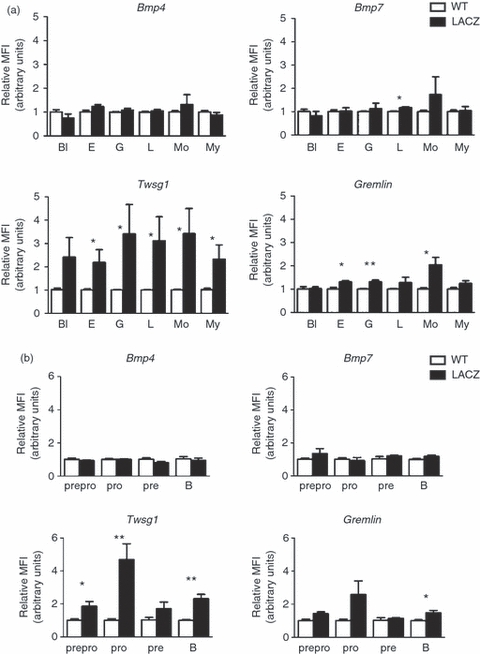
Bmp7, Twsg1 and Gremlin are expressed in haematopoietic cells in the bone marrow. (a) FDG-based quantitative analysis of lacZ gene reporter activity of Bmp4, Bmp7, Twsg1 and Gremlin by FACS on bone marrow subpopulations identified by Ly6C/CD31 staining.55 (a) or B-cell subpopulations identified by B220/CD19/cKIT/CD252 (b). (a) Significant expression for Bmp7 was observed in lymphoid cells (n = 4 mice per group), for Twsg1 in erythroid cells, granulocytes, lymphoid and myeloid cells as well as monocytes (n = 5 mice per group), for Gremlin in the erythroid cells, granulocytes and monocytes (n = 4 mice per group), while no expression of Bmp4 was detected (n = 4 mice per group). (b) Significant expression for Twsg1 was observed in pre-pro (B220+/cKIT−/CD19−), pro B220+/CD19+/cKIT+/CD25+) and B cells (B220+/CD19+/cKIT−/CD25−) whereas Gremlin expression was limited to B cells (B220+/CD19+/cKIT−/CD25−). Bl, blast-like cells; L, lymphocytes; E, erythrocytes; My, myeloid precursor cells; G, granulocytes; Mo, monocytes; MFI, mean fluorescence intensity, *P< 0·05.
A more detailed analysis of expression in various B-cell subsets revealed as expected no expression for Bmp4 but also no clear expression for Bmp7. In contrast, expression of Twsg1 was dynamic, with peak expression seen in pro B cells. Some Twsg1 expression was also visible in the pre-pro subset and in mature B cells (Fig. 5b). The expression of Gremlin was variable and significant expression was only observed in mature B cells (Fig. 5b).
Steady-state lymphopoiesis is unperturbed in Bmp7fl/Δvav::iCre mice
Subsequently, we focused our analysis on two members of the BMP signalling family, Bmp7 and Twsg1, and asked whether their deletion from all haematopoietic cells would affect steady-state lymphopoiesis in vivo. For this we generated mice lacking Bmp7 in all haematopoietic cells (Bmp7cKO) by crossing a conditional Bmp7 allele38 to the vav::iCre deleter line.40 Bmp7cKO animals had normal weight and appeared healthy. Analysis of 4- to 6-week-old mice revealed that deletion of Bmp7 had no apparent effects on thymocyte development. The numbers and percentages of progenitor DN, DP, CD4 single-positive (SP) and CD8 SP thymocyte subsets were not significantly altered when compared with littermate control mice (Fig. 6a). Analysis of the bone marrow from 6- to 8-week-old mice showed that the cell counts of blast-like cells, erythroid cells, granulocytes, lymphocytes, monocytes and myeloid cells were all normal when compared with control mice (Fig. 6b). To extend our studies we next transplanted fetal liver cells from 15.5dpc Bmp7-null or control embryos in lethal irradiated, allotypically marked recipient mice. Bmp7-deficient mice, obtained by germline deletion of the Bmp7fl/fl-allele (Bmp7Δ/Δ,38) survive beyond the embryonic stage E15.5. Transfer of Bmp7-null cells resulted in > 80% reconstitution of recipient's immune system by 7–8 weeks. Absence of Bmp7 did not result in any apparent defects of thymocyte subsets. Similarly, analysis of bone marrow cells revealed unperturbed populations of blast cells, erythroid cells, granulocytes, lymphocytes, monocytes and myeloid cells (see Supplementary material, Fig. S1). Taken together, the results obtained by a lineage-specific deletion of Bmp7 and by the transplantation of fetal Bmp7-null progenitors reveals that BMP7 secretion from any haematopoietic subset is dispensable for lymphopoiesis per se.
Figure 6.
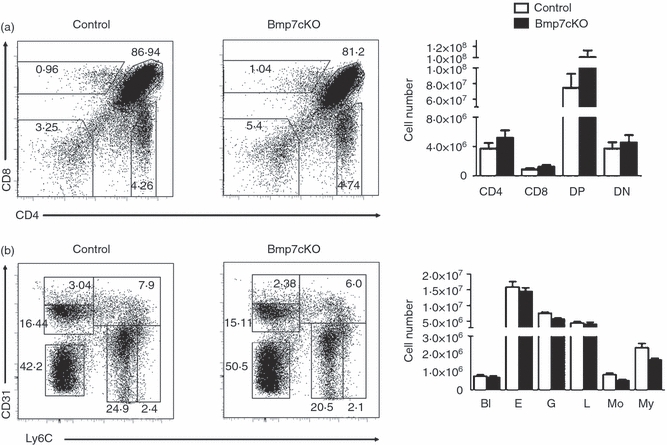
Normal lineage distribution in Bmp7cKO mice in thymus and bone marrow. Analysis of thymocyte (a) and bone marrow (b) subpopulations and graphical representation of cell numbers from Bmp7cKO and littermate control mice. (n = 4 mice per group). The results are representative for more than three independent experiments. (a) Thymocyte populations were detected by CD4/CD8 staining and gated as double-negative (DN), double-positive (DP), CD4 single-positive (SP), CD8 SP subpopulations. (b) Bone marrow populations were detected by CD31/Ly6C staining.55 Percentages and total numbers of the various subsets were unaltered (n = 4 mice per group). The results are representative for more than three independent experiments. Bl, blast-like cells; L, lymphocytes; E, erythrocytes; My, myeloid precursor cells; G, granulocytes; Mo, monocytes.
Steady-state lymphopoiesis is unperturbed in Twsg1fl/KOvav::iCre mice
Twsg1, similar to Bmp7, is expressed both by thymocytes and by thymic stroma cells. In vitro analyses have shown that Twsg1 regulates thymocyte development at the DN and DN to DP transitional stage.26,27 As Twsg1-deficient mice display defects in lymphopoiesis41 we sought to determine whether these defects are the result of a lack of Twsg1 in the lymphoycte precursor cells or the respective stroma cells. For this we generated mice lacking Twsg1 in all haematopoietic cells (Twsg1cKO) by crossing a conditional Twsg1fl/fl allele37 to the vav::iCre deleter line.40 Twsg1cKO animals appeared normal and remained healthy for up to 10–12 months. Analysis of thymocyte subsets Twsg1cKO or control mice aged 4–6 weeks by flow cytometry revealed that absence of Twsg1 in the haematopoietic cell subset did not affect thymocyte development. The DN, DP, CD4 SP and CD8 SP thymocyte subsets were not altered (Fig. 7a). This indicated that lack of stroma-derived rather than thymocyte-derived Twsg1 is responsible for the lymphoid defects observed in Twsg1-deficient mice.41 Similarly, analysis of bone marrow cells mice aged 6–8 weeks showed that the relative number of blast-like cells, erythroid cells, granulocytes, lymphocytes, monocytes and myeloid cells was not altered (Fig. 7b). Moreover, analysis of developing B cells showed that pre-pro (B220+ CD19− cKIT− CD25− IgM−), pro (B220+ CD19+ cKIT+ CD25+ IgM−), pre (B220+ CD19+ cKIT− CD25+ IgM+), immature (B220+ CD19+ cKIT− CD25− IgM+) cells remained unaltered (Fig. 7c). These data indicate that despite its abundant expression in thymocytes and B-lymphocyte precursors, lymphopoiesis is not critically dependent on Twsg1 expression in the haematopoietic compartment.
Figure 7.
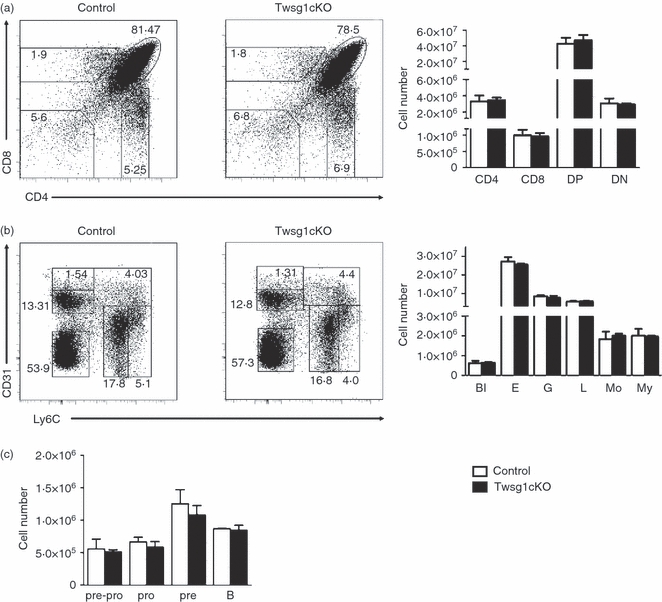
Normal lineage distribution in Twsg1cKO mice in thymus and bone marrow. Analysis of thymocyte (a) and bone marrow (b) subpopulations and graphical representation of cell numbers from Twsg1cKO and littermate control mice. (n = 3 mice per group). The results are representative for more than three independent experiments. (a) Thymocyte populations were detected by CD4/CD8 staining and gated as double-negative (DN), double-positive (DP), CD4 single-positive (SP), CD8SP subpopulations. (b) Bone marrow populations were detected by CD31/Ly6C staining.55 Percentages and cell numbers of the various subsets were unaltered (n = 4 mice per group). The results are representative for more than three independent experiments. (c) B-cell subpopulations identified by B220/CD19/cKIT/CD25/IgM staining.2 Percentages and cell numbers of the various subsets were unaltered (n = 3 mice per group). Bl, blast-like cells; L, lymphocytes; E, erythrocytes; My, myeloid precursor cells; G, granulocytes; Mo, monocytes.
Discussion
The importance of BMP signalling for the development and homeostasis of thymus and bone marrow is clearly documented.6,24,26,28,41 Several different BMPs (BMP2, BMP4, BMP5, BMP6 and BMP7) and BMP antagonists (Noggin, Gremlin, Twsg1 and Chordin) are expressed both in thymus and the bone marrow. Using LacZ reporter mice for several BMP/BMP antagonists in combination with histochemistry we have mapped the expression of Bmp4, Bmp7, Gremlin and Twsg1 in the thymus and developing lymphocytes revealing highly unique expression pattern for every gene analysed. Bmp4 was mainly expressed along vessels of the cortical region as well as in the sub-capsular region of the thymus but not in the medullary area, where single-positive T cells reside. In contrast Bmp7 expression was observed both in the cortex and in the medulla, in epithelial cells, dendritic cells, as well as some developing lymphocytes. Both Twsg1 and Gremlin were abundantly expressed in cortex and medulla. Expression of both molecules was noted in epithelial cells and dendritic cells. As expected, Twsg1 was also found in developing thymocytes. It is noteworthy that Twsg1 expression in the cortex was not uniform but was confined to distinct areas. This could indicate a hitherto unrecognized functional compartmentalization of the thymic cortex. Twsg1 might also differentially affect CD4 and CD8 T cells. We frequently observed CD3+ T cells in close vicinity to Twsg1+ medullary stroma cells, but we never saw this for CD8+ T cells. Though we mapped expression for only a few BMP/BMP antagonists, this limited survey was sufficient to indicate an unexpected complexity of BMP signalling in the adult thymus where multiple BMP signalling networks regulate thymus homeostasis and thymocyte development. With respect to the thymocytes, BMP/BMP antagonist expression appeared compartmentalized in cortical and medullary areas of the thymus suggesting that BMP signals might affect all stages of T-cell development.
Relatively little is known about how BMP signals regulate the various aspects of thymocyte differentiation.42In vitro experiments have established that BMP2/4 negatively affect the transition from the DN2 to the DN3 and from the DN to the DP stage. Addition of exogenous BMP antagonists such as Noggin or Chordin/Twisted Gastrulation reversed these effects.26,27 Hence, the balance between BMP/BMP antagonists might regulate T-cell development within the various thymic microenvironments in vivo. Thereby, BMP signals could be acting on thymocytes directly or indirectly via the thymic stroma.
One prominent example involving direct signalling of BMPs is their regulation of Id (Inhibitor of differentiation) gene expression.43 The Ids inhibit the E-proteins E47 and E12, two important regulators of thymocyte development, and have been mapped downstream of TCR signalling.44 Over-expression of a dominant negative form of Id3 in human T lineage precursor cells blocks early T-cell development.45 In addition, the observation that the BMP antagonist Twsg1 is up-regulated following pre-TCR engagement26 might also be regarded as evidence for a direct effect of BMP signals on developing thymocytes. As the degradation of E-proteins is regulated by Notch signalling,46 Id/E protein activity might be an integration platform of various major signalling pathways.
Futhermore, BMPs appear to be important regulators of the thymic stroma per se6 acting mainly indirectly by engaging several cellular signalling pathways. This can be achieved in part through regulating FoxN1 gene expression as was shown for the embryonic thymic stroma.47 Whether this mechanism also operates in the adult thymus needs to be shown. Comparisons with other organs would indicate that BMP signalling can interact with all major developmental signalling pathways such as Notch, wnt/b-catenin, Hh and FGF. All these pathways have established roles in thymus and thymocyte development on their own,6–9 but comparatively little is known on reciprocal interactions between these pathways. Interestingly, BMP4 reportedly can modulate FGF7/FGF1024 in the thymic stroma. In addition, BMP signals might also interfere with cytokine signalling, as shown for BMP4, which negatively regulates interleukin-7-mediated development of thymic progenitor cells.28
With respect to BMP/BMP antagonist expression in developing thymocytes only Bmp7 and Twsg1 were detected. They were expressed in all thymocyte subsets investigated, and for both expression was strongest in DN thymocyte subsets. Twsg1 expression in DN thymocyte subsets had been reported previously.26,28 In this report we show that Twsg1 is also expressed in DP, CD4SP, and CD8SP thymocytes. Twsg1 can be induced in DP thymocytes in a TCR-dependent manner,26 so its expression might reflect their activation. Twsg1 was also dynamically expressed in various subsets of developing B cells. The highest expression was observed in pro B cells, which like the DN2 thymocyte population are known to require cytokine survival signals, most prominantly interleukin-7.48,49 As BMP4 can counteract this interleukin-7-induced proliferation and differentiation,28Twsg1 expression in these subsets could serve to overcome a BMP-mediated proliferative block, in a similar manner to its role in regulating the DN to DP transition in thymocytes.26Twsg1 was also expressed in all other haematopoietic subsets analysed, indicating a function for BMP signalling also for their development.
The expression of Bmp7, which was strongest in the DN3/DN4 thymocyte subsets, could not be confirmed in thymocytes or bone marrow cells using the Bmp7-lacZ reporter mouse.34 Possible explanations for this might be low expression and/or insufficient sensitivity of the lacZ reporter line caused by the incidental disruption of a lymphocyte specific enhancer when inserting the lacZ gene into the Bmp7 locus or alternatively, by the silencing of promoter structures instigated by the presence of the lacZ gene.
In the bone marrow, BMP signalling has mainly been associated with a role in regulating the stem cell-niche synapse.50 The expression of Bmp7, Twsg1 and Gremlin in developing haematopoietic cells would therefore suggest that Bmp signalling regulates the maturation of all haematopoietic cell lineages. This might be direct by regulating their development or indirect by providing signals to their niche or to the close cellular environment.
In the thymus, developing lymphocytes need to interact with thymic stroma cells for a functional organ to develop.29 Hence, the necessity for reciprocal cross-talk is well established in this organ. Thymic epithelial cells are constantly regenerated from a pool of stem/progenitor cells in the adult thymus making the thymus a more dynamic structure than previously assumed.51 It has been shown that BMP signals maintain the expression of the critical FoxN1 gene in embryonic thymic stroma.47 Currently, it is not known whether in the adult thymus FoxN1 expression is also regulated by BMP signals, and if it is, what is the identity and source of this BMP signal. It will be of particular interest to establish whether lymphocyte-derived BMP signals participate in this process.
Bmp7-deficient mice are not viable38,52,53 and the in vivo requirements for Twsg1 are background dependent. Whereas Twsg1-deficient mice are viable and show impaired lymphocyte development on some genetic backgrounds,41 about 44% of Twsg1 null mutants on the C57BL/6 background die in utero and display craniofacial malformations of variable severity (ref. 37, and own observations). Conditional deletion of Bmp7 and Twsg1 in all haematopoietic cells using the vav::iCre resulted in viable and healthy mice with normal cellularity of the lymphoid organs. We also analysed 10- to 12-month-old Bmp7cKO and Twsg1cKO mice to assess potential late-onset effects, but still did not observe any alterations to the haematopoietic compartments (data not shown). This indicated that bone-marrow stroma-derived Twsg1 rather than haematopoietic cell-derived Twsg1 might be responsible for the lymphoid defects observed in Twsg1 null mice.41
To extend these results we transplanted Bmp7-deficient fetal liver cells into lethally irradiated wild-type recipients, which revealed that Bmp7 was even dispensable for the development of embryonic haematopoietic stem cells. Taken together, these data suggest that although Twsg1 and Bmp7 are dynamically expressed in lymphoid and some myeloid cells, they appear to be redundant for steady-state lymphoid and myeloid development. How can this be reconciled with published in vitro data where Twsg1 has been shown to counteract BMP2/4, which are both negative regulators of lymphopoiesis?26–28 One obvious possibility is their compensation from other cellular sources or functional redundancy. Stromal cells also produce Twsg1 and BMP7. This compartment is seemingly the critical contributor of Twsg1 in vivo given the overall undisturbed lymphopoiesis in Tswg1fl/flvav::iCre animals. Since Bmp7-deficient animals die in late gestation or shortly after birth38 we have no information on its role for T-cell or B-cell development. Some preliminary analysis of fetal Bmp7-deficient thymi indicates altered thymocyte development in the absence of Bmp7 (O. Passa and D. Graf, unpublished results). Compensation by other BMPs, such as BMP5 or BMP6 that belong to the same BMP subgroup (glass-bottom-boat orthologues) is also a possibility. In contrast, a functional compensation by BMP2/4 (decapentaplegic orthologues) is less likely, as BMP7 itself did not recapitulate the inhibitory effects of BMP2/4 on thymocyte proliferation and differentiation.26 Why would developing lymphocytes express BMP/BMP antagonists if their function appears to be redundant? Our analysis of Twsg1 and Bmp7 deficiency was restricted to steady-state lymphoid development. Even if lymphoid development per se is not affected, the functional properties of the mature B and T cells might be altered in their absence. For instance, B-cell-derived Twsg1 is not required for B-cell development per se, but is involved in regulating T-cell-independent plasma B-cell production and function.54 Hence, BMP signalling might fine-tune lymphocyte development, which could be achieved by altering cytokine or TCR/BCR signalling thresholds. As a consequence, absence of these molecules might affect repertoire selection or recruiting into different functional subsets. On the other hand, thymic epithelial cells might also be targets of lymphocyte-derived BMP signals. Both cortical and medullary epithelial cells are continuously replenished from epithelial stem/precursor cells.51 It is therefore feasible that lymphocyte-derived BMP signals are part of the thymocyte–thymic stroma cross-talk and contribute to the maintenance of a functional thymus.
Acknowledgments
This work was supported by the Joint Research and Technology Programme between the Greek Ministry for Technology and Development and the British Council (to DG and AJP) and the Association for International Cancer Research (to DG). DG is grateful to the Biomedical Sciences Research Center Alexander Fleming, at which the project was initiated and a substantial amount of the experiments were performed.
Disclosures
The authors have no financial conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Normal lineage distribution of Bmp7Δ/Δ cells after bone marrow transplantation of E15.5 fetal liver Bmp7Δ/Δ cells.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4:278–89. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–16. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 3.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Vicente R, Swainson L, Marty-Gres S, De Barros SC, Kinet S, Zimmermann VS, Taylor N. Molecular and cellular basis of T cell lineage commitment. Semin Immunol. 2010;22:270–5. doi: 10.1016/j.smim.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zetterblad J, Qian H, Zandi S, et al. Genomics based analysis of interactions between developing B-lymphocytes and stromal cells reveal complex interactions and two-way communication. BMC Genomics. 2010;11:108. doi: 10.1186/1471-2164-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleul CC, Boehm T. BMP signaling is required for normal thymus development. J Immunol. 2005;175:5213–21. doi: 10.4049/jimmunol.175.8.5213. [DOI] [PubMed] [Google Scholar]
- 7.Siggins SL, Nguyen NY, McCormack MP, Vasudevan S, Villani R, Jane SM, Wainwright BJ, Curtis DJ. The Hedgehog receptor Patched1 regulates myeloid and lymphoid progenitors by distinct cell-extrinsic mechanisms. Blood. 2009;114:995–1004. doi: 10.1182/blood-2009-03-208330. [DOI] [PubMed] [Google Scholar]
- 8.Tanigaki K, Honjo T. Regulation of lymphocyte development by Notch signaling. Nat Immunol. 2007;8:451–6. doi: 10.1038/ni1453. [DOI] [PubMed] [Google Scholar]
- 9.Timm A, Grosschedl R. Wnt signaling in lymphopoiesis. Curr Top Microbiol Immunol. 2005;290:225–52. doi: 10.1007/3-540-26363-2_10. [DOI] [PubMed] [Google Scholar]
- 10.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 11.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–78. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–8. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 13.Wagner DO, Sieber C, Bhushan R, Borgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3 doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189:1139–48. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durand C, Robin C, Bollerot K, Baron MH, Ottersbach K, Dzierzak E. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc Natl Acad Sci U S A. 2007;104:20838–43. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–44. [PubMed] [Google Scholar]
- 17.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–9. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265–76. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- 19.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 20.Nickel J, Sebald W, Groppe JC, Mueller TD. Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev. 2009;20:367–77. doi: 10.1016/j.cytogfr.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 22.Zavadil J, Brezinova J, Svoboda P, Zemanova Z, Michalova K. Smad5, a tumor suppressor candidate at 5q31.1, is hemizygously lost and not mutated in the retained allele in human leukemia cell line HL60. Leukemia. 1997;11:1187–92. doi: 10.1038/sj.leu.2400750. [DOI] [PubMed] [Google Scholar]
- 23.Patel SR, Gordon J, Mahbub F, Blackburn CC, Manley NR. Bmp4 and Noggin expression during early thymus and parathyroid organogenesis. Gene Expr Patterns. 2006;6:794–9. doi: 10.1016/j.modgep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Tsai PT, Lee RA, Wu H. BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood. 2003;102:3947–53. doi: 10.1182/blood-2003-05-1657. [DOI] [PubMed] [Google Scholar]
- 25.Cejalvo T, Sacedon R, Hernandez-Lopez C, et al. Bone morphogenetic protein-2/4 signalling pathway components are expressed in the human thymus and inhibit early T-cell development. Immunology. 2007;121:94–104. doi: 10.1111/j.1365-2567.2007.02541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graf D, Nethisinghe S, Palmer DB, Fisher AG, Merkenschlager M. The developmentally regulated expression of Twisted gastrulation reveals a role for bone morphogenetic proteins in the control of T cell development. J Exp Med. 2002;196:163–71. doi: 10.1084/jem.20020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hager-Theodorides AL, Outram SV, Shah DK, Sacedon R, Shrimpton RE, Vicente A, Varas A, Crompton T. Bone morphogenetic protein 2/4 signaling regulates early thymocyte differentiation. J Immunol. 2002;169:5496–504. doi: 10.4049/jimmunol.169.10.5496. [DOI] [PubMed] [Google Scholar]
- 28.Varas A, Sacedon R, Hidalgo L, et al. Interplay between BMP4 and IL-7 in human intrathymic precursor cells. Cell Cycle. 2009;8:4119–26. doi: 10.4161/cc.8.24.10149. [DOI] [PubMed] [Google Scholar]
- 29.van Ewijk W, Hollander G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583–91. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 30.Graf D, Timmons PM, Hitchins M, et al. Evolutionary conservation, developmental expression, and genomic mapping of mammalian Twisted gastrulation. Mamm Genome. 2001;12:554–60. doi: 10.1007/s0033501-0005-x. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Rovira T, Chalaux E, Massague J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–85. doi: 10.1074/jbc.M106826200. [DOI] [PubMed] [Google Scholar]
- 32.Engel I, Murre C. The function of E- and Id proteins in lymphocyte development. Nat Rev Immunol. 2001;1:193–9. doi: 10.1038/35105060. [DOI] [PubMed] [Google Scholar]
- 33.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–36. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godin RE, Takaesu NT, Robertson EJ, Dudley AT. Regulation of BMP7 expression during kidney development. Development. 1998;125:3473–82. doi: 10.1242/dev.125.17.3473. [DOI] [PubMed] [Google Scholar]
- 35.Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet. 2003;34:303–7. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 36.Gazzerro E, Deregowski V, Stadmeyer L, Gale NW, Economides AN, Canalis E. Twisted gastrulation, a bone morphogenetic protein agonist/antagonist, is not required for post-natal skeletal function. Bone. 2006;39:1252–60. doi: 10.1016/j.bone.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 37.Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O'Connor MB. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev Biol. 2004;267:374–86. doi: 10.1016/j.ydbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Zouvelou V, Passa O, Segklia K, Tsalavos S, Valenzuela DM, Economides AN, Graf D. Generation and functional characterization of mice with a conditional BMP7 allele. Int J Dev Biol. 2009;53:597–603. doi: 10.1387/ijdb.082648vz. [DOI] [PubMed] [Google Scholar]
- 39.de Bruijn MF, van Vianen W, Ploemacher RE, Bakker-Woudenberg IA, Campbell PA, van Ewijk W, Leenen PJ. Bone marrow cellular composition in Listeria monocytogenes infected mice detected using ER-MP12 and ER-MP20 antibodies: a flow cytometric alternative to differential counting. J Immunol Methods. 1998;217:27–39. doi: 10.1016/s0022-1759(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 40.de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–25. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 41.Nosaka T, Morita S, Kitamura H, et al. Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol Cell Biol. 2003;23:2969–80. doi: 10.1128/MCB.23.8.2969-2980.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Licona-Limon P, Soldevila G. The role of TGF-beta superfamily during T cell development: new insights. Immunol Lett. 2007;109:1–12. doi: 10.1016/j.imlet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002;2002 doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- 44.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–86. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 45.Heemskerk MH, Blom B, Nolan G, Stegmann AP, Bakker AQ, Weijer K, Res PC, Spits H. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J Exp Med. 1997;186:1597–602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nie L, Xu M, Vladimirova A, Sun XH. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 2003;22:5780–92. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soza-Ried C, Bleul CC, Schorpp M, Boehm T. Maintenance of thymic epithelial phenotype requires extrinsic signals in mouse and zebrafish. J Immunol. 2008;181:5272–7. doi: 10.4049/jimmunol.181.8.5272. [DOI] [PubMed] [Google Scholar]
- 48.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 51.Anderson G, Jenkinson EJ, Rodewald HR. A roadmap for thymic epithelial cell development. Eur J Immunol. 2009;39:1694–9. doi: 10.1002/eji.200939379. [DOI] [PubMed] [Google Scholar]
- 52.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 53.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 54.Tsalavos S, Segklia K, Passa O, Petryk A, O'Connor MB, Graf D. Involvement of twisted gastrulation in T cell-independent plasma cell production. J Immunol. 2011;186:6860–70. doi: 10.4049/jimmunol.1001833. [DOI] [PubMed] [Google Scholar]
- 55.de Bruijn MF, Slieker WA, van der Loo JC, Voerman JS, van Ewijk W, Leenen PJ. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol. 1994;24:2279–84. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


