Abstract
Pulmonary hypertension in the neonate is associated with multiple underlying problems such as respiratory distress syndrome, meconium aspiration syndrome, congenital diaphragmatic hernia, bronchopulmonary dysplasia, sepsis, or congenital heart disease. Because of the heterogeneous group of disorders, the therapeutic approach and response often depends on the underlying disease. In many of these conditions, there is evidence that cyclic nucleotide signaling and specifically phosphodiesterases (PDEs) are disrupted. PDE inhibitors represent an emerging class of pulmonary vasodilators in adults. Studies are now under way to evaluate the utility, efficacy, and safety of such therapies in infants with pulmonary hypertension.
Keywords: Bronchopulmonary dysplasia, cAMP, cGMP, Congenital diaphragmatic hernia, Nitric oxide, Persistent pulmonary hypertension of the newborn, Phosphodiesterase, Prostacyclin
1 Phosphodiesterases in the Pathophysiology of Neonatal Pulmonary Hypertension
1.1 Normal Pulmonary Vascular Transition
Pulmonary hypertension is normal during fetal life. Because the placenta, not the lung, serves as the organ of gas exchange, only 10% of the cardiac output is circulated through the pulmonary vascular bed. In utero, pulmonary pressures are equivalent to systemic pressures due to elevated pulmonary vascular resistance. As gestation and fetal lung growth progress, rapid vascular growth increases the number of small pulmonary arteries within the lung by tenfold, preparing the lungs to accept the dramatic increase in blood flow that occurs at birth (Levin et al. 1976). Despite this increase in vascular surface area, pulmonary vascular resistance actually increases with gestational age when corrected for lung or body weight. These findings indicate that vascular constriction must play a key role in maintaining high pulmonary vascular tone during fetal life. There are multiple pathways involved in maintaining high pulmonary vascular tone in utero. Some of the known pulmonary vasoconstrictors include hypoxia (a defining feature of fetal development), as well as endothelin-1 (ET-1), thromboxane, acidosis, and various mediators of inflammation (Lakshminrusimha and Steinhorn 1999).
As gestation progresses, the mediators of pulmonary vasodilation become more dominant. In particular, pulmonary expression of endothelial nitric oxide (NO) synthase (eNOS), inducible NO synthase (iNOS), and NO production increase near the time of birth (Abman et al. 1990; Shaul et al. 2002). Coincident increases in soluble guanylate cyclase (sGC) activity promote increased vascular cGMP, which then leads to vasorelaxation via decreasing intracellular calcium (Bloch et al. 1997). Another potentially important vasodilatory pathway in the fetal lung is the prostacyclin pathway. Cyclooxygenase (COX) is the rate-limiting enzyme that generates prostacyclin from arachadonic acid. COX-1 in particular is upregulated in late gestation (Brannon et al. 1994, 1998), leading to an increase in prostacyclin production in late gestation and early postnatal life (Brannon et al. 1994; Leffler et al. 1984). Prostacyclin upregulates adenylyl cyclase to increase intracellular cAMP levels, which then lead to vasorelaxation.
Phosphodiesterases (PDEs) counteract these cAMP and cGMP vasodilatory pathways, and as described extensively within this text, they comprise a super-family of enzymes that includes 11 different PDE families with specific tissue and cellular distributions (Conti and Beavo 2007; Lugnier 2006). The prevalent PDE within the lung is PDE5, although there are significant amounts of PDE1, PDE3, and PDE4 as well (Maclean et al. 1997). PDE5, a cGMP-specific PDE, was initially characterized in bovine lung and has since been found in multiple other tissues (Loughney et al. 1998). In rats, PDE5 expression and activity steadily increase through the end of gestation, peak on day of life one, and then drop dramatically into adulthood, strongly suggesting developmental regulation (Sanchez et al. 1998). In contrast, in neonatal lambs, PDE5 activity and expression appear to acutely decrease within 1 h after birth and then rise again at 4–7 days of life (Farrow et al. 2008a; Hanson et al. 1998a; Okogbule-Wonodi et al. 1998; Sanchez et al. 1998). Furthermore, PDE5 activity is higher in pulmonary arteries than pulmonary veins in lambs, suggesting location-dependent as well as developmental regulation (Okogbule-Wonodi et al. 1998). As the primary enzyme responsible for regulating cGMP, PDE5 potentially represents the most important regulator of NO-mediated vascular relaxation in the normal pulmonary vascular transition after birth (Abman et al. 1990; Lakshminrusimha and Steinhorn 1999).
Unlike PDE5, less is known about the developmental regulation of other cGMP PDEs in the fetal and neonatal lung. PDE1, a dual specificity PDE, consists of three isoforms in mammals; PDE1A and PDE1B have higher affinity for cGMP but hydrolyze both cyclic nucleotides with similar efficacy. In contrast, PDE1C hydrolyzes cGMP and cAMP with equal affinity and rate. All three isoforms of PDE1 have been described in the pulmonary vasculature of various animals and in human pulmonary artery smooth muscle cells (Evgenov et al. 2006). While their presence has been documented in older animals and adult humans, there has been little study of PDE1 expression or activity in the perinatal period. A recent study in neonatal mice indicates that PDE1A mRNA decreased postnatally in normoxia, but was increased by exposure to hyperoxia for 21 days, although PDE1A protein expression remained unchanged (Woyda et al. 2009). Thus, regulation of PDE1 in the neonatal pulmonary vasculature is unclear, and further investigation is needed.
As described above, prostacyclin–cAMP signaling operates in parallel to the NO–cGMP pathway for perinatal pulmonary vasodilation and is regulated in part by cAMP-hydrolyzing PDEs such as PDE3 and PDE4. Our group recently published that PDE3A expression and activity in the resistance pulmonary arteries increase dramatically by 24 h after birth. These results were surprising and unexpected, as we would have predicted that similar to PDE5, PDE3 activity would decrease after birth to facilitate increased cAMP levels (Chen et al. 2009). This increase may be acting to establish cAMP-containing regulatory regions within the pulmonary vascular smooth muscle cell after birth, although it is unclear what role PDE3 has in normal pulmonary vascular transition after birth.
Even less is known about the perinatal regulation of PDE4, which has strong specificity for cAMP hydrolysis. PDE4 in the lung has been most extensively studied in the airway smooth muscle (Fan Chung 2006). However, more recent studies have demonstrated the presence of PDE4 in adult human pulmonary artery smooth muscle cells and that exposure to hypoxia increases expression of several PDE4 isoforms without impacting total PDE4 activity (Millen et al. 2006). Thus, while no one has specifically examined PDE4 around the time of birth, it is plausible to hypothesize that PDE4 might be differentially regulated between the relatively hypoxic in utero environment and the normoxic extrauterine environment.
At birth, a rapid and dramatic decrease in pulmonary vascular resistance allows half of the combined ventricular output to be redirected from the placenta to the lung, leading to an eight- to tenfold increase in pulmonary blood flow. The stimuli that seem to be most important are lung inflation with a gas, a decrease in carbon dioxide tension, and an increase in oxygen tension. Each of these stimuli will independently decrease PVR and increase pulmonary blood flow, with the largest effects seen when the two events occur simultaneously. For instance, oxygen directly and indirectly stimulates the activity of both eNOS and COX-1 immediately after birth, leading to increased levels of the vasodilators, NO and prostacyclin (Shaul et al. 1992; Shaul and Wells 1994; Steinhorn et al. 1994). Shear stress is also known to regulate the synthesis of NO in the fetal circulation. During transition, the initial increase in pulmonary blood flow in response to ventilation or oxygenation likely leads to increased shear stress in the vasculature, which further potentiates NO production (Uematsu et al. 1995). In contrast, PDE5 expression and activity fall after birth in the pulmonary vasculature, further accentuating upstream effects leading to increased cGMP and vasodilation (Farrow et al. 2008a; Sanchez et al. 1998). It is unclear which specific signals cause PDE5 to fall as part of the normal transition. However, if events in utero and at the time of birth impair these critical transition steps, they may lead to elevated pulmonary pressures and the symptomatic infant with persistent pulmonary hypertension of the newborn (PPHN).
1.2 Pathophysiology of Persistent Pulmonary Hypertension of the Newborn
When the normal cardiopulmonary transition fails to occur, the result is PPHN. PPHN describes a syndrome characterized by common pathophysiologic features including sustained elevation of pulmonary vascular resistance and hypoxemia due to right-to-left extrapulmonary shunting of blood flow across the ductus arteriosus or foramen ovale. PPHN affects 2–6 per 1,000 live births or approximately 10% of all infants admitted to neonatal intensive care and is accompanied by an 8–10% risk of death and significant short-term and long-term morbidity (Walsh-Sukys et al. 2000). The physiologic findings of PPHN may be found in association with a wide range of cardiopulmonary disorders such as meconium aspiration, sepsis, pneumonia, asphyxia, congenital diaphragmatic hernia (CDH), respiratory distress syndrome, and others. Pathological findings include pulmonary vascular remodeling and smooth muscle hyperplasia, often in the absence of significant lung parenchyma pathology (Haworth 1988; Murphy et al. 1981). PPHN can largely be thought of as one of three types: (1) the abnormally constricted pulmonary vasculature, which is the most common type and includes diagnoses such as meconium aspiration syndrome (MAS), respiratory distress syndrome, and sepsis; (2) the structurally abnormal vasculature, which is often termed idiopathic PPHN; or (3) the hypoplastic vasculature such as is seen in CDH or alveolar capillary dysplasia, a rare malformation of lung development. The pathophysiology of each type is dependent on the point in gestation when the normal transition to extrauterine life fails. Thus, since the underlying pathophysiology differs, different pulmonary vasodilators may be more or less successful for treatment and a thorough understanding of the pathways that are disrupted in each condition will be key to determine the most appropriate therapy.
1.2.1 Meconium Aspiration Syndrome
The most common cause of PPHN is meconium aspiration syndrome (MAS), which affects 25,000–30,000 infants with 1,000 deaths annually in the United States (Gelfand et al. 2004). In these cases, the infant passes meconium while still in utero, usually in response to stressful stimuli. Affected infants aspirate the meconium into their airways, where it can impede ventilation, cause severe pneumonitis, and induce lung inflammatory changes. While a fair bit is known about the parenchymal disease associated with MAS, there is much less known about the utility of specific pulmonary vasodilators. In the NINOS trial, 51% of the infants had MAS as the underlying cause of their PPHN. In that study, treatment with inhaled nitric oxide (iNO) decreased the combined outcome of death or cardiopulmonary bypass support (known as ECMO), suggesting that modulation of the NO–cGMP pathway may be a useful treatment modality for infants with MAS (Group 1997). However, little data exist to address whether the PDEs are specifically dysregulated in MAS. Interestingly, a recent study demonstrated that sildenafil is a potent pulmonary vasodilator in a neonatal piglet model of MAS (Shekerdemian et al. 2002, 2004).
1.2.2 Idiopathic PPHN
Idiopathic PPHN is the second most common etiology of PPHN and is classically described in near-term (>34 weeks gestation) and term newborns (Konduri 2004; Walsh-Sukys et al. 2000). Autopsy studies of fatal PPHN demonstrate severe hypertensive structural remodeling with vessel wall thickening and smooth muscle hyperplasia even in newborns who die shortly after birth, suggesting that many cases of severe disease are associated with chronic intrauterine stress. In these patients, the vascular smooth muscle extends to the level of the intra-acinar arteries, which does not normally occur until much later in the postnatal period (Haworth 1988; Murphy et al. 1981). The severity of vascular remodeling does not allow the pulmonary vasculature in these infants to appropriately vasodilate in response to birth-related stimuli, and they will present with profound hypoxemia and clear lung fields on X-ray, leading many to refer to this as “black-lung” PPHN (Farrow et al. 2005; Konduri 2004; Lakshminrusimha and Steinhorn 1999).
Because idiopathic PPHN is characterized by “pure” vascular disease, it is probably the best studied of the various causes of neonatal pulmonary hypertension, and many groups have worked to characterize the underlying fetal and neonatal pathophysiology that might lead to abnormal remodeling of the fetal and early neonatal pulmonary vasculature. For instance, a well-known cause of idiopathic PPHN is constriction of the fetal ductus arteriosus in utero from exposure to nonsteroidal anti-inflammatory drugs (NSAIDs) during the third trimester (Manchester et al. 1976). A fetal lamb model was developed by surgically closing the ductus in utero, which induces rapid increases in fetal pulmonary artery pressure, pulmonary vascular remodeling, and a subsequent failure to transition to extrauterine life (Morin 1989; Wild et al. 1989).
Decreased expression and activity of eNOS have been documented in infants with PPHN, as well as in the ductal-ligation lamb model (Shaul et al. 1997; Villanueva et al. 1998). Increased production of reactive oxygen species (ROS) by multiple sources leads to vasoconstriction, smooth muscle hypertrophy, and NOS dysfunction (Brennan et al. 2003; Konduri et al. 2003). Further, activity of vascular sGC is diminished in PPHN lambs (Steinhorn et al. 1995), and activity of PDE5 is increased (Hanson et al. 1998b), both of which lead to decreased cGMP concentrations (Tzao et al. 2001).
While prostacyclin appears to be important in the normal pulmonary vascular transition, much less is known about potential dysregulation of the prostacyclin pathway in PPHN (Konduri 2004; Lakshminrusimha et al. 2009a). Our group has recently demonstrated decreased expression of the prostacyclin synthase (PGIS) and the prostacyclin IP receptor in PPHN lambs, leading to reduced vasodilation to prostacyclin analogues. Pretreatment with milrinone, a PDE3 inhibitor, restores relaxations to prostanoids to levels similar to that seen in control fetuses. While fetal levels of PDE3 are not altered by PPHN, PPHN suppresses the normal rise in PDE3 expression and activity following delivery and mechanical ventilation (Chen et al. 2009; Lakshminrusimha et al. 2009a). Interestingly, nitric oxide increases PDE3 expression and activity in these lambs, demonstrating the interrelated nature of cGMP and cAMP pathways in PPHN.
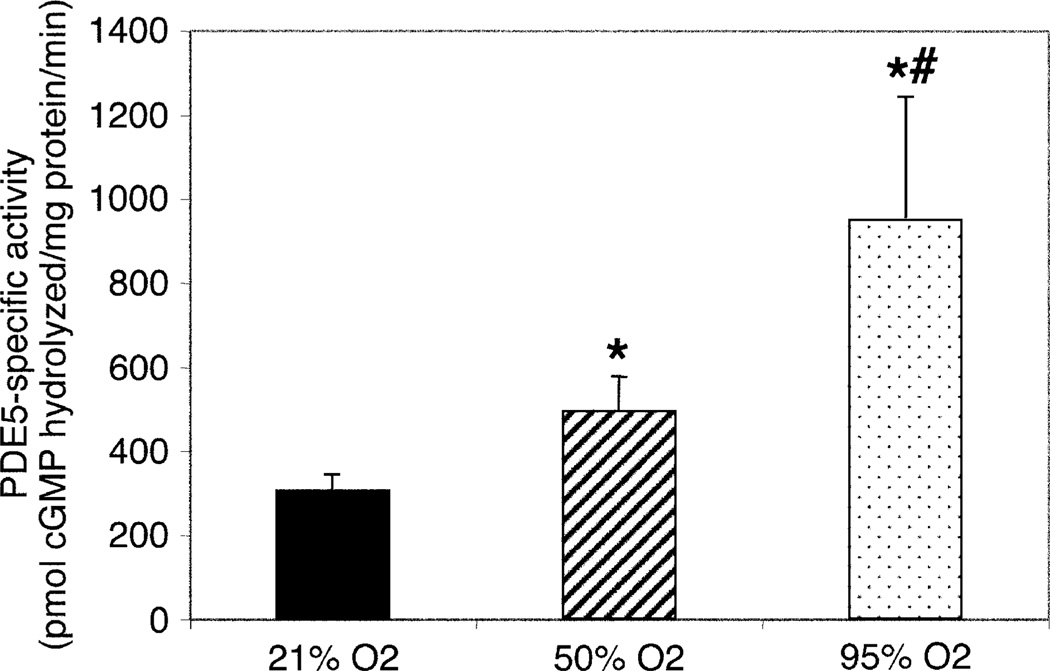
Because neonatal PPHN is associated with severe hypoxemia, the use of high oxygen concentrations, up to 100% oxygen, is typically considered as a first-line therapy in infants with PPHN (Farrow et al. 2005; Tiktinsky and Morin 1993). However, the use of oxygen may greatly exaggerate oxidative stress in multiple cellular compartments of the diseased vasculature. Recent data suggest that hyperoxia may diminish vascular responses to endogenous and exogenous nitric oxide in both the normal and remodeled pulmonary vasculature, indicating that ROS may inactivate NO or other enzymes responsible for mediating its vascular effects (Lakshminrusimha et al. 2007a, 2009b). The mechanisms by which ROS lead to pulmonary vasoconstriction and diminished NO responsiveness is certainly complicated, but emerging data indicate multiple targets in the pulmonary vascular regulatory pathways, including the PDEs. We recently published that exposing normal fetal pulmonary artery smooth muscle cells (FPASMC) to hyperoxia for 24 h leads to decreased cGMP response to exogenous NO. We further demonstrated that exposure to hyperoxia for 24 h increases PDE5 mRNA and protein expression as well as increased level of PDE5 phosphorylation and activity. We also noted a dose–response effect of hyperoxia on PDE5 activity, with a stepwise increase noted in PDE5 activity as the cells were treated with 21, 50, and 95% O2 (Fig. 1). Inhibition of the hyperoxia-induced PDE5 activity with sildenafil was sufficient to partially rescue the cGMP response to exogenous NO, further indicating that PDE5 is a critical regulator of cGMP in the context of hyperoxia (Farrow et al. 2008a).
Fig. 1.
Hyperoxia increases PDE5 activity in Ovine FPASMCs. FPASMCs were exposed to 21% O2–5% CO2, 50% O2–5% CO2, or 95% O2–5% CO2 for 24 h, and total protein was harvested. PDE5-specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total milligrams of protein (200 µM cGMP substrate in assay; 100 nM sildenafil for inhibition). Data are shown as mean ± SEM (n = 8; read in duplicate). *P < 0.05 vs. 21% O2, #P < 0.05 vs. 50% O2. Reproduced with permission from Farrow et al. (2008a)
As might be expected, exposure to hyperoxia for 24 h led to increased oxidative stress within the FPASMC. As such, we hypothesized that ROS may serve as critical mediators in the crosstalk between oxygen and PDE5. In support of that, a single dose of an exogenous oxidant, hydrogen peroxide (H2O2), was sufficient to induce long-lasting changes in PDE5 expression, phosphorylation, and activity, which mirrored those seen after exposure to hyperoxia. Similarly, the changes in PDE5 expression and activity as well as the decreased cGMP responsiveness in hyperoxia were all reversed with pretreatment with a chemical antioxidant, N-acetyl-cysteine (NAC). This confirms that ROS, in general, and H2O2, in particular, are sufficient to induce significant increases in PDE5 expression and activity in the pulmonary artery smooth muscle cell, which may promote vasoconstriction, poor NO response, and vascular remodeling (Farrow et al. 2008a).
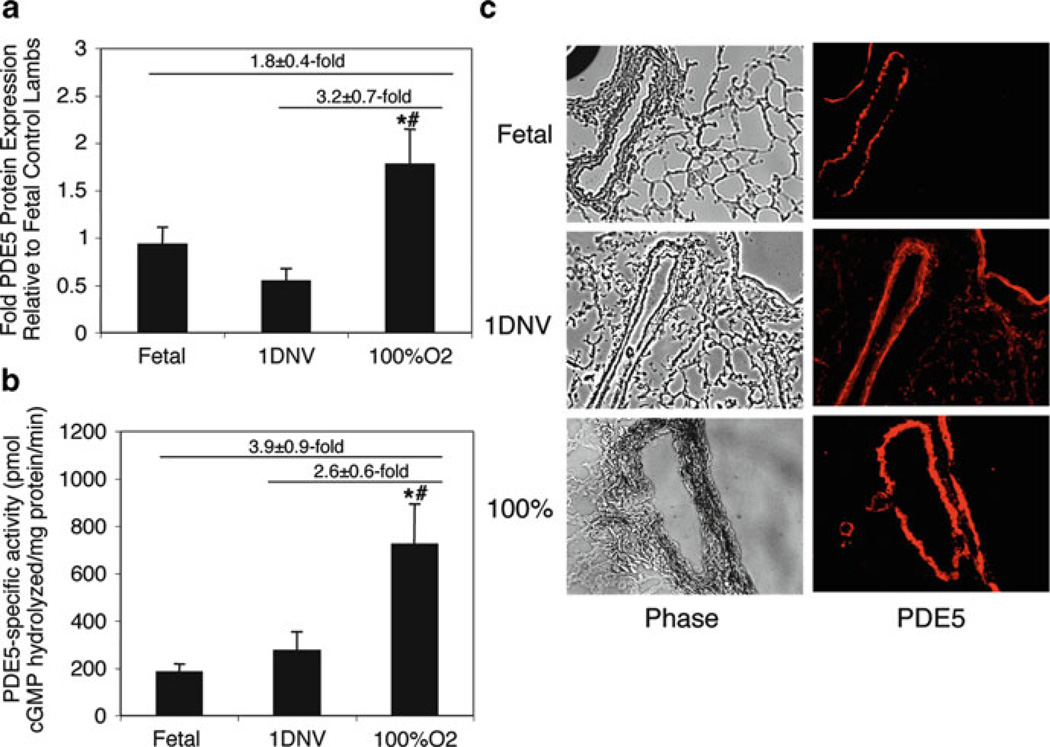
In order to better understand the impact of ROS on the intact neonatal pulmonary vasculature, we recently ventilated both healthy control and PPHN lambs immediately after birth with 100% oxygen, in order to simulate the clinical scenario seen with a human infant with severe PPHN. Resuscitation of healthy lambs with 100% oxygen significantly exaggerated pulmonary arterial contractile responses to norepinephrine compared to vessels from animals resuscitated with 21% oxygen (Lakshminrusimha et al. 2006). Use of 100% oxygen ventilation significantly increased pulmonary vascular PDE5 expression and activity (Fig. 2) (Farrow et al. 2008a), indicating that PDE5 activity is an important mediator in the vascular response to oxygen and hyperoxia. More recently, we reported that ventilation of PPHN lambs with 100% oxygen results in pulmonary vasoconstriction and diminished pulmonary vascular relaxation to inhaled NO (Lakshminrusimha et al. 2009b). Very recent studies indicate that these abnormal responses are mediated in part by a greatly exaggerated increase in PDE5 activity (Farrow et al. 2010a, b).
Fig. 2.
Ventilation of healthy neonatal sheep with 100% O2 induces PDE5 protein expression and activity. Ovine lung parenchyma and resistance PAs from healthy lambs ventilated with 100% O2 were harvested after 24 h and compared with both fetal lambs (Fetus) and healthy 1-day nonventilated lambs (1DNV). (a) PA PDE5 protein expression was analyzed via Western blot, with β-actin normalization. Data are shown as mean ± SEM. *P < 0.05 vs. fetal lambs, #P < 0.05 vs. 1-day nonventilated lambs. (b) Lung PDE5-specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total milligrams of protein (200 µM cGMP substrate in assay; 100 nM sildenafil for inhibition). Data are shown as mean ± SEM. *P < 0.05 vs. fetal lambs, #P < 0.05 vs. 1-day nonventilated lambs. (c) PDE5 expression is localized within the PA smooth muscle and nearby airways. The left column shows phase-contrast images (×10) of frozen lamb lung sections. The right column shows the corresponding sections stained for PDE5, with immunofluorescence shown as red. Reproduced with permission from Farrow et al. (2008a)
Ventilation of healthy control lambs with either 21 or 100% oxygen blunts the increase in PDE3 expression and activity normally seen in the healthy spontaneously breathing 1-day lambs, suggesting that PDE3 may be dysregulated by mechanical forces associated with ventilation rather than by hyperoxia (Chen et al. 2009). This dysregulation of PDE3 expression and activity may lead to a disruption of the normal cAMP signaling within the pulmonary vascular smooth muscle during this critical transition time. Recent studies have shown that the PDE3 inhibitor milrinone, when used in conjunction with either iNO or iloprost, can help to restore normal vascular responses (Chen et al. 2009; Lakshminrusimha et al. 2009a).
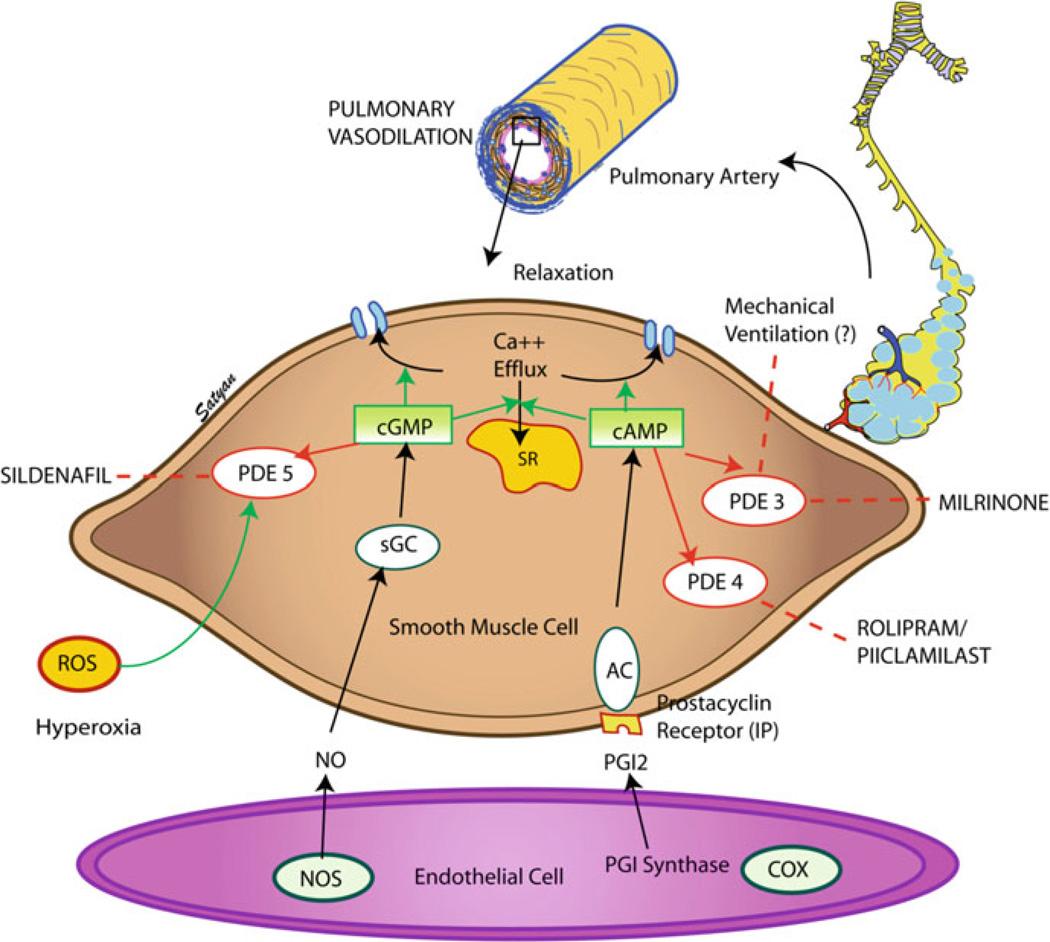
Thus, idiopathic PPHN is a complex pathological process induced by disruption of multiple signaling pathways, including the NO–cGMP–PDE5 and prostacyclin–cAMP–PDE3 pathways, leading to vasoconstriction and structural remodeling of the vasculature. Postnatal treatment with high levels of oxygen exacerbates these abnormalities, leading to decreases in cGMP and cAMP and impaired vasodilation. Inhibitors of PDE5 and PDE3, such as sildenafil and milrinone, represent novel and particularly attractive new therapies for these infants (Fig. 3) (Lakshminrusimha and Steinhorn 2009).
Fig. 3.
Mechanism of action of phosphodiesterase inhibitors in PPHN. PDEs play a critical role in regulating cGMP and cAMP within the vascular smooth muscle. Elevations in cGMP and cAMP lead to vasorelaxation by decreasing intracellular calcium via decreasing calcium efflux from the sarcoplasmic reticulum and increasing calcium efflux from the cell. PDEs hydrolyze cGMP and cAMP, thereby preventing cyclic nucleotide-mediated vasorelaxation. Thus, PDE inhibitors such as sildenafil and milrinone represent promising therapies for neonates with PPHN resistant to traditional therapy with inhaled nitric oxide. Dashed lines represent inhibition. Figure kindly provided by Dr. Satyan Lakshminrusimha
1.2.3 Congenital Diaphragmatic Hernia
CDH is a serious birth defect that includes disordered development of the diaphragm and a variable degree of pulmonary hypoplasia. It occurs in 1 of every 2,000–4,000 live births and accounts for 8% of all major congenital anomalies. Severe CDH develops early in the course of lung development, and as a result airway divisions and alveolarization may be significantly impaired. Because development of the pulmonary arterial system parallels development of the bronchial tree, fewer arterial branches are observed in CDH. Further, abnormal medial muscular hypertrophy is observed as far distally as the acinar arterioles. Pulmonary capillary blood flow is decreased because of the small cross-sectional area of the pulmonary vascular bed, and flow may be further decreased by abnormal pulmonary vasoconstriction (de Buys Roessingh and Dinh-Xuan 2009; Farrow et al. 2005).
There is no ideal animal model in which the disruption in the diaphragm occurs at the same stage of lung development as is seen in human fetuses. The most widely utilized is the nitrofen-induced rat model of CDH. In this model, reduced lung eNOS activity and expression has been described, thereby pinpointing the eNOS–cGMP pathway as important in the pathogenesis of CDH and its accompanying pulmonary hypertension (Karamanoukian et al. 1996). Another group has shown that response to exogenous nitric oxide and a cGMP analogue, 8-Br-cGMP, is impaired in resistance pulmonary arterioles of the CDH animals. Of note, pretreatment of CDH resistance pulmonary arterioles with the PDE5 inhibitor zaprinast completely restored their vasodilatory response to 8-Br-cGMP (Vukcevic et al. 2005), suggesting that PDE5 may also be involved with the pathogenesis of pulmonary hypertension in the infant with CDH.
1.3 Pathophysiology of Pulmonary Hypertension in the Older Infant
There are two other major classes of infants who develop pulmonary hypertension – former preterm infants who develop pulmonary hypertension as a result of their underlying lung disease and infants, both term and preterm, who develop pulmonary hypertension as a result of their congenital heart disease. In both cases, the disease process begins during the neonatal period, but symptoms may not become manifest until the infant is several months old. Further, the resulting pulmonary hypertension is not only a disease of altered vasoreactivity, but it also involves significant vascular remodeling. The role of PDEs in the pathophysiology of these infants is just beginning to be elucidated.
1.3.1 Bronchopulmonary Dysplasia and Cor Pulmonale
Bronchopulmonary dysplasia (BPD) is a common complication of preterm birth that affects approximately 30% of infants (or roughly 10,000 babies per year) with extreme prematurity (typically defined as a birthweight <1,000 g). BPD results in significant long-term morbidity in childhood, including poor lung function, diminished growth, and impaired neurodevelopment (Jobe and Bancalari 2001; Walsh et al. 2006). A recently recognized complication of moderate or severe BPD is pulmonary hypertension and right-sided heart failure, or cor pulmonale. While the overall risk of BPD clearly correlates with gestational age and birthweight, it remains unclear why some infants develop mild or severe disease. Even less is known about why some infants develop pulmonary hypertension and how to appropriately treat these infants. Poor outcomes, including mortality, are distressingly common (Jobe and Bancalari 2001; Khemani et al. 2007; Mourani et al. 2008).
Recent clinical trials have laid the foundation for the use of iNO for prevention of BPD (Ballard et al. 2006; Kinsella et al. 2006). Smaller case series also suggest that the use of iNO may also improve oxygenation in those BPD infants with established pulmonary hypertension (Mourani et al. 2008). Additionally, premature babies are forced to live in an environment with higher oxygen concentrations than would be encountered during fetal development. Not surprisingly, emerging evidence indicates that oxidative stress may produce significant lung parenchymal and vascular injury (Farrow et al. 2008a, b, 2010a, b; Lakshminrusimha et al. 2006, 2007a, b; Thebaud et al. 2005; Vento et al. 2001). As discussed above, PDE5 expression and function may be adversely affected by ROS-mediated events (Farrow et al. 2008a, 2010a, b).
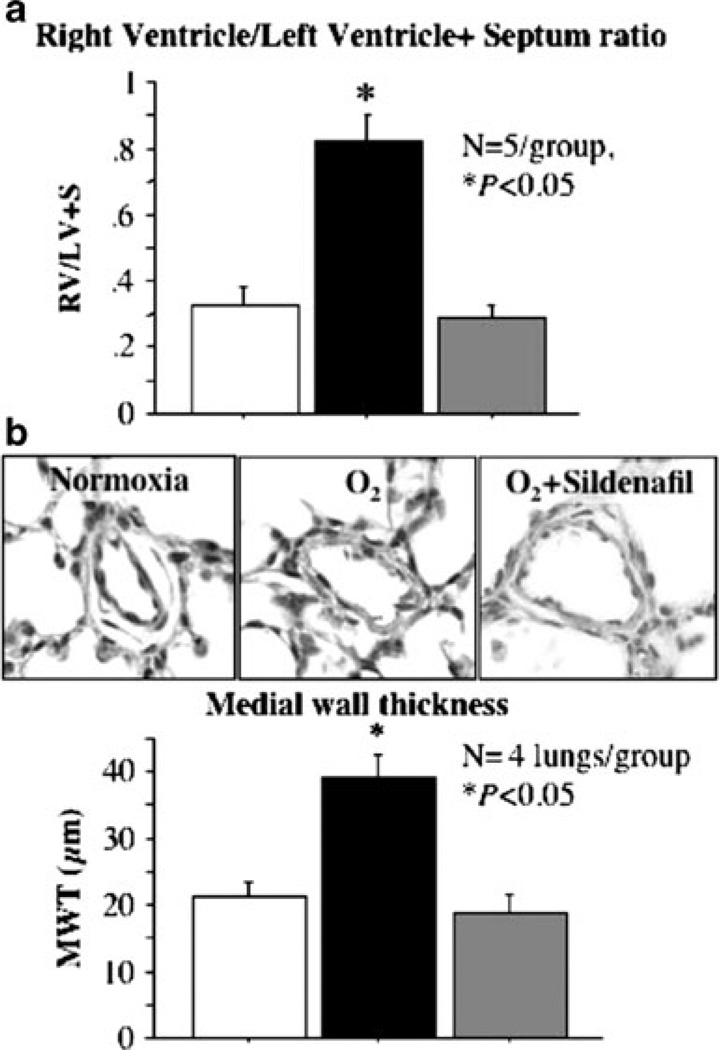
Consistent with this hypothesis, three studies address the role of cGMP and PDE5 in models of preterm lung disease – one study in preterm lambs and two studies in neonatal rats. In the preterm lamb model of BPD, treatment with a cGMP analogue that is not hydrolyzable by PDE5 decreased pulmonary vascular resistance, which would indirectly suggest that PDE5 plays a role in the elevated pulmonary vascular resistance seen with BPD (Bland et al. 2003). Perhaps more intriguing are the results seen in the rat model of BPD, induced by hyperoxia. In that model, treatment of the neonatal rats with sildenafil resulted in decreased pulmonary vascular resistance, decreased right ventricular hypertrophy (RVH), and decreased medial wall thickness of pulmonary arteries. This study also showed that sildenafil improved lung alveolarization, suggesting that PDE5 may play a critical role in both alveolar and vascular development (Fig. 4) (Ladha et al. 2005). A more recent study in the rat model extends these studies and demonstrated that treatment with sildenafil prior to hyperoxia exposure increased lung cGMP levels and improved survival as well as lung alveolarization and angiogenesis. In another arm of that study, rescue treatment with sildenafil (administration of drug 6 days after initiation of hyperoxia) significantly decreased pulmonary vessel medial wall thickness and reduced RVH (de Visser et al. 2009).
Fig. 4.
Sildenafil reduces pulmonary hypertension associated with oxygen-induced BPD. (a) Right ventricular hypertrophy. Hyperoxia-exposed rats had significant right ventricular hypertrophy (RVH) as indicated by the increase in RV/LV+S ratio compared with normoxic controls. Sildenafil treatment reduced RVH (sildenafil 100 mg/kg subcutaneously daily during hyperoxia exposure). (b) Medial wall thickness. A representative picture of pulmonary arteries is shown displaying a thickened medial arterial wall in hyperoxic rat lungs as compared with normoxic controls. Sildenafil treatment significantly reduced the % medial wall thickness as compared with untreated hyperoxic pulmonary arteries (sildenafil 100 mg/kg subcutaneously daily during hyperoxia exposure). Reprinted with permission of the American Thoracic Society. Copyright American Thoracic Society; Ladha et al. (2005); Official Journal of the American Thoracic Society, Diane Gern, Publisher
Interesting new data have recently emerged implicating a role for PDE4 in the pathogenesis of BPD. In one study (de Visser et al. 2008), preterm rats were exposed to room air, hyperoxia, or hyperoxia with either of the PDE4 inhibitors, rolipram or piclamilast (Houslay et al. 2005). PDE4 inhibition prolonged median survival and decreased lung inflammation and vascular leakage as well as decreased markers of inflammation (de Visser et al. 2008). Another model of BPD, produced by prolonged exposure of mice to hyperoxia, results in decreased alveolarization and increased septal wall thickness (Woyda et al. 2009). Pretreatment with a PDE4 inhibitor decreased septal wall thickness and increased total airspace area, suggesting that PDE4 may be of critical importance in neonatal lung development (Woyda et al. 2009).
1.3.2 Congenital Heart Disease
Congenital heart disease, particularly that associated with increased pulmonary arterial blood flow from left-to-right shunts, increased right-sided pressures from nonrestrictive septal defects and aorto-pulmonary shunts, and increased pulmonary venous congestion, can lead to remodeling of the pulmonary vascular bed with associated pulmonary hypertension (Hoffman et al. 1981). The risk of developing pulmonary hypertension in these patients is dependent on many factors including the age of the patient, the type of congenital heart disease, and the degree of pulmonary overcirculation. Endothelial dysfunction has been hypothesized to play a major role in this vascular dysfunction and remodeling. Adult patients with pulmonary hypertension have impaired endothelium-dependent pulmonary vasodilation and decreased eNOS expression (Giaid and Saleh 1995). Children with pulmonary hypertension due to congenital heart disease are also thought to have endothelial dysfunction, and they have also been described to have extension of smooth muscle cells to peripheral pulmonary arteries, neointimal formation due to smooth muscle cell migration, medial hypertrophy, and plexigenic lesion formation (Rabinovitch et al. 1978, 1984, 1986).
A number of studies in a juvenile lamb model of pulmonary hypertension due to chronic pulmonary overcirculation have indicated its pathophysiology includes abnormal NO–cGMP–PDE5 signaling and excess production of ROS and endothelin (Black et al. 1998, 2000, 2001, 2003; Lakshminrusimha et al. 2007b; Steinhorn et al. 2001). In this model (Reddy et al. 1996), an aortopulmonary shunt is placed in fetal lambs during the last week of gestation, followed by spontaneous delivery. The lambs have impaired endothelium-dependent vasorelaxation by 4 weeks of age (Reddy et al. 1996), associated with complex vascular changes including increased pulmonary vascular eNOS expression and downstream derangements such as increased pulmonary sGC protein expression and increased PDE5 protein expression and activity (Black et al. 1998, 2001). Further studies demonstrate that antioxidants such as superoxide dismutase normalized vascular reactivity in the shunt vessels and that the ROS in these lambs likely are the result of uncoupled NOS (Lakshminrusimha et al. 2007b; Steinhorn et al. 2001).
Other studies have explored the impact of various treatment strategies on the NO–cGMP–PDE5 pathway in lambs with congenital heart disease. Inhaled NO treatment of shunted lambs decreases lung eNOS expression without changing NOS activity, decreases sGC protein expression, and has no effect on PDE5 protein expression (Ross et al. 2005). However, acute withdrawal of the iNO rapidly and dramatically increases pulmonary vascular resistance by 45% and decreases in cGMP levels (Ross et al. 2005). These findings would suggest that the PDE5 in these lambs becomes the dominant determinant of cGMP concentration after withdrawal of iNO (Ross et al. 2005). An additional study attempted to address the role of cAMP in rebound pulmonary hypertension upon iNO withdrawal (Thelitz et al. 2004). Treatment of shunt lambs with iNO for 24 h decreased lung cAMP concentrations, both during iNO treatment and upon acute withdrawal of iNO. Concomitant treatment with milrinone, a PDE3 inhibitor, normalized cAMP concentrations during iNO treatment and upon acute withdrawal. Milrinone also prevented the increase in pulmonary vascular resistance seen upon acute iNO withdrawal (Thelitz et al. 2004).
The increased pulmonary vascular resistance seen in these shunted lambs upon withdrawal of iNO is consistent with the well-known phenomenon of rebound pulmonary hypertension seen in human infants upon acute withdrawal of iNO (Atz et al. 1996). Taken together, these studies suggest that iNO may alter expression and activity of PDEs, such as PDE3 and PDE5, and that inhibitors of PDE3 and PDE5 may be of clinical utility to treat rebound pulmonary hypertension upon iNO withdrawal (Ross et al. 2005; Thelitz et al. 2004).
2 PDE Inhibitors in Neonatal Pulmonary Hypertension
The initial treatment of the neonate with pulmonary hypertension depends in part on the underlying disorder. Therapy often includes aggressive support of cardiac function and perfusion with volume and inotropic agents to enhance cardiac output and systemic O2 transport. While most infants require mechanical ventilation to allow for lung recruitment, an important goal is to avoid postnatal lung injury, which worsens the degree of pulmonary hypertension (Farrow et al. 2005).
When supportive treatment is insufficient, the only FDA-approved pulmonary vasodilator in neonates is iNO. Multicenter randomized, placebo-controlled, blinded trials of iNO in term and near-term infants with PPHN demonstrated that iNO significantly decreased the need for ECMO in newborns with PPHN (Clark et al. 2000; Group 1997). However, up to 40% of infants do not respond or sustain their response to iNO, and it does not reduce mortality, duration of mechanical ventilation, or length of hospitalization. Follow-up studies to 12–24 months also show that iNO does not significantly decrease the incidence of chronic lung disease or adverse neurodevelopmental sequelae (Clark et al. 2003; Group 2000).
In addition to the problem of inadequate response to iNO, many infants will acutely develop “rebound” pulmonary hypertension upon its discontinuation (Atz et al. 1996; Farrow et al. 2005). As noted above, this finding was first described in young infants receiving inhaled NO after surgery for congenital heart disease, although the phenomenon frequently occurs in infants with PPHN as well. Rebound pulmonary hypertension tends to be most severe if discontinuation occurs when infants are breathing higher concentrations of iNO, but may also occur even after more gradual iNO weaning. It is important for clinicians to understand that rebound pulmonary hypertension can occur even in infants who were apparent nonresponders to iNO therapy, particularly after treatment has been delivered for more than an hour. While rebound pulmonary hypertension typically responds to reinstitution of iNO, it represents a significant limitation of this therapy (Atz et al. 1996; Farrow et al. 2005).
Additional pulmonary vasodilators are urgently needed for infants with pulmonary hypertension. Inhibition of PDEs represents a potentially powerful and novel way to achieve safe, sustained, and even selective pulmonary vasodilation in these infants.
2.1 PDE5 Inhibitors
PDE5 is by far the best studied of the PDEs for involvement in the pathophysiology of neonatal pulmonary hypertension. Its dysregulation has been implicated in most of the major causes of neonatal pulmonary hypertension including PPHN, CDH, BPD, and congenital heart disease. The PDE5 inhibitor sildenafil has been shown to be an effective treatment in adult pulmonary arterial hypertension (Galie et al. 2005) and is now marketed as Revatio for this purpose. Not surprisingly, PDE5 inhibitors have been among the first PDE inhibitors used in neonates. While many have proposed their use to augment iNO therapy, there is some evidence to suggest that PDE5 inhibitors may serve as effective vasodilators in their own right.
2.1.1 Dipyridamole
One of the earliest drugs used to manipulate the pulmonary circulation was the nonspecific PDE5 inhibitor dipyridamole (Persantin) (al-Alaiyan et al. 1996; Dukarm et al. 1998; Ivy et al. 1998). In neonatal lambs with PPHN, dipyridamole significantly decreased pulmonary vascular resistance and pulmonary blood pressure, and increased pulmonary blood flow. Unfortunately, it was not selective for the pulmonary vasculature, and it decreased systemic blood pressure to a similar degree (Dukarm et al. 1998). In human neonates, there are a handful of case reports reporting the use of dipyridamole to facilitate iNO weaning in infants with PPHN, congenital heart disease, and CDH. In two case reports, treatment with dipyridamole facilitated iNO weaning in infants with PPHN, and in both cases, the infants survived (al-Alaiyan et al. 1996; Worwag et al. 2000). In infants with CDH, successful treatment with dipyridamole has been reported, but improvement was transient in at least two cases (Buysse et al. 2001; Thebaud et al. 1999). The largest report of dipyridamole use was in infants who required postoperative iNO therapy for pulmonary hypertension after repair of congenital heart disease. In a series of 23 consecutive children receiving iNO postoperatively, seven developed rebound pulmonary hypertension when iNO was acutely stopped. In those seven, dipyridamole attenuated the rise in pulmonary pressure after acute withdrawal of iNO (Ivy et al. 1998). None of these case reports described a significant decrease in systemic blood pressure similar to that observed in the PPHN lamb model (Dukarm et al. 2005).
2.1.2 Sildenafil
Sildenafil has been demonstrated to acutely decrease pulmonary vascular resistance and improve pulmonary blood flow in a neonatal porcine model of MAS (Shekerdemian et al. 2002, 2004). Chronic use of sildenafil decreased medial wall thickness and RVH in a rat model of BPD (Ladha et al. 2005). Like dipyridamole, one of the earliest clinical uses of sildenafil was to facilitate weaning from iNO in infants with congenital heart disease following corrective surgery. In an initial case series, enteral sildenafil increased circulating cGMP and allowed two of three infants to wean from iNO without rebound pulmonary hypertension (Atz and Wessel 1999). Two subsequent studies have expanded these initial observations. Oral sildenafil facilitated iNO discontinuation in seven infants (ages 3 days to 21 months) who had previously failed to wean from iNO (Lee et al. 2008). Namachivayam et al. delivered enteral sildenafil (n = 15) or placebo (n = 14) to a total of 29 infants with critical illness (most with congenital heart disease), who were breathing 2 ppm iNO in preparation for a discontinuation trial. Ten out of 14 patients (71%) receiving placebo experienced rebound pulmonary hypertension after iNO was stopped. In contrast, administration of a single dose of oral sildenafil (0.4 mg/kg) prevented rebound pulmonary hypertension in all 15 treated patients. The authors also noted a significant reduction in the duration of mechanical ventilation and ICU length of stay in the group that received sildenafil (Namachivayam et al. 2006).
Some data raise specific concerns regarding the use of sildenafil in combination with iNO. In piglets with meconium aspiration, intravenous sildenafil in combination with iNO enhanced the decrease in pulmonary artery pressure and pulmonary vascular resistance, but also produced systemic hypotension and decreased oxygenation (Shekerdemian et al. 2004). Similarly, in neonates after repair of ventricular or atrioventricular septal defects, systemic hypotension and decreased oxygenation were noted when sildenafil and iNO were given concurrently (Stocker et al. 2003). It is possible that sildenafil in combination with iNO may increase pulmonary perfusion to underventilated lung segments, thereby leading to worsening VQ mismatch and decreasing oxygenation. Furthermore, when given intravenously, sildenafil may not be selective to the pulmonary vascular bed. It is possible that a drop in systemic blood pressure may be poorly tolerated if combined with diminished oxygenation due to worsened VQ mismatch. We have shown that idiopathic PPHN is associated with increased PDE5 expression and activity in the pulmonary vascular bed (Farrow et al. 2008a, 2010a, b). Thus, those infants may be better able to tolerate the combined therapy of iNO and intravenous sildenafil.
The clinical use of sildenafil has recently been reported in infants with PPHN, including one small, randomized controlled trial with oral sildenafil, and one pilot pharmacokinetic, pharmacodynamic trial with intravenous sildenafil (Baquero et al. 2006; Steinhorn et al. 2009). As mentioned previously, the only FDA-approved pulmonary vasodilator for infants is iNO. However, iNO therapy is currently expensive and unavailable in many parts of the world, leading to much higher mortality from PPHN in those regions. For this reason, the use of oral sildenafil as the primary treatment for severe PPHN was studied in term and near-term infants in Colombia. This study randomized 13 infants (>35 5/7 weeks gestation and age <3 days) with an oxygenation index greater than 25, indicating severe PPHN. In the sildenafil treatment group, a significant improvement in oxygenation was observed by 24 h and six of the seven infants survived. In contrast, the infants in the placebo group did not improve their oxygenation and only one of the six infants survived (Baquero et al. 2006). Despite these encouraging results, significant concerns exist regarding giving sildenafil enterally to infants who may have compromised intestinal perfusion with unknown and inconsistent rates of gastrointestinal absorption.
Two recently published papers described the results of a multicenter pilot trial examining the safety and pharmacokinetics of intravenous sildenafil in term and near-term neonates with PPHN (Mukherjee et al. 2009; Steinhorn et al. 2009). Thirty-six neonates (>34 weeks gestation) were enrolled at a mean age of 34 ± 17 h when they had moderate to severe pulmonary hypertension with an oxygenation index >15 (Mukherjee et al. 2009; Steinhorn et al. 2009). Infants with congenital anomalies including CDH were excluded. Infants received IV sildenafil administered within eight progressive, “step-up” dosing groups, and the study also determined the optimal loading dose and duration. Infants received sildenafil for a minimum of 48 h. After that time, sildenafil was discontinued once infants had been successfully weaned from iNO for at least 1 h, or after 7 days (168 h). Based on data from adults, clearance via the hepatic CYP3A4 and CYP2C9 enzymes was expected. In the study, sildenafil clearance in neonates increased threefold between day of life one and the end of the first week of life, likely reflecting postnatal maturation of this CYP system. Hence, postnatal age at the time of sildenafil initiation has a significant impact on drug clearance. Similarly, significant increases in sildenafil concentration should be expected when it is used concomitantly with CYP3A4 inhibitors such as cimetidine and erythromycin (Mukherjee et al. 2009).
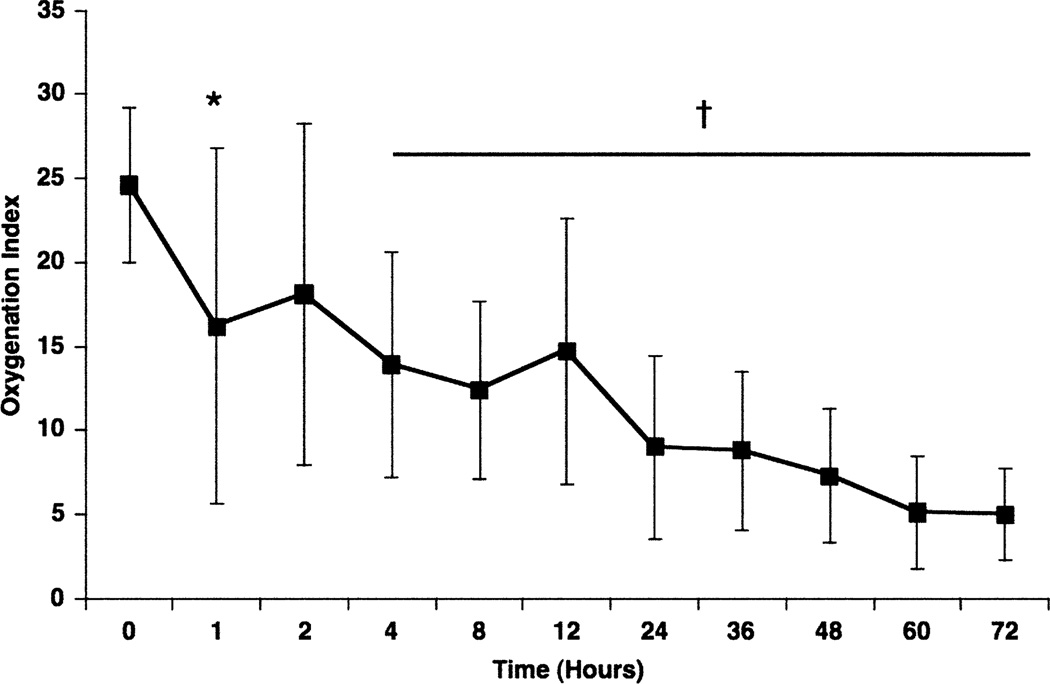
Hypotension was the most commonly observed adverse effect. One infant was withdrawn from the study because the sildenafil loading infusion (scheduled to be given over 5 min) had to be stopped after 2 min due to systemic hypotension. Hypotension was not observed when the loading dose was delivered over 3 h. An additional infant died due to bilateral tension pneumothoraces, not related to sildenafil infusion. For the remaining 34 infants, oxygenation improved significantly through the course of the sildenafil infusion, with no significant changes in systemic blood pressure. Only one infant required cannulation for extracorporeal life support. Of the seven infants enrolled without prior use of iNO, all experienced a significant improvement in oxygenation within 4 h after sildenafil administration (Fig. 5). Only one of the seven infants required iNO, and the other six infants improved and survived to hospital discharge without requiring either iNO or ECMO (Steinhorn et al. 2009). Unlike the previously mentioned studies, sildenafil used in combination with iNO did not significantly decrease systemic blood pressure or oxygenation. We speculate that it is because there is increased PDE5 expression and activity in the pulmonary vessels of infants with PPHN (Farrow et al. 2008a, 2010a, b). Future studies are planned to further evaluate efficacy of IV sildenafil in neonates with PPHN. Of note, the intravenous form of sildenafil was FDA-approved in December of 2009 for adults with pulmonary arterial hypertension.
Fig. 5.
Response to sildenafil intravenous infusion without iNO. Seven infants were enrolled before the need for iNO. Oxygenation index (OI) was improved by 1 h (24.6 ± 4.6 to 16.1 ± 9.9; *P = 0.0502), with significant and sustained improvement by 4 h after the initiation of sildenafil (14.7 ± 6.4, p = 0.0088). Of the seven infants who received sildenafil only, the majority (n = 5) were in cohorts 6–8, meaning that they received the highest maintenance infusion dose of 1.64 mg/kg/day, and differed only in the approach to the loading dose. Of these seven infants, only one infant required additional treatment with iNO, and that infant was in cohort 4, receiving loading and maintenance doses that were approximately 20% of the final dose tested in trial. Reprinted from Steinhorn et al. (2009) with permission from Elsevier
Smaller case reports and case series have examined the efficacy of oral sildenafil in infants with CDH and BPD. In one case report, oral sildenafil facilitated weaning from iNO in a 7-week-old infant with CDH, although the infant ultimately died (Keller et al. 2004). In another series of seven infants with CDH, oral sildenafil improved right cardiac output and decreased pulmonary vascular resistance as measured by echocardiography, and five of the seven infants survived (Noori et al. 2007). A larger, double-blind, randomized controlled trial of oral sildenafil in infants with CDH and severe pulmonary hypertension is ongoing (NCT00133679). Infants are recruited between 10 and 42 days of age if they continue to have a significant FiO2 requirement, if they need ECMO beyond 10 days of age, or if they have a pulmonary artery pressure >2/3 systemic pressure beyond 14 days of age. Target enrollment is 32 infants, who will receive placebo or sildenafil for 5 weeks. The primary outcome is pulmonary artery pressure on echocardiogram at the end of the treatment period.
Sildenafil is an attractive therapeutic option for infants with chronic pulmonary hypertension because of its relative ease of administration and apparent low toxicity. A recent case series examined the effect of oral sildenafil in 25 infants and children with pulmonary hypertension due to chronic lung disease, who initiated sildenafil treatment at <2 years of age (14–673 days). Most patients (88%) achieved hemodynamic improvement after a median treatment interval of 40 days, and the majority of infants receiving iNO could be weaned off. Five patients died after initiation of sildenafil treatment, but none died from refractory pulmonary hypertension or right heart failure. This important pilot study suggests that sildenafil is safe for infants with pulmonary hypertension due to chronic lung disease and indicates that further studies are warranted (Mourani et al. 2009). Because sildenafil improved lung alveolarization in neonatal rats, a small double-blind randomized controlled trial of oral sildenafil in the prevention of BPD is ongoing (NCT00431418). The target population is extremely preterm infants <28 weeks gestation who still require ventilatory support at 7 days of age. This study should also provide important initial data about the safety of sildenafil in young preterm infants.
2.2 PDE3 Inhibitors
The PDE3 inhibitor milrinone is most commonly used in pediatric patients for its inotropic effects(Chang et al. 1995; Hoffman et al. 2003; Zaccolo and Movsesian 2007). However, by raising cAMP in the pulmonary vasculature, milrinone can potentially act as a pulmonary vasodilator and improve oxygenation in patients with poor response to iNO. In neonatal lambs with PPHN, one of the unexpected results of iNO was a dramatic increase in PDE3 activity to levels similar to those observed in normal 1-day lambs. Further, milrinone was found to significantly relax pulmonary arteries isolated from PPHN lambs ventilated with iNO (Chen et al. 2009). In infants with PPHN, there have been two small case series of treatment with milrinone. In both series, the infants were already receiving iNO at the time that the milrinone was started, and significant improvements in oxygenation were observed with milrinone treatment (Bassler et al. 2006; McNamara et al. 2006). Similarly, in pediatric patients (n = 46, average age 5 years) undergoing a Fontan procedure, postoperative use of milrinone in conjunction with iNO resulted in greater improvements in the transpulmonary gradient and arterial oxygen saturation versus those children treated with iNO alone or milrinone alone (Cai et al. 2008a, b). These clinical case series all support an interesting potential role for milrinone in the treatment of neonatal pulmonary hypertension, especially when used in conjunction with iNO. However, more study is needed to determine optimum dosing and timing of therapy initiation for milrinone in these infants.
2.3 PDE1 and PDE4 Inhibitors
To date, there have been no trials or case reports of PDE1 or PDE4 inhibitors in neonates with pulmonary hypertension. However, data from adult patients and animal models would suggest that this should be an area of investigation for future potential therapies. For instance, zaprinast, initially thought to be a selective PDE5 inhibitor, has recently been described to have significant inhibitory activity against PDE1. This agent was found to enhance relaxations to iNO in neonatal lambs with PPHN (Thusu et al. 1995). Increased PDE1C mRNA and protein have been described in resistance pulmonary arteries and pulmonary artery smooth muscle cells derived from lung specimens of human pulmonary arterial hypertension patients (Murray et al. 2007; Schermuly et al. 2007). Thus, a PDE1C inhibitor might have clinical utility across all of the different types of neonatal pulmonary hypertension. Similarly, PDE4 inhibitors have reached clinical trials in adults with asthma and chronic obstructive pulmonary disease (Fan Chung 2006). In light of the recent publications that PDE4 inhibition is beneficial in neonatal mice with BPD, it is reasonable to hypothesize that PDE4 inhibitors might be clinically useful in the patients with pulmonary hypertension due to BPD.
3 Conclusions
Neonatal pulmonary hypertension is a clinical syndrome that affects approximately 10% of all infants admitted to neonatal intensive care units. It has multiple etiologies that involve complex alterations in multiple signaling pathways. As such, no one therapy is likely to be appropriate or effective in every patient. There is growing evidence from vascular smooth muscle cells and various animal models that PDEs play a critically important role in regulating pulmonary vascular tone in the neonatal period. Two early clinical trials of sildenafil have been completed in infants with PPHN with encouraging results, and double-blind randomized controlled trials are under way to assess the effectiveness of sildenafil in CDH and BPD. There is new clinical evidence to suggest that milrinone may be useful in patients with neonatal pulmonary hypertension, particularly when used in conjunction with inhaled NO. However, more data are needed from both preclinical and clinical studies to know how to select the best combination of PDE inhibitors for specific disease states and age groups. Until such studies have been conducted and evaluated, the use of PDE inhibitors for neonatal pulmonary hypertension should be confined to appropriately designed clinical trials (Shah and Ohlsson 2007).
Acknowledgments
The authors are supported by NIH grants HL086715 (KNF) and HL54705 (RHS).
Abbreviations
- BPD
Bronchopulmonary dysplasia
- CDH
Congenital diaphragmatic hernia
- COX
Cyclooxygenase
- eNOS
Endothelial nitric oxide synthase
- ET-1
Endothelin-1
- FPASMC
Fetal pulmonary artery smooth muscle cells
- H2O2
Hydrogen peroxide
- iNOS
Inducible nitric oxide synthase
- iNO
Inhaled nitric oxide
- MAS
Meconium aspiration syndrome
- NAC
N-acetyl-cysteine
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PPHN
Persistent pulmonary hypertension of the newborn
- PDE
Phosphodiesterase
- PGIS
Prostacyclin synthase
- ROS
Reactive oxygen species
- RVH
Right ventricular hypertrophy
- sGC
Soluble guanylate cyclase
Contributor Information
Kathryn N. Farrow, Department of Pediatrics, Division of Neonatology, Northwestern University Feinberg School of Medicine, 310 E. Superior St., Morton 4-685D, Chicago, IL 60611, USA, k-farrow@northwestern.edu
Robin H. Steinhorn, Division of Neonatology, Children’s Memorial Hospital and Northwestern University, 2300 Children’s Plaza #45, Chicago, IL 60611, USA, r-steinhorn@northwestern.edu
References
- Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259:H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- al-Alaiyan S, al-Omran A, Dyer D. The use of phosphodiesterase inhibitor (dipyridamole) to wean from inhaled nitric oxide. Intensive Care Med. 1996;22(10):1093–1095. [PubMed] [Google Scholar]
- Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91(1):307–310. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- Atz AM, Adatia I, Wessel DL. Rebound pulmonary hypertension after inhalation of nitric oxide. Ann Thorac Surg. 1996;62(6):1759–1764. doi: 10.1016/s0003-4975(96)00542-5. [DOI] [PubMed] [Google Scholar]
- Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355(4):343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117(4):1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- Bassler D, Choong K, McNamara P, Kirpalani H. Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biol Neonate. 2006;89(1):1–5. doi: 10.1159/000088192. [DOI] [PubMed] [Google Scholar]
- Black SM, Fineman JR, Steinhorn RH, Bristow J, Soifer SJ. Increased endothelial NOS in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol. 1998;275(5 Pt 2):H1643–H1651. doi: 10.1152/ajpheart.1998.275.5.H1643. [DOI] [PubMed] [Google Scholar]
- Black SM, Bekker JM, Johengen MJ, Parry AJ, Soifer SJ, Fineman JR. Altered regulation of the ET-1 cascade in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res. 2000;47:97–106. doi: 10.1203/00006450-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Black SM, Sanchez LS, Mata-Greenwood E, Bekker JM, Steinhorn RH, Fineman JR. sGC and PDE5 are elevated in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1051–L1057. doi: 10.1152/ajplung.2001.281.5.L1051. [DOI] [PubMed] [Google Scholar]
- Black SM, Mata-Greenwood E, Dettman RW, Ovadia B, Fitzgerald RK, Reinhartz O, Thelitz S, Steinhorn RH, Gerrets R, Hendricks-Munoz K, Ross GA, Bekker JM, Johengen MJ, Fineman JR. Emergence of smooth muscle cell endothelin B-vasoconstriction in lambs with experimental congenital heart disease and increased pulmonary blood flow. Circulation. 2003;108:1646–1654. doi: 10.1161/01.CIR.0000087596.01416.2F. [DOI] [PubMed] [Google Scholar]
- Bland RD, Ling CY, Albertine KH, Carlton DP, MacRitchie AJ, Day RW, Dahl MJ. Pulmonary vascular dysfunction in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L76–L85. doi: 10.1152/ajplung.00395.2002. [DOI] [PubMed] [Google Scholar]
- Bloch KD, Filippov G, Sanchez LS, Nakane M, de la Monte SM. Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol. 1997;272:L400–L406. doi: 10.1152/ajplung.1997.272.3.L400. [DOI] [PubMed] [Google Scholar]
- Brannon TS, North AJ, Wells LB, Shaul PW. Prostacyclin synthesis in ovine pulmonary artery is developmentally regulated by changes in cyclooxygenase-1 gene expression. J Clin Invest. 1994;93:2230–2235. doi: 10.1172/JCI117220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon TS, MacRitchie AN, Jaramillo MA, Sherman TS, Yuhanna IS, Margraf LR, Shaul PW. Ontogeny of cyclooxygenase-1 and cyclooxygenase-2 gene expression in ovine lung. Am J Physiol. 1998;274:L66–L71. doi: 10.1152/ajplung.1998.274.1.L66. [DOI] [PubMed] [Google Scholar]
- Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- Buysse C, Fonteyne C, Dessy H, De Laet MH, Biarent D. The use of dipyridamole to wean from inhaled nitric oxide in congenital diaphragmatic hernia. J Pediatr Surg. 2001;36(12):1864–1865. doi: 10.1053/jpsu.2001.28873. [DOI] [PubMed] [Google Scholar]
- Cai J, Su Z, Shi Z, Zhou Y, Xu Z, Liu J, Chen L, Xu Z, Yu X, Ding W, Yang Y. Nitric oxide in conjunction with milrinone better stabilized pulmonary hemodynamics after Fontan procedure. Artif Organs. 2008a;32(11):864–869. doi: 10.1111/j.1525-1594.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- Cai J, Su Z, Shi Z, Zhou Y, Xu Z, Liu J, Chen L, Xu Z, Yu X, Ding W, Yang Y. Nitric oxide and milrinone: combined effect on pulmonary circulation after Fontan-type procedure: a prospective, randomized study. Ann Thorac Surg. 2008b;86(3):882–888. doi: 10.1016/j.athoracsur.2008.05.014. discussion 882–888. [DOI] [PubMed] [Google Scholar]
- Chang AC, Atz AM, Wernovsky G, Burke RP, Wessel DL. Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med. 1995;23(11):1907–1914. doi: 10.1097/00003246-199511000-00018. [DOI] [PubMed] [Google Scholar]
- Chen B, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Russell JA, Farrow KN, Steinhorn RH. Regulation of phosphodiesterase 3 in the pulmonary arteries during the perinatal period in sheep. Pediatr Res. 2009;66(6):682–687. doi: 10.1203/PDR.0b013e3181bce574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342(7):469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- Clark RH, Huckaby JL, Kueser TJ, Walker MW, Southgate WM, Perez JA, Roy BJ, Keszler M. Low-dose nitric oxide therapy for persistent pulmonary hypertension: 1-year follow-up. J Perinatol. 2003;23(4):300–303. doi: 10.1038/sj.jp.7210908. [DOI] [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- de Buys Roessingh AS, Dinh-Xuan AT. Congenital diaphragmatic hernia: current status and review of the literature. Eur J Pediatr. 2009;168(4):393–406. doi: 10.1007/s00431-008-0904-x. [DOI] [PubMed] [Google Scholar]
- de Visser YP, Walther FJ, Laghmani EH, van Wijngaarden S, Nieuwland K, Wagenaar GT. Phosphodiesterase-4 inhibition attenuates pulmonary inflammation in neonatal lung injury. Eur Respir J. 2008;31(3):633–644. doi: 10.1183/09031936.00071307. [DOI] [PubMed] [Google Scholar]
- de Visser YP, Walther FJ, Laghmani el H, Boersma H, van der Laarse A, Wagenaar GT. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res. 2009;10:30. doi: 10.1186/1465-9921-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukarm RC, Morin FC, 3rd, Russell JA, Steinhorn RH. Pulmonary and systemic effects of the phosphodiesterase inhibitor dipyridamole in newborn lambs with persistent pulmonary hypertension. Pediatr Res. 1998;44(6):831–837. doi: 10.1203/00006450-199812000-00002. [DOI] [PubMed] [Google Scholar]
- Dukarm RC, Steinhorn RH, Russell JA, Lakshminrusimha S, Swartz D, Cummings JJ. Selective type 5 phosphodiesterase inhibition alters pulmonary hemodynamics and lung liquid production in near term fetal lambs. J Appl Physiol. 2005;99(6):2331–2336. doi: 10.1152/japplphysiol.00120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgenov OV, Busch CJ, Evgenov NV, Liu R, Petersen B, Falkowski GE, Petho B, Vas A, Bloch KD, Zapol WM, Ichinose F. Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L723–L729. doi: 10.1152/ajplung.00485.2004. [DOI] [PubMed] [Google Scholar]
- Fan Chung K. Phosphodiesterase inhibitors in airways disease. Eur J Pharmacol. 2006;533(1–3):110–117. doi: 10.1016/j.ejphar.2005.12.059. [DOI] [PubMed] [Google Scholar]
- Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol. 2005;29(1):8–14. doi: 10.1053/j.semperi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008a;102(2):226–233. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008b;295(6):L979–L987. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010a;299(1):L109–L116. doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow KN, Wedgwood S, Lee KJ, Czech L, Gugino SF, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol. 2010b doi: 10.1016/j.resp.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- Gelfand SL, Fanaroff JM, Walsh MC. Controversies in the treatment of meconium aspiration syndrome. Clin Perinatol. 2004;31(3):445–452. doi: 10.1016/j.clp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333(4):214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- Group, N.I.N.O.S. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. New Eng J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- Group, N.I.N.O.S. Inhaled nitric oxide in term and near-term infants: neurodevelopmental follow-up of the neonatal inhaled nitric oxide study group (NINOS) J Pediatr. 2000;136(5):611–617. doi: 10.1067/mpd.2000.104826. [DOI] [PubMed] [Google Scholar]
- Hanson KA, Burns F, Rybalkin SD, Miller JW, Beavo J, Clarke WR. Developmental changes in lung cGMP phosphodiesterase-5 activity, protein, and message. Am J Respir Crit Care Med. 1998a;158(1):279–288. doi: 10.1164/ajrccm.158.1.9711042. [DOI] [PubMed] [Google Scholar]
- Hanson KA, Ziegler JW, Rybalkin SD, Miller JW, Abman SH, Clarke WR. Chronic pulmonary hypertension increases fetal lung cGMP phosphodiesterase activity. Am J Physiol. 1998b;275(5 Pt 1):L931–L941. doi: 10.1152/ajplung.1998.275.5.L931. [DOI] [PubMed] [Google Scholar]
- Haworth SG. Pulmonary vascular remodeling in neonatal pulmonary hypertension. Chest. 1988;93:133S–138S. [PubMed] [Google Scholar]
- Hoffman JI, Rudolph AM, Heymann MA. Pulmonary vascular disease with congenital heart lesions: pathologic features and causes. Circulation. 1981;64(5):873–877. doi: 10.1161/01.cir.64.5.873. [DOI] [PubMed] [Google Scholar]
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107(7):996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10(22):1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Ivy DD, Kinsella JP, Ziegler JW, Abman SH. Dipyridamole attenuates rebound pulmonary hypertension after inhaled nitric oxide withdrawal in postoperative congenital heart disease. J Thorac Cardiovasc Surg. 1998;115(4):875–882. doi: 10.1016/S0022-5223(98)70369-1. [DOI] [PubMed] [Google Scholar]
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- Karamanoukian HL, Peay T, Love JE, Abdel-Rahman E, Dandonna P, Azizkhan RG, Glick PL. Decreased pulmonary nitric oxide synthase activity in the rat model of congenital diaphragmatic hernia. J Pediatr Surg. 1996;31(8):1016–1019. doi: 10.1016/s0022-3468(96)90076-7. [DOI] [PubMed] [Google Scholar]
- Keller RL, Hamrick SE, Kitterman JA, Fineman JR, Hawgood S. Treatment of rebound and chronic pulmonary hypertension with oral sildenafil in an infant with congenital diaphragmatic hernia. Pediatr Crit Care Med. 2004;5(2):184–187. doi: 10.1097/01.pcc.0000113266.26638.ad. [DOI] [PubMed] [Google Scholar]
- Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355(4):354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- Konduri GG. New approaches for persistent pulmonary hypertension of newborn. Clin Perinatol. 2004;31(3):591–611. doi: 10.1016/j.clp.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Konduri GG, Ou J, Shi Y, Pritchard KA. Decreased association of hsp90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H204–H211. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005;172:750–756. doi: 10.1164/rccm.200503-510OC. [DOI] [PubMed] [Google Scholar]
- Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol. 1999;26(3):601–619. [PubMed] [Google Scholar]
- Lakshminrusimha S, Steinhorn RH. Phosphodiesterase inhibitors in the management of persistent pulmonary hypertension of the newborn (PPHN) eNeonatal Review. 2009;6(12) http//www.hopkinscme.edu/ofp/eNeonatalReview/Newsletters/2009/0909.html. [Google Scholar]
- Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59(1):137–141. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, Morin FC., 3rd Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007a;62(3):313–318. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Wiseman D, Black SM, Russell JA, Gugino SF, Oishi P, Steinhorn RH, Fineman JR. The role of nitric oxide synthase-derived reactive oxygen species in the altered relaxation of pulmonary arteries from lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol. 2007b;293(3):H1491–H1497. doi: 10.1152/ajpheart.00185.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Porta NF, Farrow KN, Chen B, Gugino SF, Kumar VH, Russell JA, Steinhorn RH. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2009a;10(1):106–112. doi: 10.1097/PCC.0b013e3181936aee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, Russell JA, Steinhorn RH. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res. 2009b;66(5):539–544. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Hillier SC, Knoderer CA. Use of sildenafil to facilitate weaning from inhaled nitric oxide in children with pulmonary hypertension following surgery for congenital heart disease. J Intensive Care Med. 2008;23(5):329–334. doi: 10.1177/0885066608321389. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Hessler JR, Green RS. The onset of breathing at birth stimulates pulmonary vascular prostacyclin synthesis. Pediatr Res. 1984;18:938–942. doi: 10.1203/00006450-198410000-00006. [DOI] [PubMed] [Google Scholar]
- Levin DL, Rudolph AM, Heymann MA, Phibbs RH. Morphological development of the pulmonary vascular bed in fetal lambs. Circulation. 1976;53:144–151. doi: 10.1161/01.cir.53.1.144. [DOI] [PubMed] [Google Scholar]
- Loughney K, Hill TR, Florio VA, Uher L, Rosman GJ, Wolda SL, Jones BA, Howard ML, McAllister-Lucas LM, Sonnenburg WK, Francis SH, Corbin JD, Beavo JA, Ferguson K. Isolation and characterization of cDNA encoding PDE5A, a human cGMP-binding, cGMP-specific 3′, 5′-cyclic nucleotide phosphodiesterase. Gene. 1998;216:139–147. doi: 10.1016/s0378-1119(98)00303-5. [DOI] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109(3):366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283(2):619–624. [PubMed] [Google Scholar]
- Manchester D, Margolis HS, Sheldon RE. Possible association between maternal indomethacin therapy and primary pulmonary hypertension of the newborn. Am J Obstet Gynecol. 1976;126:467–469. doi: 10.1016/0002-9378(76)90640-2. [DOI] [PubMed] [Google Scholar]
- McNamara PJ, Laique F, Muang-In S, Whyte HE. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care. 2006;21(2):217–222. doi: 10.1016/j.jcrc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Millen J, MacLean MR, Houslay MD. Hypoxia-induced remodelling of PDE4 isoform expression and cAMP handling in human pulmonary artery smooth muscle cells. Eur J Cell Biol. 2006;85(7):679–691. doi: 10.1016/j.ejcb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Morin FC., 3rd Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res. 1989;25:245–250. doi: 10.1203/00006450-198903000-00005. [DOI] [PubMed] [Google Scholar]
- Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121(2):317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154(3):379–384. doi: 10.1016/j.jpeds.2008.09.021. 384 e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Dombi T, Wittke B, Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009;85(1):56–63. doi: 10.1038/clpt.2008.177. [DOI] [PubMed] [Google Scholar]
- Murphy JD, Rabinovitch M, Goldstein JD, Reid LM. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981;98(6):962–967. doi: 10.1016/s0022-3476(81)80605-1. [DOI] [PubMed] [Google Scholar]
- Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, Insel PA. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L294–L303. doi: 10.1152/ajplung.00190.2006. [DOI] [PubMed] [Google Scholar]
- Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174(9):1042–1047. doi: 10.1164/rccm.200605-694OC. [DOI] [PubMed] [Google Scholar]
- Noori S, Friedlich P, Wong P, Garingo A, Seri I. Cardiovascular effects of sildenafil in neonates and infants with congenital diaphragmatic hernia and pulmonary hypertension. Neonatology. 2007;91(2):92–100. doi: 10.1159/000097125. [DOI] [PubMed] [Google Scholar]
- Okogbule-Wonodi AC, Ibe BO, Yue BW, Hsu S, Raj JU. Phosphodiesterase activity in intrapulmonary arteries and veins of perinatal lambs. Mol Genet Metab. 1998;65(3):229–237. doi: 10.1006/mgme.1998.2756. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Haworth SG, Castaneda AR, Nadas AS, Reid LM. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation. 1978;58(6):1107–1122. doi: 10.1161/01.cir.58.6.1107. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Keane JF, Norwood WI, Castaneda AR, Reid L. Vascular structure in lung tissue obtained at biopsy correlated with pulmonary hemodynamic findings after repair of congenital heart defects. Circulation. 1984;69(4):655–667. doi: 10.1161/01.cir.69.4.655. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Bothwell T, Hayakawa BN, Williams WG, Trusler GA, Rowe RD, Olley PM, Cutz E. Pulmonary artery endothelial abnormalities in patients with congenital heart defects and pulmonary hypertension A correlation of light with scanning electron microscopy and transmission electron microscopy. Lab Invest. 1986;55(6):632–653. [PubMed] [Google Scholar]
- Reddy VM, Wong J, Liddicoat JR, Johengen M, Chang R, Fineman JR. Altered endothelium-dependent responses in lambs with pulmonary hypertension and increased pulmonary blood flow. Am J Physiol. 1996;271(2 Pt 2):H562–H570. doi: 10.1152/ajpheart.1996.271.2.H562. [DOI] [PubMed] [Google Scholar]
- Ross GA, Oishi P, Azakie A, Fratz S, Fitzgerald RK, Johengen MJ, Harmon C, Hendricks-Munoz K, Xu J, Black SM, Fineman JR. Endothelial alterations during inhaled NO in lambs with pulmonary hypertension: implications for rebound hypertension. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L27–L35. doi: 10.1152/ajplung.00144.2004. [DOI] [PubMed] [Google Scholar]
- Sanchez LS, de la Monte SM, Filippov G, Jones RC, Zapol WM, Bloch KD. Cyclic-GMP-binding, cyclic-GMP-specific phosphodiesterase (PDE5) gene expression is regulated during rat pulmonary development. Pediatr Res. 1998;43(2):163–168. doi: 10.1203/00006450-199802000-00002. [DOI] [PubMed] [Google Scholar]
- Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115(17):2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- Shah PS, Ohlsson A. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD005494.pub2. CD005494. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Wells LB. Oxygen modulates nitric oxide production selectively in fetal pulmonary endothelial cells. Am J Respir Crit Care Med. 1994;11:432–438. doi: 10.1165/ajrcmb.11.4.7522486. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Campbell WB, Farrar MA, Magness RR. Oxygen modulates prostacyclin synthesis in ovine fetal pulmonary arteries by an effect on cyclooxygenase. J Clin Invest. 1992;90:2147–2155. doi: 10.1172/JCI116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC., 3rd Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 1997;272:L1005–L1012. doi: 10.1152/ajplung.1997.272.5.L1005. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Afshar S, Gibson LL, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC. Developmental changes in nitric oxide synthase isoform expression and nitric oxide production in fetal baboon lung. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1192–L1199. doi: 10.1152/ajplung.00112.2002. [DOI] [PubMed] [Google Scholar]
- Shekerdemian LS, Ravn HB, Penny DJ. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2002;165(8):1098–1102. doi: 10.1164/ajrccm.165.8.2107097. [DOI] [PubMed] [Google Scholar]
- Shekerdemian LS, Ravn HB, Penny DJ. Interaction between inhaled nitric oxide and intravenous sildenafil in a porcine model of meconium aspiration syndrome. Pediatr Res. 2004;55(3):413–418. doi: 10.1203/01.PDR.0000112033.81970.C2. [DOI] [PubMed] [Google Scholar]
- Steinhorn RH, Morin FC, 3rd, VanWylen DG, Gugino SF, Giese EC, Russell JA. Endothelium-dependent relaxations to adenosine in juvenile rabbit pulmonary arteries and veins. Am J Physiol. 1994;266:H2001–H2006. doi: 10.1152/ajpheart.1994.266.5.H2001. [DOI] [PubMed] [Google Scholar]
- Steinhorn RH, Russell JA, Morin FC., 3rd Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1995;268:H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- Steinhorn RH, Russell JA, Lakshminrusimha S, Gugino SF, Black SM, Fineman JR. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001;280(1):H311–H317. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, Wessel DL. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155(6):841–847. doi: 10.1016/j.jpeds.2009.06.012. e1. [DOI] [PubMed] [Google Scholar]
- Stocker C, Penny DJ, Brizard CP, Cochrane AD, Soto R, Shekerdemian LS. Intravenous sildenafil and inhaled nitric oxide: a randomised trial in infants after cardiac surgery. Intensive Care Med. 2003;29(11):1996–2003. doi: 10.1007/s00134-003-2016-4. [DOI] [PubMed] [Google Scholar]
- Thebaud B, Saizou C, Farnoux C, Hartman JF, Mercier JC. Dypiridamole, a cGMP phosphodiesterase inhibitor, transiently improves the response to inhaled nitric oxide in two newborns with congenital diaphragmatic hernia. Intensive Care Med. 1999;25(3):300–303. doi: 10.1007/s001340050839. [DOI] [PubMed] [Google Scholar]
- Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112(16):2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- Thelitz S, Oishi P, Sanchez LS, Bekker JM, Ovadia B, Johengen MJ, Black SM, Fineman JR. Phosphodiesterase-3 inhibition prevents the increase in pulmonary vascular resistance following inhaled nitric oxide withdrawal in lambs. Pediatr Crit Care Med. 2004;5(3):234–239. doi: 10.1097/01.pcc.0000124021.25393.2d. [DOI] [PubMed] [Google Scholar]
- Thusu KG, Morin FC, 3rd, Russell JA, Steinhorn RH. The cGMP phosphodiesterase inhibitor zaprinast enhances the effect of nitric oxide. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1605–1610. doi: 10.1164/ajrccm.152.5.7582302. [DOI] [PubMed] [Google Scholar]
- Tiktinsky MH, Morin FC., 3rd Increasing oxygen tension dilates fetal pulmonary circulation via endothelium-derived relaxing factor. Am J Physiol. 1993;265(1 Pt 2):H376–H380. doi: 10.1152/ajpheart.1993.265.1.H376. [DOI] [PubMed] [Google Scholar]
- Tzao C, Nickerson PA, Russell JA, Gugino SF, Steinhorn RH. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr Pulmonol. 2001;31(2):97–105. doi: 10.1002/1099-0496(200102)31:2<97::aid-ppul1016>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371–C1378. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107(4):642–647. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- Villanueva ME, Zaher FM, Svinarich DM, Konduri GG. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res. 1998;44:338–343. doi: 10.1203/00006450-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Vukcevic Z, Coppola CP, Hults C, Gosche JR. Nitrovasodilator responses in pulmonary arterioles from rats with nitrofen-induced congenital diaphragmatic hernia. J Pediatr Surg. 2005;40(11):1706–1711. doi: 10.1016/j.jpedsurg.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, Blackmon L, Jobe A. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006;117(3 Pt 2):S52–S56. doi: 10.1542/peds.2005-0620I. [DOI] [PubMed] [Google Scholar]
- Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile L, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- Wild LM, Nickerson PA, Morin FC., 3rd Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res. 1989;25:251–257. doi: 10.1203/00006450-198903000-00006. [DOI] [PubMed] [Google Scholar]
- Worwag S, Mulla H, Luyt D, Firmin RK. Dipyridamole in the treatment of a neonate with persistent pulmonary hypertension. J R Soc Med. 2000;93(2):77–78. doi: 10.1177/014107680009300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyda K, Koebrich S, Reiss I, Rudloff S, Pullamsetti SS, Ruhlmann A, Weissmann N, Ghofrani HA, Gunther A, Seeger W, Grimminger F, Morty RE, Schermuly RT. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. Eur Respir J. 2009;33(4):861–870. doi: 10.1183/09031936.00109008. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100(11):1569–1578. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]