Abstract
Relapse of castration-resistant prostate cancer (CRPC) that occurs after androgen deprivation therapy of primary prostate cancer can be mediated by reactivation of the androgen receptor (AR). One important mechanism mediating this AR reactivation is intratumoral conversion of the weak adrenal androgens DHEA and androstenedione into the AR ligands testosterone and dihydrotestosterone (DHT). DHEA and androstenedione are synthesized by the adrenals through the sequential actions of the cytochrome P450 enzymes CYP11A1 and CYP17A1, so that CYP17A1 inhibitors such as abiraterone are effective therapies for CRPC. However, the significance of intratumoral CYP17A1 and de novo androgen synthesis from cholesterol in CRPC, and the mechanisms contributing to CYP17A1 inhibitor resistance/relapse, remain to be determined. We report that AR activity in castration-resistant VCaP tumor xenografts can be restored through CYP17A1-dependent de novo androgen synthesis, and that abiraterone treatment of these xenografts imposes selective pressure for increased intratumoral expression of CYP17A1, thereby generating a mechanism for development of resistance to CYP17A1 inhibitors. Supporting the clinical relevance of this mechanism, we found that intratumoral expression of CYP17A1 was markedly increased in tumor biopsies from CRPC patients after CYP17A1 inhibitor therapy. We further show that CRPC cells expressing a progesterone responsive T877A mutant AR are not CYP17A1 dependent, but that AR activity in these cells is still steroid dependent and mediated by upstream CYP11A1 dependent intraturmoral pregnenolone/progesterone synthesis. Together, our results indicate that CRPCs resistant to CYP17A1 inhibition may remain steroid dependent and therefore responsive to therapies that can further suppress de novo intratumoral steroid synthesis.
Keywords: prostate cancer, androgen receptor, CYP17A1, steroid synthesis, androgen deprivation therapy
Introduction
The majority of prostate cancers (PCa) express androgen receptor (AR) at high levels and respond to androgen deprivation therapies (ADT, surgical or medical castration), but patients usually relapse within 2–3 years. Significantly, these relapsed tumors (castration resistant prostate cancer, CRPC) express AR and androgen regulated genes at high levels, indicating that AR transcriptional activity has been at least partially reactivated despite castrate serum androgen levels (1–3). Multiple mechanisms may contribute to this AR reactivation (4–6), but one significant mechanism is increased synthesis of testosterone and dihydrotestosterone (DHT) by the tumor cells from weak androgens produced by the adrenal glands (intratumoral androgen synthesis). Normal prostate contains the enzymatic machinery to convert weak androgens produced by the adrenal glands, including androstenedione, dehydroepiandrosterone (DHEA), and DHEA-sulfate (DHEA-S, which circulates at high levels), into the physiological high affinity AR ligands testosterone and DHT, so prostatic testosterone and DHT levels decline less in response to castration than do levels in serum (7–9). Moreover, intraprostatic androgen levels in castrated men with locally recurrent CRPC are further elevated relative to serum levels, while tissue testosterone levels in metastatic CRPC may actually be higher than in prostate prior to castration (10–13). Consistent with these observations, expression of enzymes that convert DHEA and androstenedione to testosterone and DHT is increased in CRPC, with the greatest increase being in AKR1C3, the prostate enzyme that reduces androstenedione to testosterone (2, 13).
While these findings strongly support increased conversion of weak adrenal androgens to testosterone and DHT as a mechanism contributing to AR reactivation in CRPC, it has been less clear whether these tumors can produce physiologically significant levels of androgens de novo from cholesterol and thereby become independent of circulating adrenal androgens. Transcripts encoding enzymes required for de novo synthesis of steroids from cholesterol (including CYP11A1 and CYP17A1) can be detected in PCa cell lines, and studies in the LNCaP PCa cell line have found de novo steroid synthesis using radioactive acetate, but the levels of testosterone were extremely low and androgen levels were not clearly adequate to restore AR activity (14, 15). One study of patient samples obtained through “warm autopsies” found that CYP17A1 mRNA was increased in metastatic CRPC, suggesting increased de novo androgen synthesis (13). However, increased CYP17A1 was not observed in other studies of local or metastatic CRPC (1, 2, 16).
Clinical responses to adrenalectomy provided the initial indication that adrenal derived androgens were contributing to CRPC (17). Subsequent studies using ketoconazole, which decreases adrenal androgens by inhibiting the enzyme CYP17A1 that is required for DHEA and androstenedione synthesis, have yielded substantial response rates and further support a role for adrenal androgens in CRPC (18). A more potent and selective CYP17A1 inhibitor, abiraterone, has yielded higher and more durable responses in CRPC patients (19–21), and recently received FDA approval based on a phase III clinical trial that showed a survival advantage. Unfortunately, these abiraterone treated patients generally relapse within one year, and the recurrent tumors again express high levels of the AR regulated PSA gene, indicating that AR has once again been reactivated. Significantly, adrenal gland resistance to abiraterone does not appear to be a resistance mechanism as serum DHEA, androstenedione, or testosterone levels are not increased at the time of relapse, and there have been no reports of alternative resistance mechanisms in these patients. In this study we show that AR activity in CRPC can be driven by CYP17A1 dependent de novo androgen synthesis, and that treatment with CYP17A1 inhibitors selects for tumor cells expressing increased levels of CYP17A1, providing a mechanism for the development of resistance to CYP17A1 inhibitors.
Materials and Methods
Cell culture and xenografts
LNCaP and C4-2 cells were cultured in RPMI-1640 with 10% FBS, VCaP cells were in DMEM with 10% FBS, and VCS2 cells were in DMEM with 8% charcoal-dextran stripped serum (CSS)/2% FBS (Hyclone, Logan, UT). The former cells (LNCaP, C4-2, and VCaP) were from ATCC and express AR and AR regulated genes, while the derivation of VCS2 is described here. For androgen stimulation assays, cells were grown to 50–60% confluence in medium with 5% CSS for 3 days prior to treatment. Castration resistant VCaP xenografts were established as described (22). To generate xenografts in female scid mice, relapsed tumors from males were dispersed and injected in 50% Matrigel. Abiraterone (provided as abiraterone acetate) was from Cougar Biotechnology. RNA and protein were extracted from frozen sections, with viable tumor confirmed by H&E staining on adjacent sections. Intratumoral androgens were measured by mass spectroscopy as described previously (9).
RT-PCR and immunoblotting
RNA from bone marrow biopsies replaced with tumor in patients with CRPC were obtained as described previously (2). Additional snap frozen samples were obtained under an IRB approved protocol by CT guided bone marrow biopsy from CRPC patients treated with ketoconazole (1200 mg/d) and dutasteride (0.5 mg/d) for one month, or who were relapsing after responding for >1 year. Quantitative real-time RT-PCR amplification was with Taqman one-step RT-PCR reagents and results were normalized to co-amplified 18S RNA or GAPDH. Data shown are representative of at least 3 experiments, and significant differences are indicated. Primers and probes are in supplementary materials (Supplementary Figure S16). Proteins were extracted by boiling for 15 min in 2% SDS and detected by blotting with anti-ERG (Santa Cruz), anti-PSA (BioDesign), anti-AR (Upstate), anti-AKR1C3 (23), anti-CYP11A1 (Abcam), anti-β-actin (Abcam), or anti-β-tubulin (Upstate). Band intensities relative to loading controls on low exposure gels were determined and normalized to the no treatment controls. Gels shown are representative of at least 3 independent experiments.
Immunohistochemistry (IHC) and immunofluorescence
Paraffin sections underwent antigen retrieval and were then blocked using 5% goat serum and avidin blocking solution (Vector). Anti-AR (Santa Cruz), anti-ERG (Santa Cruz), anti-PSA (Abcam), or anti-Ki67 (Dako) were then added overnight at 4°C, followed by biotinylated secondary antibody and streptavidinhorseradish peroxidase (HRP) (Vector). Sections compared in each figure were stained at the same time and photographed under identical conditions.
Chromatin immunoprecipitation (ChIP)
Cells were formalin fixed, lysed and sonicated to break the chromatin into 500–800 bp fragments. Anti-AR or rabbit IgG (Santa Cruz) were used to precipitate chromatin and qRT-PCR was used to analyze binding to the PSA enhancer or an irrelevant locus (primers are in supplementary Figure S13).
Results
VCaP PCa cells have substantial basal AR transcriptional activity
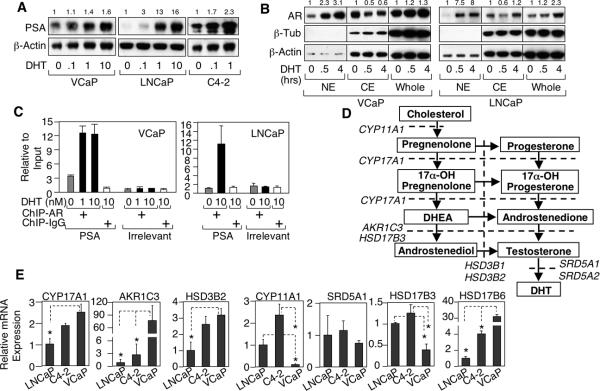
VCaP cells, derived from a vertebral metastasis in a patient with CRPC, express wild-type AR and AR-regulated genes such as PSA and the TMPRSS2:ERG fusion gene (24). Although androgen responsive, VCaP cells, like C4-2 cells (derived from a LNCaP xenograft that relapsed after castration), express substantial basal levels of the AR regulated PSA gene when cultured in steroid depleted serum (Figure 1A). This basal AR activity was not from residual androgens in the serum, as it was similarly observed in serum free medium (Supplemental Figure S1). VCaP also had substantial nuclear AR under these conditions, consistent with the basal PSA expression, which was increased by DHT (Figure 1B). ChIP further showed that substantial AR in VCaP cells, but not LNCaP cells, was bound to the androgen responsive element (ARE) in the PSA gene enhancer in the absence of exogenous androgens (Figure 1C).
Figure 1.
VCaP cells have substantial basal AR activity. A, VCaP, LNCaP, or C4-2 cells grown in medium with 5% charcoal-dextran stripped fetal bovine serum (CSS) were treated with 0, 0.1, 1, or 10 nM DHT for 24h and then immunoblotted for PSA and β-actin (loading control). The number on top of each lane is band intensity compared to loading control, which is then normalized to the untreated lane. B, VCaP or LNCaP cells in CSS medium were treated with 10 nM DHT for 0, 0.5 or 4h and then fractionated into nuclear extracts (NE) or cytoplasmic extracts (CE). NE, CE or whole cell lysates were immunoblotted for AR, β-tubulin (cytoplasm control), or β-actin (loading control). Blots shown were from the same gel. C, VCaP or LNCaP cells in CSS medium were treated with 0, 1, or 10 nM DHT for 4h and then subjected to ChIP assay. The DNA fragments were PCR amplified and normalized to input to measure binding to the PSA enhancer ARE or irrelevant site (negative control). D, androgen synthesis pathway. E, LNCaP, C4-2, or VCaP cells were subjected to quantitative real time RT-PCR (qRT-PCR) to measure CYP17A1, AKR1C3, HSD3B2, CYP11A1, SRD5A1, HSD17B3, or HSD17B6 (major enzyme mediating the regeneration of DHT from its reduced metabolite). Equal amounts of cellular RNA were coamplified with 18S RNA as an internal control.
To determine whether de novo (from cholesterol) steroid synthesis may contribute to basal this AR activity in VCaP or C4-2 cells, we next accessed the expression of steroid biosynthetic enzymes (Figure 1D). Transcripts encoding each enzyme were readily detected and moderately higher in C4-2 versus LNCaP, while VCaP had markedly higher expression of AKR1C3 and HSD17B6, but lower CYP11A1 compared to C4-2 and LNCaP cells (Figure 1E). Together these findings suggested that basal AR activity in VCaP and C4-2 cells may be mediated by CYP17A1 dependent de novo androgen synthesis.
Basal AR activity in VCaP cells and relapsed xenografts is CYP17A1 dependent
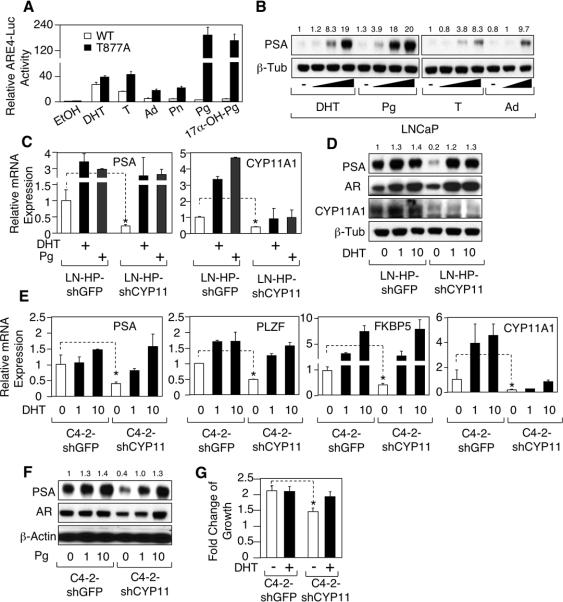
Significantly, a CYP17A1 inhibitor (ketoconazole) decreased basal (in steroid depleted medium) PSA expression in VCaP cells, but not C4-2 cells (Figure 2A). This block could be overcome by an androgen precursor that is downstream of CYP17A1, androstenedione, indicating that it was due to CYP17A1 blockade and not a toxic effect (Figure 2B). Abiraterone, a more specific CYP17A1 inhibitor, similarly decreased basal PSA and ERG (from the AR regulated TMPRSS2:ERG fusion gene) expression in VCaP cells, but not in C4-2 cells, further indicating that basal AR activity in VCaP, but not C4-2, was CYP17A1 dependent (Figure 2C). AR protein in VCaP cells was also decreased by abiraterone, consistent with the decreased stability of the unliganded AR. Confocal microscopy further showed that AR nuclear expression in VCaP cells in the absence of exogenous androgens was blocked by abiraterone (Supplemental Figure S2). Abiraterone also blocked PSA expression in VCaP cells induced by progesterone, an upstream CYP17A1 substrate, confirming the CYP17A1 dependent conversion of progesterone to physiologically significant levels of androgens (Supplemental Figure S3). In contrast, PSA expression stimulated by androstenedione (downstream of CYP17A1) was not blocked by abiraterone (Figure 2D).
Figure 2.
Basal AR activity in VCaP is dependent on CYP17A1. A, VCaP, LNCaP, or C4-2 cells in CSS medium were treated with 0, 2, or 5 μM ketoconazole (Keto) for 24h and then immunoblotted. B, VCaP cells in CSS medium were treated with 0, 1, 10, or 100 nM androstenedione (Ad) with or without 2 μM ketoconazole for 24h. C, VCaP or C4-2 cells in CSS medium were treated with 0, 2, or 5 μM abiraterone (Abir) for 24h. D, VCaP cells in CSS medium were treated with 0 or 100 nM androstenedione with or without 2 μM abiraterone and then immunoblotted or (E) subjected to qRT-PCR. F, VCaP cells grown for 2 days in CSS medium were treated with abiraterone (5 μM) and androstenedione (25 nM) for 24h and then harvested for steroid measurements. Each bar represents a biological replicate. G, tissue samples of recurrent VCaP xenogafts were taken from each mouse pre-treatment and post-treatment with abiraterone (0.5 mg/2d for 8 days) and then subjected to RT-PCR (n=4) or (H) immunohistochemistry. I, testosterone or DHT levels in recurrent VCaP xenograft tumors were measured pre- or post-abiraterone treatment. DHEA and androstenedione levels were below the level of detection pre- and post-treatment.
RT-PCR further established that abiraterone was decreasing expression of AR-regulated transcripts encoding PSA, TMPRSS2, ERG, PLZF, and FKBP5, and that this block could be bypassed by androstenedione (Figure 2E). Finally, to confirm that abiraterone was reducing intracellular androgen synthesis, we measured androgen levels in untreated versus abiraterone treated VCaP cells cultured in steroid depleted medium. As shown in figure 2F, abiraterone decreased DHT and testosterone levels, but did not prevent the increase in DHT and testosterone when androstenedione was added to the medium. We conclude from these results that VCaP cells synthesize testosterone and DHT de novo at levels that are adequate to drive AR transcriptional activity.
Rodent adrenal glands express low or undetectable levels of CYP17, so serum levels of testosterone and androgen precursors are extremely low in castrated male and in female mice (25). In a previous study we showed that VCaP xenografts that relapsed after castration had restored AR transcriptional activity and had increased expression of androgen synthetic genes including AKR1C3, but CYP17A1 was not consistently increased (22). To assess the effects of inhibiting CYP17A1 in vivo, castration resistant VCaP xenografts were transplanted into female mice. Established tumors in each mouse were biopsied, and the mice were then treated with 0.5 mg abiraterone ip every other day for 8 days. Comparison of mRNA from pre- and post-treatment tumors showed that abiraterone decreased the expression of multiple androgen-regulated genes (PSA, TMPRSS2, and ERG), but did not decrease AR mRNA, consistent with decreased AR transcriptional activity (Figure 2G). Immunohistochemistry also indicated decreased PSA protein and decreased nuclear ERG and AR after abiraterone treatment (Figure 2H and Supplemental Figure S4). Moreover, Ki67-positive cells were greatly decreased in the abiraterone treated tumors (62% mean decrease), indicating that tumor growth was suppressed (Figure 2G and Supplemental Figure S5). Measurement of androgen levels in tumors pre- and post treatment confirmed that testosterone and DHT were decreased by abiraterone (Figure 2I).
Basal AR activity in VCaP cells and castration resistant VCaP xenografts is AKR1C3 dependent
Previously we showed that AKR1C3, the prostatic enzyme mediating reduction of androstenedione to testosterone, was increased in human CRPC biopsies relative to primary PCa (2), and that its expression was increased in VCaP xenografts that had relapsed after castration (22). AKR1C3 mRNA was also much higher in VCaP than LNCaP or C4-2 cells (Figure 1E), while at the protein level AKR1C3 was only detected in VCaP cells (Figure 3A). As specific AKR1C3 inhibitors are not yet available, we generated stable AKR1C3 knock-down cells by infecting VCaP cells with an AKR1C3 shRNA lentivirus. PSA in the absence of DHT was dramatically decreased in VCaP-shAKR1C3 cells, and could be restored by DHT (Figure 3B). In contrast, androstenedione, a very weak direct AR agonist, only minimally increased PSA in the VCaP-shAKR1C3 cells (Figure 3C), confirming that AKR1C3 was mediating the synthesis of physiologically significant levels of testosterone from androstenedione. Although not selective, the nonsteroidal anti-inflammatory drug indomethacin at micromolar concentrations can also inhibit AKR1C3 (26, 27). Similarly to the effect of shAKR1C3, indomethacin decreased basal and androstenedione stimulated PSA expression in VCaP cells, but not in C4-2 cells (Figure 3D and Supplemental Figure S6). RT-PCR confirmed that indomethacin was decreasing mRNA levels of PSA, TMPRSS2, and PLZF, and that this effect could be bypassed by DHT (Figure 3E).
Figure 3.
Basal AR activity in VCaP is AKR1C3 dependent. A, LNCaP, C4-2, or VCaP cells were immunoblotted for AKR1C3 or β-tubulin (loading control). B and C, VCaP cells stably infected with lentivirus expressing either GFP shRNA or AKR1C3 shRNA (Open Biosystems) in CSS medium were treated with 10 nM DHT or 100 nM androstenedione (Ad) for 24h, and then immunoblotted. D, VCaP or C4-2 cells in CSS medium were treated with 0, 20, or 40 μM indomethacin for 24h. E, VCaP cells in CSS medium were treated with 0, 20, or 40 μM indomethacin with or without 10nM DHT and subjected to qRT-PCR. F, mice bearing relapsed VCaP xenografts were treated with indomethacin for 2 weeks (~0.25 mg per day in drinking water) and tissue samples taken pre- and post-therapy from tumors (n=5) were analyzed by RT-PCR as indicated or (G) by immunohistochemistry for PSA, ERG, AR, and Ki67. H, testosterone and DHT levels in recurrent xenograft tumors in transplanted female scid mice (n=3) were measured pre- or post-indomethacin (Indo) treatment.
To examine the effects of inhibiting AKR1C3 in vivo in castration resistant VCaP xenografts, we castrated another series of male mice bearing VCaP xenografts. Consistent with previous results, quantitative RT-PCR of tumor samples taken pre- and post-castration and at relapse showed renewed expression of AR regulated genes and increased expression of AR mRNA in the castration resistant VCaP xenografts (Supplemental Figure S7). Immunohistochemistry on serial biopsies also confirmed that cytoplasmic expression of AKR1C3 protein was rapidly increased after castration (although this increase clearly was not adequate to restore AR activity) and was further increased in the relapsed tumors (Supplemental Figure S8).
To inhibit AKR1C3 in these castrated male mice bearing relapsed VCaP xenografts, we then treated the mice for 2 weeks with indomethacin, which resulted in decreased PSA, ERG, and TMPRSS2 mRNA expression (Figure 3F). AR mRNA was slightly decreased, although this was not significant and was not seen in vitro in VCaP cells (Supplemental Figure S9). Immunohistochemistry also indicated decreased PSA and ERG protein, decreased nuclear expression of ERG and AR, and a significant decrease in Ki67-positive cells (mean 31% decrease in Ki67 positive cells) in response to indomethacin (Figure 3G and Supplemental Figures S10 and S11). Finally, consistent with AKR1C3 inhibition, testosterone and DHT levels were decreased in the treated tumors (Figure 3H). It should be noted that abiraterone was more effective than indomethacin in suppressing androgen levels, but was less effective at reducing expression of AR regulated genes (Figures 2I versus 3F). This could reflect some additional activities of indomethacin, but also may be related to other factors including the in vivo half-life of the drugs and transcripts.
AR reactivation in castration resistant VCaP cell line is dependent on de novo androgen synthesis
While the above results indicated that AR activity in the relapsed VCaP xenografts was being driven by CYP17A1 and AKR1C3 dependent de novo intratumoral androgen synthesis, we could not rule out completely the possibility that the tumors were generating androgens from very low levels precursor steroids in the serum. To address this point, we used a relapsed VCaP xenograft to generate a castration resistant VCaP cell line (VCS2). Significantly, AR transcriptional activity in steroid-depleted medium, as assessed by PSA expression, was markedly increased in these VCS2 cells compared to the parental VCaP cells, and was only weakly further induced by DHT (Figure 4A). It should be noted that the decrease in AR protein levels after 24 hours of DHT treatment in both VCaP and VCS2 cells is due to a decrease in AR gene transcription mediated by the agonist-liganded AR (data not shown).
Figure 4.
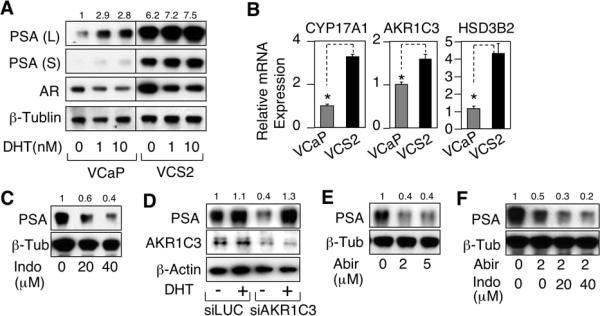
Inhibition of CYP17A1 and AKR1C3 suppresses AR activity in a catration-resistant VCaP cell line. A, the VCS2 cells generated from a relapsed VCaP xenograft were passaged in culture in 8% CSS/2% FBS medium. VCaP and VCS2 cells were switched to CSS medium for 3 days, then treated for 1 day with 0, 1, or 10 nM DHT, and proteins were then immunoblotted. Long (L) and short (S) exposures are shown for PSA. PSA protein is quantified relative to VCaP with no added DHT. B, VCaP or VCS2 cells were subjected to qRT-PCR to measure CYP17A1, AKR1C3, or HSD3B2. C, VCS2 cells in CSS medium were treated for 24h with 0, 20, or 40 μM indomethacin and then immunoblotted. D, VCS2 cells in CSS medium were transfected with 20 nM AKR1C3 siRNA (Dharmacon) for 2d and then treated for 24h with vehicle (ethanol) or 10 nM DHT and immunoblotted for PSA and AKR1C3. E, VCS2 cells in CSS medium were treated for 24h with 0, 2, or 5 μM abiraterone and then immunoblotted. F, VCS2 cells in CSS medium were treated for 24h with abiraterone (2 μM) and indomethacin (20 or 40 μM) as indicated and then immunoblotted (note longer exposure compared to E).
Consistent with this increased basal AR activity, expression of AR and androgen synthetic enzymes were increased in the VCS2 cells (Figure 4A and 4B). An increase in CYP17A1 protein was also confirmed by immunoblotting (Supplemental Figure S12). PSA expression in the VCS2 cells could be markedly decreased by indomethacin (Figure 4C) and by AKR1C3 siRNA (Figure 4D), confirming that the high level basal PSA expression was AKR1C3 dependent. Basal PSA expression was also markedly suppressed by abiraterone (Figure 4E), and could be even further suppressed by combined abiraterone and indomethacin treatment (Figure 4F), confirming that reactivated AR activity in these castration resistant tumors is dependent on enhanced CYP17A1 and AKR1C3 dependent intratumoral de novo androgen synthesis.
Intratumoral CYP11A1 dependent pregnenolone/progesterone synthesis drives CYP17A1 independent AR activity in cells expressing progesterone responsive T877A mutant AR
In contrast to VCaP cells, C4-2 cells express very low levels of AKR1C3 and their basal AR activity was not blocked by CYP17A1 or AKR1C3 inhibitors (see Figures 1–3). Significantly, LNCaP and C4-2 cells express a mutant AR (T877A) that can be stimulated by certain AR antagonists and steroids including progesterone that do not substantially activate wildtype AR (Figure 5A) (28, 29). Indeed, both DHT and progesterone strongly stimulated endogenous AR activity in LNCaP cells, and were more potent than androstenedione or testosterone (Figure 5B). These observations, in conjunction with increased levels of CYP11A1 mRNA (Figure 1E) and protein (Supplemental Figure S13) in C4-2 cells, suggested that basal AR activity in C4-2 cells may be driven by progesterone, which is synthesized from cholesterol upstream of CYP17A1 by the sequential actions of CYP11A1 and HSD3B1 or HSD3B2 (see Figure 1D).
Figure 5.
Basal activity of T877A mutant AR in LNCaP and C4-2 is dependent on CYP11A1. A, COS-7 cells in 5% CSS medium were transfected with an androgen responsive element regulated luciferase reporter (ARE4-Luc) and wild-type AR or T877A mutant AR. Cells were then treated with vehicle (ethanol), DHT, testosterone (T), androstenedione (Ad), pregnenolone (Pn), progesterone (Pg), or 17α-OH-progesterone (17α-OH-Pg) (10 nM for each treatment). Reporter activity was normalized to the cotransfected CMV-Renilla-Luc. B, LNCaP cells in 5% CSS medium were treated with vehicle (−), with 0.1, 1, or 10 nM DHT, progesterone, or testosterone, or with 1 or 10 nM androstenedione and then immunoblotted. C, high-passage number LNCaP cell line (>50, LN-HP) was stably infected with lentivirus expressing either GFP shRNA or CYP11A1 shRNA (Open Biosystems), then treated with vehicle (ethanol), 10 nM DHT, or 10 nM progesterone, and analyzed by qRT-PCR for PSA and CYP11A1. D, LN-HP cells infected with GFP or AKR1C3 shRNA lentivirus were treated with 0, 1, 10 nM DHT and immunoblotted. E, C4-2 cells stably infected with lentivirus expressing either GFP shRNA or CYP11A1 shRNA were treated with 0, 1, 10 nM DHT and analyzed by RT-PCR or (F) treated with 0, 1, 10 nM progesterone and immunoblotted. G, numbers of C4-2-shGFP or C4-2-shCYP11A1 cells cultured in 5% CSS medium and treated with or without DHT were measured using MTT assay after 7 days.
To test this hypothesis, we first examined high passage number LNCaP cells (LN-HP cells) that express substantial PSA in steroid depleted medium. As shown in figure 5C, stable infection with a CYP11A1 shRNA lentivirus markedly decreased basal PSA mRNA, and this was prevented by DHT or progesterone. Interestingly, although CYP11A1 has not been characterized as an AR regulated gene, its expression also was increased by progesterone and DHT. Immunoblotting confirmed the marked decrease in basal PSA in response to CYP11A1 shRNA, which could be restored with DHT (Figure 5D). In C4-2 cells, basal levels of AR regulated transcripts (PSA, PLZF, and FKBP5) and PSA protein expression were similarly decreased by CYP11A1 shRNA, and could be restored by DHT or progesterone (Figure 5E and F). Finally, CYP11A1 shRNA decreased C4-2 cells growth in steroid depleted medium and restored the ability of DHT to androgen stimulate their growth (Figure 5G). Together these findings indicate that CYP11A1 dependent intratumoral synthesis of progesterone is driving the high levels of AR activity in these cells with a T877A mutant AR.
Increased CYP17A1 dependent intratumoral de novo androgen synthesis mediates resistance to CYP17A1 inhibitors
To gain insight into possible mechanisms of acquired resistance to CYP17A1 inhibition, we treated a series of mice bearing castration resistant VCaP xenografts with abiraterone. All mice responded initially (see Figure 2), and there was no substantial or consistent change in CYP17A1 expression in the responding xenografts from mice sacrificed after 8 days of treatment (Figure 6A). We then biopsied another group of castration resistant VCaP xenografts and then treated with abiraterone until relapse based on renewed rapid growth after 4–6 weeks. PSA expression, which declined initially in response to abiraterone, returned to levels that were close to or above baseline, indicating restored AR activity (Figure 6B). Immunohistochemistry also showed strong nuclear AR expression in the relapsed tumors, consistent with AR reactivation (Supplemental Figure S14). Changes in the expression of AKR1C3 and AR were modest and variable (Figure 6B), and no AR ligand binding domain mutations were detected by cDNA sequencing (not shown). In contrast, CYP17A1 expression was increased in all relapsed xenografts, with increases of >10-fold in 2 of the xenografts (Figure 6B). This selection for tumors cells with elevated CYP17A1 in response to abiraterone treatment indicates that upregulation of CYP17A1 provides a growth advantage and is a mechanism that can contribute to the development of abiraterone resistance.
Figure 6.
CYP17A1 inhibition in CRPC selects for increased CYP17A1. A, mice bearing recurrent VCaP xenografts were treated with abiraterone for a short period (n=4, 0.5 mg/d for 8 days by i.p. injection) or (B) for extended periods until relapse (n=6, 0.1 mg/ml in drinking water, which was changed every 3 days, for 4–6 weeks). RNA extracted from tumor samples pre- or post-treatment was analyzed by qRT-PCR with GAPDH coamplified as an internal control. The change of gene expression was presented as Log2(fold change). C, Affymetrix microarray expression data for CYP17A1 and CYP11A1 in 27 primary tumors (no hormonal therapy) and 29 CRPC bone marrow metastases. D, expression of CYP17A1, AKR1C3, and CYP11A1 were assessed by qRT-PCR in 29 CRPC bone marrow biopsy tumor samples, 3 relapsed castration resistant VCaP xenografts (VCaP-CR), 3 relapsed castration resistant LNCaP xenografts (LNCR), 2–3 bone marrow biopsy tumor samples each from 6 ketoconazole-treated patient (P1–P6), and 4 bone marrow biopsy samples from CRPC patients that contained only normal bone marrow (NBM), with GAPDH amplified as an internal control.
We reported previously that mRNA for enzymes mediating conversion of weak adrenal androgens (DHEA and androstenedione) to testosterone and DHT (including HSD17B2, AKR1C3, and SRD5A1) were increased in metastatic CRPC clinical samples (2). There was also an increase in median CYP11A1, but CYP17A1 was not increased (Figure 6C), suggesting that these tumors may not carry out significant de novo androgen synthesis or that CYP17A1 is not rate limiting. To address these alternatives, we used quantitative real time RT-PCR to directly compare expression of CYP17A1 in CRPC clinical samples versus castration resistant VCaP xenografts. Significantly, median CYP17A1 levels in the CRPC clinical samples and castration resistant VCaP xenografts (VCaP-CR) were comparable, while median CYP11A1 was higher in the CRPC samples, indicating that many or most of the clinical samples expressed these enzymes at levels that were consistent with physiologically significant levels of androgen synthesis and AR reactivation (Figure 6D).
While our data indicate that CYP17A1 mRNA is not consistently increased in CRPC, this may reflect the availability of circulating weak androgens produced by the adrenal glands. Therefore, to determine whether suppression of adrenal gland steroid synthesis in CRPC patients through treatment with a CYP17A1 inhibitor selects for tumor cells with increased CYP17A1, we next examined tumor samples obtained from bone marrow biopsies in CRPC patients treated with high dose ketoconazole (CYP17A1 inhibitor) and dutasteride (5α-reductase inhibitor) (30). Biopsies from four patients obtained after one month of treatment (P1–P4), when serum PSA levels were declining, and from two patients who were relapsing after responding to ketoconazole for > 1 year, all showed levels of CYP17A1 mRNA that were markedly higher than the median expression in biopsies from CRPC patients who were not being treated with a CYP17A1 inhibitor (Figure 6D) (each circle reflects the level measured in an independent biopsy taken from the same region). In contrast, there were no apparent further increases in AKR1C3 or CYP11A1 expression.
Discussion
Normal prostate expresses enzymes that can maintain high intraprostatic androgen levels by converting weak adrenal androgens (DHEA and androstenedione) into testosterone and DHT, and it is becoming clear that increased intratumoral expression of these enzymes is a major mechanism contributing to AR reactivation in CRPC. However, it has not been clear whether CRPC can synthesize de novo physiologically significant levels of androgens. In this study we showed that AR activity in castration resistant VCaP xenografts was driven by CYP17A1 and AKR1C3 dependent intratumoral de novo androgen synthesis. Moreover, we found that CYP17A1 expression in CRPC clinical samples was comparable to the levels in these castration resistant VCaP xenografts, supporting the conclusion that CRPC cells can mediate levels of de novo androgen synthesis that are adequate to restore AR transcriptional activity. Interestingly, many of the CRPC tumors expressing the lowest levels of CYP17A1 had higher levels of CYP11A1 (Supplemental Figure S15), suggesting that modest increases in either enzyme, in conjunction with the substantial levels of adrenal derived DHEA that remain after surgical or medical castration, may be adequate to support AR activity in CRPC prior to therapy with a CYP17A1 inhibitor.
We further showed that treatment of castration resistant VCaP xenografts with a CYP17A1 inhibitor, abiraterone, selected for relapsed tumors with increased CYP17A1 expression. Significantly, tumor biopsies from CRPC patients treated with ketoconazole (a CYP17A1 inhibitor) and dutasteride (a type 1 and 2 5α-reductase inhibitor) for one month or until relapse similarly had markedly increased levels of CYP17A1 relative to the levels in CRPC prior to CYP17A1 inhibitor therapy. Together these findings indicate that treatment of CRPC patients with a CYP17A1 inhibitor, by markedly decreasing the levels of circulating weak androgens, generates strong selective pressure for increasing CYP17A1 expression and intratumoral de novo steroid synthesis. While biopsy samples from patients failing abiraterone are not yet available for analysis, we predict that many of these tumors will also have marked increases in CYP17A1 and may have become dependent on increased intratumoral de novo steroid synthesis. Importantly, such tumors may respond to higher doses of abiraterone or to the addition of agents suppressing other steps in androgen synthesis. In support of this approach, results from two recent small trials indicate that responses to ketoconazole may be enhanced by the addition of dutasteride (5α-reductase inhibitor) (29, 41), although it remains to be determined whether dutasteride or related drugs can enhance the efficacy of more potent CYP17A1 inhibitors such as abiraterone.
Previous studies carried out primarily in LNCaP PCa cells and xenografts showed that cholesterol synthesis and metabolism were increased as tumors progressed after androgen deprivation, and that these cells expressed the enzymes required for de novo androgen synthesis from cholesterol (14, 15, 31–33). Studies using radiolabeled acetic acid in relapsed LNCaP xenografts explants further showed detectable DHT synthesis (although progesterone was found at higher levels and testosterone was low or not clearly detected), but whether this DHT was adequate to drive AR activation was not determined (14). Consistent with the relatively high level expression of CYP11A1 and progesterone synthesis in LNCaP cells, we found that basal AR activity in LNCaP and C4-2 cells was CYP11A1 dependent, but CYP17A1 independent, indicating that intratumoral synthesis of progesterone is driving activity of the T877A mutant AR in these cells. We showed previously that treatment with the AR antagonist flutamide selects for tumor cells expressing mutant ARs (including the T877A mutation) with altered ligand binding domains that are activated by flutamide (29, 34, 35). Therefore, our data suggest that increased levels of progesterone (or other steroids that accumulate in response to CYP17A1 inhibition) may select for such ligand binding domain mutations in a subset of patients.
While these data support de novo intratumoral steroid synthesis as a mechanism contributing to AR reactivation in CRPC and resistance to CYP17A1 inhibitors, additional mechanisms that sensitize AR to very low androgen levels or render it ligand independent, including alternative splicing that truncates the AR ligand binding domain (36–41), may also make substantial contributions. Consistent with the ligand dependence of basal AR activity in the VCaP and castration resistant VCaP cells, we have not yet observed lower molecular weight isoforms of AR protein (not shown). However, it is possible that very low levels of these isoforms could function as coactivators, and that alternative AR splicing may emerge as a significant contributor to restoration of AR activity in tumors that relapse after CYP17A1 inhibitor therapy. In any case, further molecular analyses of CYP17A1 inhibitor resistant/relapsed tumors will be critical to identify mechanisms of resistance in individual patients and to develop and deploy individualized therapies that target these resistance mechanisms.
Supplementary Material
Acknowledgments
We thank Cougar Biotechnology for providing abiraterone acetate and Trevor Penning (University of Pennsylvania) for the AKR1C3 antibody.
Financial support: Supported by grants to S.P.B. from the NIH (R01 CA111803 and Prostate SPORE P50 CA090381), DOD Prostate Cancer Research Program (PC060807), and a Challenge Grant from the Prostate Cancer Foundation. C.C. was supported by postdoctoral award from the DOD Prostate Cancer Research Program (PC08115) and a Career Developmetn Award from the Prostate SPORE (P50 CA090381).
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 3.Mendiratta P, Mostaghel E, Guinney J, Tewari AK, Porrello A, Barry WT, et al. Genomic strategy for targeting therapy in castration-resistant prostate cancer. J Clin Oncol. 2009;27:2022–9. doi: 10.1200/JCO.2008.17.2882. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 5.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458–62. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–6. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–6. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 9.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 10.Geller J, Albert JD, Nachtsheim DA, Loza D. Comparison of prostatic cancer tissue dihydrotestosterone levels at the time of relapse following orchiectomy or estrogen therapy. J Urol. 1984;132:693–6. doi: 10.1016/s0022-5347(17)49829-6. [DOI] [PubMed] [Google Scholar]
- 11.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 12.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 15.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–20. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–64. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney EM, Harrison JH. Bilateral adrenalectomy for palliative treatment of prostatic cancer. J Urol. 1972;108:936–8. doi: 10.1016/s0022-5347(17)60911-x. [DOI] [PubMed] [Google Scholar]
- 18.Yap TA, Carden CP, Attard G, de Bono JS. Targeting CYP17: established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8:449–57. doi: 10.1016/j.coph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69:4937–40. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 20.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai C, Wang H, Xu Y, Chen S, Balk SP. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res. 2009;69:6027–32. doi: 10.1158/0008-5472.CAN-09-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Loberg RD, St John LN, Day LL, Neeley CK, Pienta KJ. Development of the VCaP androgen-independent model of prostate cancer. Urol Oncol. 2006;24:161–8. doi: 10.1016/j.urolonc.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schroder FH. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992;50:857–61. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]
- 26.Lovering AL, Ride JP, Bunce CM, Desmond JC, Cummings SM, White SA. Crystal structures of prostaglandin D(2) 11-ketoreductase (AKR1C3) in complex with the nonsteroidal anti-inflammatory drugs flufenamic acid and indomethacin. Cancer Res. 2004;64:1802–10. doi: 10.1158/0008-5472.can-03-2847. [DOI] [PubMed] [Google Scholar]
- 27.Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol. 2008;75:484–93. doi: 10.1016/j.bcp.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–40. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 29.Fenton MA, Shuster TD, Fertig AM, Taplin ME, Kolvenbag G, Bubley GJ, et al. Functional characterization of mutant androgen receptors from androgen-independent prostate cancer. Clin Cancer Res. 1997;3:1383–8. [PubMed] [Google Scholar]
- 30.Taplin ME, Regan MM, Ko YJ, Bubley GJ, Duggan SE, Werner L, et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7099–105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 32.Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, Gleave ME, et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64:2212–21. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 33.Bauman DR, Steckelbroeck S, Peehl DM, Penning TM. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology. 2006;147:5806–16. doi: 10.1210/en.2006-0627. [DOI] [PubMed] [Google Scholar]
- 34.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 35.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–5. [PubMed] [Google Scholar]
- 36.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.