Abstract
Takotsubo cardiomyopathy, also called stress-induced cardiomyopathy, usually occurs in patients with severe emotional or physiologic stress. The prognosis is favorable, and the wall motion abnormlities normalize within weeks. However, stress-induced cardiomyopathy is rarely assosicated with left ventricular thrombus and thromboembolic complications. Here, we report a case of stress-induced cardiomyopathy with left ventricular thrombus that embolized to cause cerebral infarction.
Keywords: Takotsubo cardiomyopathy, Thrombus, Cerebral infarction
Introduction
Takotsubo cardiomyopathy, also called 'stress-induced cardiomyopathy', has clinical features that resemble an acute coronary syndrome, such as chest pain, ST-segment changes observed on electrocardiography, mild elevation of serum cardiac enzymes, and transient left ventricular (LV) dysfunction with marked apical dyskinesis and ballooning; however, angiographic features of significant coronary artery disease are absent.1-5) The general prognosis is considered to be favorable, although some investigators have reported cases with various complications, including death.4),6)
LV thrombus is a known complication of stress-induced cardiomyopathy.7-9) However, the clinical significance and therapy of LV thrombus in stress-induced cardiomyopathy remain unclear. Authors experienced a 76-year-old woman who had embolic cerebral infarction following LV thrombus with stress-induced cardiomyopathy. Therefore, we report this case with review of literature.
Case
A 76-year-old woman, with a past medical history of hypertension and diabetes mellitus, visited the emergency department for worsening nausea and abdominal discomfort. On admission, her mental status was alert, the blood pressure was 90/60 mmHg, respiratory rate was 22 per minute, pulse rate was 110 per minute, and temperature was 38.1℃. Serum creatinin was 2.94 mg/dL. The serum liver enzyme and bilirubin levels were also elevated. Endoscopic retrograde cholangio-pancreatography revealed suppurative cholangitis, which was treated by biliary stenting.
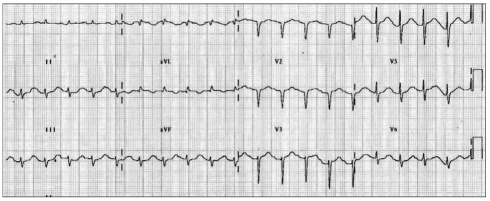
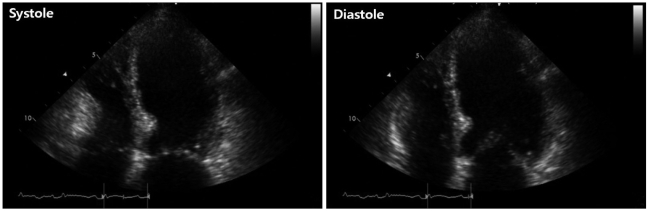
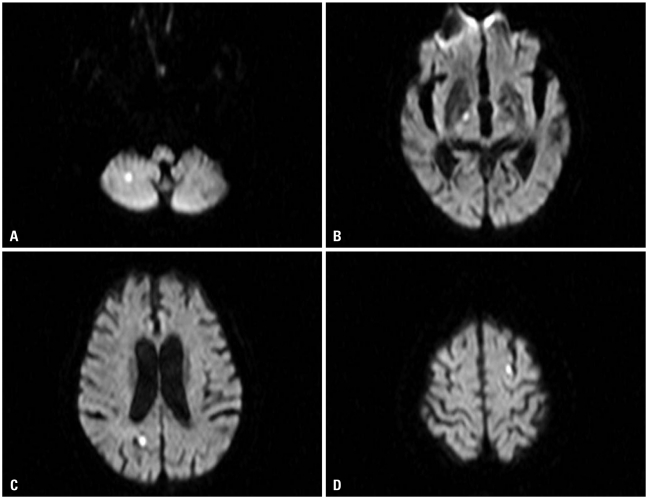
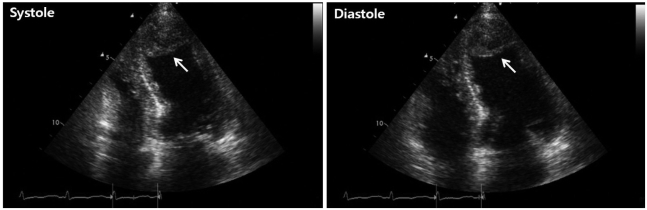
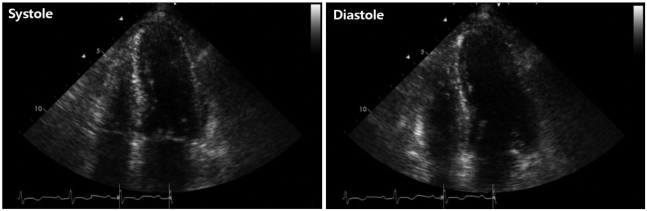
On admission, the electrocardiogram showed an abnormal pathologic Q wave in leads V1-2 and a prolonged QT interval (Fig. 1). She did not complain chest pain or shortness of breath. The troponin T level was elevated at 0.29 ng/dL (reference level, < 0.01 ng/dL), while creatine kinase (CK) and CK-MB levels were normal. Transthoracic echocardiography (TTE) revealed that wall motion was abnormal with mid and apical akinesis, and the ejection fraction (EF) was estimated to be 12% (Fig. 2). Stress-induced cardiomyopathy was diagnosed, and supportive therapy for infection and LV dysfunction was initiated. After biliary stenting and antibiotics therapy, her general conditions were recovered. However, ten days after admission, her mental state was changed into semicoma state. Brain magnetic resonance imaging revealed multiple brain embolic infarction (Fig. 3). Echocardiography was repeated to detect the intracardiac embolic source. TTE revealed mild improvement of the LV systolic function (EF 44%), with a 24 × 25 mm sized thrombus in the LV apex (Fig. 4).
Fig. 1.
An electrocardiogram showing an abnormal Q wave in the anterior precordial leads and a prolonged QT interval.
Fig. 2.
Initial transthoracic echocardiographic image in the apical 4-chamber view showing left ventricular apical ballooning and dyskinesis.
Fig. 3.
Diffusion image of magnetic resonance imaging showed multiple diffusion restrictive lesions in right cerebellar hemisphere (A), right internal capsule (B), right occipital lobe (C), and left parietal lobe (D).
Fig. 4.
Transthoracic echocardiographic image obtained after cerebral infarction developed, shows a 24 × 25 mm thrombus (arrow) in the left ventricular apex.
Low molecular weight heparin and warfarin therapy was started. Three days later, her metal state became alert. TTE performed 7 days after the start of anticoagulant therapy revealed a near normal LV wall motion (EF 55%) and complete resolution of the apical thrombus (Fig. 5).
Fig. 5.
Transthoracic echocardiographic image obtained after 1 week of anticoagulation therapy shows near normal left ventricular wall motion and complete resolution of the apical thrombus.
Discussion
Takotsubo cardiomyopathy (stress-induced cardiomyopathy) is a relatively novel cardiac syndrome characterized by peculiar transient LV dysfunction. Approximately 1-3% of the patients with stress-induced cardiomyopathy show symptoms that initially mimic acute coronary syndrome.3),5) In this case, we did not performed coronary angiography, but we could tentatively diagnose as stress-induced cardiomyopathy because of the absent of cardiovascular symptom and no serial changes of cardiac biomarkers in septic patient.
Despite the favorable prognosis, certain serious complications have been reported in patients with stress-induced cardiomyopathy, such as acute decompensated heart failure, ventricular arrhythmia, LV rupture, and LV thrombus.4),7-12) Thrombus formation in such cases was probably related to transient apical asynergy combined with increased sympathetic activation, which alters the coagulation cascade.
To date, the true incidence and clinical significance of LV thrombus and the related embolic outcomes in patients with stress-induced cardiomyopathy have not been fully established. Haghi et al.11) reported an 8% incidence of LV thrombus in the study population, but a much lower incidence of accompanying embolic complications. They concluded that LV thrombus can occur at the initial presentation or any time later during the disease. In our patient, the initial echocardiogram showed only apical ballooning and akinesia without any evidence of LV apical thrombus; however, thrombus formation occurred after a week and led to cerebral infarction. In a systematic review, de Gregorio et al.13) found that among 15 Takotsubo cardiomyopathy patients with ventricular thrombosis, 5 patients suffered from thromboembolic events, 3 of whom developed stroke. Therefore, physicians should be aware of this complication.
The current treatment of stress-induced cardiomyopathy consists of supportive care and standard treatments for LV systolic dysfunction. The role of anticoagulation therapy has not yet been defined. To the best of our knowledge, there are no published guidelines for the management of stress-induced cardiomyopathy with LV thrombus. However, some reports mention that short-term anticoagulation therapy with heparin and warfarin for several weeks resolved LV thrombus.8),9),11) In this case, LV thrombus was resolved after 1 week of anticoagulation therapy.
From our review, we conclude that patients with stress-induced cardiomyopathy appear to be at a significant risk for development of thrombus and subsequent stroke because of the marked apical wall motion abnormality. Thus, all patients with stress-induced cardiomyopathy should be evaluated for the presence of a ventricular thrombus. We cannot observe the LV thrombus even the patients with depressed LV function; however, we can observe the LV thrombus even the LV function was more improved in follow up echocardiography. The patient did not be followed by echocardiography during 10 days, so we cannot clarify when the LV thrombus was developed. The physicians have to keep in mind that frequent echocardiographic follow up should be needed in the cases with stress-induced cardiomyopathy not only a period of markedly reduced LV function but also after clinically improvement.
Although no specific data exist regarding the role of anticoagulation in stress-induced cardiomyopathy, short-term anticoagulation therapy has been indicated as a treatment for patients with LV thrombus. Further research is needed to determine the true incidence of LV thrombus and the role of short-term anticoagulant therapy in patients with stress-induced cardiomyopathy with LV thrombus.
References
- 1.Lee JW, Kim JY. Stress-induced cardiomyopathy: the role of echocardiography. J Cardiovasc Ultrasound. 2011;19:7–12. doi: 10.4250/jcu.2011.19.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 3.Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation. 2007;115:e56–e59. doi: 10.1161/CIRCULATIONAHA.106.669341. [DOI] [PubMed] [Google Scholar]
- 4.Akashi YJ, Tejima T, Sakurada H, Matsuda H, Suzuki K, Kawasaki K, Tsuchiya K, Hashimoto N, Musha H, Sakakibara M, Nakazawa K, Miyake F. Left ventricular rupture associated with Takotsubo cardiomyopathy. Mayo Clin Proc. 2004;79:821–824. doi: 10.4065/79.6.821. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Bang DW, Ahn JH, Park SH, Oh HS, Yoon YJ, Hyon MS, Kim SK, Kwon YJ. Three cases of stress induced transient LV dysfunction: stress induced cardiomyopathy. J Korean Soc Echocardiogr. 2005;13:83–86. [Google Scholar]
- 6.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–341. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 7.Iengo R, Marrazzo G, Rumolo S, Accadia M, Di Donato M, Ascione L, Tuccillo B. An unusual presentation of "tako-tsubo cardiomyopathy". Eur J Echocardiogr. 2007;8:491–494. doi: 10.1016/j.euje.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida T, Hibino T, Fujimaki T, Oguri M, Kato K, Yajima K, Ohte N, Yokoi K, Kimura G. Tako-tsubo cardiomyopathy complicated by apical thrombus formation: a case report. Int J Cardiol. 2009;132:e120–e122. doi: 10.1016/j.ijcard.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Tibrewala AV, Moss BN, Cooper HA. A rare case of tako-tsubo cardiomyopathy complicated by a left ventricular thrombus. South Med J. 2006;99:70–73. doi: 10.1097/01.smj.0000197509.72076.21. [DOI] [PubMed] [Google Scholar]
- 10.Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 11.Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in tako-tsubo cardiomyopathy assessed with echocardiography. QJM. 2008;101:381–386. doi: 10.1093/qjmed/hcn017. [DOI] [PubMed] [Google Scholar]
- 12.Tobar R, Rotzak R, Rozenman Y. Apical thrombus associated with Takotsubo cardiomyopathy in a young woman. Echocardiography. 2009;26:575–580. doi: 10.1111/j.1540-8175.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- 13.de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol. 2008;131:18–24. doi: 10.1016/j.ijcard.2008.05.060. [DOI] [PubMed] [Google Scholar]