Abstract
The monomer composition of the esterified part of suberin can be determined using gas chromatography-mass spectroscopy technology and is accordingly believed to be well known. However, evidence was presented recently indicating that the suberin of green cotton (Gossypium hirsutum cv Green Lint) fibers contains substantial amounts of esterified glycerol. This observation is confirmed in the present report by a sodium dodecyl sulfate extraction of membrane lipids and by a developmental study, demonstrating the correlated accumulation of glycerol and established suberin monomers. Corresponding amounts of glycerol also occur in the suberin of the periderm of cotton stems and potato (Solanum tuberosum) tubers. A periderm preparation of wound-healing potato tuber storage parenchyma was further purified by different treatments. As the purification proceeded, the concentration of glycerol increased at about the same rate as that of α,ω-alkanedioic acids, the most diagnostic suberin monomers. Therefore, it is proposed that glycerol is a monomer of suberins in general and can cross-link aliphatic and aromatic suberin domains, corresponding to the electron-translucent and electron-opaque suberin lamellae, respectively. This proposal is consistent with the reported dimensions of the electron-translucent suberin lamellae.
Suberin and cutin are insoluble, lipophilic biopolymers. Together with complex mixtures of soluble lipids, they form the protective layers of higher plants: the cuticle of the epidermis and the suberin layers of the periderm and the exodermis. These cell layers are diffusion barriers for water and other small, polar compounds (Schönherr, 1976, 1982; Soliday et al., 1979; Vogt et al., 1983; Riederer and Schreiber, 1995; Kerstiens, 1996; Schreiber et al., 1996). Cuticle, exodermis, and periderm also constitute the first constitutive barrier against penetration of the plant body by pathogens (Kolattukudy 1977, 1985, 1987, 1996; Kolattukudy and Köller, 1983; Yang et al., 1993). Wounding triggers suberization irrespective of the natural protective covering of a plant organ (Kolattukudy and Soliday, 1985). However, the complex mixtures of waxy components of a cuticle are a dynamic system, and physical healing of regions depleted in their wax may be possible (Grace and van Gardingen, 1996).
Suberins and cutins are considered to be closely related polyesters composed of fatty acids, differing in their chain length and their substitution patterns, along with smaller amounts of long-chain alcohols and hydroxycinnamic acids (Kolattukudy and Agrawal, 1974; Kolattukudy 1980a, 1980b, 1984; Holloway, 1983, 1984). In his tentative suberin and cutin models, Kolattukudy (1980a) proposed that suberin, in contrast to cutin, contains a lignin-like aromatic domain, covalently linked to the aliphatic suberin domain by ester bonds. The presence of an aromatic suberin domain was supported by the nondegradative technique of solid-state 13C-NMR spectroscopy, using partially purified suberin preparations from wound-healing potato tuber slices (Garbow et al., 1989; Stark et al., 1989, 1994). However, no proof was obtained for the proposed aromatic-aliphatic linkages in suberin. Using solid-state 13C-NMR spectroscopic analysis of isotopically enriched and enzymatically purified wound-healing potato suberin, Bernards et al. (1995) presented evidence that the polyaromatic domain of suberin is composed mainly of covalently linked hydroxycinnamic acids rather than of the corresponding alcohols found in lignin. The chemical-shift data for isotopically labeled resonances was consistent with significant nonester covalent cross-linking between the phenolic units in suberin. However, it is not known in which particular cell wall domain these hydroxycinnamic acids are located (Bernards and Lewis, 1998). Smaller amounts of syringyl and guaiacyl lignin monomers were identified in potato wound-healing periderm, and their inter-unit bonding patterns were determined after thioacidolysis, a procedure that cleaves alkylaryl ether linkages (Borg-Olivier and Monties, 1993; Lapierre et al., 1996). The lignin is thought to be located in the middle lamellae and the primary walls of the periderm (Esau, 1977; Fahn, 1990; Lulai and Morgan, 1992; Thomson et al., 1995).
As seen in transmission electron micrographs, the suberin layers are lamellated. The surface parallel lamellation is apparently not affected by extractions with hot organic solvents (for review, see Ryser and Holloway, 1985). The chemical basis of the lamellation is not known (Schmidt and Schönherr, 1982). In many species, perhaps in the majority, the outer layer of the cuticle also has a lamellate construction (Jeffree, 1996). In suberin the electron-translucent lamellae are more consistent in thickness than the electron-opaque ones and measure about 3 nm (Falk and El Hadidi, 1961; Schmutz et al., 1996). It is generally assumed that the waxes are confined to the electron-translucent lamellae. On the basis of inhibitor experiments, it was proposed that the constant thickness of these lamellae is determined by perpendicularly oriented aliphatic suberin monomers covalently linked to glycerol (Schmutz et al., 1996).
To date two model systems have been used to study the monomer composition, structure, and biogenesis of suberin layers: (a) the ABA-induced formation of a wound periderm at the surface of tissue slices of potato (Solanum tuberosum L.) tuber storage parenchyma (Kolattukudy and Dean, 1974), and (b) green cotton (Gossypium hirsutum cv Green Lint) fibers harvested from plant-grown seeds or from ovules cultured in vitro (Ryser et al., 1983; Ryser and Holloway, 1985). The green cotton fiber phenotype is conditioned by a single dominant gene locus Lg (Kohel, 1985).
A caffeoyl-fatty acid-glycerol ester was isolated from the wax of green cotton fibers (Schmutz et al., 1993, 1994b), and evidence was presented indicating that glycerol is also an important constituent of cotton fiber suberin (Schmutz et al., 1993). The immediate significance of this finding was obvious: The aliphatic monomers of cotton fiber suberin can only form linear polymers on their own, whereas glycerol allows the formation of a three-dimensional cross-linked network, as suggested by the lamellar ultrastructure and insolubility of suberin.
In the present study we show that the glycerol in the suberin of green cotton fibers is not a contaminant from the wax or from membrane lipids and accumulates synchronously with the aliphatic suberin monomers. Comparable amounts of glycerol were also determined in the stem periderm of cotton plants. During the purification of the suberin of the wound-healing periderm of potato tubers, glycerol accumulated at the same rate as α,ω-dicarboxylic fatty acids, the most diagnostic suberin monomers (Matzke and Riederer, 1991). These results suggest that glycerol may be a monomer of suberins in general. The molar concentrations of glycerol in the examined tissues are sufficient to propose a structural role for glycerol in suberin.
MATERIALS AND METHODS
Plant Material
Cotton (Gossypium hirsutum L.) cv St 406 and a genotype (Lg) with green fibers (obtained from GERDAT, Bouaké, Ivory Coast) were grown in a greenhouse at 25°C to 30°C during the day and at 19°C to 22°C at night. Under these conditions secondary wall formation in the fibers started at 20 to 25 d after anthesis and the fruit capsules opened at 60 to 70 d after anthesis.
Cotton fibers were detached with tweezers from ovules at different time points after anthesis. The periderm was isolated from the lower part of the main stem of the cotton cultivar with white fibers (St 406) by scraping with a razor blade. Seeds of cv St 406 were evacuated in distilled water to a pressure of 20 to 27 kPa to remove air, soaked overnight at 4°C in distilled water, and dissected to separate the chalazal region from the remaining seed coat and the nucellar cuticle fraction, containing collapsed nucellar cells, the nucellar cuticle, and the fringe layer, the inner epidermis of cotton seed coats (Ryser et al., 1988). The isolated tissues were then immediately frozen in liquid N2, ground to a fine powder with a mortar and pestle, and finally lyophilized and weighed.
Extraction of the Purified Cell Walls of Cotton Fibers and Tissues
The lyophilized cell walls were extracted three times with hexane at about 40°C (2 × 5 h and 1 × 16 h), three times with chloroform:methanol (2:1, v/v) at about 60°C (2 × 5 h and 1 × 16 h), and finally two times with methanol at about 70°C (2 × 5 h) in closed Pyrex tubes. In some experiments an additional extraction was performed with boiling SDS (2% [w/v] in distilled water) for 4 h before the standardized solvent extractions. After the SDS extraction the fibers were washed on glass fiber filters (GF/A, Whatman) with distilled water.
Purification of the Wound-Healing Periderm of Potato Tuber Parenchyma
According to previously published procedures (Stark and Garbow, 1992; Pacchiano et al., 1993), fresh potatoes (Solanum tuberosum L. cv Russet Burbank) were cleaned and peeled under sterile conditions. The tubers were cut into 2- × 20- × 30-mm sections and aerated in a dark incubator at 25°C for 7 d. The brown layer of wound periderm on the surface of the potato discs was collected by blade peeling to streamline the removal of unsuberized storage parenchyma cells (Bernards et al., 1995). Unsuberized cell wall materials were removed by standard cellulase (ICN) and pectinase (Sigma) enzymatic treatments. Subsequently, soluble lipids and wax were removed using a 2:1 (v/v) mixture of methylene chloride:methanol and exhaustive extraction with a Soxhlet apparatus (Fisher Scientific) for 48 h. Finally, the suberin was extracted with 1,4-dioxane:water (96:4) at room temperature overnight (Ralph et al., 1995) to remove soluble lignins and residual sugars. The resulting pieces of suberized periderm tissue were powdered at liquid N2 temperature in a freezer mill. Dry suberin samples were obtained by thorough drying in a Speed Vac concentrator (Savant Instruments, Farmingdale, NY) at 60°C for more than 2 h until there was no further weight loss. Typically, 1 kg of potato yielded approximately 2 g of dry suberin.
NMR Spectroscopy
All solid-state, cross-polarization, magic-angle spinning NMR spectra were acquired on a Unity plus spectrometer (Varian Instruments, Palo Alto, CA) operating at a 1H frequency of 300.001 MHz and a 13C frequency of 75.445 MHz. The experiments were conducted with a 7-mm magic-angle spinning probe (Varian Instruments) at a regulated temperature of 25°C. Typically, 150 mg of suberin (dry weight) was used. The 90° pulses for 1H and 13C were both set to 5.5 μs. The rotor-spinning speed was maintained at 3000 ± 2 Hz by a speed controlling device (Varian). All 13C chemical shifts were referenced to tetramethylsilane via hexamethylbenzene as a secondary substitution reference.
Depolymerization Procedure and GC-MS
The chloroform:methanol extracts and the exhaustively extracted cell wall residues were depolymerized by acid-catalyzed transesterification with 5% (w/v) HCl in methanol for 16 h (extracts) or for 40 h (cell wall residues) at 50°C (Holloway, 1984). The hydrolyzed cell wall residues were washed on glass-fiber filters with methanol and chloroform:methanol (2:1, v/v), lyophilized, and weighed. After addition of 5% (w/v) aqueous NaCl solution to the filtrates and the hydrolyzed chloroform:methanol extracts, the aqueous layers were removed for the determination of glycerol and the organic layers dried over Na2SO4. Salt precipitates were removed by filtration. The solvent was evaporated and the resulting components converted to their corresponding trimethyl silyl ethers. The same treatment was performed for potato powders. GC analysis was performed using N2 as the carrier gas at 1 mL min−1 on a SE 52 Permabond column (25 m, i.d. 0.33 mm, film thickness 0.5 μm; Macherey and Nagel, Duren, Germany) at a temperature of 140°C for 2 min and then elevated to 280°C at 4°C min−1. The ionizing energy of the MS was 70 eV. Before transesterification 1-pentadecanol was added as the internal standard to the samples. A mixture of ferulic acid, C16 α,ω-alkanedioic acid, C16 ω-hydroxyalkanoic acid, C18:1 alkanoic acid, and 1-pentadecanol was used as the external standard.
Determination of Glycerol
The aqueous layer obtained after transesterification was concentrated under reduced pressure, as necessary, and the glycerol was quantitated enzymatically (Eggstein and Kuhlmann, 1974) with a commercial test combination (Boehringer Mannheim). The test couples glycerokinase (EC 2.7.1.30) with pyruvate kinase (EC 2.7.1.40) and lactate dehydrogenase (EC 1.1.1.27). Finally, the consumption of NADH is measured at 340 nm. Glycerokinase phosphorylates glycerol (Km = 60 μmol; pH 9.8; 25°C) and dihydroxyacetone. However, the conversion of dihydroxyacetone is slow. After transesterification, dihydroxyacetone can no longer be measured with the test combination and reduces the measurable concentrations of glycerol, probably by forming stable homo- and heterodimers. Each test was made with at least two different volumes of the transesterification reaction mixture to check the accuracy of the method. In the absence of glycerokinase, no significant amounts of NADH were consumed with the exception of one sample (the chalazal region of the seed coat), where a corresponding correction was made. Glycerol was also identified by TLC in alkaline hydrolysates of exhaustively extracted green cotton fibers (Schmutz et al., 1993). Other polyols such as mannitol or erythritol could not be identified in the hydrolysates (A. Schmutz and U. Ryser, unpublished results).
RESULTS
Association of Bound Glycerol with the Suberin of Green Cotton Fibers
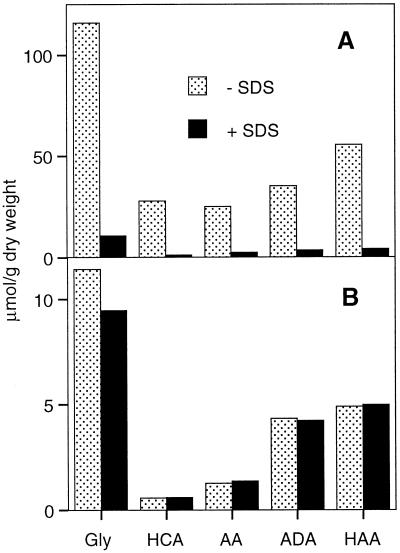
The wax fraction of green cotton fibers contains about 10 times more bound glycerol than the suberin fraction (Schmutz et al., 1993), yet the monomer composition and the chain-length distribution of the fatty acids were reported to be surprisingly similar in the wax and in suberin (Schmutz et al., 1996). An estimation of a possible contamination of the suberin fraction with wax components was therefore not possible. In a different approach, the possibility of a contamination of cotton fiber suberin with glycerides of the wax was tested by extracting the purified cell walls of the green fibers with boiling SDS before the conventional wax extraction with organic solvents. After acid-catalyzed transesterification of the extracts and the cell wall residues, the liberated glycerol was determined enzymatically, and the other wax and suberin monomers were characterized by GC-MS. A single SDS treatment removed the major wax monomers almost quantitatively (Fig. 1A), whereas the same treatment had no significant effect on the amount of suberin monomers, including glycerol (Fig. 1B). This observation indicates clearly that glycerol is covalently bound to suberin and is not a contaminant due to unextracted wax or membrane lipids.
Figure 1.
Effect of an extraction with boiling SDS (2%, w/v) on the glycerol content of the wax and suberin fractions of green cotton fibers. A, Wax fraction; B, suberin fraction. After the SDS extraction, the fibers were dewaxed with organic solvents as usual. The remaining wax (A) and suberin (B) were then depolymerized by acid-catalyzed transesterification and the monomer concentration was determined as described in Methods. Gly, Glycerol; HCA, hydroxycinnamic acids; AA, alkanoic acids, ADA, α,ω-alkanedioic acids; HAA, ω-hydroxyalkanoic acids.
Accumulation of Bound Glycerol and Other Suberin Monomers during the Development of Green Cotton Fibers
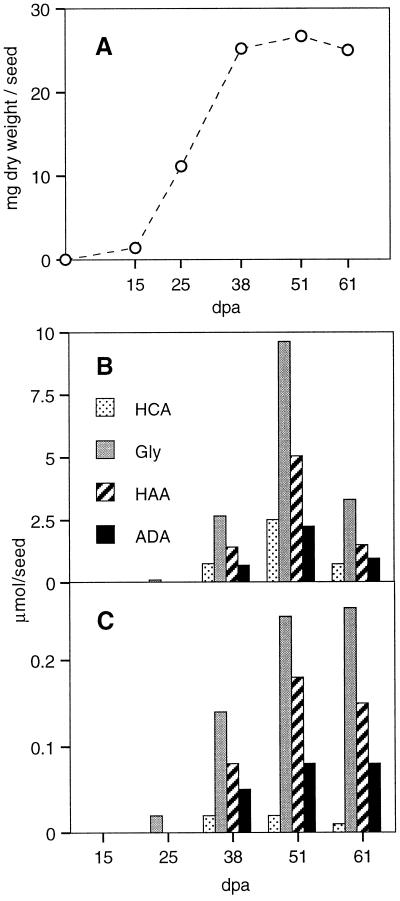
The accumulation of the major suberin and wax monomers, determined after acid-catalyzed transesterification, was compared with the increase of fiber dry weight, a good indicator of secondary wall cellulose deposition. During the time period of primary wall formation, up to 16 d after anthesis, the rate of dry weight accumulation remained low. It then increased by a factor of about 10 at the onset of secondary wall formation at about 20 d after anthesis (Fig. 2A).
Figure 2.
Accumulation of glycerol and other wax and suberin monomers during the development of green cotton fibers (mean values of two independent experiments). A, Accumulation of cell wall dry weight. Secondary wall deposition began at about 20 d after anthesis, as indicated by the increased rate of dry weight deposition. B, Accumulation of glycerol, hydroxycinnamic acids, and alkanoic acids in the wax fraction. C, Accumulation of glycerol, hydroxycinnamic acids, and alkanoic acids in the suberin fraction. dpa, Days postanthesis; HCA, hydroxycinnamic acids; Gly, glycerol; HAA, ω-hydroxyalkanoic acids; ADA, α,ω-alkanedioic acids.
In the wax, secondary wall formation is accompanied by the accumulation of glycerol, ω-hydroxyalkanoic acids, alkanoic acids, α,ω-alkandioic acids, and hydroxycinnamic acids (Fig. 2B), as well as smaller amounts of alkanols and long-chain alkanoic acids (Fig. 3A). The accumulation of the wax monomers is clearly delayed compared with dry weight accumulation. A reduction of the amount of wax monomers by more than 50% was observed in the oldest fibers, analyzed shortly before opening of the fruit capsules. The observed reduction may be due to a partial degradation of the wax during the phase of fiber maturation.
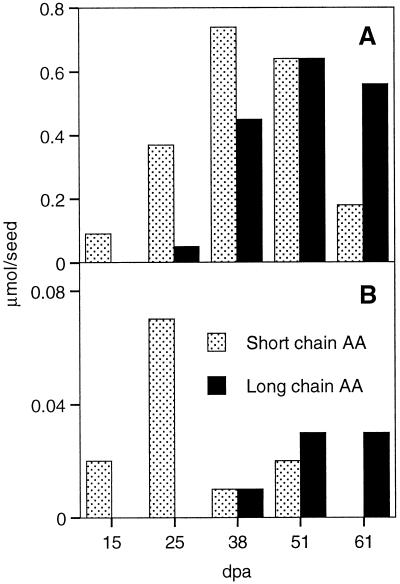
Figure 3.
Accumulation of short- (≤C18) and long-chain (>C18) alkanoic acids in the wax and suberin fractions of green cotton fibers (mean values of two independent experiments). A, Wax; B, suberin. dpa, Days postanthesis; AA, alkanoic acids.
In suberin, glycerol, ω-hydroxyalkanoic acids, and α,ω-alkandioic acids accumulate during secondary wall formation (Fig. 2C), as do smaller amounts of long-chain alkanoic acids (Fig. 3B). Only traces of alkanols and hydroxycinnamic acids were determined in the suberin fraction. As already observed for the wax monomers, the accumulation of suberin monomers was delayed compared with dry weight accumulation. This delay may be explained by the observation that the first secondary wall layer is always cellulosic, and is thicker in some fibers than in others. In contrast to the situation in the wax, no significant reduction in the amounts of monomers was observed in the suberin fraction of the oldest fibers.
Short-chain (C16 and C18) alkanoic acids accumulated in the wax and in the suberin fraction at the beginning of secondary wall formation and then declined again, whereas the longer acids (C20–C26) accumulated during secondary wall formation (Fig. 3), suggesting that the short-chain alkanoic acids do not represent genuine suberin monomers.
The Glycerol Content of the Stem Periderm and of Different Tissues of Cotton Seeds
Stem periderm and three different parts of mature cotton seeds of cv St 406 were isolated as described in Methods. The chalazal region and a nucellar cuticle fraction were separated from the remaining seed coat. The nucellar cuticle fraction is composed of collapsed nucellar cells, the nucellar cuticle, and the innermost epidermis of the cotton seed coat, the so-called fringe layer (Ryser et al., 1988). In the chalazal region the inner integument is appreciably thicker than in the rest of the seed coat. The thickened region, forming a kind of plug, was isolated in mature, hydrated seeds by separating it from the rest of the seed coat and the fringe layer. A large portion of the cell walls of the chalazal region could be stained with phloroglucinol-HCl, probably indicating that these walls are lignified. Only the innermost, central cells of the chalazal region were suberized in the studied cultivar, as determined by staining with a mixture of the lipophilic Sudan III and IV dyes (results not shown).
The purified cell wall powders of the different tissues were exhaustively extracted with hot organic solvents. The wax fractions and the polyester part of the cell wall residues were then depolymerized by acid-catalyzed transesterification. Glycerol was determined enzymatically, and the suberin and cutin monomers were characterized by GC-MS.
The glycerol content of the wax and suberin fractions, together with the GC-MS analyses of the suberin fractions, are summarized in Table I. It is important to remember that complex tissues were analyzed and that not all of the compounds detected were necessarily derived from cutin or suberin, but may be derived from other cell wall layers or from incompletely removed cellular contents. Smaller amounts of hydroxybenzoic acids were determined in the seed coats and in the chalaza, probably derived from the lignified cell walls observed in these tissues by phloroglucinol-HCl staining.
Table I.
Glycerol and aromatic and aliphatic suberin and cutin monomers released after acid-catalyzed transesterification of extractive free stem periderm and of three different tissues of seeds of a cotton cultivar with white fibers (St 406)
| Compound | Seed Coat | Chalaza | Stem Periderm | Nucellar Cuticlea |
|---|---|---|---|---|
| μmol/g dry wt | ||||
| Glycerol in the wax fraction | 2.90 | 1.01 | 8.54 | 76.44 |
| Glycerol in the suberin fraction | 2.75 | 0.83 | 73.88 | 6.84 |
| Hydroxycinnamic acids | ||||
| p-Coumaric acid | 1.97 | – | 0.96 | – |
| Caffeic acid | 4.56 | – | 0.51 | 0.02 |
| Ferulic acid | 0.56 | 0.14 | 4.66 | 0.14 |
| 4-Hydroxybenzoic acid | 0.07 | 1.78 | – | – |
| 3,4-Dihydroxybenzoic acid | – | 0.22 | – | – |
| 4-Hydroxy,3-methoxybenzoic acid | 0.13 | – | – | – |
| Total | 7.29 | 2.14 | 6.13 | 0.16 |
| Alkanols | ||||
| C14 | 0.04 | 0.05 | 0.26 | – |
| C20 | – | – | 0.54 | – |
| C22 | 0.54 | – | 8.58 | – |
| C24 | 0.05 | – | 5.90 | – |
| Total | 0.63 | 0.05 | 15.28 | 0 |
| Alkanoic and alkenoic acids | ||||
| C14:0 | – | – | – | 0.12 |
| C16:0 | 0.27 | 0.77 | 1.12 | 3.24 |
| C18:0/1/2/3 | 0.33 | 0.84 | 1.89 | 6.43 |
| C20:0 | – | – | 2.00 | 0.17 |
| C22:0 | 0.16 | 0.32 | 5.93 | 0.26 |
| C24:0 | – | 0.12 | 5.43 | – |
| C26:0 | – | 0.15 | 0.15 | – |
| Total | 0.76 | 2.20 | 16.52 | 10.22 |
| α,ω-Alkane and alkenedioic acids | ||||
| C16:0 | – | 0.02 | 14.54 | 0.05 |
| C18:1 | 0.21 | 0.55 | 32.53 | – |
| C20:0 | – | – | 4.09 | – |
| C22:0 | 0.59 | 0.04 | 2.61 | – |
| C24:0 | 0.06 | 0.06 | 0.09 | – |
| Total | 0.86 | 0.67 | 53.86 | 0.05 |
| α-Hydroxyalkanoic acids | ||||
| C20 | – | 0.06 | – | – |
| C22 | 0.11 | 0.16 | – | – |
| C23 | 0.10 | 0.27 | – | – |
| C24 | 0.23 | 0.43 | 0.27 | – |
| C25 | 0.10 | 0.18 | – | – |
| Total | 0.54 | 1.10 | 0.27 | 0 |
| ω-Hydroxyalkanoic and alkenoic acids | ||||
| C16:0 | 0.06 | 0.02 | 9.44 | 0.37 |
| C18:1 | 0.12 | 0.12 | 25.16 | 2.88 |
| C20:0 | 0.16 | 0.03 | 4.50 | – |
| C22:0 | 1.36 | 0.20 | 9.71 | – |
| C24:0 | 0.09 | 0.14 | 0.63 | – |
| 9 or 10,16-Dihydroxy-hexadecanoic acid | – | – | 3.61 | 0.38 |
| 9,10,18-Trihydroxy-octadecanoic acid | 0.10 | 0.39 | 1.16 | 5.82 |
| Total | 1.89 | 0.91 | 54.21 | 9.45 |
| Coefficient Db | +4.67 | +2.10 | +5.44 | −0.18 |
For comparisons, the glycerol content of the wax fraction is also indicated.
Results of a single experiment; the other values correspond to the mean of two independent experiments.
Discriminant score D, according to the method of Matzke and Riederer (1991). Negative scores are indicative of cutin and positive scores of suberin.
Cotton seed coats and green cotton fibers have a qualitatively similar suberin monomer composition. However, the seed coats have been found previously to contain higher amounts of 1-alkanols (Ryser and Holloway, 1985). This observation was confirmed in the present study. In addition, larger amounts of hydroxycinnamic acids and to a lesser extent of α-hydroxyalkanoic acids were determined in the seed coats (results not shown). Among the hydroxycinnamic acids of the seed coat (Table I), caffeate was the most prominent monomer (38.4%), followed by p-coumarate (16.6%) and ferulate (4.7%). C18 α,ωalkenedioic acid was determined in the hydrolysates of the seed coats of the cotton cultivar with white fibers (St 406), but this monomer was not found in the fibers and seed coats of cv Green Lint.
The fatty acid composition of the transesterification reaction mixtures of the four tissues (seed coat, stem periderm, chalazal region, and nucellar cuticle) was clearly different. Using the discriminant score of Matzke and Riederer (1991) to distinguish suberin and cutin, typical suberins dominate in the seed coat, the chalaza, and the periderm, whereas the nucellar cuticle fraction contains cutin, as expected (bottom of Table I). It can be deduced from these results that the chalazal suberin was not significantly contaminated with nucellar cutin or with suberin from the seed coats, as the predominating chain length of the α,ω-dicarboxylic and ω-hydroxy fatty acids in the chalazal suberin was C18, whereas in the seed coat and in cotton fibers chain lengths of C22 predominated.
The suberin of the stem periderm contained about 74 μmol/g glycerol, 15 μmol/g alkanols, 54 μmol/g α,ω-dicarboxylic fatty acids, and 54 μmol/g ω-hydroxy fatty acids. Because the wax of the stem periderm contained about 10 times less glycerol than the cell wall residue of the same tissue, the glycerol determined in the suberin cannot be attributed to wax contamination. Assuming that the monobasic alkanoic and alkenoic acids with chain lengths of up to C18 are not bona fide suberin or cutin monomers, a conservative estimate can be made concerning a possible contamination of the purified cell wall fractions with membrane lipids or triglycerides. Less than 11% of the glycerol could be attributed to membrane lipids in the seed coat fraction, and less than 2% in the periderm, whereas in the chalaza this value could reach 97%. Accordingly, the contamination of the nucellar cuticle with triglycerides from contaminating endosperm cells must be less than 50%. It should be stressed, however, that in addition to the results presented above for green cotton fiber suberin, no clear-cut scientific evidence is available to rule out the possibility that short-chain alkanoic and alkenoic fatty acids may be bona fide suberin monomers.
As in the transesterification reaction mixtures of green cotton fibers (Fig. 1), roughly equimolar concentrations of glycerol and of the sum of the α,ω-dicarboxylic and ω-hydroxy fatty acids were recovered in the transesterification reaction mixtures of exhaustively extracted cell wall powders of the periderm of cotton stems and of suberized and cutinized tissues of cotton seeds.
Glycerol Accumulation during the Purification of the Suberin of Wound-Healing Potato Tuber Storage Parenchyma
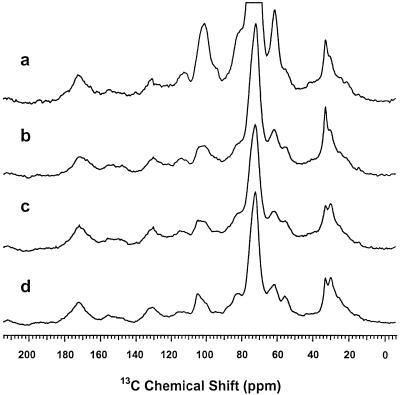
In a preliminary experiment, 1.2% (of the dry weight) of bound glycerol was determined in the purified periderm of steam-cooked potato tubers (Schmutz et al., 1993). In the present experiment, 0.47% glycerol was determined in the periderm of wound-healing potato tuber storage parenchyma, and 1.04% in the purified suberin preparation. Solid-state 13C-NMR spectroscopy (Fig. 4) was used to monitor the purification steps as follows: The cold-water-washed crude periderm preparation was further purified by enzymatic treatments to remove polysaccharides; the exhaustive solvent extraction was used to remove waxes; and the dioxane:water extraction was used to remove soluble lignins and residual sugars.
Figure 4.
Solid-state 13C-NMR spectra showing the progressive purification of the suberin from the wound-healing periderm of potato tuber slices. a, Cold-water-washed periderm preparation showing resonances from suberin, wax, and cell wall components. b, Periderm preparation treated with cellulase and pectinase, drastically reducing carbohydrate peaks centered at 72 ppm. c, Suberin fraction after exhaustive extraction of the wax with organic solvents, reducing mainly the methylene peaks at 33 ppm. d, Suberin fraction after dioxane:water extraction to remove residual sugars and soluble lignins, resulting in a better definition of the carbohydrate peaks. The glycerol resonances expected at 66 and 75 ppm were obscured by large cell wall peaks near 72 ppm and possibly by signals from suberin esters of primary alcohols at 65 ppm.
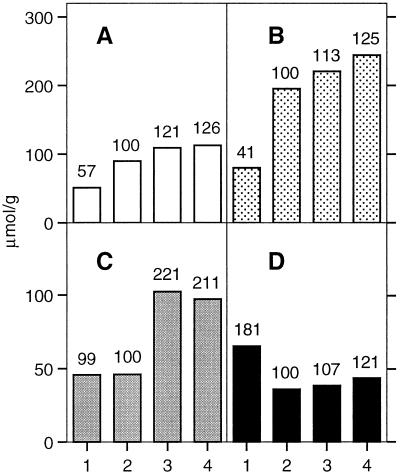
The crude suberin preparation and the residues obtained after the three purification steps were each depolymerized by acid-catalyzed transesterification, and the concentration of the liberated suberin monomers was determined as described above. The results of this experiment are summarized in Figure 5. Glycerol is present in all four suberin fractions, and its proportion increases at about the same rate as the α,ω-dicarboxylic fatty acids, the most diagnostic suberin monomers (Matzke and Riederer, 1991). In the purified suberin fraction (after dioxane:water extraction), 113 mm glycerol, 244 mm α,ω-dicarboxylic fatty acids, and 97 mm ω-hydroxy fatty acids were determined per gram dry weight. The ω-hydroxy fatty acids accumulate at different rates than glycerol and α,ω-dicarboxylic fatty acids during suberin purification. The reason for this difference is not known. The concentration of hydroxycinnamic acids drops markedly after the extraction with pectinase and cellulase and accumulates subsequently at about the same rate as glycerol and the α,ω-alkanedioic acids. This result indicates that hydroxycinnamic acids are covalently linked to the cell wall layers removed by enzymatic treatments, as well as to suberin. In a control experiment unsuberized tissue from potato tuber slices was purified and depolymerized exactly as the suberized periderm preparations. No glycerol was detected in these preparations. This observation rules out the possibility that glycerol or glycerol-containing compounds could artifactually fix to cell walls or starch during the purification procedures.
Figure 5.
Concentration of the major suberin monomers during the purification of suberin from the periderm of wound-healing potato tuber slices. A, Glycerol; B, α,ω-dicarboxylic acids; C, ω-hydroxy fatty acids; D, hydroxycinnamic acids. The values in A are means of three determinations, and the values in B to D are means of two determinations. The isolated periderm was purified as in Figure 4 by 1, a cold water wash; 2, cellulase and pectinase treatments; 3, exhaustive extractions with organic solvents; and 4, extraction with dioxane:H2O. The figures above the bars indicate the relative changes in percent, with the enzyme-treated periderm sample corresponding to 100%.
Making a conservative estimate as above, and assuming that all of the monobasic fatty acids with chain lengths of up to C18 are derived from membrane lipids, less than 18% of the glycerol in the potato periderm could be accounted for by membrane lipids (results not shown). Thus, the presence of glycerol was confirmed in sterile suberin preparations from wound-healing potato tubers, and the observed glycerol concentrations were compatible with a structural role of glycerol in potato suberin.
DISCUSSION
Kügler (1884; cited by Gilson, 1890) obtained the first preliminary evidence for the presence of bound glycerol in the cork of oak. After extraction of the wax with boiling chloroform, boiling ethanol, and water, suberin was depolymerized with boiling ethanolic KOH for 48 h. The resulting extract contained 2.65% glycerol and 30% fatty acids. Kügler concluded that suberin is a triglyceride-like substance, rendered insoluble by the presence of cellulose molecules. Gilson (1890) confirmed the presence of glycerol in crude oak cork preparations using improved analytical methods. However, he did not quantify the glycerol content of his preparations and he firmly rejected the idea that suberin is a triglyceride, as proposed by Kügler. His conclusion was based on the absence of cellulose in the microscopically thin suberin layers and the different solubilities of suberin and triglycerides in organic solvents. Ribas and Blasco (1940) showed that extractions with water, ethanol, ether, or chloroform for 5 h in a Soxhlet apparatus did not change the glycerol content (6.2% of the dry weight) of a finely powdered oak cork preparation, and high concentrations of bound glycerol were later reported also to occur in exhaustively extracted Douglas fir bark (4.65%) by Hergert and Kurth (1952) and again in stopper cork (4.7%) by Rosa and Pereira (1994). However, Ribas (1942, 1952) was the first to propose that suberin may be a water-insoluble polyester that can be easily saponified. According to Ribas, the ease of saponification also explains why other authors (von Schmidt, 1904; Zetsche and Rosenthal, 1927), using prolonged extractions with weakly alkaline solutions before saponification, concluded that glycerol was not a suberin monomer.
With the advent of GC-MS for the identification of organic compounds, suberin and cutin monomers, or their methylesters, were extracted after the depolymerization step into organic solvents before further derivatization and injection into the GC system, leaving small polar molecules in the aqueous phase. Therefore, at most traces of glycerol derivatives can be detected in such chromatograms.
The aliphatic monomer composition of the suberin of green cotton fibers allows only the formation of linear polymers (Ryser at al., 1983; Yatsu et al., 1983). Similar observations were made for most suberins, as well as for some cutins (Kolattukudy, 1980a; Matzke and Riederer, 1991). Following the ideas outlined by Kolattukudy, we decided to study in more detail the phenolic components of cotton fiber suberin. The resulting purification of a caffeoyl-fatty acid-glycerol ester from the wax of green cotton fibers by Schmutz et al. (1994b) then prompted us to test the idea that glycerol may be a suberin monomer, connecting aliphatic and aromatic suberin constituents.
Taking white cotton fibers as a control tissue, it was readily shown that glycerol is specifically associated with the suberin layers of the green fibers (Schmutz et al., 1993, 1996). Changes in the glycerol concentration of the wax and suberin fractions induced by two specific inhibitors of suberin biosynthesis were correlated with changes in the concentrations of the aliphatic suberin monomers (Schmutz et al., 1993, 1994a, 1994b, 1996).
Using boiling SDS for the extraction of the wax of green cotton fibers, we show in this work that the glycerol content of suberin is not a contaminant from the wax or from membrane lipids. A developmental study of differentiating green cotton fibers clearly indicates that glycerol is incorporated into suberin at the same rate as the established monomers, ω-hydroxyalkanoic acids and α,ω-alkanedioic acids. Therefore, it can be excluded that glycerol becomes fixed artifactually to the suberin layers only during the phase of programmed cell death and dehydration of the fibers.
A comparison of the glycerol content of the periderm of cotton stems and of wound-healing potato tubers, in relation to the molar concentration of other suberin monomers, is consistent with our previous hypothesis that glycerol may be a monomer of suberins in general. In addition, the glycerol content of the wax of these suberins is low, indicating again that the glycerol determined in the suberin fraction cannot be considered as a contaminant from the wax. As in green cotton fibers, glycerol occurs in these tissues in sufficient molar proportions to play the expected structural role in suberin. This possibility could be further tested by the isolation, purification, and chemical analysis of suberin oligomers.
Glycerol may have a structural role in suberin, linking the aliphatic and aromatic suberin domains, corresponding to the electron-translucent and electron-opaque suberin lamellae, respectively. This hypothesis is supported by the dimensions of the electron-translucent suberin lamellae of green cotton fibers (Schmutz et al., 1996). The esterified C22 acids representing 96% of the aliphatic suberin monomers with two functional groups (α,ω-alkanedioic and ω-hydroxyalkanoic acids) are about 2.95 nm long. The thickness of the electron-translucent suberin lamellae, determined after tilting the suberin layers into a position parallel to the electron beam with a computer-controlled precision goniometer, corresponded to 3.4 ± 0.3 nm. Therefore, the electron-translucent suberin layers are somewhat thicker than the extended fatty acid chains; they could easily incorporate perpendicularly oriented glycerol molecules, cross-linking the extended aliphatic suberin monomers in the electron-translucent lamellae to the aromatic monomers confined to the electron-opaque suberin layers. Alternatively, glycerol might simply function as a plasticizer in the wax fraction and be more or less randomly incorporated into suberin. However, the ultrastructure of the suberin layers suggest that suberin is not a random polymer. The observed lamellation is typical of block copolymers. These polymers, exhibiting different types of ultrastructure, are characterized by the presence of two covalently bound compounds differing in their polarities. The linkages between the two compounds inhibit phase separation, which typically occurs in mixtures of lipids of different polarity (Strobl, 1996).
In conclusion, convincing experimental evidence is presented that glycerol is a suberin monomer, occurring in sufficient molar concentrations to play a structural role in the polymer. The three hydroxyl groups of glycerol are ideally suited to link and stabilize the three axes of lamellated structures. Therefore, the identification of glycerol as a suberin monomer will probably help us to fully understand the chemical basis of the lamellated ultrastructure of this biopolymer. The identification of a new suberin monomer also creates an argument for a reevaluation of the biochemical pathways leading to the biosynthesis of suberin and associated waxes (von Wettstein-Knowles, 1993) as also proposed by Davin and Lewis (1992).
ACKNOWLEDGMENTS
We thank Martine Schorderet for technical assistance and Ruth Bosshard for cultivating the cotton plants.
Footnotes
This work was supported by the Swiss National Science Foundation (grant nos. 31-39648.93 and 31-49305.96 to U.R.) and by the U.S. National Science Foundation (grant nos. MCB-9406354 and MCB-9728503 to R.E.S.).
LITERATURE CITED
- Bernards MA, Lewis NG. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry. 1998;47:915–933. doi: 10.1016/s0031-9422(98)80052-6. [DOI] [PubMed] [Google Scholar]
- Bernards MA, Lopez ML, Lewis NG. Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem. 1995;270:7382–7386. doi: 10.1074/jbc.270.13.7382. [DOI] [PubMed] [Google Scholar]
- Borg-Olivier O, Monties B. Lignin, suberin, phenolic acids and tyramine in the suberized, wound-induced potato periderm. Phytochemistry. 1993;32:601–606. [Google Scholar]
- Davin LB, Lewis NG (1992) Phenylpropanoid metabolism: biosynthesis of monolignols, lignans and neolignans, lignins and suberins. In HA Stafford, RK Ibrahim, eds, Recent Advances in Phytochemistry: Phenolic Metabolism in Plants. Plenum Press, New York, pp 325–375
- Eggstein M, Kuhlmann E (1974) Triglycerides and glycerol. In HU Bergmeyer, ed, Methods of Enzymatic Analysis, Ed 2, Vol 4. Verlag Chemie, Weinheim, Germany/Academic Press, New York, pp 1825–1831
- Esau K. Plant Anatomy, Ed 2. New York: John Wiley & Sons; 1977. [Google Scholar]
- Fahn A. Plant Anatomy, Ed 4. Oxford, UK: Pergamon Press; 1990. [Google Scholar]
- Falk H, El Hadidi MN (1961) Der Feinbau der Suberinschichten verkorkter Zellwände. Z Naturforsch 16b: 134–137
- Garbow JR, Ferrantello LM, Stark RE. Nuclear magnetic resonance study of suberized potato cell wall. Plant Physiol. 1989;90:783–787. doi: 10.1104/pp.90.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E. La subérine et les cellules du liège. La Cellule. 1890;6:63–117. [Google Scholar]
- Grace J, van Gardingen PR (1996) Plant cuticles under challenge. In G Kerstiens, ed, Plant Cuticles. BIOS Scientific Publishers, Oxford, UK, pp 319–329
- Hergert HL, Kurth EF. The chemical nature of the cork from Douglas-fir bark. TAPPI. 1952;35:59–66. [Google Scholar]
- Holloway PJ. Some variations in the composition of suberin from the cork layers of higher plants. Phytochemistry. 1983;22:495–502. [Google Scholar]
- Holloway PJ. Cutins and suberins, the polymeric plant lipids. In: Mangold HK, editor. Handbook Series in Chromatography, Section G: Lipids and Technical Lipid Derivatives, Vol 1. Boca Raton, FL: CRC Press; 1984. pp. 321–345. [Google Scholar]
- Jeffree CE (1996) Structure and ontogeny of plant cuticles. In G Kerstiens, ed, Plant Cuticles. BIOS Scientific Publishers, Oxford, UK, pp 33–82
- Kerstiens G (1996) Diffusion of water vapour and gases across cuticles and through stomatal pores presumed closed. In G Kerstiens, ed, Plant Cuticles. BIOS Scientific Publishers, Oxford, UK, pp 121–134
- Kohel RJ. Genetic analysis of fiber color variants in cotton. Crop Sci. 1985;25:793–797. [Google Scholar]
- Kolattukudy PE (1977) Lipid polymers and associated phenols, their chemistry, biosynthesis, and role in pathogenesis. In FA Loewus, VC Runeckles, eds, Recent Advances in Phytochemistry, Vol 11: The Structure, Biosynthesis, and Degradation of Wood. Plenum Press, New York, pp 185–246
- Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980a;208:990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE. Cutin, suberin, and waxes. In: Stumpf PK, editor. The Biochemistry of Plants. A Comprehensive Treatise, Vol 4. Lipids: Structure and Function. London: Academic Press; 1980b. pp. 571–645. [Google Scholar]
- Kolattukudy PE. Biochemistry and function of cutin and suberin. Can J Bot. 1984;62:2918–2933. [Google Scholar]
- Kolattukudy PE. Enzymatic penetration of the plant cuticle by fungal pathogens. Annu Rev Phytopathol. 1985;23:223–250. [Google Scholar]
- Kolattukudy PE. Lipid-derived defensive polymers and waxes and their role in plant-microbe interaction. In: Stumpf PK, editor. The Biochemistry of Plants. A Comprehensive Treatise, Vol 9. Lipids: Structure and Function. London: Academic Press; 1987. pp. 291–314. [Google Scholar]
- Kolattukudy PE (1996) Biosynthetic pathways of cutin and waxes, and their sensitivity to environmental stresses. In G Kerstiens, ed, Plant Cuticles. BIOS Scientific Publishers, Oxford, UK, pp 83–108
- Kolattukudy PE, Agrawal VP. Structure and composition of aliphatic constituents of potato tuber skin (suberin) Lipids. 1974;9:682–691. [Google Scholar]
- Kolattukudy PE, Dean BB. Structure, gas chromatographic measurement, and function of suberin synthesized by potato tuber tissue slices. Plant Physiol. 1974;54:116–121. doi: 10.1104/pp.54.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE, Köller W (1983) Fungal penetration of the first line defensive barriers of plants. In JA Calow, ed, Biochemical Plant Pathology. John Wiley and Sons, New York, pp 79–100
- Kolattukudy PE, Soliday CL (1985) Effects of stress on the defensive barriers of plants. In JL Key, T Kosuge, eds, Cellular and Molecular Biology of Plant Stress. UCLA Symposia on Molecular and Cellular Biology. New Series, Vol 22. Alan R. Liss, New York, pp 381–400
- Kügler X (1884) Über das Suberin. Inaugurale Dissertation der mathematischen und naturwissenschaftlichen Fakultät der Kaiser-Wilhelms-Universität, Strassburg, Germany
- Lapierre C, Pollet B, Négrel J. The phenolic domain of potato suberin: structural comparison with lignins. Phytochemistry. 1996;42:949–953. [Google Scholar]
- Lulai EC, Morgan WC. Histochemical probing of potato periderm with neutral red: a sensitive cytofluorochrome for the hydrophobic domain of suberin. Biotech Histochem. 1992;67:185–195. doi: 10.3109/10520299209110065. [DOI] [PubMed] [Google Scholar]
- Matzke K, Riederer M. A comparative study into the chemical constitution of cutins and suberins from Picea abies (L.) Karst., Quercus robur L. and Fagus silvatica L. Planta. 1991;185:233–245. doi: 10.1007/BF00194066. [DOI] [PubMed] [Google Scholar]
- Pacchiano RA, Sohn W, Chlanda VL, Garbow JR, Stark RE. Isolation and spectral characterization of plant cuticle polyesters. J Agric Food Chem. 1993;41:78–83. [Google Scholar]
- Ralph J, Grabber JH, Hatfield RD. Lignin-ferulate cross-links in grasses: active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydr Res. 1995;275:167–178. [Google Scholar]
- Ribas I. Revista Espanola de Quimica Aplicada. 1942;2:25–28. [Google Scholar]
- Ribas I. Etude sur la constitution chimique du liège. Industrie Chimie. 1952;68:333–350. [Google Scholar]
- Ribas I, Blasco E. Investigaciones sobre el corcho. II. Determinacion cuantitativa de la glicerina existente. An R Soc Esp Fis Quim. 1940;36:248–254. [Google Scholar]
- Riederer M, Schreiber L (1995) Waxes: the transport barriers of plant cuticles. In RJ Hamilton, ed, Waxes: Chemistry, Molecular Biology and Functions. Oily Press, Dundee, Scotland, pp 131–156
- Rosa ME, Pereira H. The effect of long term treatment at 100°C–150°C on structure, chemical composition and compression behaviour of cork. Holzforschung. 1994;48:226–232. [Google Scholar]
- Ryser U, Holloway PJ. Ultrastructure and chemistry of soluble and polymeric lipids in cell walls from seed coats and fibres of Gossypium species. Planta. 1985;163:151–163. doi: 10.1007/BF00393501. [DOI] [PubMed] [Google Scholar]
- Ryser U, Meier H, Holloway PJ. Identification and localization of suberin in the cell walls of green cotton fibres (Gossypium hirsutum L., var. green lint) Protoplasma. 1983;117:196–205. [Google Scholar]
- Ryser U, Schorderet M, Meier H. Ultrastructure of the “fringe-layer,” the innermost epidermis of cotton seed coats. Protoplasma. 1988;147:81–90. [Google Scholar]
- Schmidt HW, Schönherr J. Development of plant cuticles: occurrence and role of non-ester bonds in cutin of Clivia miniata Reg. leaves. Planta. 1982;156:380–384. doi: 10.1007/BF00397478. [DOI] [PubMed] [Google Scholar]
- Schmutz A, Buchala A, Jenny T, Ryser U (1994a) The phenols in the wax and the suberin polymer of green cotton fibres and their functions. In M Geibel, D Treutter, W Feucht, eds, International Symposium on Natural Phenols in Plant Resistance, Vol 1. International Society for Horticultural Science, Leuren, Belgium. Acta Hortic 381: 269–275
- Schmutz A, Buchala AJ, Ryser U. Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-associated fatty acid elongases. Plant Physiol. 1996;110:403–411. doi: 10.1104/pp.110.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz A, Jenny T, Amrhein N, Ryser U. Caffeic acid and glycerol are constituents of the suberin layers in green cotton fibres. Planta. 1993;189:453–460. doi: 10.1007/BF00194445. [DOI] [PubMed] [Google Scholar]
- Schmutz A, Jenny T, Ryser U. A caffeoyl-fatty-acid-glycerol ester from wax associated with green cotton fibre suberin. Phytochemistry. 1994b;36:1343–1346. [Google Scholar]
- Schönherr J. Water permeability of isolated cuticular membranes: the effect of cuticular waxes on diffusion of water. Planta. 1976;131:159–164. doi: 10.1007/BF00389989. [DOI] [PubMed] [Google Scholar]
- Schönherr J. Resistance of plant surfaces to water loss: transport properties of cutin, suberin and associated waxes. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of Plant Physiology: New Series, Vol 12B: Physiological Plant Ecology. Berlin: Springer Verlag; 1982. pp. 153–179. [Google Scholar]
- Schreiber L, Kirsch T, Riederer M (1996) Diffusion through cuticles: principles and models. In G Kerstiens, ed, Plant Cuticles. BIOS Scientific Publishers, Oxford, UK, pp 109–119
- Soliday CL, Kolattukudy PE, Davis RW. Chemical and structural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosum L.) Planta. 1979;146:607–614. doi: 10.1007/BF00388840. [DOI] [PubMed] [Google Scholar]
- Stark RE, Garbow JR. Nuclear magnetic resonance relaxation studies of plant polyester dynamics. 2. Suberized potato cell wall. Macromolecules. 1992;25:149–154. [Google Scholar]
- Stark RE, Sohn W, Pacchiano RA, Al-Bashir MA, Garbow JR. Following suberization in potato wound periderm by histochemical and solid-state 13C nuclear magnetic resonance methods. Plant Physiol. 1994;104:527–533. doi: 10.1104/pp.104.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark RE, Zlotnik-Mazori T, Ferrantello LM, Garbow JR. Molecular structure and dynamics of intact plant polyesters: solid-state NMR studies. In: Lewis NG, Paice MG, editors. Plant Cell Wall Polymers: Biogenesis and Biodegradation. ACS Symposium Series, Vol 399. Washington, DC: American Chemical Society; 1989. pp. 214–229. [Google Scholar]
- Strobl GR. The Physics of Polymers. Berlin: Springer Verlag; 1996. [Google Scholar]
- Thomson N, Evert RF, Kelman A. Wound healing in whole potato tubers: a cytochemical, fluorescence, and ultrastructural analysis of cut and bruise wounds. Can J Bot. 1995;73:1436–1450. [Google Scholar]
- Vogt E, Schönherr J, Schmidt HW. Water permeability of periderm membranes isolated enzymatically from potato tubers (Solanum tuberosum L.) Planta. 1983;158:294–301. doi: 10.1007/BF00397330. [DOI] [PubMed] [Google Scholar]
- von Schmidt M. Zur Kenntnis der Korksubstanz. II. Über den vermeintlichen Glyceridcharakter der Korksubstanz. Monatsh Chem. 1904;25:302–310. [Google Scholar]
- von Wettstein-Knowles PM. Waxes, cutin, and suberin. In: Moore TS, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 127–166. [Google Scholar]
- Yang G, Espelie KE, Todd JW, Culbreath AK, Pittman RN, Demski JW. Cuticular lipids from wild and cultivated peanuts and the relative resistance of these peanut species to fall armyworm and thrips. J Agric Food Chem. 1993;41:814–818. [Google Scholar]
- Yatsu LY, Espelie KE, Kolattukudy PE. Ultrastructural and chemical evidence that the cell wall of green cotton fibers is suberized. Plant Physiol. 1983;135:521–524. doi: 10.1104/pp.73.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche F, Rosenthal G. Untersuchungen über den Kork. Helv Chim Acta. 1927;10:346–374. [Google Scholar]