Abstract
Aims
We investigated the expression of claudins 18 and 10 in a large set of primary lung carcinomas.
Methods and results
Immunohistochemical expression of claudin 18 was seen in 12.7 % and claudin 10 in 12.5 % of lung carcinomas. Their expression significantly associated with each other (p<0.001). The expression of claudin 18 and 10 was most prominent in lung adenocarcinomas which displayed positivity in 21.2% and 23.4 % of cases. Female patients had more often claudin 18 and 10 positive tumors, also separately in adenocarcinomas. Interestingly, claudin 10 (p=0.036) and claudin 18 (p=0.001) were more common in tumours of nonsmokers. In adenocarcinomas claudin 18 predicted a better survival (p=0.032). In Cox multivariate analysis, claudin 18 had an independent prognostic value (p=0.027).
Conclusion
The results show that both claudins are most commonly expressed in lung adenocarcinomas and they are more occasionally detected in other histological tumour types. Curiously, female patients and non-smokers express these claudins more commonly suggesting that they may play a part in the carcinogenesis of tobacco unrelated carcinoma. Claudin 18 associated with a better survival in lung adenocarcinoma and had an independent prognostic value and may thus be used in the evaluation of patient prognosis.
Keywords: Lung, carcinoma, claudin, smoker
Introduction
Claudins are tight junctional proteins present in epithelial, mesothelial and endothelial cells [1]. They influence the electrical and solute permeability of the paracellular space, regulate cellular polarity, segregate the apicolateral cell membrane from other parts and contribute to the cells’ defence against external pathogens [2-4]. They are attached to scaffolding proteins, such as ZO-1, ZO-2 and ZO-3 by their carboxyterminal PDZ domains [4]. Through these they are connected to the actin microfilament network of the cells and also influence cellular proliferation and apoptosis [4]. There are 27 claudins known and the presence and relations of individual claudins in tight junctions determine the permeability of the paracellular space [2, 5].
Different claudins may be over- or underexpressed in cancer [6]. Claudin 7, for instance is upregulated in thyroid carcinomas, squamous cell carcinomas of the tongue, chromophobe renal cell carcinomas, prostate carcinomas and ovarian epithelial carcinomas while diminished expression has been reported in breast carcinomas and hepatoblastomas [6]. Same claudins may also influence patient prognosis in different ways. In breast carcinoma, claudin 7 downregulation is associated with a worse prognosis and aggressiveness of low claudin 1 expression in lung adenocarcinoma patients predicted a shorter survival and over-expression of claudin 1 inhibited cancer cell dissociation, migration and invasion [9,10]. Claudins may also be used as markers in the the tumors while in ovarian epithelial carcinoma overexpression of claudin 7 harbours a poor prognosis [7, 8]. In oral squamous cell carcinoma claudin 1 overexpression associates with tumors of aggressive types while in lung adenocarcinoma patients it predicted a better survival and overexpression of claudin 1 inhibited cancer cell dissociation, migration and invasion [9, 10]. Claudins may also be used as markers in the differential diagnosis of tumors. Due to the different expression patterns of claudins 3 and 4 in malignant mesothelioma and adenocarcinoma these claudins may be used to distinguish metastatic pleural adenocarcinoma and mesothelioma [11, 12]. Claudins show also promise for specific molecular targeted cancer treatment. Chemically modified clostridium perfringens toxin may modulate tight junctions containing claudins 3 and 4 thus allowing a better penetrance of chemotherapeutic drugs in cancer tissues [13].
Claudin 18 is a gene producing two splice variants, claudin 18A and 18B. The former is present in the gastrointestinal tract while the latter is present in the lung [14]. The claudin 18 gene appears to be highly conserved between different species [15]. In cancer tissues, claudin 18 expression has been detected in gastric, pancreatic and colon cancer [16-19]. In pancreatic cancer claudin 18 was detected in a high percentage of cases while non-neoplastic tissues were negative [19]. Its expression was, however, lower in colon cancer where only 4 % of cases were positive [17]. Interestingly, claudin 18 expression is also increased in ulcerative colitis and has been reported to be downregulated in gastric intestinal metaplasia compared to non-neoplastic tissues but upregulated in Barrett's esophagus [16, 20, 21].
Claudin 10 gene is able to produce 6 different splice variants [22]. Claudin 10 expression has been detected in human kidney, hepatobiliary tract and in the lung [23-25]. Interestingly, claudin 10 is a potential marker of Clara cells in the lung [25]. The influence of claudin 10 on paracellular permeability appears to depend on the splice variant and cell type in question [22]. In hepatocellular carcinoma, claudin 10 expression is associated with a worse survival and in cell line studied such overexpression was associated with a more invasive phenotype [26].
This study was undertaken to evaluate the expression of claudins 10 and 18 in different types of lung tumors, and to evaluate their relation to the clinical data of the patients. A large set of primary lung tumors, consisting of 271 cases were studied for the expression of claudins 18 and 10.
Materials and methods
Materials
The tumors studied were retrieved from the archives of Department of Pathology, University of Oulu. They consisted of 289 surgical samples of lung cancer which had been fixed in formalin and embedded in paraffin. The diagnosis of the tumor cases had been determined according to the WHO International Classification of lung and pleural tumors [27]. Two tumor regions were chosen from each sample and these were incorporated into microarray blocks (Beecher Instruments, Silver Spring, MD, USA). The presence of metastases and the stage of the tumors were determined at the time of operation. Survival data was obtained from the Finnish Cancer Registry and the clinical data from patient records. Due to exhaustion of some cases in the processing of array blocks the number of cases analyzed for each tumor histotype may be different.
Immunostaining
The rabbit polyclonal antibodies for claudin 18 and 10 were purchased from Zymed (Claudin 10 Cat.nro 38-8400 and claudin 18 cat.nro 38-8000 detecting claudin 18 both in lung and stomach). Before application of the primary antibodies, the sections were heated in a microwave oven in 10 mM citrate buffer, pH 6.0, for 10 minutes. After a 60-minute incubation with the primary antibody (dilution 1:100 for both), a biotinylated secondary anti-rabbit antibody and Histostain-SP kit (Zymed Laboratoris Inc) was used. For all the immunostaining, the color was developed by diamino-benzidine, where after the sections were lightly counterstained with hematoxylin and mounted with Eukitt (Kindler, Freiburg, Germany).
Negative control stainings were carried out by substituting non-immune serum and PBS for the primary antibodies.
The immunostaining for claudins was assessed as follows:
-= less than 5 % immunostaining present
+= less than 50 % of cells positive
++= more than 50 % of cells positive
In the evaluation for claudins 10 and 18, membrane bound positivity was considered significant.
Statistical methods
The statistical analyses were performed with SPSS for Windows software (SPSS, Chicago, IL, USA). Continuous data were compared using analysis of variance (ANOVA) followed by two-tailed t-tests. Categorical data were compared using Fisher's exact. Survival-data was analyzed using the Kaplan-Meier method with the use of the log-rank test and only deaths with lung tumor as the primary cause of death were considered. P-values less than 0.05 were considered statistically significant.
Ethical considerations
The study was approved by the ethical committee of Northern Ostrobothnia Hospital District. The study protocol was accepted by the Finnish Natiolegal board. The survival data was obtained from the Finnish Cancer Register after receiving permission from the Ministry of Health and Social Welfare.
Results
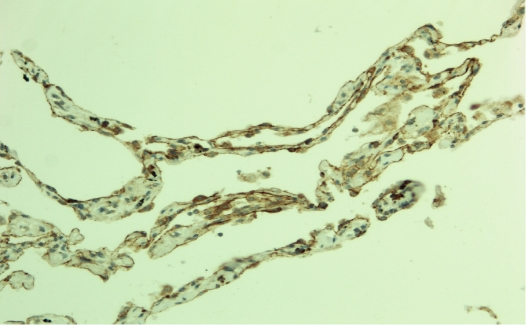
In non-neoplastic lung tissue claudin 18 was seen at the membranes of both type 1 and type 2 pneumocytes (Figure 1). In areas with pneumocyte proliferation, expression of claudin 18 was intensified. Claudin 10 was weakly expressed in pneumocytic cells of the alveoli. Bronchial cells appeared not to express either claudin 18 or claudin 10.
Figure 1.
Claudin 18 in lung alveoli. Immunopositivity is present around and beneath alveolar pneumo-cytes.
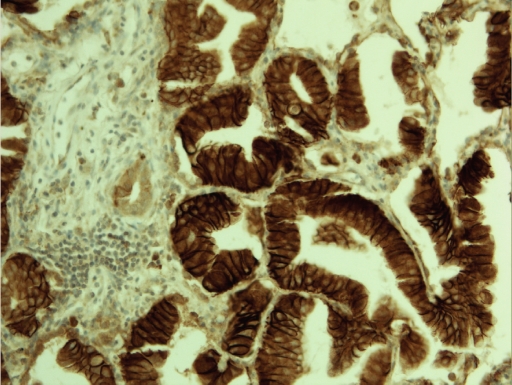
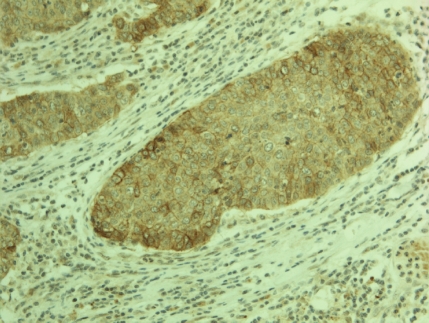
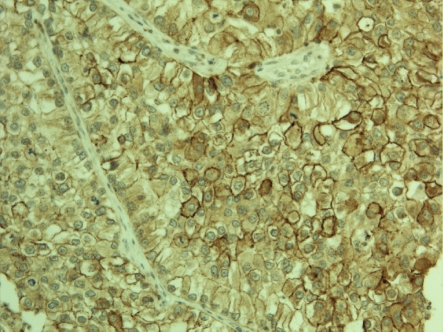
Claudin 18 positivity was observed in 34/268 (12.7 %) (Figure 2) and claudin 10 in 34/271 (12.5 %) (Figure 3 and 4) tumors. Interestingly, there was a strong association between claudin 18 and 10 immunoreactivity, 13 cases displaying both claudins (p<0.001). Squamous cell carcinomas expressed claudin 18 in 9/122 (7 %), adenocarcinomas in 23/108 (21 %) (bronchioloalveolar carcinomas in 5/9), small cell carcinomas in 1/11 (9%), large cell carcinomas in 1/8 (12 %), while adenosquamous and carcinoid tumors displayed no positivity. Claudin 10 positivity was seen in 7/122 (6 %) squamous cell, 26/111 (23 %) adenocarcinomas (bronchioloalveolar carcinomas in 3/10), and 1/13 (7 %) adenosquamous carcinomas while carcinoid tumors, small cell carcinomas and large cell carcinomas displayed no positivity. Adenocarcinomas displayed significantly more positivity for claudin 18 and claudin 10 than other tumors (p<0.001 for both). Separately, they also expressed more frequently these markers compared to squamous cell carcinomas (p=0.004, p=0.0005, respectively). No association was found between claudin 18 and 10 and tumor size (p=0.17, p=0.88), nodal metastases (p=0.12, p=0.74) or distant metastases (p=0.77, p=0.85). Curiously, female patients had more commonly tumors with claudin 10 expression (p=0.001) and such a trend was also observed with claudin 18 (p=0.06). Interestingly, both claudin 10 (p=0.036) and claudin 18 (p=0.001) were more commonly found in nonsmokers. In line with this, patient packyears were significantly lower in patients with claudin 18 (p=0.044) or claudin 10 positive (p=0.007) tumors. In the whole lung tumor material neither claudin 18 nor claudin 10 expression associated with survival of the patients (p=0.69, p=0.76, respectively).
Figure 2.
Intensive membrane bound claudin 18 expression is seen in lungadenocarcinoma.
Figure 3.
Membrane bound positivity for claudin 10 is seen in this case of a pulmonary squamous cell carcinoma.
Figure 4.
Membrane bound claudin 10 immunoposi-tivity is present in a proportion of tumor cells in this case of a pulmonary adenocarcinoma.
However, if only adenocarcinomas were studied, patients displaying claudin 18 in their tumor had a better survival (p=0.032) (Figure 5). In Cox multivariate analysis, claudin 18 had an independent prognostic value (p=0.027). No such association was seen with claudin 10 (p=0.69). In adenocarcinomas, the association between expression of claudin 18 and 10 still prevailed (p=0.001) and the association between gender and claudin 10 was still present (p=0.006) and similarly, the same tendency for claudin 18 (p=0.094) was present. However, the association between packyears was only seen for claudin 18 (p=0.045) even though non-smokers still represented a significant group in claudin 18 (p=0.009) and claudin 10 (p=0.038) positive tumors. There were significantly more claudin 18 positive tumors in the grade I group (p=0.033) and with claudin 10 the association was near significant (p=0.051). No significant association was found with claudin 10 and 18 and TNM status of the tumors.
Figure 5.
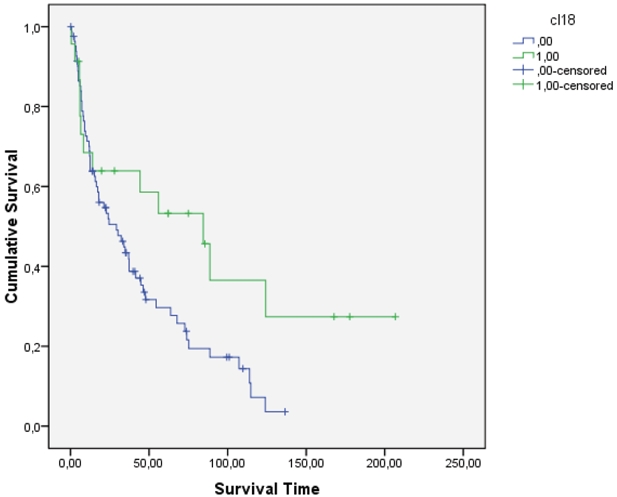
In adenocarcinomas expressing claudin 18 the survival of patients was significantly better than in the other group (p=0.032, log rank).
Of tumors metastatic to the lung 5/51 (9 %) expressed claudin 18. The positive cases represented kidney adenocarcinoma, rectum adenocarcinoma and ovarian adenocarcinoma. Of claudin 10, 2/51 (4 %) cases were positive, the positive cases representing rectal and kidney adenocarcinoma.
Discussion
Claudins are tight junctional proteins regulating the paracellular permeability of epithelial, endothelial and mesothelial cells [1-4]. In tumor tissues their expression is many times deranged and different claudins may either be under- or overexpressed depending on the location or the histological type of tumor [1, 6]. Such deranged expression is partly obscure but may depend on alterations in the expression of oncogenic genes, EMT related transcription factors, protein kinases and changes in the regulation of tight junctional scaffolding proteins which may also activate tumor proliferation or differentiation [4]. Claudin expression may, however, parallel the differentiation of the ancestor cells of the tumor thus making them possible markers to use in differential diagnosis of some tumors, such as differentiation of mesothelioma from adenocarcinoma [11, 12].
Claudin 18 is a member of the claudin family present as two splice variants [14]. Claudin 18A is expressed in the gastrointestinal tract and claudin 18B in the lung [14]. Such a specific location of claudin 18 makes it a putative marker for tumors derived from these locations. Indeed, claudin 18 expression has been found in the majority of gastric and pancreatic cancers while expression is lower in colon carcinoma being only 4 % [14-19]. The expression of claudin 18 in lung tumors has not previously been studied. We found its expression in 12.7 % of lung tumors. Its expression in our set of metastatic tumors to the lung was, however, 9 % thus making claudin 18 unreliable to distinguish lung tumors from adenocarcinomas metastatic to the lung. Such evaluation, however, depends partly on the origin of metastatic cases. We, however, observed claudin 18 positivity also in adenocarcinomas derived from the ovary and kidney. Thus the expression of claudin 18 may be broader and not only restricted to lung and gastrointestinal tract neoplasms.
While claudin 18 is expressed in alveolar cells of the lung, it might be expected that claudin 18 would be mainly present in lung adenocarcinomas and mostly in bronchioloalveolar carcinomas. According to the recent suggestion of lung adenocarcinoma classification bronchioloalveolar carcinoma represents an in situ type of carcinoma taking origin primarily from pneumocytic cells [28]. In line with this concept 5/9 bronchioloalveolar carcinomas expressed claudin 18 which was significantly more frequently than observed in all adenocarcinomas. Adenocarcinomas, on the other hand, expressed more frequently claudin 18 than other histological types of lung tumors. Expression was, however, seen in a few squamous cell carcinomas, and even in one small and large cell carcinoma suggesting that such ectopic expression is possible in tumors not directly derived from alveolar cells.
Interestingly, however, claudin 18 predicted a better survival in adenocarcinomas and it had an independent prognostic value. Claudin 18 may thus be used as a prognostic indicator in lung adenocarcinoma. Claudin 18 was also more frequently present in tumors of nonsmokers thus underlining its role as an indicator of a tobacco unrelated carcinogenic pathway in lung carcinogenesis. In pancreatic cancer, claudin 18 is upregulated by protein kinase C which again is dependent on transcription factors like AP-1 [29]. In non-small cell lung carcinoma AP-1 is overexpressed and regulated by c-jun and blockage of AP-1 leads to diminished proliferation of cancer cells [30, 31]. AP-1 by itself is, however, induced by tobacco smoke [32] and its overexpression in lung carcinoma cannot thus be a significant factor stimulating claudin 18 overexpression in lung adenocarcinoma.
Claudin 10 gene is able to form six splice variants [22, 23]. Claudin 10a is unique to the kidney while claudin 10b is detected in several tissues [23]. These splice variants influence the first extracellular domain of claudin 10 and thus affect paracellular permeability [23]. The other splice variants influence exon 1a and exon 4 of the claudin 10 gene [23]. Claudin 10 expression has been detected in the kidney tubular cells and its expression has a prognostic value in hepatocellular carcinoma [23, 26]. Claudin 10 has also been introduced as a marker of Clara cells of the lung [25]. The fact that claudin 10 is mainly present in lung adenocarcinomas is also in line with this fact since a proportion of adenocarcinomas are derived from these cells [28]. It is not, however, excluded that claudin 10 expression could also partly be related to pneumocytic features of the tumors. After all, there was a significant association between claudin 10 and 18 expression in lung tumors.
Like with claudin 18, a small proportion of squamous cell carcinomas were claudin 10 positive. There were also other similarities; claudin 10 was more often found in female patients, and its expression was more common in grade 1 tumors. Both had also an association with a non-smoker status of the patients. Thus, claudin 10 expression also appears to be related to the carcinogenesis of lung tumors not associated with tobacco smoking. It is known that adenocarcinomas and lung carcinomas of women and not related to tobacco smoking harbor more often EFGR mutations leading to EFGR overexpression [33]. In Madin Darby kidney II cells EGFR activation was followed by increased expression of claudins 1, 3 and 4 [34]. Even though such experiments have not yet been performed with claudins 10 and 18, EGFR activation could be one possible factor behind the association of claudins 10 and 18 to non-smokers’ status and histologic type.
In conclusion, our results show that expression of claudins 18 and 10 associate with each other and are both expressed mainly in lung adenocarcinoma. Their expression is also characteristic of lung tumors of non-smokers suggesting that they may somehow be involved in lung carcinogenesis not associated with smoking. Claudin 18 appeared to have an independent prognostic value in adenocarcinoma making it a putative marker of a better prognosis in these tumors.
Acknowledgments
The study was supported by the Finnish Anti-Tuberculosis Association.
References
- 1.Soini Y. Expression of claudins 1, 2, 3, 4, 5 and 7 in various types of tumours. Histopathology. 2005;46:551–60. doi: 10.1111/j.1365-2559.2005.02127.x. [DOI] [PubMed] [Google Scholar]
- 2.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Med Electron Microsc. 2003;36:147–56. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- 4.Soini Y. Claudins in lung diseases. Respiratory Res. 2011;12:70. doi: 10.1186/1465-9921-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, Tsukita S. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–12. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;7 doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–33. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 8.Kim CJ, Lee JW, Choi JJ, Choi HY, Park YA, Jeon HK, Sung CO, Song SY, Lee YY, Choi CH, Kim TJ, Lee JH, Kim BG, Bae DS. High claudin-7 expression is associated with a poor response to platinum-based chemotherapy in epithelial ovariancarcinoma. Eur J Cancer. 2011;47:918–25. doi: 10.1016/j.ejca.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Dos Reis PP, Bharadwaj RR, Machado J, Macmillan C, Pintilie M, Sukhai MA, Perez-Ordonez B, Gullane P, Irish J, Kamel-Reid S. Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer. 2008;113:3169–80. doi: 10.1002/cncr.23934. [DOI] [PubMed] [Google Scholar]
- 10.Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, Tsai MS, Chang GC, Wu CH, Wu YY, Lee YC, Hong TM, Yang PC. Claudin-1 is a metastasis suppressor and correlateswith clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 2009;179:123–33. doi: 10.1164/rccm.200803-456OC. [DOI] [PubMed] [Google Scholar]
- 11.Soini Y, Kinnula V, Kahlos K, Pääkkö P. Claudins in differential diagnosis between mesothelioma and metastatic adenocarcinoma of the pleura. J Clin Pathol. 2006;59:250–4. doi: 10.1136/jcp.2005.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinberg L, Holth A, Fridman E, Schwartz I, Shih IeM, Davidson B. The diagnostic role of claudins in serous effusions. Am J Clin Pathol. 2007;127:928–37. doi: 10.1309/V025QRN3R9CJGNPX. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z, Xu X, McClane B, Zeng Q, Litkouhi B, Welch WR, Berkowitz RS, Mok SC, Garner EI. C -Terminus of Clostridium perfringens Enterotoxin Downregulates CLDN4 and Sensitizes Ovarian Cancer Cells to Taxol and Carboplatin. Clin Cancer Res. 2011;17:1065–1074. doi: 10.1158/1078-0432.CCR-10-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano K, Imaeda T, Niimi T. Transcriptional activation of the human claudin 18 gene promoter through two AP-1 motifs in PMA-stimulated MKN 45 gastric cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G336–G34. doi: 10.1152/ajpgi.00328.2007. [DOI] [PubMed] [Google Scholar]
- 15.Türeci O, Koslowski M, Helftenbein G, Castle J, Rohde C, Dhaene K, Seitz G, Sahin U. Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene. 2011;481:83–92. doi: 10.1016/j.gene.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda Y, Semba S, Ueda J, Fuku T, Hasuo T, Chiba H, Sawada N, Kuroda Y, Yokozaki H. Gastric and intestinal claudin expression at the invasive front of gastric carcinoma. Cancer Sci. 2007;98:1014–9. doi: 10.1111/j.1349-7006.2007.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda M, Sentani K, Noguchi T, Hinoi T, Okajima M, Matsusaki K, Sakamoto N, Anami K, Naito Y, Oue N, Yasui W. Immunohistochemical analysis of colorectalcancer with gastric phenotype: claudin-18 is associated with poor prognosis. Pathol Int. 2010;60:673–80. doi: 10.1111/j.1440-1827.2010.02587.x. [DOI] [PubMed] [Google Scholar]
- 18.Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624–34. doi: 10.1158/1078-0432.CCR-08-1547. [DOI] [PubMed] [Google Scholar]
- 19.Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, Schulick R, Winter J, Sharma R, Maitra A, Goggins M, Hruban RH. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. 2008;32:188–96. doi: 10.1097/PAS.0b013e31815701f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, Orlando RC. Claudin-18: a dominant tight junction protein in Barrett's esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1106–13. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 21.Zwiers A, Fuss IJ, Leijen S, Mulder CJ, Kraal G, Bouma G. Increased expression of the tight junction molecule claudin-18 A1 in both experimental colitis and ulcerative colitis. Inflamm Bowel Dis. 2008;14:1652–9. doi: 10.1002/ibd.20695. [DOI] [PubMed] [Google Scholar]
- 22.Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Müller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci. 2009;122:1507–17. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- 23.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splicevariants of claudin-10 in the kidney create paracellular pores with different ionselectivities. Am J Physiol Renal Physiol. 2006;291:F1288–99. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 24.Németh Z, Szász AM, Tátrai P, Németh J, Gyorffy H, Somorácz A, Szíjártó A, Kupcsulik P, Kiss A, Schaff Z. Claudin-1, -2, -3, -4, -7, -8, and -10 protein expression in biliary tract cancers. J Histochem Cytochem. 2009;57:113–21. doi: 10.1369/jhc.2008.952291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, Stripp BR. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. Am J Respir Cell Mol Biol. 2009;40:340–8. doi: 10.1165/rcmb.2007-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ip YC, Cheung ST, Lee YT, Ho JC, Fan ST. Inhibition of hepatocellular carcinoma invasion by suppression of claudin-10 in HLE cells. Mol Cancer Ther. 2007;6:2858–67. doi: 10.1158/1535-7163.MCT-07-0453. [DOI] [PubMed] [Google Scholar]
- 27.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology & Genetics. Lyon: WHO Classification of tumours. IARC Press; 2004. Tumours of the Lung, Pleura, Thymus and heart. [Google Scholar]
- 28.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistubal, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International association for the study of lung cancer/american thoracicsociety/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito T, Kojima T, Yamaguchi H, Kyuno D, Kimura Y, Imamura M, Takasawa A, Murata M, Tanaka S, Hirata K, Sawada N. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA methylation in human pancreatic cancer cells. J Cell Biochem. 2011;112:1761–72. doi: 10.1002/jcb.23095. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi J, Kinoshita I, Shimizu Y, Oizumi S, Nishimura M, Birrer MJ, Dosaka-Akita H. Simultaneous blockade of AP-1 and phosphati-dylinositol 3-kinase pathway in non-small cell lung cancer cells. Br J Cancer. 2008;99:2013–9. doi: 10.1038/sj.bjc.6604782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu Y, Kinoshita I, Kikuchi J, Yamazaki K, Nishimura M, Birrer MJ, Dosaka-Akita H. Growth inhibition of non-small cell lung cancer cells by AP-1 blockade using a cJun dominant-negative mutant. Br J Cancer. 2008;98:915–22. doi: 10.1038/sj.bjc.6604267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YT, He B, Wang YZ. Exposure to cigarette smoke upregulates AP-1 activity and induces TNF-alpha overexpression in mouse lungs. Inhal Toxicol. 2009;21:641–7. doi: 10.1080/08958370802322596. [DOI] [PubMed] [Google Scholar]
- 33.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 34.Singh AB, Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem. 2004;279:3543–52. doi: 10.1074/jbc.M308682200. [DOI] [PubMed] [Google Scholar]