Abstract
The metalloproteinases (MMP) 11 and 12 have been shown to be expressed in cervical cancer (CC). In order to extend our previous results, these MMPs were evaluated in cervical precursor lesions. One hundred seventeen cervical scrapes: thirty-six normal, thirty-six Low grade squamous lesions (LSIL), thirty-six High grade (HSIL), nine CC; and, also ninety-nine paraffin-embedded cervical lesions: fifteen normal cervices, thirty eight LSIL, sixteen HSIL, and five CC were collected. The samples were analyzed for relative expression by real time RT-PCR or immunohistochemistry assay. We were able to identify a relative increased expression of MMP11 in 75% and 78% from LSIL and HSIL samples, respectively. While MMP12 expression was 64% and 75% in LSIL and HSIL, respectively. Positive samples for MMP11 expression were also positive for MMP12 expression and also increased according to illness progression. In the tissues, MMP11 or MMP12 expression was observed in the cytoplasm of the neoplastic cells, while in the normal epithelium was absent. The reaction was always stronger for MMP12 than MMP11. MMP11 expression was present in 77% and 66% of LSIL and HSIL, while MMP12 expression was 73% and 68%. There was a relationship between MMP11 or MMP12 expression and HPV infection. Our data are showing a relationship between diagnostic of precursor lesions and the MMP11 and 12 expressions, suggesting that their expression could be an early event in the neoplastic lesions of the cervix and could have clinical significance.
Keywords: MMP11, MMP12, cervical lesions, RT-PCR, immunohistochemistry, HPV
Introduction
Cervical cancer (CC) is the second most frequent neoplasm around the world; and the second cause of death by cancer in Mexican women [1]. It is known that cervical precursor lesions called Low grade Squamous Intraepithe-lial Lesions (LSIL) or High grade (HSIL) practically present the same risk factors as for CC, by instance the HPV persistent infection, smoking, oral contraceptives consumption, multiparity, etc [2,3].
One of the most studied steps of carcinogenesis is the invasion and metastasis based on the knowledge that cancer cells have the ability to migrate from origin and metastasize to surrounding or distant organs. In the microenviron-ment of the cancer cells, is needed the degradation of the extracellular matrix (ECM) and endo-thelial cell basement membrane, which the matrix metalloproteinases or MMP's are participating [4]. This process is essential for invasion and metastasis phenomena. It is known, that the MMP2, MMP9, and MMP14 are involved in the cervical carcinogenesis process [5-7] but others members of MMPs such as the MMP11 and MMP12 are less studied in this neoplasm.
The MMPs are a family of approximately 24 metalloendopeptidases that cleave the protein components of the ECM with a central role in the tissue remodeling and degradation but also, they have the regulation role of growth factors and their receptors, cytokines and chemokines, adhesion receptors and cell surface proteoglycans, and a variety of enzymes. This family of proteins has a significant role in the control of cellular interactions as in normal development, uterine and mammary involution, or pathological conditions, such as inflammation and cancer [8].
MMP11 or stromelisin-3 is encoded by mmp11 gene in the chromosome 22 q11.23. This protease was initially identified in the fibroblastic cells surrounding invasive cancer cell of breast carcinoma [9, 10]. For MMP12 or human macrophage metalloelastase is encoded in the chromosome 11q22.3. It has a great variety of substrates as elastin, collagenase type IV, plasminogen, endostatin, etc [10, 11].
Our group previously reported the overexpression of MMP11 and MMP12 in CC suggesting the participation of these proteases in this type of cancer [12]. In order to extend the role of these proteases, the goal in the present study was to search the MMP11 and MMP12 expression in cervical scrapes from cervical precursor lesions.
Materials and methods
Samples collection
Patients with normal and abnormal cervical smears were recruited with informed consents and ethical approval from the local institute (IMSS Mexico). All the patients were attended in the colposcopy clinics from Hospital Ginecologia and Obstetricia No. 3 or No. 4, IMSS at Mexico City. They were examined colposcopically with biopsies when indicated. According to WHO criteria, they were classified as CC, HSIL, LSIL and normal tissues. In general, three hundred forty cervical scrapes samples were collected. For each patient, the first smear was made for routine cytological examination. The remaining material from the first spatula (cervix brush) and an additional scrape were collected in Trizol reagent (Invitrogen, CA, USA) for MMPs detection. The RNAs were purified and then quantized in a NanoDrop Spectrophotometer ND-1000, and resolved in agarose gel ethidium bromide stained. The cases analyzed were not previously treated and corresponded to thirty-six normal, thirty-six LSIL and thirty-six HSIL and 9 invasive carcinomas scrapes. Furthermore, ninety-nine paraffin-embedded tissues were also collected including: fifteen normal samples, twenty-five “normal” adjacent to a lesion as special group, thirty-eight LSIL, sixteen HSIL and 5 CC. “Normal” adjacent tissue was considered as the epithelium ≥1 cm far away from the lesion. Hematoxylin-eosin stained sections were evaluated for a pathologist to select areas of neoplasm to construct the tissue microarray.
Semiquantitative real time RT-PCR
To detect the expression of MMP11 and MMP12 in the cervical specimens, the samples were analyzed by using real time RT-PCR assay. For relative quantitation, the reactions were performed using Taqman One-Step RT-PCR Master Mix Reagents Kit 4309169 (Applied Biosystems Co., CA, USA) in the 7500 Fast Real-Time PCR System (Applied Biosystem Co.). The RT-PCR was performed with 200 ng of total RNA with a first step for cDNA synthesis for 30 min at 48°C followed up by enzyme inactivation step of 10 min at 95°C, after, were carried out 40 cycles of 15 sec at 95°C and an extension was made at 60°C by 1 min. To detect the fluorescent signal we used the predeveloped Taqman Gene Expression Assays Hs00968291_m 1 for MMP11, Hs00899669_m1 for MMP12, and 4333764 for GAPDH as internal control (the sequence primers belongs to Applied Biosystems Co.). The relative change in expression was calculated using the comparative CT method [13].
Immunohistochemistry assay
The tissue microarray (TMA) was constructed as described [14] including ninety-nine cases. Core samples were taken using 0.6 mm2 blunt-tip needles and placed on the recipient microarray block using a Tissue Microarrayer (Chemicon Co., MA, USA). Tumors were represented with 2 spots of redundancy, which has been shown to provide a sufficiently representative sample. Sections of 4 um were deparaffinized and rehydrated in graded ethanol series until water. After that, the antigen retrieval was done using Trilogy buffer (Cell Marque CA, USA) 1X for 15 min in a pressure cooker. Endogenous peroxidase inactivation was carried out with H2O2 10% in methanol solution for 30 min at room temperature and the slides were washed in phosphate buffer solution, non specific reactions were blocked with bovine serum at 5% on the slides for 30 min. Excess medium were decanted and tissues were incubated with the primary antibody (anti-MMP11 Biomeda Corp., CA, USA, cat. V10221, and anti-MMP12 Santa Cruz Biotechnology Inc., CA, USA, cat. sc-12361 dilutions 1:100 and anti-p16 Cell Marque Corp. cat. CMA801 ready to use) at 4°C overnight. For the development of the reaction the Mouse/ Rabbit Immunodetector HRP/DAB (Bio SB Inc. CA, USA) on the slides was used. Finally, slides were washes and hematoxylin counterstained. The reaction was evaluated under a light microscope as a brown precipitated, results were evaluated as positive or negative by visual assessment.
HPV sequences
Human papillomavirus detection was carried out by polymerase chain reaction (PCR) using two sets of primers. First, to identify HPV16 DNA sequences the E6 primers of HPV16 were used [15]. After 5 minutes of denaturation at 94°C, 100ng of DNA were subjected to 40 amplification cycles with the following conditions: 94°C for 30 sec, 55°C for 30 sec, 72°C 30 sec and a final extension step of 10 min at 72°C. The negative samples for the first PCR, were then subjected to second PCR with the universal GP5+/GP6+ primers for L1 gene of HPV [16] with an initial denaturation of 94°C for 4min, followed to 40 amplification cycles, 94°C for 1min, 40°C for 2 min, 72°C for 2min and a final extension of 72 °C for 10 min. The amplification products were visualized in an agarose gel ethidium bromide stained.
Statistical analysis
The statistical analysis between the MMPs expression and the clinicopathological features was performed using the statistical software SPSS v15. We used the X2 test to find any correlation between MMPs expression and clinicopathological features.
Results
The study design is summarized in Figure 1. In general, 230 patients were selected in the colposcopy clinic, 110 from out patient clinic. After colposcopic examination and histopathology analysis, the samples were classified as CC, HSIL and LSIL. Unfortunately, 29.1% of the patients diagnosed with any cervical lesion did not accept to participate in the present study, thus those samples were not subjected to this analysis. Then, the samples were selected according to RNA quality. In this case, 21.1% of the samples were eliminated because high RNA degradation or lack of RNA. This percentage of the samples due to RNA quality could indicate that most of the scrape samples were taken with acceptable precision and quality. Finally, after colposcopic examination and histopathology analysis, the samples studied were 37 HSIL and 79 LSIL. Thirty-six scrapes collected from women with normal cytology at out patient clinic served as control. To get our purpose we selected thirty-six samples of each group (normal, LSIL and HSIL) and nine CC.
Figure 1.
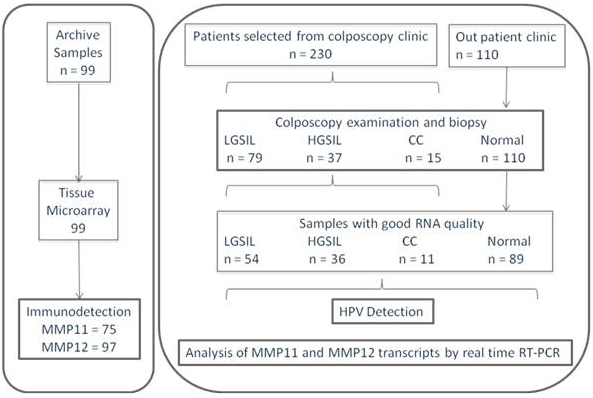
Summary of the study design.
Detection of MMP11 and MMP12 transcripts in cervical scrapes from precursor lesions
In order to detect the presence of MMP11 and 12 transcripts in cervical epithelium scrapes, each RNA sample was subjected to relative real time RT-PCR. The MMP11 expression was present in 75% and 78% of LSIL and HSIL samples, while MMP12 expression was 64% and 75%, respectively. The CCs were positive for both MMPs as expected. The analysis showed relative increments of MMP11 of 1.26, 1.67 and 2.90 in LSIL, HSIL and CC (p=0.05), respectively; while MMP12 were 2.34, 4.58 and 5.49 in LSIL, HSIL and CC (p=0.08), respectively (Figure 2; the increments were respect to MMP11 or MMP12 expression from the normal cervical scrapes). Interestingly, positive samples for MMP11 expression were also positive for MMP12 expression (p=0.000), and also increased according to precursor lesions. Thus, it is clear that MMP12 expression is almost onefold more than MMP11 expression.
Figure 2.
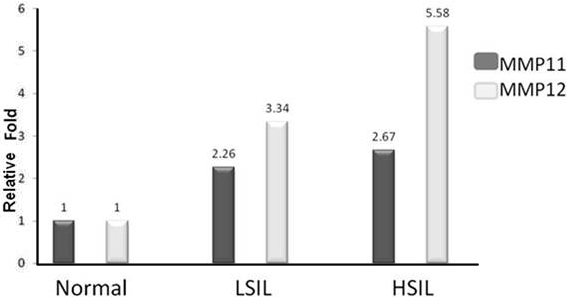
Relative quantitation of MMP11 and MMP12 expression in cervical scrapes by using real icalscrapesbyusingreal time RT-PCR. The MMP11 shows MMP12 a significant increase expression according with the illness progression compared to the normal samples. MMP12 shows an increase expression according with the illness progression compared to the normal samples. The MMP11 and MMP12 expression values in normal cervical smears are represented as 1.
Expression of MMP11 and MMP12 in the cervical precursor lesions
For immunohistochemistry assays the expression of MMP11 and MMP12 was confirmed in the samples. Two groups of samples, were observed: immunopositive and negative staining. Positive immunoreaction was detected in the cytoplasm of the cells with homogeneous staining. The invasive samples were positive as expected.
In this context, MMP11 expression was observed in the cytoplasm of the neoplastic cells, while in the normal epithelium was absent. Similar results were observed for MMP12 detection (Figure 3). In each positive sample, the MMP12 immunoreaction was always stronger than MMP11 immunoreaction. MMP11 expression was present in 77% and 66% of LSIL and HSIL samples, while MMP12 expression was 73% and 68%, respectively. These data suggest that the increased expression of these MMPs could be found either in scrapes or tissue and is quite similar, although the low percentage seen in the HSIL tissues could be influenced by the number of samples. We also included “normal” samples adjacent to an epithelial lesions showing MMP11 immunoreactivity in 27% and 28% for MMP12, suggesting that this epithelium could not be totally “normal”.
Figure 3.
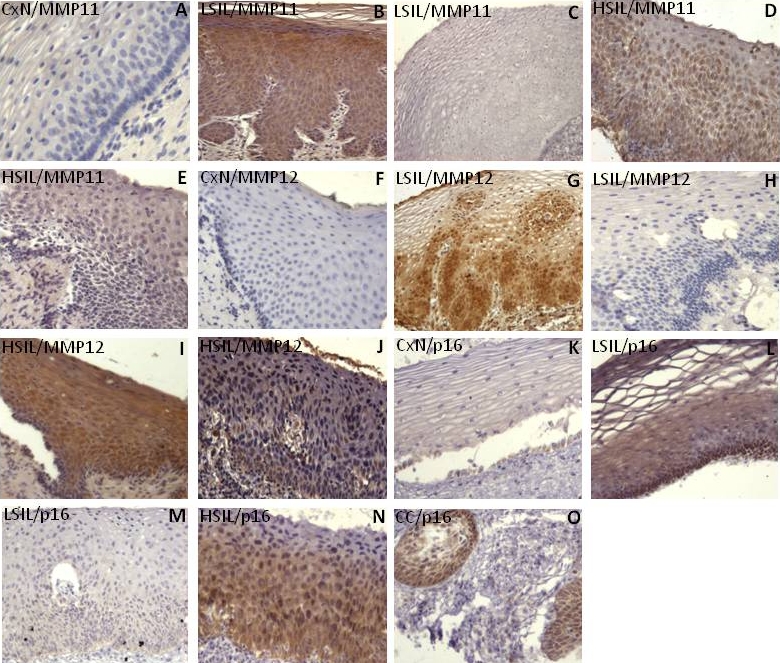
MMP11 and MMP12 immunodetection in cervical precursors lesions. The positive reaction is visualized as a brown color in the neoplastic cells. Immunodetection of MMP11 in normal cervix (A) as negative; a positive LSIL (B); a negative LSIL (C); positive HSIL (D), and negative HSIL (E). Immunodetection of MMP12 in normal cervix (F) negative; positive LSIL (G); negative LSIL (H); and positive HSIL (I, J). We also detected the p16 protein in normal cervix (K) as negative; positive LSIL (L); negative LSIL (M); positive HSIL (N), and a positive CC (O). Micrographs A-M and O at 20X original magnification; D and N at 40X original magnification.
HPV infection in the cervical smears
The HPV DNA sequences were found in the 30.5% of normal samples, 38.8% in LSIL samples, 71.4% in HSIL, and in 88.8% of carcinoma samples. In order of frequency, the HPV types were found 16, 33, 51, 70, 53, 67, 39, and 45 and some of them not identified. For the HPV sequences not identified, the electropherogram showed several curves suggesting multiple HPVs types in the samples (16.6%).
Analysis between MMP11 and MMP12 expression and clinicopathologic variables
To determine any correlation between MMPs and some clinicopathologic variables, the data were subjected to statistical analysis (see Table 1). It was found that the relative increased of both MMP11 and MMP12 expression is associated with the diagnostic (p=0.049; p=0.008, respectively) or HPV (p=0.000 for both). The correlation between the diagnosis and the clinicopathologic variables is shown in Table 2. As expected, it was also observed a correlation between diagnosis and HPV (p=0.001), and diagnosis with smoking (p=0.054). These data could suggest a close relationship between cervical lesion (HPV positive) and MMPs expression.
Table 1.
Association between the MMPs expression and the clinicopathological variable
| Clinicopathologic variables | n | MMP11 expression | P value | n | MMP12 expression | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| negative | positive | negative | positive | ||||||
| Pregnancies | <3 | 90 | 42 | 22 | 0.318 | 93 | 37 | 29 | 0.572 |
| >3 | 15 | 11 | 15 | 12 | |||||
| births | <3 | 66 | 34 | 22 | 0.383 | 67 | 32 | 25 | 0.490 |
| >3 | 5 | 5 | 5 | 5 | |||||
| HPV | Positive | 97 | 24 | 22 | 0.049* | 100 | 20 | 29 | 0.008* |
| Negative | 36 | 15 | 34 | 17 | |||||
| Diagnosis | Normal | 100 | 36 | 0 | 0.000* | 101 | 36 | 0 | 0.000* |
| LSIL | 18 | 14 | 12 | 22 | |||||
| HSIL | 8 | 24 | 12 | 22 | |||||
| Menarche | <10 | 90 | 4 | 1 | 0.435 | 93 | 4 | 2 | 0.603 |
| 11-14 years | 46 | 30 | 6 | 3 | |||||
| > 15 | 7 | 2 | 6 | 3 | |||||
| Onset of | <18 years | 90 | 19 | 10 | 0.478 | 93 | 18 | 13 | 0.472 |
| sexual activity | >18 years | 38 | 23 | 34 | 28 | ||||
| Sexual partner | <3 | 89 | 38 | 20 | 0.432 | 92 | 35 | 27 | 0.476 |
| number | >3 | 19 | 12 | 16 | 14 | ||||
| Oral contra- | <1year | 20 | 2 | 0 | 0.632 | 20 | 2 | 0 | 0.347 |
| ceptive use | > 1year | 14 | 4 | 10 | 8 | ||||
| Smoking | Yes | 87 | 9 | 8 | 0.175 | 89 | 7 | 10 | 0.133 |
| No | 48 | 22 | 6 | 43 | 29 | ||||
| Alcoholism | Yes | 84 | 2 | 1 | 0.727 | 86 | 1 | 2 | 0.412 |
| No | 53 | 28 | 47 | 36 | |||||
| History of can- | Yes | 82 | 18 | 8 | 0.429 | 83 | 15 | 10 | 0.436 |
| cer | no | 36 | 20 | 32 | 26 | ||||
Expression changes adjusted to the normal samples. HPV: Human Papillomavirus; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; smoking: at least seven cigarettes/week.
Statistical significance
Table 2.
Association between the Diagnosis and the clinicopathological variables
| Variables | n | Diagnostic | ||||
|---|---|---|---|---|---|---|
| Normal | LSIL | HSIL | ||||
| HPV | Positive | 105 | 11 | 14 | 26 | 0.001* |
| Negative | 23 | 22 | 9 | |||
| Scholar | Illiterate | 94 | 0 | 1 | 2 | 0.269 |
| Elementary | 2 | 9 | 8 | |||
| High school | 13 | 9 | 6 | |||
| Bachelor | 8 | 11 | 10 | |||
| College | 7 | 3 | 5 | |||
| Pregnancies | ≤3 | 98 | 27 | 22 | 21 | 0.242 |
| 〉3 | 6 | 10 | 12 | |||
| Births | ≤3 | 71 | 21 | 20 | 19 | 0.232 |
| 〉3 | 1 | 5 | 5 | |||
| Menarche | ≤ 10 years | 98 | 3 | 0 | 3 | 0.102 |
| 11-14 years | 27 | 31 | 24 | |||
| ≥15 years | 3 | 1 | 6 | |||
| Onset of sexual | 〈 18 years | 98 | 12 | 11 | 11 | 0.996 |
| activity | ≥ 18 years | 22 | 21 | 21 | ||
| Sexual partner | 〈3 | 97 | 19 | 23 | 21 | 0.372 |
| number | ≥3 | 14 | 8 | 12 | ||
| Oral contraceptive | 〈1year | 21 | 1 | 1 | 0 | 0.738 |
| use | ≥1 year | 9 | 6 | 4 | ||
| Smoking | Yes | 93 | 5 | 4 | 10 | 0.054 |
| No | 28 | 28 | 18 | |||
| Alcoholism | Yes | 90 | 0 | 1 | 2 | 0.307 |
| No | 32 | 29 | 26 | |||
| History of cancer | Yes | 88 | 10 | 5 | 12 | 0.127 |
| No | 22 | 23 | 16 | |||
Statistical significance
Discussion
There is sufficient evidence about the participation of matrix metalloproteinases in the carcinogenesis process in a great variety of tumors as in breast, colon, bladder, brain, and ovary (for review see 17).
The stepwise inter-relationship between precancerous lesions and CC samples have been postulated by several groups [18-20]. Theoretically, early detection of precancerous lesions by massive cancer screening programs based on the use of the cervical Pap smears could result in a total prevention of invasive malignant disease. Despite the wide acceptance of screening program of CC, in Mexico, more than 12,000 woman are diagnosed with CC, from them 50% will die per year. Thus, there are clearly deficiencies in the current system of screening. Due to high number of false negatives or positives there is a need of supplementary cervical screening techniques.
Specifically, there are few reports of MMP2, MMP9 and MT1-MMP in cervical precursor lesions [6, 18] showing that MMP9 and MT1-MMP increase their expression according to the illness progression, while the MMP2 its not associated to this event; however, there is lack information of a relationship between MMPs expression and the diagnostic. Previously we have reported the presence of increased expression of MMP11 and MMP12 in CC, but, it is unknown if their expression is present in cervical precursor lesions. In this scenario, in the present work we were able to identify a significantly increased the MMP11 and MMP12 expression in cervical scrapes from cervical precursor lesions. Interestingly, the MMP11 expression levels were lower than MMP12 levels, suggesting potentially a strong molecular activity of mmp12 gene than mmp11, or maybe more stable transcript or protein half time.
MMP11 expression showed a significative increased relative fold change which is correlated with the type of lesion suggesting that this MMP11 protein could participate in the neo-plastic epithelial process. A MMP11 increased expression has been reported in several human invasive tumors as renal, gastric, breast [21-23], as well as in some precursors lesions from breast and oral cancer [23, 24]. Thus, our results are supported by these previous studies, suggesting that MMP11 expression could be associated for cervical cancer progression.
In the case of the MMP12, there are few reports about its participation in cancer. An increased expression of MMP12 has been observed in some tumors as tongue, larynx, gastric, vulva, liver and also for CC [25-29]. In the present work, we are showing that MMP12 expression could also be associated for CC progression.
Our present data are showing that MMP11 and 12 co-expression is correlated with cervical precursor lesions probably regulated by different molecular mechanism.
Interestingly, MMP11 and MMP12 proteins expression were also correlated with HPV infection. For instance, it has been reported that MMP9 expression is induced by the E2 protein of HPV16 via MEK1-ERK1/2-AP [30]. It is known that HPV status in precursor lesions is in episomal form, where the E2 gene is intact. According to an analysis of the sequence promoter of the MMP11 and MMP12, there is an absence of motif response for viral proteins (data not shown), but an AP-1 motif is found, thus a probable activation by this motif could be possible. However, our results could suggest that some viral proteins as E2, E6 and E7 could trans-activate indirectly to MMP11 and MMP12.
In the early steps of carcinogenesis are present E6 and E7 viral expression in low levels, and these proteins levels are increased in CC with a lack of E2 gene. In this case, our results could support the idea that E6 and E7 proteins could activate indirectly the MMP11 and MMP12 expression. Very recently, we have demonstrated that E2/HPV16 can activate indirectly to MMP13 [31]. These results could involve an indirectly activation of some MMPs via HPV infection.
Other important data in the present work was the frequency of HPV types in the samples. The HPV16 was the most frequent type followed by the types 33, 51, 70, these viral types not previously reported in America. These results are very important in terms of epidemiological studies because the emerging of infrequent HPV types and probably could have an impact to respect vaccination programs. Of course, this number of samples is too small to draw any conclusion.
Some implications about MMP11 and 12 expressions in the cervical precursor lesions could be important in several aspects. At present, it is clear that HPV-DNA testing is the approved screening tool for HPV infection as main etiological factor for CC, while, Pap smear is related for cytology looking for cellular changes. In this case, both tools have their own fortitude. The present data could support another molecular tool for cervical neoplasia diagnosis. Taking all together and in order to discard any suspicious colposcopic image (clinically) or ASCUS lesion (cytology) or any epithelial change related to an immortalization or transformation process, MMP11 and MMP12 detection could help to have a better clinical significance and robust diagnosis. In retrospective analysis (from cytological diagnosis), the detection of MMP11 or MMP12 could provide interesting data.
At present, it is unknown if these MMPs expression are related to clinical features of tumor as invasiveness, or survival time in CC and its precursor lesions. In order to address the suggestions, it is necessary to extend the monitoring of the patients.
Finally, we postulated that epithelial cells from cervical scrapes would reflect the MMP11 or MMP12 inmunoreactivities seen in the tissue sections. MMP11 and MMP12 expression in the cervical scrapes cells would indicate the presence of dysplatic changes in the cervix.
Acknowledgments
This work was partially supported by 69719 and 87244 Fondos Sectoriales en SALUD grants from Mexican Council of Science and Technology (CONACyT). AV and RP were recipient of scholarship from the CONACyT and IMSS.
References
- 1.Wentzensen N, Klug SJ. Early Detection of Cervical Carcinomas: finding an overall approach. Dtsch Arztebl Int. 2008;105:627–622. doi: 10.3238/arztebl.2008.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins D. Histopathology and cytopathology of cervical cancer. Disease Markers. 2007;23:199–212. doi: 10.1155/2007/874795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arends MJ, Buckley CH, Wells M. Aetiology, pathogenesis, and pathology of cervical neoplasia. J Clin Pathol. 1998;51:96–103. doi: 10.1136/jcp.51.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libra M, Scalisi A, Vella N, Clementi S, Sorio R, Stivala F, Spandidos DA, Mazzaino C. Uterine cervical carcinoma: Role of matrix metalloproteinases (Review) Int J Oncol. 2009;34:897–903. doi: 10.3892/ijo_00000215. [DOI] [PubMed] [Google Scholar]
- 5.Sheu BC, Lien HC, Ho HN, Lin HH, Chow SN, Huang SC, Hsu SM. Increased Expression and Activation of Gelatinolytic Matrix Metalloproteinases is Associated with the Progression and Recurrence of Human Cervical Cancer. Cancer Res. 2003;63:6537–6542. [PubMed] [Google Scholar]
- 6.Zhai Y, Hotary KB, Nan B, Bosch FX, Muñoz N, Weiss SJ, Cho KR. Expression of Membrane Type 1 Matrix Metalloproteinase is Associated with Cervical Carcinoma Progression and Invasion. Cancer Res. 2005;65:6543–6550. doi: 10.1158/0008-5472.CAN-05-0231. [DOI] [PubMed] [Google Scholar]
- 7.Wang PH, Ko JL, Tsai HT, Yang SF, Han CP, Lin LY, Chen GD. Clinical significance of matrix metalloproteinase-2 in cancer of uterine cervix: A semiquantitative study of immunoreactivities using tissue array. Gynecol Oncol. 2008;108:533–542. doi: 10.1016/j.ygyno.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix Metalloproteinases: Biologic Activity and Clinical Implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 9.Anglard P, Melot T, Guérin E, Thomas G, Basset P. Structure and Promoter Characterization of the Human Stromelysin-3 Gene. J Biol Chem. 1995;270:20337–20344. doi: 10.1074/jbc.270.35.20337. [DOI] [PubMed] [Google Scholar]
- 10.Folgueras AR, Pendás AM, Sánchez LM, López-Otín C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 11.Belaaouaj A, Shipley JM, Kobayashi DK, Zimonjic DB, Popescu N, Silverman GA, Shapiro SD. Human Macrophage Metalloelastase Genomic organization, chromosomal localization, gene linkage, and tissue-specific expression. J Biol Chem. 1995;270:14568–14575. doi: 10.1074/jbc.270.24.14568. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez-Ortiz G, Pina-Sánchez P, Vazquez K, Duenas A, Taja L, Mendoza P, Garcia JA, Salcedo M. Overexpression of cathepsine f, matrix metalloproteinases 11 and 12 in cervical cancer. BMC Cancer. 2005;5:68–75. doi: 10.1186/1471-2407-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2ΔΔct Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo A, Piña P, Guerrero G, Lazos M, Salcedo A simple method for the construction of small format tissue arrays. J Clin Pathol. 2003;56:144–146. doi: 10.1136/jcp.56.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terris MK, Peehl DM. Human papillomavirus detection by polymerase chain reaction in benign and malignant prostate tissue is dependent on the primer set used. Urol. 1997;50:150–156. doi: 10.1016/S0090-4295(97)00126-X. [DOI] [PubMed] [Google Scholar]
- 16.De Roda AM, Wallboomers JM, Van Den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 17.Roy R, Yang J, Moses MA. Matrix Metalloproteinases as novel Biomarkers and Potential Therapeutic Targets in Human Cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.No JH, Jo H, Kim SH, Park IA, Kang D, Lee CH, Han SS, Kim JW, Park NH, Kang SB, Song YS. Expression of MMP-2, MMP-9, and Urokinase-type Plasminogen Activator in Cervical Intra-epithelial Neoplasia. Ann NY Acad Sci. 2009;1171:100–104. doi: 10.1111/j.1749-6632.2009.04898.x. [DOI] [PubMed] [Google Scholar]
- 19.Schröpfer A, Kammerer U, Kapp M, Dietl J, Feix S, Anacker J. Expression pattern of matrix metalloproteinases in human gynecological cancer cell lines. BMC Cancer. 2010;10:553–564. doi: 10.1186/1471-2407-10-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Miller C, Mosher R, Deeds J, Morrissey M, Bryant B, Yang D, Meyer R, Cronin F, Gostout BS, Smith-McCune K, Schlegel R. Identification of Cervical Cancer Markers by cDNA and Tissue Microarrays. Cancer Res. 2003;63:1927–1935. [PubMed] [Google Scholar]
- 21.Perret AG, Clemencon A, Li G, Tostain J, Peoc'h M. Differential expression of prognostic markers in histological subtypes of papillary renal cell carcinoma. BJU Int. 2008;102:183–187. doi: 10.1111/j.1464-410X.2008.07605.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang YH, Deng H, Li WM, Zhang QY, Hu XT, Xiao B, Zhu HH, Geng PL, Lu YY. Identification of Matrix Metalloproteinase 11 as a Predictive Tumor Marker in Serum Based on Gene Expression Profiling. Clin Cancer Res. 2008;14:74–81. doi: 10.1158/1078-0432.CCR-07-1179. [DOI] [PubMed] [Google Scholar]
- 23.Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora S, Kaur J, Sharma C, Mathur M, Bahadur S, Shukla NK, Deo SV, Ralhan R. Stromelysin 3, Ets-1, and Vascular Endothelial Growth Factor Expression in Oral Precancerous and Cancerous Lesions: Correlation with Microvessel Density, Progression, and Prognosis. Clin Cancer Res. 2005;11:2272–2284. doi: 10.1158/1078-0432.CCR-04-0572. [DOI] [PubMed] [Google Scholar]
- 25.Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, Mao L, Wong DT, Zhou X. Transcriptomic dissention of tongue squamous cell carcinoma. BMC Genomics. 2008;9:69–80. doi: 10.1186/1471-2164-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma LJ, Li W, Zhang X, Huang DH, Zhang H, Xiao JY, Tian YQ. Differential gene expression profiling of laryngeal squamous cell carcinoma by laser capture microdissection and complementary DNA microarray. Arch Med Res. 2009;40:114–123. doi: 10.1016/j.arcmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Li YM, Xu GQ. The expression of human macrophage metalloelastase mRNA in gastric cancer cell lines and tissues and its clinical significance. Zhonghua Zhong Liu Za Zhi. 2007;29:830–832. [PubMed] [Google Scholar]
- 28.Kerkelä E, Ala-aho R, Klemi P, Grénman S, Shapiro SD, Kähäri VM, Saarialho-Kere U. Metalloelastase (MMP-12) expression by tumor cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J Pathol. 2002;198:258–269. doi: 10.1002/path.1198. [DOI] [PubMed] [Google Scholar]
- 29.Gorrin Rivas MJ, Arij S, Furutani M, Harada T, Mizumoto M, Nishiyama H, Fujita J, Imamura M. Expression of human macrophage metalloelastase gene in hepatocellular carcinoma: correlation with angiostatin generation and its clinical significance. Hepatol. 1998;28:986–993. doi: 10.1002/hep.510280413. [DOI] [PubMed] [Google Scholar]
- 30.Mühlen S, Behren A, Iftner T, Simon C. Influence of HPV16 E2 and its localization on the expression of matrix metalloproteinase-9. Int J Oncol. 2010;37:337–345. doi: 10.3892/ijo_00000682. [DOI] [PubMed] [Google Scholar]
- 31.Ramírez-Salazar E, Centeno F, Nieto K, Valencia-Hernández A, Salcedo M, Garrido E. HPV16 E2 could act as down-regulator in cellular genes implicated in apoptosis, proliferation and cell differentiation. Virol J. 2011;8:247–257. doi: 10.1186/1743-422X-8-247. [DOI] [PMC free article] [PubMed] [Google Scholar]


