Abstract
This study addresses the morphopathogenesis of Achilles tendinosis, using a rat model and presenting quantitative analysis of time-dependent histological changes. Thirty Wistar rats were used, randomly split in experimental and control groups. Animals of the experimental group were submitted to a treadmill running scheme. Five animals of each group were euthanized at four, eight and sixteen weeks. Achilles tendons were collected and processed routinely for histopath sections. Slides were stained by Hematoxylin-Eosin, Picrosirius Red, Alcian Blue, AgNOR, TUNEL and evaluated morphometrically. Cellular density decreased slightly along the time and was higher in the experimental group than in controls at fourth, eighth and sixteenth weeks. Fiber microtearing, percentual of reticular fibers and glycosaminoglycans content increased along the time and were higher in experimental group than in controls at all-time intervals. AgNOR labeling here interpreted as a marker of transcription activity was higher in the experimental groups than in controls at all-time intervals. Apoptotic cells were more frequent and diffusely distributed in tendinosis samples than in control groups. These results suggest that as mechanical overload is becoming chronic, cellular turnover and matrix deposition increases leading to tendinosis. The combination of staining techniques and morphometry used here to describe the evolution of lesions occurring in a rat model system has proved to be suited for the study of induced Achilles tendinosis.
Keywords: Tendinosis, Achilles tendon, animal model, overuse, morphometry
Introduction
Tendinopathy is the clinical term frequently used to designate all tendons injuries, leaving the terms tendinitis, tendinosis and/or paratendinitis more for histological diagnosis [1]. This divergence reinforces that more studies are required to clarify, elucidate and unify nomenclature of tendon lesions. Nevertheless, it is well known that acute lesions with reactive inflammatory infiltrate behave differently than chronic degenerative lesions in terms of disease evolution and pathology.
Tendinosis afflicts almost 14% of the elite athletes [2]. Considering the high incidence and the fact that non-surgical treatment is usually long [3], a more precise description of the tendi-nosis∼ pathogenesis is necessary [4, 5]. To better accomplish that, animal inducting models should be systemized.
Repetitive microtraumas due to overuse and mechanical overload have been proposed as etiologic factors in tendinosis. Clinically, there is tenderness to palpation, pain on resisted movements and decreased activity [6]. Diagnostic imaging shows alterations in Ultra-Sound and Magnetic Nuclear Resonance imaging. Macro-scopically tendinosis usually presents mucoid degeneration, with a friable and non-structured pattern with a light brown color. Microscopically collagen fibres are disorganized and show microtearing [6-10]. Although many studies have focused on tendinosis, no one has attempted to evaluate morphometrically the events in pathogenesis.
Experimental models are frequently used and have great value in the scientific scenario, because they allow biochemical, histological, cellular and temporal analyses [11]. Further, the success of the physical and/or pharmacological treatments may be assessed directly and indirectly using these animal models. An ideal animal model should include as criteria: (1) appropriate tissue type modelled, (2) accurate simulation of the injury conditions, (3) reproduction in animal tissues of lesions similar to human diseased tissues and finally (4) ease of application [6, 12].
This study's goal was to evaluate Achilles tendinosis pathogenesis, using a rat overuse induction model with active muscle contraction through repetitive running. This is the first attempt to evaluate morphometrically the progression of cellularity, collagen, glycosaminoglycans and microtearing in tendinosis.
Materials and methods
Thirty male Wistar rats, 11-12 weeks of age (adults), weighting 220-250 grams, were randomly split in an experimental (n=15) and a control groups (n=15). Five animals from each group were euthanized by inhaling CO2 after four, eight and sixteen weeks (approved by The Experimental Animal Ethic Committee of Universidade Federal de Minas Gerais – Certificate 189/06).
The experimental group was subjected to a running scheme [7] which included 80 minutes daily, at 26.8 m/min (10° incline), with the first 10 minutes in a gradually increasing speed (warm-up) and last 10 minutes in a decreasing speed (to allow basal metabolism gradual return). The rats ran on a specially adapted treadmill (Weslo, Cadence 840), with capacity to eight animals. They ran inside a glass box within individual compartments, with a metallic material in the posterior part. An electrical-stimulation (IBRAMED, Neurodyn) was connected to this metallic part, using a low intensity charge, to encourage the rats to keep on running. Before the beginning of the experiment, a two-weeks training period was used with the same protocol, except that the velocity was 13.4 m/min, to allowed animals to be familiar with the equipment.
The control group did normal cage activities, such as walking, feeding and sleeping. Cages had no gadgets to allow animal exercise. Both groups had free access to water and commercial food (Nuvilab, Nuvital®).
After the euthanasia, the gastrocnemius and soleus muscles were dissected and both right and left Achilles tendons were isolated proximally at the muscle tendon junction; and distally at the calcaneus insertion. The tendons samples were then fixed in 10% neutral buffered formalin for 48-72 hours and manually processed. Tendons were embedded longitudinally in paraffin and several 5μm sections were obtained from each tendon after trimmed. Hematoxilyn-eosin (HE), Picrosirius Red, Alcian Blue, AgNOR and TUNEL staining were used on sections from each tendon.
Parameters and morphometric strategy
Microscopic images were obtained with a digital camera (Sony DSC-W7 / 7.2 Mega Pixels) connected to a light microscope (Olympus BX41) using 10x and 40x plan achromatic objectives. Images were transferred to a computer for morphometry. Densitometric quantification of the microtearing, percentual of reticular fibers and glycosaminoglycans content was accomplished with an image analyzer (Kontron KS300 version 2.0, Karl Zeiss). Cellularity was obtained by software Media Cybernetics Image Pro-Plus (Version 4.5029). All densitometric analyses were performed by the same examiner, initially, in a blind assay; however, during the evaluations the injury presence itself accused the experimental group.
Digitalized 10x images of HE stained longitudinal axis of the central region were analysed to cellularity and microtearing through densitometric quantification. The same criteria were used on both control and experimental specimens, excluding enthuses and muscle tendon junctions. Cellularity was quantified in each microscopical field, considering the total number of cells within the area occupied by the tendon, excluded the microtearing areas, according to the following formula:
The percentual of microtearing was obtained by the sum of the areas of interstitial gaps between collagen fibers divided by the area occupied by the tendon, according to the formula:
Picrosirius Red, an anionic composite that distinguishes the thickness and density of collagen fibers through coloration emitted under polarized light, was used to estimate the percentual of reticular fibers within the extra cellular matrix. While the thin dissociated fibers typical of type III collagen are greenish, the thickest and strong associated fibers of type I collagen emit colors with bigger length wave as red and yellow [13, 14]. Digitalized 10x images of Picrosirius Red stained longitudinal axis of central region were analysed using polarized light filters. Densitometric quantification of reticular fibers (most likely type III collagen) was achieved by image binarization so that areas with the same pixel density could be processed and summed. The following formula was used to quantify the areas:
Alcian Blue staining on pH 2.5 with nuclear red counterstain was used to quantify the glycosaminoglycans content within the extra cellular matrix of the tendon [5]. Digitalized 10x images of Alcian Blue stained longitudinal axis of central region were obtained and morphometry was achieved as described above to reticular fibers. The following formula was used to quantify areas of glycosaminoglycans:
Silver stained Nucleolar Organizing Regions (AgNOR) staining was used to quantify the cellular activation and DNA transcription within the tendon. In this technique, brownish granules within the nuclei indicate the presence of argyrophilic proteins (AgNORs) that can be considered as an active unit of DNA transcription [15]. Quantification of these units was achieved through digitalized images obtained with a 40x plan achromatic objective. The transcription activity was obtained as the arithmetic mean of the AgNORs in 100 selected fibroblasts in microscopical fields of the three considered intervals, in both the experimental and control specimens:
TUNEL reaction was used to detect apoptotic cells. This reaction identifies the in situ genome fragmentation, through the insertion of peroxidase labelled nucleotides by the terminal deoxynucleotidyl transferase. Labelled nucleotides are later identified as brownish clusters after revelation with diaminobenzidine [16]. A commercial kit was used (Cat # QIA33, Oncogene Research Products, Cambridge, MA, USA). Apoptotic cells were counted considering morphological characteristics of apoptosis in HE sections and TUNEL labelling.
Statistical analysis
Results are expressed either as mean ± standard error or median, as they have a normal distribution or not, according to the Kolmogorov-Smirnov test. Results obtained from data with a normal distribution were analysed by a t test of Student to compare two groups of the same time interval and by the analysis of variance (ANOVA) with a Newman Keuls post-test to compare the three intervals in experimental or control groups. Results obtained from data with a non-normal distribution were analysed by a t test non-parametric to compare two groups in the same time interval and by Mann Whitney test with a Multiple Comparison Dunn post-test to compare the three intervals in experimental or control group. Values of P<0.05 were considered significant.
Results
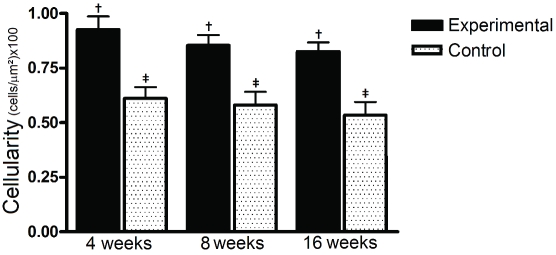
Cellularity decreased slightly along the time but was always higher in the experimental group than in controls after four weeks (4 weeks: 0.92 ± 0.06 vs 0.61 ± 0.05 - P<0.01; 8 weeks: 0.85 ± 0.04 vs 0.58 ± 0.06 - P<0.05; 16 weeks: 0.82 ± 0.04 vs 0.53 ± 0.06 - P<0.01). However, time interval (protocol evolution) did not affect significantly neither experimental nor control groups (Figure 1).
Figure 1.
Cellularity evolution in experimental and control groups, in 3 time intervals. Cellularity was always higher in the experimental group than in controls after four weeks and decreased slightly along the time. The symbols (f, $) represent significant statistical difference.
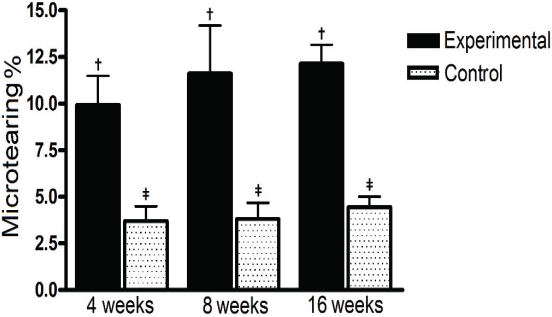
Microtearing increased slightly along the time and was already more evident in experimental group at 4 weeks. It was more intense in the experimental group in all time intervals (4 weeks: 9.93 ± 1.55 vs 3.7 ± 0.79 - P<0.01; 8 weeks: 11.6 ± 2.59 vs 3.81 ± 0.87 - P<0.01; 16 weeks: 12.13 ± 1.03 vs 4.44 ± 0.56 -P<0.001). Once again, time interval (protocol evolution) did not affect significantly neither experimental nor control groups (Figure 2).
Figure 2.
Percentual of microtearing in experimental and control groups, in 3 time intervals. Microtearing was more intense in the experimental group in all time intervals, increased slightly along the time and was already more evident in experimental group at 4 weeks. The symbols (f, $) represent significant statistical difference.
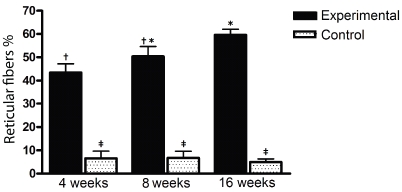
Reticular fibers were more frequent in experimental than in control group (Figure 3) in all time intervals (4 weeks: 43.4 ± 3.84 vs 6.57 ± 3.06 - P<0.001; 8 weeks: 50.45 ± 4.2 vs 6.71 ± 2.84 - P<0.0001; 16 weeks: 59.6 ± 2.41 vs 4.96 ± 1.32 - P<0.0001). Thin dissociated greenish reticular fibers, most likely type III collagen as seen by Picrosirius staining and polarized light microscopy, also increased in experimental group from the 4th to 16th weeks. However such increase was significant only between 4th and 16th weeks (P<0.05), and not between 4th and 8th or between 8th and 16th weeks. Time interval (protocol evolution) did not have a significant effect in controls. Figure 4 shows morphological evidence of increased reticular fibers percentual in experimental animals, detected as greenish, thin dissociated fibers in oposition to the red and yellow thickest and strong associated fibers as the protocol was executed (from 4th to 16th weeks).
Figure 3.
Percentual of Reticuiar Fibers (collagen deposition) in experimental and control groups, in 3 time intervals. Reticuiar fibers were more frequent in experimental than in control group in all time intervals. Time interval did have a significant effect only in between 4 and 16 weeks in experimental group. The symbols (f, $,*) represent significant statistical difference.
Figure 4.
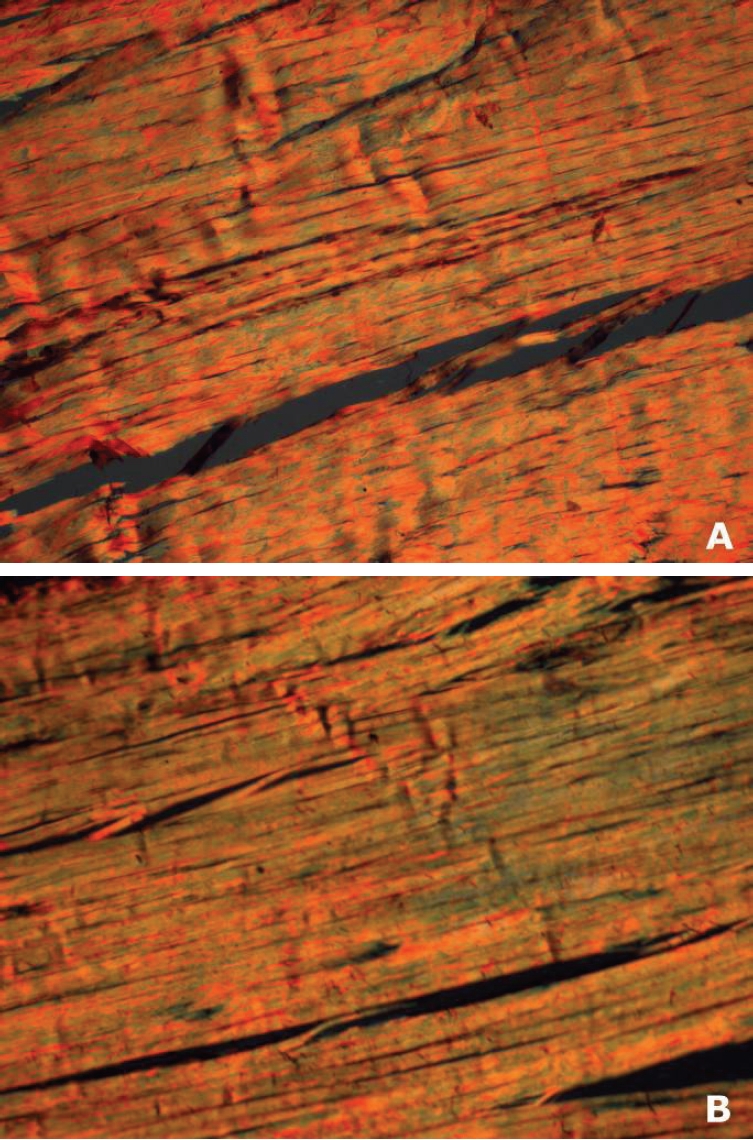
Representative histological sections (5 mm, picrossirius red under polarized light, lOx objective) of the longitudinal axis of the central region of the tendon. Experimental group. Increased reticuiar fibers as evidenced by greenish areas in two different time intervals- 4th (A) and 16th (B) weeks.
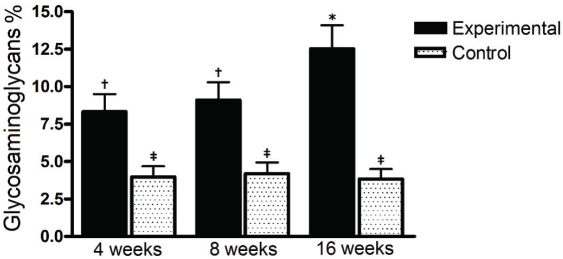
Glycosaminoglycans were detected as bluish areas in Alcian Blue stained sections. These strongly acidic sulfated mucosubstances were quantified by densitometry and showed significantly increased in all experimental animals (Figure 5) as compared to the controls, in all time intervals (4 weeks: 8.33 ± 1.17 vs 3.97 ± 0.71 - P<0.01; 8 weeks: 9.12 ± 1.17 vs 4.19 ± 0.75 - P<0.01; 16 weeks: 12.53 ± 1.56 vs 3.84 ± 0.66 - P<0.01). Experimental animals also showed a higher glycosaminoglycans contents at the 16th week than in the 4th and 8th weeks (P<0.05). Controls did not show a significant effect of time interval (protocol evolution).
Figure 5.
Percentual of glycosaminoglycans deposition in experimental and control groups, in 3 time intervals. GAGs were higher in all experimental animals than in controls, in all time intervals. The symbols (f, $,*) represent significant statistical difference.
Silver stained nucleolar organizing regions (AgNOR) increased within fibroblasts of the experimental group (median = 4 granules/ nucleus) at all time intervals in relation to control group (median = 2 granules/nucleus) (P<0.0001). Again, time interval (protocol evolution) did not have an effect on experimental nor control groups. Figure 6 show dark brownish clusters of AgNORs within the nuclei in experimental specimens, more easily identified than within nuclei of the control group at 16th week.
Figure 6.
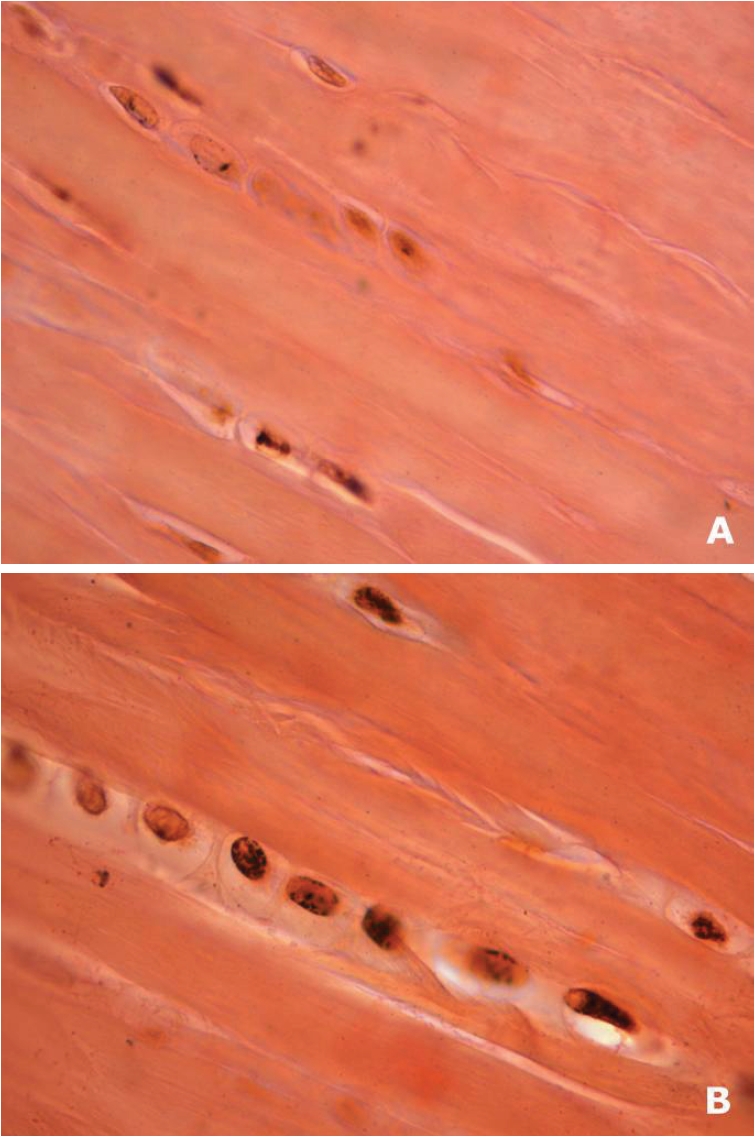
Representative histological sections (5 mm, AgNOR, lOOx objective) of the longitudinal axis of the central region of the tendon. Increased AgNORs expression seen as brownish nuclear granules in experimental (B) as compared to control (A) group at 16th week.
Apoptotic cells seemed to be more frequent and diffusely distributed in tendinosis samples than in control groups. However, in most of the TUNEL reactions, significant parts of the samples detached even from silanized slides, disabling morphometry and statistical analysis, due to the smaller number of representative images.
Discussion
The microscopical results of our study, involving cellularity, microtearing, reticular fibers and glycosaminoglycans, obtained at three different intervals (four, eight and sixteen weeks after protocol start) are in concordance with the histological patterns of tendinosis as described in humans [8, 9, 17, 18] and in other similar models [6, 7, 19].
Microscopically, collagen fibres were progressively disorganized and showed increased microtearing, besides hipercellularity with more rounded nuclei, similar to described by others [7-10]. Neovascularisation as described by Khan et al. [3] and Szomor et al. [20] was not addressed in our study, but no evidence of increased angiogenesis could be detected. It is important to confirm the absence of inflammatory cells within our samples, as described by other authors [5, 6]. Similarly, no adipous and fibrocartilaginous metaplasia were evident in opposition to described by Riley et al. [21].
Cellularity was significantly higher in the experimental compared to the control group, in all time intervals. Increased cellular density was discussed by several authors [6-10, 22]. However, in our study time interval (4, 8 and 16 weeks) did not have a significant effect on experimental nor control groups. It demonstrates that even early in the pathogenesis of a tendinosis (at 4 weeks) an increased cellular density may be present and does not change with the continued exposure to the aggressive factor.
Interstitials gaps representing microtearing were more intense in tendinosis than in control groups, at all studied time intervals. Microtearing among the collagen fibres is described by several authors [6-10, 19, 22]. However, our morphometrical approach showed a higher heterogeneity at 4th and 8th weeks in experimental group, which did not happens in control group. Therefore time interval did have a significant effect only in experimental group. These findings corroborate with the inference that processing artefacts are not involved in generating microtearing. Nevertheless, if both control and experimental samples were processed at the same time and conditions, and if an artefact may play a role, we should expect similar microtearing intensity in both groups. It is clear that microtearing was significantly higher in experimental animals than in controls and increased as time intervals increased only in that group.
Intense microtearing among the fibres may be associated with the greater percentual of reticular fibers found in the experimental group at all-time intervals. At the sixteenth week, reticular fibers (most likely type III collagen) were predominant, accounting for almost 60% of all the tendon's fibers in our study. This is consistent with the reported by Maffulli et al. [23] that fibroblasts harvested from a torn Achilles tendon produce more type III collagen than from a healthy tendon. Ireland et al. [24] and Jones et al. [25] also demonstrated a higher gene expression to type III collagen in tendinosis. That may help explain why tendinosis usually show a tendency to reduce the resistance to stress, becoming more predisposed to a partial or a total rupture.
Glycosaminoglycans increased in the experimental animals when compared to the controls, at all time intervals in our study. That was also reported by other authors [6, 7, 18]. Biochemical analyses suggest significant increased glycosaminoglycans content [5, 18]. Our morphometrical approach innovate showing that glycosaminoglycans content was increased at the 16th week compared to the 4th and 8th week. That may play a role in keepin hydration and interfi-brillar spacing beyond contribute of tendon's viscoelastic properties.
Silver stained nucleolar organizing regions increased within fibroblasts in animals with tendinosis at all-time intervals in comparison to control groups. Counting of the nucleolar organizing regions within nuclei provides a cellular activity status since it correlates with active DNA transcription [15]. Scott et al. [5] also demonstrated an increased tenocytes activity by assessing proliferation (mitosis). The AgNORs counting is consistent with the above, as increased cellularity and extracellular matrix (increased reticular fibres and glycosaminoglycans) were also found in this study.
Apoptotic cells appeared to be increased and more diffusely distributed in tendinosis samples than in control group, as described by others [17, 26]. However, only few images were available to analysis, because in most of the TUNEL reactions, significant parts of the samples detached from the silanized slides. Different protocols were attempted, but results were not considered to be adequate to carry out morphometry and statistical analysis, due to the small number of representative images. Therefore, in terms of apoptosis, there is only a qualitative description as results. Problems in immunohistochemistry processing are also reported in tendon samples by Glazebrook et al. [6]. Anyway, evidence of more frequent and diffuse apoptosis taken together with AgNORs results apparently support an increased cell turn over in experimental group.
Results obtained in this study support the hypothesis that the tendon is attempting to adapt to the persistent overuse. Cell numbers are increased (mitosis), showing higher nucleolar organizing regions (active transcription). Also cells are differentiating to produce more cellular matrix components (collagen and glycosaminoglycans) in an attempt to increase turn over and repair the tissue. Further it seems that the predominant synthesis and deposition of reticular fibers (most likely type III collagen), as an initial and faster step to substitute degenerated collagen, is associated with mechanical and biochemical deterioration, as suggested by Scott et al. [27].
Another interesting approach to explain the pathogenesis of tendinosis should consider the mecanotransduction concept, which is the capacity of answering to loads in order to keep tissue homeostasis [28, 29]. According to Provenzano et al. [30], fibroblasts show surface invaginations with collagen fibrils that can transmit force. This cellular mechanism implies that the load is capable to deform extracellular matrix and be transmitted to the cellular membranes and/or cytoskeletons. In response to this mechano-sensorial system (tensegrity), an adaptive metabolic reply is induced [31, 32]. Therefore, overuse can induce gene expression with consequent morphological alterations that may be implicated in a degenerative status, without classic necrosis and inflammation. As a suitable period of rest is not available for tendon complete repair, it is reasonable to infer that gene expression leads the tissue to degenerative alterations [33], such as the ones presented here.
Tendon injury can occur either in the midsubstance of the tendon, as in its muscular origin or at its enthuses - insertion to the bones. Enthuses show a complex architecture with obliquity of collagen fibers, tissular transitions with condrocytes and glycosaminoglycans rich regions [5]. Due to this complex conformation, in this study focus was directed only to the central region of the longitudinal axis of the tendon, easier to deal with. Also that was useful to prevent false inferences such as chondroid metaplasias and variations in glycosaminoglycans content.
Biomechanics of the quadrupeds’ gait is based on propeller posterior members and on decelerated anterior members [34]. In our protocol, a 10° uphill treadmill and a high speed were used. The uphill inclination optimized the contraction of the muscles of the posterior members in the impulse phase during running. Recently, Glazebrook et al. [6] also demonstrated that a 10° uphill treadmill was useful in inducing Achilles tendinosis in rats. In our experiment, the higher speed amplified the overloading the Achilles tendon, since the relation triceps surae/tendon is greater than the supraspinatus/tendon, according to Magnusson and Kjaer [35]. By the other hand, Huang et al. [36] used a 10° downhill treadmill, which did not generate the eccentric contraction of triceps surae. Thus the biomechanics of this gait did not reproduce the desired effect. Moreover, gravity worked in favour of the propulsion of the animals.
In conclusion our results showed that Achilles tendinosis can be induced, with little or no evidence of inflammatory reaction, by using an overuse uphill animal running model. This approach has provided new information suggesting that as mechanical overload is becoming chronic, cellular turnover and matrix deposition increases leading to tendinosis. The combination of staining techniques and morphometry used here to describe the evolution of lesions occurring in a rat model system has proved to be suited for the study of induced Achilles tendinosis.
Future research should be directed at using this model to investigate the molecular and biochemical mechanisms involved in the pathogenesis, as well as the effect of several physical and pharmacological therapies in the Achilles tendinosis.
Acknowledgments
Funds in support of this work have been provided by FAPEMIG and CNPq. RD Silva was the recipient of master's program grant. AC Vasconcelos is supported by a fellowship from CNPq, Brazil.
References
- 1.Maffulli N, Khan KM, Puddu G. Overuse Tendon Conditions: Time to Change a Confusing Terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 2.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper's knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2007;33:561–567. doi: 10.1177/0363546504270454. [DOI] [PubMed] [Google Scholar]
- 3.Khan KM, Cook JL, Bonar SF, Harcourt P, Astrom M. Histopathology of Common Tendi-nopathies: Update and Implications for Clinical Management. Sports Med. 1999;27:393–404. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 4.Maganaris CN, Narici MV, Almekinders LC, Maffulli N. Biomechanics and Pathophysiology of Overuse Tendon Injuries. Sports Med. 2004;34:1005–1017. doi: 10.2165/00007256-200434140-00005. [DOI] [PubMed] [Google Scholar]
- 5.Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte Responses to Mechanical Loading In Vivo - A Role for Local Insulin-Like Growth Factor 1 Signaling in Early Tendinosis in Rats. Arthritis Rheum. 2007;56:871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 6.Glazebrook MA, Wright JR, Jr, Langman M, Stanish WD, Lee JM. Histological Analysis of Achilles Tendons in an Overuse Rat Model. J Orthop Res. 2008;26:840–846. doi: 10.1002/jor.20546. [DOI] [PubMed] [Google Scholar]
- 7.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE, Mich AA. Overuse activity injures the supraspinatus tendon in an animal model: A histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 8.Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: Some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–249. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 10.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil Rehabil. 2008;30:1–12. doi: 10.1080/09638280701785460. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter JE, Hankenson KD. Animal models of tendon and ligament injuries for tissue engineering applications. Biomaterials. 2004;25:1715–1722. doi: 10.1016/s0142-9612(03)00507-6. [DOI] [PubMed] [Google Scholar]
- 13.Junqueira LCU, Cossermeli W, Brentani R. Picrosirius staining plus polarization microscopy, a specific method for colagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann E, Ribas-Filho JM, Malafaia O, Ribas CAPM, Nassif PAN, Filho ES, Przysiezny PE. Tracheal suture in rats with hypothyroidism: Wound healing study. Acta Cir Bras. 2009;24:282–289. doi: 10.1590/s0102-86502009000400007. [DOI] [PubMed] [Google Scholar]
- 15.Ploton D, Menager M, Jeannesson P. Improvement in the staining and the visualization of the argyrophilic proteins of nucleolar organizer regions at the optical level. Histochem J. 1986;18:5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuoheti Y, Itoi E, Pradhan RL, Wakabayashi I, Takahashi S, Minagawa H, Kobayashi M, Okada K, Shimada Y. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005;14:535–541. doi: 10.1016/j.jse.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Fu SC, Chan KM, Rolf CG. Increased deposition of sulfated glycosaminoglycans in human patellar tendinopathy. Clin J Sport Med. 2007;17:129–134. doi: 10.1097/JSM.0b013e318037998f. [DOI] [PubMed] [Google Scholar]
- 19.Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Szomor ZL, Appleyard RC, Murrel GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80–86. doi: 10.1002/jor.20009. [DOI] [PubMed] [Google Scholar]
- 21.Riley GP, Harrall RL, Constant CR, Cawston TE, Hazleman BL. Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann Rheum Dis. 1996;55:109–115. doi: 10.1136/ard.55.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warden SJ. Animal models for the study of tendinopathy. Br J Sports Med. 2007;41:232–240. doi: 10.1136/bjsm.2006.032342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from Ruptured and Tendinopathic Achilles Tendons Produce Greater Quantities of Type III Collagen than Tenocytes from Normal Achilles Tendons An In Vitro Model of Human Tendon Healing. Am J Sports Med. 2000;28:499–505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- 24.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 25.Jones GC, Corps AN, Pennington CJ, Clark IM, Edwards DR, Bradley MM. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human Achilles tendon. Arthritis Rheum. 2006;54:832–842. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- 26.Lian OB, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive Apoptosis in Patellar Tendinopathy in Athletes. Am J Sports Med. 2007;35:605–611. doi: 10.1177/0363546506295702. [DOI] [PubMed] [Google Scholar]
- 27.Scott A, Khan KM, Duronio V. IGF-I activates PKB and prevents anoxic apoptosis in Achilles tendon cells. J Orthop Res. 2005;23:1219–1225. doi: 10.1016/j.orthres.2004.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 29.Ruoslahti E. Stretching is good for a cell. Science. 1997;276:1345–1346. doi: 10.1126/science.276.5317.1345. [DOI] [PubMed] [Google Scholar]
- 30.Provenzano PP, Heisey D, Hayashi K, Lakes R, Vanderby R., Jr Subfailure damage in ligament: a structural and cellular evaluation. J Appl Physiol. 2002;92:362–371. doi: 10.1152/jappl.2002.92.1.362. [DOI] [PubMed] [Google Scholar]
- 31.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the un-der-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JHC, losifidis MI, Fu FH. Biomechanical basis for tendinopathy. Clin Orthop Relat Res. 2006;443:320–332. doi: 10.1097/01.blo.0000195927.81845.46. [DOI] [PubMed] [Google Scholar]
- 33.Kjaer M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 34.Courtine G, Roy RR, Hodgson J, McKay H, Raven J, Zhong H, Yang H, Tuszynski MH, Edgerton VR. Kinematic and EMG Determinants in Quadrupedal Locomotion of a Non-Human Primate (Rhesus. J Neurophysiol. 2005;93:3127–3145. doi: 10.1152/jn.01073.2004. [DOI] [PubMed] [Google Scholar]
- 35.Magnusson SP, Kjaer M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. Eur J Appl Physiol. 2003;90:549–553. doi: 10.1007/s00421-003-0865-8. [DOI] [PubMed] [Google Scholar]
- 36.Huang TF, Perry SM, Soslowsky LJ. The effect of overuse activity on Achilles tendon in an animal model: A biomechanical study. Ann Biomed Eng. 2004;32:336–341. doi: 10.1023/b:abme.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]