Abstract
The antibiotic novobiocin is shown to alter the sedimentation properties of human cellular DNA in alkaline sucrose. This alteration is at least partially due to increased DNA-protein binding in the cell in the presence of novobiocin. Pyrimidine dimer analysis and repair replication studies support previous reports that novobiocin inhibits repair of UV damage in human cells but we find this block to be shortlived. It is also shown that novobiocin is ineffective at blocking "long-patch" repair induced by methyl methanesulfonate as measured both by CsCl density centrifugation and the ara-C inhibition technique. However, the accumulation of breaks in MMS-treated cellular DNA in the presence of novobiocin suggests that some "short-patch" alkylation repair may be inhibited by the antibiotic. These findings are discussed in light of the proposal that novobiocin may inhibit a DNA gyrase-like activity in human as in bacterial cells.
Full text
PDF



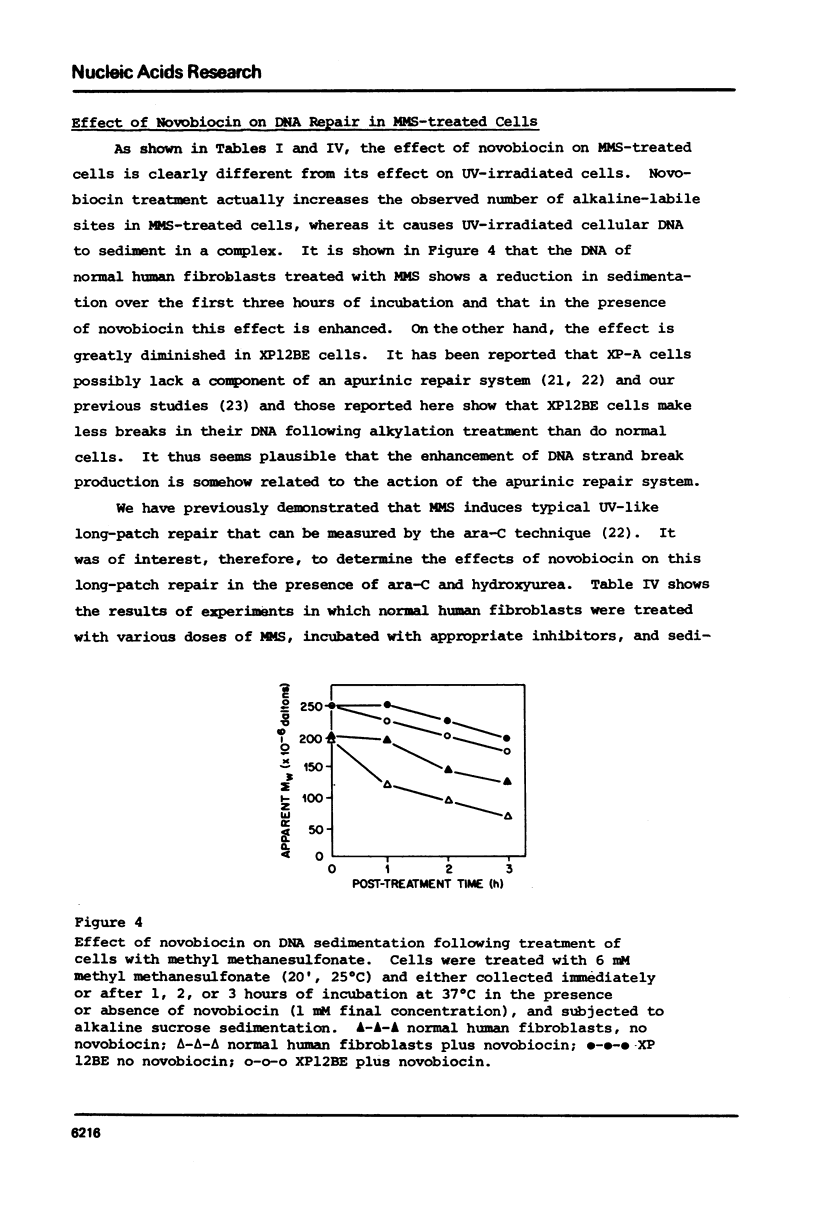

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldi M. I., Benedetti P., Mattoccia E., Tocchini-Valentini G. P. In vitro catenation and decatenation of DNA and a novel eucaryotic ATP-dependent topoisomerase. Cell. 1980 Jun;20(2):461–467. doi: 10.1016/0092-8674(80)90632-7. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Collins A., Johnson R. Novobiocin; an inhibitor of the repair of UV-induced but not X-ray-induced damage in mammalian cells. Nucleic Acids Res. 1979 Nov 10;7(5):1311–1320. doi: 10.1093/nar/7.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Snyder M. Superhelical Escherichia coli DNA: relaxation by coumermycin. J Mol Biol. 1978 Apr 5;120(2):145–154. doi: 10.1016/0022-2836(78)90061-x. [DOI] [PubMed] [Google Scholar]
- Dunn W. C., Regan J. D. Inhibition of DNA excision repair in human cells by arabinofuranosyl cytosine: effect on normal and xeroderma pigmentosum cells. Mol Pharmacol. 1979 Mar;15(2):367–374. [PubMed] [Google Scholar]
- Edenberg H. J. Novobiocin inhibition of simian virus 40 DNA replication. Nature. 1980 Jul 31;286(5772):529–531. doi: 10.1038/286529a0. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin M. F. Effect of novobiocin on DNA synthesis and structure in human lymphoblastoid cells. Biochem Biophys Res Commun. 1981 May 15;100(1):328–335. doi: 10.1016/s0006-291x(81)80100-3. [DOI] [PubMed] [Google Scholar]
- Lynch W. E., Short J., Lieberman I. The 7.1 S nuclear DNA polymerase and DNA replication in intact liver. Cancer Res. 1976 Mar;36(3):901–904. [PubMed] [Google Scholar]
- Mattern M. R., Painter R. B. Dependence of mammalian DNA replication on DNA supercoiling. II. Effects of novobiocin on DNA synthesis in Chinese hamster ovary cells. Biochim Biophys Acta. 1979 Jul 26;563(2):306–312. doi: 10.1016/0005-2787(79)90049-2. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Scudiero D. A. Dependence of mammalian DNA synthesis on DNA supercoiling. III. Characterization of the inhibition of replicative and repair-type DNA synthesis by novobiocin and nalidixic acid. Biochim Biophys Acta. 1981 Apr 27;653(2):248–258. doi: 10.1016/0005-2787(81)90160-x. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Sugino A. Novobiocin and nalidixic acid target proteins in yeast. Biochem Biophys Res Commun. 1980 Sep 16;96(1):306–312. doi: 10.1016/0006-291x(80)91215-2. [DOI] [PubMed] [Google Scholar]
- SMITH K. C. Dose dependent decrease in extractability of DNA from bacteria following irradiation with ultraviolet light or with visible light plus dye. Biochem Biophys Res Commun. 1962 Jul 3;8:157–163. doi: 10.1016/0006-291x(62)90255-3. [DOI] [PubMed] [Google Scholar]
- Snyder R. D., Carrier W. L., Regan J. D. Application of arabinofuranosyl cytosine in the kinetic analysis and quantitation of DNA repair in human cells after ultraviolet irradiation. Biophys J. 1981 Aug;35(2):339–350. doi: 10.1016/S0006-3495(81)84793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. D., Regan J. D. DNA repair in normal human and xeroderma pigmentosum group A fibroblasts following treatment with various methanesulfonates and the demonstration of a long-patch (u.v.-like) repair component. Carcinogenesis. 1982;3(1):7–14. doi: 10.1093/carcin/3.1.7. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Letters to the editor: Novobiocin-a specific inhibitor of semiconservative DNA replication in permeabilized Escherichia coli cells. J Mol Biol. 1975 Jul 25;96(1):201–205. doi: 10.1016/0022-2836(75)90191-6. [DOI] [PubMed] [Google Scholar]
- Sung S. C. Effect of novobiocin on DNA-dependent DNA polymerases from developing rat brain. Biochim Biophys Acta. 1974 Aug 15;361(1):115–117. doi: 10.1016/0005-2787(74)90214-7. [DOI] [PubMed] [Google Scholar]
- Witte I., Thielmann H. W. Extracts of xeroderma pigmentosum group A fibroblasts introduce less nicks into methyl methanesulfonate-treated DNA than extracts of normal fibroblasts. Cancer Lett. 1979 Mar;6(3):129–136. doi: 10.1016/s0304-3835(79)80023-3. [DOI] [PubMed] [Google Scholar]


