Abstract
Oxidative stress in liver cells may contribute to the etiology of hepatic diseases, as in liver cirrhosis. AP-Endonuclease1 (APE1/Ref-1) is essential for cell protection toward oxidative stress by acting as a transcriptional regulator of pro-survival genes and as a redox sensitive protein. The aim of this study was to critically analyze the various parameters governing the success of human umbilical cord blood mononuclear stem cell-based (MNCs) therapy without the use of an immunosuppressant and to investigate for the first time the expression of APE1 during thioacetamide (TAA)-induced cirrhosis and MNCs therapy in a rat model. Umbilical cord blood samples from full-term deliveries were collected. Lethal fulminant hepatic cirrhosis in rats was induced by intraperitoneal injection of thio-acetamide. MNCs were then intrahepatically transplanted. We measured APE1 expression at mRNA and protein levels, mRNA expression of TGF-β, α-SMA, STAP, CTGF, MMP-9 and TIMP-1 in a follow up study. Histopathological and immunohistochemical analyses were performed 10 weeks after intrahepatic injection of the cells. Transdifferentiated cells could be efficiently stained with antihuman hepatocytes. Interestingly, human hepatocyte-specific markers, human albumin, cytokeratin-18 and cytokeratin-19 mRNAs were detected in rat liver after 10 days of MNCs infusion. MNC transplanted by intrahepatic route, could engraft recipient liver, differentiated into functional hepatocytes, and rescued liver failure. Moreover up regulation of APE1 expression confirmed by marked immunohistochemical staining may be involved in MNCs-induced hepatocytes regeneration suggesting that maintaining high level of APE1 has protective effect as pro-survival signal.
Keywords: AP-Endonuclease1, human umbilical cord blood mononuclearstem cell, liver cirrhosis, human albumin
Introduction
Liver cirrhosis represents the final common pathological outcome for the majority of chronic liver diseases caused by a variety of factors, such as viral infections, alcohol, drugs and chemical toxicity [1-2]. It is often associated with the loss of functional liver cells, activation of hepatic stellate cells (HSCs), senescence of hepatocyte cells and accumulation of extracellular matrix, amongst other detrimental processes [3].
ROS have roles as mediators of growth signaling for induction of cell proliferation, in responses to stress, and in energy metabolism [4]. However, if ROS accumulate in the cell due to extended oxidative stress or irreparable DNA damage, they can lead to apoptotic cell death or to senescence through the p53 pathway [5]. To prevent ROS accumulation, cells have several antioxidant systems including enzymes and re-dox-sensitive molecules (Trx, APE1/Ref-1) that protect the cells from oxidative stress [6]. Activation of APE1/Ref-1 increases the binding of oxidative stress regulating transcription factors (Hif-1, p53, Ap-1). There are many studies on the relationship between p53 and APE1/Ref-1 [7-8]. Oxidized APE1/Ref-1 facilitates p53 DNA binding. In contrast, p53 is a negative regulator of APE1/Ref-1 promoter. Despite accumulating evidence for the role of APE1/Ref-1 in redox regulation, the underlying mechanism is poorly understood. Furthermore it has been shown that redox chaperone activity of APE1/Ref-1 is critical to NF-κB-mediated gene expression in human cells and is mediated through its physical association with target transcription factors. Thus, APE1/Ref-1 may play multiple roles in an anti-oxidative stress response pathway through its different biochemical activities. These findings also provide new insight into the mechanism of intracellular redox regulation [9].
As the liver has a fantastic regenerative capacity but following chronic liver damage, this regenerative capacity fails and fibrosis eventually leads to cirrhosis. The ultimate therapy for cirrhosis and end stage liver disease is liver transplantation. Cell therapy represents a unique opportunity for regenerative medicine to cure liver cirrhosis for which current treatment only alleviates symptoms or retards further disease progression. In addition, the recent possibility to derive pluripotent stem cells from somatic cells using epigenetic reprogramming has further increased the clinical interest of stem cells since induced pluripotent stem cells could render personalized cell-based therapy possible [10]. Recently, transplantation of bone marrow-derived cells including mesenchymal stem cells was reported to reduce carbon tetrachloride (CCl4)-induced liver fibrosis [11-12]. Umbilical cord blood is now an established source of transplantable hematopoietic stem cells (HSCs) that have a greater proliferative capacity, lower immunological reactivity and lower risk of graft-versus-host disease (GVHD) than those derived from adult bone marrow [13-18]. The cells are CD34+ and CD38− and their frequency is greater than that of bone marrow or peripheral blood following cytokine mobilization [19]. It has also been demonstrated that cord blood contains MSCs [20-21] that can support the in vivo expansion of HSCs and function as an accessory cell population for engraftment [22-23]. The frequency of MSCs in umbilical cord blood is low however, with MSCs successfully isolated from only a third of all samples collected [24]. Cord blood stem cells expressing baseline levels of ES cell markers such as Oct-4, Nanog, SSEA-3 and SSEA-4 have also been described [25-26].
In the present study, zenogeneic human umbilical cord blood mononuclear cells (MNCs) were used, to determine if they could be a good therapeutics for the thioacetamide-induced liver cirrhosis of rats. We attempted also to shed the light, for the first time on the possible involvement of APE1 down-regulation in the progression of liver injury to cirrhosis, and its up-regulation during human MNCs-induced regeneration in cirrhotic rats.
Materials and methods
Isolation of mononuclear cells from human umbilical cord blood and Intrahepatic transplantation procedures
Mononuclear cells (MNCs) from human umbilical cord blood (UCB) were collected from full-termed delivery women with informed consent at Alexandria University Hospital. All studies were approved by the ethical committee of Alexandria and Ain Shams Universities. At the end of the normal delivery process and after the complete separation of the placenta, blood was collected under complete aseptically technique and was transported immediately to the laboratory. The cord blood was processed as described before [24]. Briefly, cord blood was drawn directly into 50 ml tube containing 5ml PBS buffer pH 7.2 containing 2mM EDTA and stored at 4°C for 30 min. The cord blood was then diluted 3X with PBS buffer pH 7.2 containing 2 mM EDTA. Thirty five ml of the diluted cord blood was layered carefully over 15ml of Ficoll-Paque in 50 ml tube, and centrifuged at 400 xg for 35min at 20°C. The upper layer was aspirated leaving the MNCs layer un-disturbed at the inter-phase layer. The MNCs layer was then transferred carefully to a new 50ml tube. The cells were washed twice with PBS buffer then collected by centrifugation. The isolated MNCs pellets were suspended in an appropriate amount of the PBS buffer containing 0.5% bovine serum albumin (BSA) and stored at -80°C for future application.
Cirrhosis induction and treatment
Forty male Sprague-Dawley rats, weighing 100-150 g were used in all experiments. The animals were maintained under controlled temperature (25±2°C) and constant photoperiodic conditions (12:12-h daylight/darkness). The dams had free access to water and standard commercial chow. The principles of laboratory animal care were followed in all experimental protocols and were approved by ethics committee of animal research t Faculty of Medicine, Alexandria University.
After 5-days acclimation, rats were randomly divided into three major groups: Group 1 (control group) received an intraperitoneal (IP) injection of saline solution, group 2 (TAA-induced) received IP administration of thioacetamide (TAA) (200 mg/kg/body weight) twice weekly for 20 weeks. 30% ethanol solution was added to the drinking water for the last 4 weeks and group 3 (TAA-MNCs-treated) was induced as in group 2 then treated by Intrahepatic injection of human MNCs. Blood was taken from the ophthalmic venous plexus from all groups, by three rats each group on a follow up basis after TAA induction in order to monitor whether or not the liver was in state of cirrhosis. Serological and histopathological examination was performed in groups 2 and 3 during the follow up period at weeks 0, 8, 16 and 20.
Group 3 were anesthetized using ether inhalation and abdominal incision was done, the liver was exposed, the cells were injected into the liver over approximately 10∼15 seconds. Using a 26-gauge syringe, cells were injected at a dose of 5×105 cell/ rat in 0.5 ml PBS. The control group received PBS instead of cells. The abdominal incision was closed and the animals were monitored closely until recovery. Three rats were exsanquinated under ether anesthesia and sacrificed on days 0, 10, 14, 20 and 30 after MNCs administration in order to monitor the liver condition. The animals were sacrificed, the liver was excised, and tissue pieces were taken for histopathology and immunohistochemical studies from all groups at the indicated time points. Blood samples were collected from sacrificed animals by heart puncture. After coagulation, sera were collected and stored at -80°C for further analyses of ALT, AST, albumin, total protein. A small portion of the liver was removed for histochemical studies by fixation with 10% formalin. The remaining sections of the liver tissues were rapidly frozen with liquid nitrogen and stored at -80°C for extraction of total RNA, and determination of hepatic proteins and enzyme assays.
Histopathological and immunohistochemical study
The fixed liver tissues in formalin were dehydrated in ascending grades of alcohol, and cleaned by immersing the tissues in xylene for 1h (three times), followed by impregnation in melted paraffin in oven at 600°C for 1h. The specimens were embedded in paraffin and were left to solidify at room temperature. Using a rotatory microtome, multiple sections of 5μm thick were cut and mounted on clean glass slides. All sections were stained with hematoxylin and eosin (H & E) and examined for any Histopathological changes [27]. Pathological diagnosis of each liver specimen was assessed and graded from 0 to V by two pathologists in a blinded manner according to the criteria described by Wang et al. [28]. To investigate the hepatic differentiation of cord blood cells intra-hepatically injected into the liver of cirrhotic rats and their liver engraftment, we traced human MNCs cells by immunostaining analysis with antihuman hepatocyte antibody (Clone OCHIE 5; DACO) as the primary antibody which reacts only with human hepatocytes but not with rat hepa-tocytes. In addition we analyzed APE1 expression using rabbit Polyclonal anti-APE1. The sections were deposited on charged glass slides. Slides were dried completely and deparaffinized and rehydrated in xylene and grades of alcohol, the slides were exposed to epitope retrieval by placing them in boiling water containing 0.01 ml/L sodium citrate buffer (pH 6) for 15 min. Followed by quenching of endogenous peroxidase with 1% hydrogen peroxide (H202) for 10 minutes. The primary antibody was applied for 24h in humid chamber. The primary antibody was detected using ABC complex and developed with DAB as substrate the slides were counter-stained with hematoxylin, mounted in DPX and covered with cover slips.
Preparation of nuclear, cytosolic and total cell extracts
Part of frozen liver tissues was homogenized in lysis buffer (50 mM HEPES solution pH 7.4 containing 1% (v/v) Triton X-100, 4mM EDTA, Cocktail protease inhibitor), then freezed in ethanol/ dry ice bath. Cycles of thawing/ freezing were repeated twice. The collected total cell extracts were stored at -80°C.
Nuclear extract was prepared as described by Schreiber et al. method [29]. 500 mg of frozen tissue was homogenized in ice-cold extraction buffer A (0.6% NP-40, 150 mM NaCl, 10mM HEPES pH 6.9, 1mM EDTA, 0.5 mM phenyl-methylsulfonyl fluoride). The homogenate was centrifuged at 2,000 rpm for 30 s at 0°C. After incubation for 5 min on ice, the supernatant was centrifuged at 5,000 rpm for 5 min at 0°C. The cytoplasmic fraction was collected and stored at -80°C. The precipitate was resuspended in extraction buffer B (25% glycerol, 420 mM NaCl, and 20 mM HEPES pH 7.9, 1.2 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and protease inhibitors), kept on ice for 20 min then centrifuged at 12,000 rpm for 25 min at 0°C. The supernatant was transferred to a new tube and stored at -80°C.
RNA isolation and RT-PCR analyses
Total RNA was extracted from frozen liver tissues according to Chomczynski and Sacchi procedure [30] using GStractTM RNA Isolation Kit II Guanidinium Thiocyanate Method. Alterations in the steady -state mRNA levels of genes relevant to hepatic stellate cells (HSCs) activation and cirrhosis development in TAA-induced rats either by elevated expression (TIMP, TGF-β, CTGF, α-SMA, STAP, p53 and p21) or by reduced expression (albumin, MMP-9, Mn-SOD and APE1) were determined using reverse-transcriptase PCR analysis. Expression of human genes (albumin, CK-18, CK-19, and GAPDH) were detected by RT-PCR in the liver of MNCs-treated rats.
Using one-step RT-PCR (RT/PCR Master Mix. Gold Beads, BIORON) reaction, the cDNA was synthesized and used for amplification of target gene(s) using specific primer sets. Primer sequences of rat target genes and expected PCR product size for are; TIMP-1-F-GCCCCAACCCA CCCACAGA; R-TTTGCAAGGGATG GCTGAACAG (405 bp), MMP-9- F-CCACCGAG CTATCCACTCAT-R-GTCCGGTTTCAGCATGTTTT (159 bp), Mn-SOD-F-CTGAGGAGAGCAGCGGTCGT-R-CTTGGCCAGC GCCTCGTGGT (200 bp), CTGF-F- ATCCCTGCGAC CCACACAAG-R-CAACTGCTTTGGAAGGACTCGC (450 bp), TGFβ-1-F-CGGGAAGC AGTGCCAGAA-R-TGCTCCACAGTTGACTTGAATCTC (273 bp), α-SMA -F-ACTGGGACGACATGGAAAAG-R-CATCTCCAGAGT CCAGCACA (280 bp), STAP-F-ATGGAGAAAGTGC CGGGCGAC-R-TGGCCCTGAAGAGGGCAGTGT (340 bp), Rat-albumin- F-GCTC AGAGACTGCCC TGTGT-R-ACAAGGTTTGGCCCC TCAGT (400), p53 -F-CACAGTCGGATATGAGCATC-R-GTCGTCCAGAT ACTCAGCAT (600 bp), p21- F- G TGAGACACCA-GAGTGACAGA-R-ACAGCGATATCGAGACACTCA (400 bp), APE1-F-CTGCCTGGACTCTCTCATCAAT AC-R-CCTCATCGCCTATGCCGTAAG (200) and the house keeping gene GAPDH-F-TGCTGGTGCTG AGTATGTCG-R-ATTGAGAGCAATGCCAGCC(646).
Primers and products size of human target genes are; albumi: F-CCAGGAAGACATCCTTTGC; R-CCTGAGCCAGA GATTTCC (329 bp), CK 18: F-TGGTCACCACACAGTCTGCT-R-CCAAGGCATCACCA AGATTA (347 bp), CK 19: F-AGGTGGATTCCGCTC CGGGCA-R-ATCTTCCTGTCCCTCGAGCA (460 bp) and the house keeping gene GAPDH: F-GTCTT CTCCACCATGGAGAAGGCT; R-CATGCCAGTGAG CTTCCCGTTCA (395bp).
Real-time PCR analysis of APE1 was performed at real time facility at Mubarak City using cyper-green master mix reagent and the same primers that have been used for the reverse transcriptase-PCR.
Western blotting
According to the method described by Burnette [31], APE1 and p53 immunoblots were performed on prepared cytosolic and total cell extracts respectively. Briefly, 50 μg protein lysates from each sample were separated on 12% SDS-PAGE then transferred to a nitrocellouse membrane. Primary antibody to APE1 (rabbit Polyclonal anti-APE1) and p53 (sc-DO-1, Santa Cruz Biotechnology) was used. Then incubated with HRP-conjugated secondary antibodies (Santa Cruz; diluted). Assay of anti-oxidant versus prooxidant parameters
Lipid peroxidation assay
Liver was homogenized in ice cold buffer (50 mM potassium phosphate, pH 7.5). Then centrifuge at 4000 rpm for 15 min. Supernatant was used for lipid peroxidation assays according to Ohkawa et al. [32] method. Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) in acidic medium to form thiobarbituric acid reactive product the absorbance of pink color can be measured at 534nm.
Determination of reduced glutathione (GSH) level, glutathione peroxidase (GPx) and glutathione-s-transferase (GST) activities
Part of liver tissue (10% W/V) was washed with saline solution, minced and homogenized in ice-cold 1.15% KCL- 0.01M sodium phosphate buffer, pH 7.4. The supernatant was stored at -80°C to be used for determination of GSH, Gpx and total protein contents. GSH was determined described by Ellman [33]. GPx was determined accordingto Rotruck et al. [34] method.
GST activity was analyzed in liver homogenate prepared by liver homogenized in ice cold buffer (100 mM potassium phosphate, pH 7.0 containing 2 mM EDTA) per gram tissue. Then centrifuged and the supernatant was removed and stored at -80°C until used. GST was assayed according to Habig et al. [35] method.
For assay of Catalase activity liver was homogenized in 5-10 ml cold buffer (50mM potassium phosphate, pH 7.4, 1mM EDTA and 1ml/L Triton X-100) per gram tissue. Then centrifuged and the supernatant was used. Catalase activity was assayed according to Aebi [36] procedure.
Detection of hydroxyproline content in liver hydroxyproline (hyp) assay
A colorimetric assay was performed as described before [37]. Briefly, liver sections (0.5 g) were hydrolyzed for 20 h using 6 mol/L HCl at 100°C. The precipitate is dissolved in water, and centrifuged to remove any impurities. Samples were incubated for 10 min in 0.05 mol/L chloramine-T (Fisher, Fair Lawn, NJ, USA) at room temperature, followed by 15-min incubation in Ehrlich's-perchloric acid solution at 65°C. Sample absorbencies were assessed at 560 nm and resulting values compared to a Hyp standard curve. Each sample was assayed in duplicate. The Hyp content was expressed as micrograms per gram of wet liver.
Cytokines determination
IL-6, TNF-α, TGF-β, and PDGF levels were assayed in total cell extracts prepared form liver tissues and sera samples. Cytokines levels were determined using commercially available kits. The analyses were performed according to the manufactures’ guidelines. IL-6 level assayed using BioLegend ELISA MAX™ Set Standard Rat IL-6 Kit (Cat.430502). TNF-α was determined using Rat TNF-α Prprotech kit (cat# 900-K25). Analysis of TGF-β and PDGF were analyzed using Quantikine kit (Cat# MB100B and Cat# MBB00 respectively). Nuclear factor-kB p50/65 DNA-binding activity in nuclear extracts of hepatic tissue samples was evaluated. Analysis was performed according to manufacturer's protocol guidelines using commercial Kit (NF-kB p50/65 Transcription Factor Assay Colorimetric. Cat. No. SGT510 Themicon International Inc. Temecula, CA, USA).
Statistical analysis
All experiments were repeated two or more times independently and typical graphs are presented in some cases data are expressed as mean ± SD. data were analyzed by student's test and difference was considered significant from control when P<0.05. ANOVA test was used to compare the statistical difference between groups. The results considered significant when p<0.01.
Results
Histological analyses
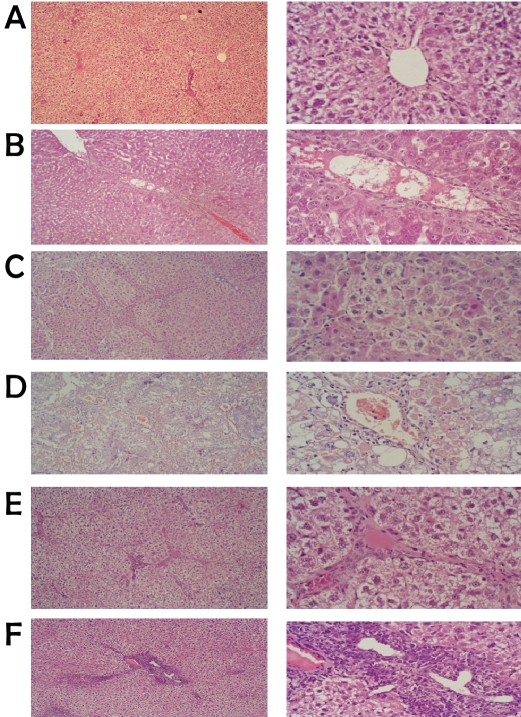
Representative microscopic photographs of liver sections stained with H & E stain of TAA-induced and human MNCs-treated groups are shown in Figure 1. We assessed the degree of liver insult and progression to cirrhosis (score) according to the pathological index described by Wang et al. [28]. Stained and examined liver sections of TAA-induced and control rats were graded from score 0 (at week zero, control), III (at week 8), IV (at week 16) and from V to VI, (at week 20). At score V-VI the TAA-administration was stopped and human MNCs treatment was started.
Figure 1.
Representative microscopic photographs of control versus TAA-induced rats by H & E staining (low-power on the left and high-power on the right). a) mock-treated showing normal hepatocytes. b) TAA-induction for 8 weeks, c) 16 weeks showed small nodules with degenerative hepatocytes, absence of sinusoid, an increase of fibrous tissue thickness septa, expansion of portal tract with hepatic central vein and hepatic nodules increase (cirrhosis). d) After 20 weeks of TAA induction the results showed marked macro vesicular steatosis changes of hepatocytes as well as congested sinusoidal structures and complete cirrhosis. e) Rat liver paraffin section after 2 weeks of MNCs treatment showing decrease in fibrotic thickness septa and appearance of apoptotic cells (shrinkage and dark color nucleus). f) Photograph of rat liver paraffin section after 4 weeks of MNCs treatment showing the appearance of discontinuous septa, presence of apoptotic cells and liver return to normal architecture.
As shown in Figure 1 the mock treated group, control, microscopic photograph indicated normal architecture of complete hepatic lobules without neither fibroplasia nor inflammatory cells infiltration. Following 8 weeks of TAA administration the results showed thick fibrous septa surround the hepatic central vein, the hepatic nodules with disorganized hepatic architecture, absence of sinusoid and large hepatocytes with various sizes of nuclei. After 20 weeks of TAA induction the results showed marked macro vesicular steatosis changes of hepatocytes as well as congested sinusoidal structures and complete cirrhosis. Treatment of cirrhotic rats with intra-hepatic injection of human mononuclear cells (MNCs) for 2 weeks indicated significant decrease in fibrotic thickness septa and appearance of apoptotic cells. Furthermore after 4 weeks of MNCs treatment we observed the appearance of discontinuous septa, presence of apoptotic cells and liver returned to normal architecture as shown in Figure 1.
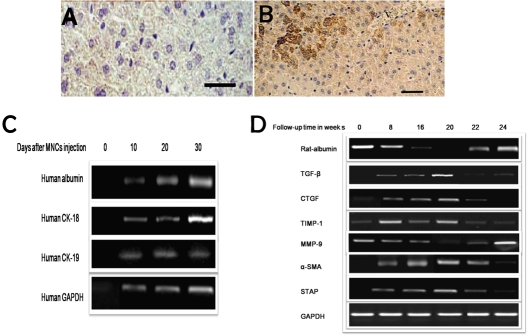
In this study, we injected mononuclear cells (MNCs) derived from umbilical cord blood into the liver of immunocompetent rats after induction of cirrhosis without immunosupression. The histological sections didn't show significant inflammatory aggregates indicating that the graft was not rejected. Histopathological examination also showed significantly decreased fibrosis and marked liver regeneration as well as better restoration of architecture as shown in Figure 1. At week 4 of MNCs injection, liver sections of transplanted animals were stained positive for anti-human hepatocytes antibodies (Figure 3A and B), which stains only human hepatocytes, while none of the control animals showed such staining.
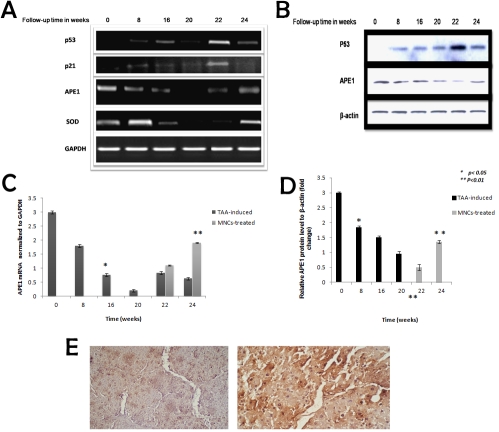
Figure 3.
The alterations in APE1, p53 mRNA and protein levels as well as the expression profile of cirrhosis-related versus anti-cirrhosis genes in cirrhotic versus human MNCs-treated rats. RT-PCR analysis of p53, p21, APE1 and SOD levels of TAA-administered rats indicated pathological hepatocytes damage with reduced level of p53 protein and mRNA up to week 20. MNCs induced HSCs apoptosis with significant elevation in p53 mRNA level at weeks 22 (b). Change in p21 mRNA level was used as positive control for p53 activation. APE1 mRNA and protein levels were significantly reduced during the time course of HSCs activation. MNCs-treatment induced HSCs inhibition and up-regulation of APE1 expression as shown in Figures a & b. Alterations in SOD mRNA level were used to assess the general decline in the hepatocytes total antioxidant capacity. Moreover, SOD gene expression has been reported to be down-regulated by p53 protein. GAPDH was used as an internal control. β-actin level was used as internal control in western blotting analysis. c) Real time-PCR analysis of APE1 levels indicated significant reduction during TAA-induced cirrhosis development versus control and siginfcant elevation in its level in treated versus self-recovery weeks. e) Immunohistochemical analysis of APE1 in treated liver at week 22 indicated marked cytoplasmic expression. MNCs treatment induced elevation in APE1 level at week 24. * represent the statistical difference from control and ** represents the statistical significance between groups.
Sera levels of ALT and AST activities were increased significantly during the TAA-induced cirrhosis time-course. On the contrary at weeks 22 & 24 of receiving MNCs injection a reduction in AST and ALT activities was observed compared to rats received empty vehicle. In addition, an overall improvement in liver synthetic function were observed by the significant elevation in sera total protein and albumin levels in MNCs-treated versus TAA-induced group that did not receive treatment in parallel to the elevation in albumin mRNA level (data are not shown).
Expression of human genes in MNCs-treated rat's liver
Expression of human genes albumin, cytokeratin-18 (CK-18), cytokeratin-19 (CK-19) and GAPDH were investigated in the cirrhotic rat's liver at days 0, 10, 20 and 30 after MNCs injection. Interestingly we observed time-dependent increase in the expression of human albumin, CK-18, CK-19 and GAPDH in total RNA isolated from MNCs-treated cirrhotic rats’ liver. RT-PCR results at zero time indicate the expression profile of human genes before MNCs treatment in the samples obtained from rats with liver cirrhosis at week 20 (Figure 2C).
Figure 2.
Detection of human cells transplantation into injured rat liver by immunohistochemistry and expression of human genes albumin, CK-18, CK-19 and GAPDH in rat liver. (A) Section of liver of the control animal shows negative stain for anti-human antibody.(B) section of liver of animals treated with MNCs for four weeks shows positive stain for antihuman hepatocytes. (C) RT-PCR analysis of total mRNA isolated from liver of rats treated by intra-hepatic injection of MNCs at follow up time of 0, 10, 20 and 30 days following MNCs injection indicated time-dependent expression of human albumin, CK-18 CK-19 and GAPDH. (D) RT-PCR analysis of alterations in hepatic stellate cells (HSCs) activation-related genes (TGF-β, CTGF, TIMP-1, MMP-9, α-SMA and STAP) during TAA-induction time course (from 0 to 20 weeks) and after treatment with MNCs injection at weeks 22 and 24. HSCs activation was confirmed by marked elevation in TGF-β, CTGF, TIMP-1, α-SMA and STAP as well as marked reduction in MMP-9 mRNA level. MNCs treatment induced HSCs apoptosis, hepatocytes regeneration and reduction in HSCs-activation related genes.
Chronic damage usually favors fibrogenesis over fibrolysis, with an upregulation of tissue inhibitors of MMPs (TIMPs) [38]. The major hepatic ECM producing cells are myofibroblasts that either derived from activated hepatic stel- late cells (HSC) or perivascular fibroblasts [39].
When treated with TAA, the cirrhotic livers exhibited a marked increase in extracellular matrix (ECM) content and induced expression of HSCs-activation related genes. Expression profile of cirrhosis-related versus anti-cirrhosis genes by reverse transcriptase (RT-PCR) analysis during the cirrhosis-induction period in a time-course study at weeks 0, 8, 16, and 20 was carried out on RNA isolated from experimental rat's liver tissues. The results indicated significant elevation in TGF-β (consistent with the elevation in protein level measured in liver total cell extracts and sera samples), CTGF, TIMP-1, α-SMA and STAP levels were observed during the cirrhosis induction period. Treatment of cirrhotic rats by human MNCs injection at weeks 22 and 24 is shown to inhibit HSCs-activation related genes and cirrhosis-promoting genes with hepatocytes regeneration (Figure 2D). On the contrary and as it has been reported that during chronic damage there is usually imbalance between TIMPs and MMPs, marked reduction in MMP-9 mRNA expression level was observed that has been abrogated by MNCs treatment.
Human MNCs induce up-regulation of APE1 expression and reduce HSCs-activation related genes
The APE1 and p53 mRNA and protein levels in cirrhotic versus MNCs-treated rats were analyzed by RT-PCR and western blotting. TAA-administration induced pathological hepatocytes damage accompanied with reduced level of p53 as shown in Figures 4A and 4B. MNCs injection inhibited HSCs activation and liver regeneration as confirmed by induction of HSCs apoptosis and the appearance of apoptotic bodies (as represented in histological results). In addition to, a marked elevation in p53 mRNA level was observed at week 22. P53 protein positively activates p21 promoter and induces p21 gene expression therefore we evaluated the change in p21 mRNA level as positive control for transcriptional activity p53 (Figures 3A and 3B). On the contrary SOD gene expression has been reported to be down-regulated by p53 therefore it has been used as a negative control for p53 transcriptional activity. SOD mRNA level was reduced significantly in consistent with the general decline in SOD activity detected in total liver homogenate during the course of cirrhosis development (Figure 3A). In the present study we evaluated for the first time the possible modulation of APE1 mRNA and protein levels during the course of cirrhosis development. Because APE1 has been considered pro-survival protein, its down regulation was reported to be associated with cell death. APE1 protein levels in the cytoplasmic fractions were reduced significantly during HSCs-activation time course (at weeks 8, 16 and 20) then its level was significantly elevated and up-regulated during human MNCs-induced hepatocytes regeneration. GAPDH was used as an invariant internal control for results validation (Figures 3A, 3B and 3D). Change in APE1 mRNA level was confirmed by real-time PCR analysis of the induced versus treated rats (Figure 3C). Moreover, immunohistochemical analysis of APE1 expression of MNCs-treated rats at week 22 indicated strong cytoplasmic expression of APE1 consistent with the western blotting analysis results as shown in Figure 3E.
Figure 4.
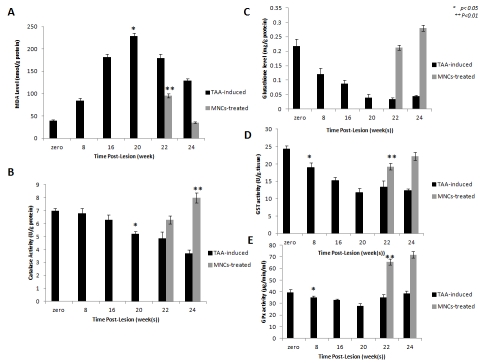
Antioxidant versus pro-oxidant capacity of TAA-induced versus human MNCs-treated rats. a) MDA level was significantly (p < 0.05) elevated in TAA-induced rats versus control (at 0 time). MNCs injection improved the TAA-induced oxidative stress as indicated by significant reduction (p < 0.05) in MDA level at weeks 22 and 24. c) Marked reduction in GSH was observed followed by human elevation in GSH level after MNCs treatment induced significant, b, d, and e) Catalase, GST and GPx activities in TAA-induced were reduced (p<0.05). Treatment of rats with cirrhotic liver by injection of human MNCs, increased the antioxidant enzymes activities significantly (p <0.05). The statistical significance was indicated by *from control and **between MNCs-treated versus mock-treated rats.
MNCs-induce elevation in total antioxidant capacity of cirrhotic rats’ liver
Total anti- versus pro-oxidant parameters including MDA and GSH levels, as well as the activities of catalase, GPx, and GST were determined. All parameters were assayed in the liver homogenates of the experimental rats of groups 1, 2, and 3 that has been collected at weeks 0, 8, 16 and 20 of TAA-administration and at weeks 22 and 24 of MNCs injection. MDA level was increased during TAA administration. GSH as well as the activities of the enzymes catalase, GPx, and GST were reduced significantly during the time course of TAA-induction. Intra-hepatic injection of human MNCs restored gradually hepatocytes function with an overall improvement of total antioxidant capacity compared to mock-treated rats. A significant elevation in catalase, GPx, and GST activities as well as GSH level accompanied with significant reduction in MDA level in the liver tissues of MNCs-treated rats for 2 and 4 weeks (weeks 22 and 24) was observed (Figure 4).
Human MNCs treatment attenuates pro-inflammatory cytokines production
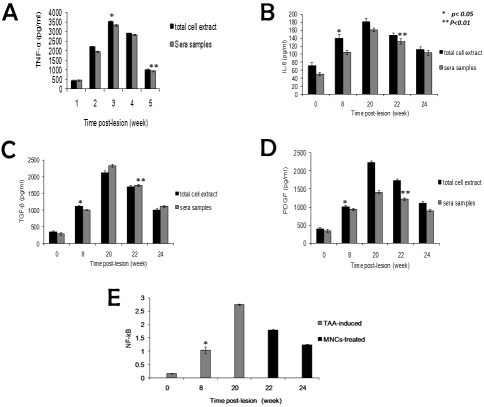
In the present study we investigate the expression level of the pro-inflammatory cytokines TGF -β, TNF-α, IL-6, and PDGF in liver total cell extracts and sera samples collected from sacrificed rats from all experimental groups. Furthermore NF-κB was determined in nuclear extracts. The most prominent profibrogenic cytokine is transforming growth factor β which suppresses inflammation, but drives fibrogenic gene expression in these Myofibroblasts [39]. Therefore we determined TGF- β expression at mRNA and protein levels. TAA-induced HSCs activation induced significant increase in TGF-β, TNF-α, IL-6, and PDGF in both total cell extracts and sera samples in time-dependent manner with progression of TAA-induced liver cirrhosis at weeks 8, 16 and 20 as shown in Figures 4 and 5. In addition to NF-κB is a redox-sensitive transcription factor which has been implicated in stimulating an increase in inflammation in the injured liver was significantly elevated in total cell extracts of TAA-induced rats at different time points (Figure 5). Human MNCs treatment of injured liver abrogated HSCs-mediated inflammatory cytokines production as demonstrated by gradual significant reduction in TGF-β, TNF-α, IL-6, PDGF and NF-κB levels at weeks 22 and 24 (Figure 5).
Figure 5.
Time course study of the alterations in pro-inflammatory cytokines TNF-α, TGF-β, IL-6 and PDGF levels in sera and liver total cell extracts as well as NF-κB in total cell extracts of TAA-induced versus human MNCs-treated rats. Our results indicated significant elevation (p< 0.05) in TNF-α, TGF-β (a & b), IL-6 and PDGF levels (c &d) (p< 0.05) during the course of TAA-administration. Human MNCs injection induced HSCs apoptosis and significantly reduced (p<0.05) their levels at weeks 22 and 24. e) Data indicate significant elevation (p<0.05) in NF-κB during the follow up period of cirrhosis development and MNCs injection showed significant reduction (p <0.05) in NF-κB at weeks 22 and 24.* used to indicate the significance from control, and ** to indicate the significance between groups.
Discussion
Cirrhosis is a progressive liver disease and is marked by the gradual destruction of liver tissue over time. Persistent injuries lead to hepatic scarring (fibrosis), which, if unopposed, leads to cirrhosis and demise of liver function. Cell therapy has emerged as a novel approach for the treatment of many human degenerative diseases. The aim of this study was to investigate the value of cord blood (CB) cell therapy approach to the reduction of hepatic cirrhosis.
Considering the growing use of (CB) for hematological reconstitution, its widespread availability and the potential use in non-hematopoietic related-diseases, it is an attractive source for tissue regeneration [40-41]. For the treatment of liver injuries, the collection of stem cells from CB of a placental umbilical cord stump is simpler compared to the more complex collection from bone marrow or from embryonic human liver. Furthermore, a paramount advantage of CB stem cells over adult stem cells is that they possess a primitive ontogeny and have not been exposed to immunologic challenge. This rather naïve immune system of CB cells may play a significant role in reduced rejection after their transplantation into a mismatched host [40].
In the present work, we have performed a chronic study in which experimental cirrhosis model was effectively induced by thioacetamide combined with alcohol in drinking water during the last 4 weeks of treatment. This experimental model of liver cirrhosis induced in rats by administration of TAA reproduces many of the histological and biochemical alterations that appear in the human disease [42-43]. The TAA model was selected for the study because of these similarities. Even the malnutrition exhibited by human patients was present in the cirrhotic rats, as evidenced by the weight of the rats.
Thioacetamide could directly damage hepatocytes, thus leading to fatty degeneration and necrosis of hepatocytes. During formation of cirrhosis, the body weight of rats was dramatically reduced within the first 5 wk (the mean loss body mass was 35.40 g ± 8.34 g in the first 4 wk.) and then slowly increased within 5-9 wk (increased by 19.44 g ± 6.78 g in the later 4 wk). From wk 8, the body weight of rats slightly increased in treatment group with no obvious change in control group, showing that rats become adapted to toxicity.
Twenty weeks after developing hepatic cirrhosis based on histological, tissue and serum analyses, we injected the animals with human cord blood mononuclear cells intrahepatically. The general conditions of rats improved after infusion of human embryonic mononuclear cells within ten days, suggesting that mononuclear cells can improve liver cirrhosis. Consistent with other studies [44-45] MNCs administration could repair injured liver by reducing inflammation, collagen deposition and remodeling. In the current study, transplanted mononuclear cells could degrade collagen fibers and improve liver fibrosis.
Xenotransplantation of the human UCB-MNCs to rats, intrahepatically, was examined by the histopathology, immunohistochemistry and clinical biochemistry.
The results of the histopathological analysis showed that the MNCs-administered groups exhibited a major reduction in collagen fiber to 396.8 ± 116.3 mg/g liver in the treatment group compared to 566.0 ± 237.4 mg/g liver in control group. It was suggested that the MNCs could replace the damaged hepatocytes of the recipient and could decompose the surrounding collagen fiber through cytokines, such as insulin -like growth factor-1 (IGF-1) as previously reported [44]. The mechanism involved should be further investigated.
In the present study, we used the well-established model of rat liver cirrhosis to gain further insight into the potential role of CTGF in liver fibrogenesis. After liver injury, HSCs undergo a complex transformation or activation process, by which the cells change from quiescent cells to activated myofibroblast-like cells [46]. α-SMA is a cytoskeletal protein that a bio-marker of HSC activation [46]. Analysis of mRNA level revealed that, in MNCs-treated livers, HSC activation is prevented and only very low levels of α-SMA are detected, which further supports that MNCs can inhibit TAA-induced liver cirrhosis.
Consistent with histological findings the mRNA level of cirrhosis-promoting genes; CTGF, TIMP-1, α-SMA and STAP were elevated significantly as shown by RT-PCR analysis. Inversely, the mRNA level of anti-cirrhotic gene MMP-9 was reduced significantly.
A strong body of evidence suggests a crucial role of reactive oxygen species (ROS) in development of chronic liver disease, and evidence of oxidative stress has been detected in almost all clinical and experimental conditions of chronic liver diseases with different etiology and progression rate of fibrosis [47-48]. The increase of ROS, associated with the disease development and induced by developing parenchymal damage, promotes oxidative stress damage to proteins, lipids, and DNA [49], as well as activation of transcription factors such as NF-κB, STAT3, and AP-1 that are either involved in cell-survival pathway [50] or specifically activated in hepato-cellular carcinoma [51-52].
In our study, the histopathology of liver tissue of rats treated by MNCs was significantly improved, including the decrease of hepatocellular necrosis, inflammatory infiltration, and fibroplasias and the resolution of fibro-septa. The effect of MNCs on oxidative stress was confirmed by the oxidative stress parameters, including GSH, catalase, GPx, GST and MDA, by biochemical assays. It was found in this study that MNCs elevated the activity of GPx, catalase and GSH, GST levels, four antioxidant enzymes, and significantly decreases MDA levels, the products of lipid peroxidation, in rat liver cirrhosis.
APE1/Ref-1 is a multifunctional protein representing a central factor during cell response to oxidative stress through the modulation of transcriptional activation, as in the case of AP-1 [53], NF-κB [54], and DNA repair functions.
To our knowledge, this is the first time to report that altered expression of APE1, a redox sensitive protein, is involved in liver insult from fibrosis followed by cirrhosis. Change in APE1 gene expression during liver cirrhosis development is correlated with induced-hepatic stellate cells (HSCs) activation and consequent pathological hepatocytes cell damage. The mechanism by which elevated APE1 level protects cells against induced inflammation is not fully understood. Protein expression of APE1/Ref-1 was significantly higher during the first 3 weeks of thioacetamide treatment then started to decline during the 4th week. Again after 4 week of treatment (week 24) p53 started to decline with elevation of APE1. This is the typical response of p53 and APE1 in normal liver. This is consistent with our previous studies [8]. MNCs intrahepatic injection induced HSCs apoptosis as shown by histological analysis. Daily oral administration of E3330 (10-100 mg/kg) during 4 week of treatment significantly attenuated the liver regeneration
Tissue inhibitor metalloproteinase-1 (TIMP-1) is produced by Kupffer cells, HSCs, and myofibroblasts in the liver, but most part of it is produced mainly by activated HSCs [55-57]. The results of this study suggest that in rat liver cirrhosis, MNCs significantly increase the expression of TIMP at mRNA level.
TGF-β plays a key role in the activation of HSC and extracellular matrix remodeling in liver fibrosis [58]. In our study, MNCs may exert its effect on oxidative stress to prevent the progression of liver cirrhosis via inhibition of the TGF-β pathway and the level of NF-κB. This was confirmed by the significant inhibition of the expression of TGF-β at mRNA level by MNCs treatment. Our experimental results suggest that in rat liver cirrhosis, MNCs significantly decrease the levels of NF-κB.
PDGF is a major factor that stimulates HSC stimulation and migration [59]. TNF-α and IL-6 have been documented as “pro-inflammatory cytokines” in various liver disease states [3, 60]. In TAA rats, elevated hepatic levels of TNF-α and IL-6 indicated an inflammatory state in the rat liver subjected to TAA. Our results confirm and agree with Duan et al. [61] work where activation of Kupffer cells results in production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6. TNF-a activates the nuclear factor-kappa B (NF-κB) transcription factor in macrophages and hepatocytes. This response leads to secretion of IL-6, mainly from Kupffer cells, which in turn activates the transcription factor signal transducer and activator of transcription (STAT) 3 in hepatocytes.
RT-PCR, ELISA, and immunochemistry showed abundant amounts of human liver-specific mRNA and protein species in the rat liver and serum within 2 weeks after transplantation, when the animals were sacrificed. Detection of human liver protein generated by MNCs-derived hepatocytes in the serum of an animal model is in full agreement with Mark Zern group study [62]. Of note, there was no signal for AFP, suggesting that the cells were well-differentiated and/or become more differentiated in vivo, and we found no tumors in the livers of rats that received MNCs up to 8-week experimental period.
It remains unclear about the long-term fate of the engraftment, and some unexpected effects may be encountered during their application. Large-scale controlled and double-blinded clinical trials are required before this cell transplantation becomes a regular therapy.
Conclusion
In conclusion, APE1/Ref-1 is a multifunctional protein playing a central role in cellular response to oxidative stress, involving both control of gene expression and maintenance of genome stability and its down-regulation may be involved in the pathogenesis of liver injury. MNCs-induced hepatocytes regeneration restored APE1 expression. Finally, our study is the first to demonstrate the possible protective effect of APE1 against oxidative stress-induced hepatocytes damage during severe liver insult and suggest that maintaining high level of APE1 expression could prevent and/or inhibit the progression of induced liver damage.
Acknowledgments
This work was partially supported by BA/CSSP postdoctoral research grant for the year 2010, as well as from FP7 project number 245807 "N€uromed". We are grateful to Professor Marc Landry, Université Bordeaux Segalen, Chef d'équipe « Mécanismes centraux de sensibilisa-tion à la douleur» for his advice and support.
The costs of publication of this article were defrayed by the STDF project number 513 of Dr Marie Moftah. We thank excellent technical assistance of Dr. Ehsan Hassan at Pathology Department, National Hepatology and Tropical Medicine Research Institute, Cairo, Egypt for performing Histopathological and immunohistochemical analyses.
References
- 1.Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086–3098. doi: 10.3748/wjg.15.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Possamai LA, Antoniades CG, Anstee QM, Quaglia A, Vergani D, Thursz M, Wendon J. Role of monocytes and macrophages in experimental and human acute liver failure. World J Gastroenterol. 2010;16:1811–1819. doi: 10.3748/wjg.v16.i15.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Lewis DA, Yi Q, Travers JB, Spandau DF. UVB-induced senescence in human keratinocytes requires a functional insulin-like growth factor-1 receptor and p53. Mol Biol Cell. 2008;19:1346–1353. doi: 10.1091/mbc.E07-10-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 7.Seemann S, Hainaut P. Roles of thioredoxin reductase 1 and APE/Ref-1 in the control of basal p53 stability and activity. Oncogene. 2005;24:3853–3863. doi: 10.1038/sj.onc.1208549. [DOI] [PubMed] [Google Scholar]
- 8.Zaky A, Busso C, Izumi T, Chattopadhyay R, Bassiouny AR, Mitra S. Regulation of the human AP-endonuclease (APE1/Ref-1) expression by the tumor suppressor p53 in response to DNA damage. Nucleic Acids Res. 2008;36:1555–1566. doi: 10.1093/nar/gkm1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozue A, Satoshi H, Yasuaki K, Yuji O, Iwao S, Yuki Y, Tadashi W, Hiroshi H. A new APE1/ Ref-1-dependent pathway leading to reduction of NF-iB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Research. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adrian K, Teo K, Vallier L. Emerging use of stem cells in regenerative medicine. Biochem J. 2010;428:11–23. doi: 10.1042/BJ20100102. [DOI] [PubMed] [Google Scholar]
- 11.Zhao DC, Lei JX, Chen R, Yu WH, Zhang XMSN, Xiang Li P. Bone marrow-derived mesenchy-mal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc. Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005;105:3786–3792. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- 15.Schoemans H, Theunissen K, Maertens J, Boogaerts M, Verfaillie C, Wagner J. Adult umbilical cord blood transplantation: a comprehensive review. Bone Marrow Transplant. 2006;38:83–93. doi: 10.1038/sj.bmt.1705403. [DOI] [PubMed] [Google Scholar]
- 16.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang WYK, Samuel M, Tan D, Koh LP, Lim W, Linn YC. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444–453. doi: 10.1016/j.bbmt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Broxmeyer H. Umbilical cord transplantation: epilogue. Semin Hematol. 2010;47:97–103. doi: 10.1053/j.seminhematol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pappa K, Anagnou N. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med. 2009;4:423–433. doi: 10.2217/rme.09.12. [DOI] [PubMed] [Google Scholar]
- 20.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Secco M, Zucconi E, Vieira NM, Fogaça LLQ, Cerqueira A, Carvalho MDF, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood. Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- 22.Javazon E, Beggs K, Flake A. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen C. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 24.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cell. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Wang H, Mazzone T. Identification of stem cells from human umbilical cord blood with embryonic and hematopoietic characteristics. Exp Cell Res. 2006;312:2454–2464. doi: 10.1016/j.yexcr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. Journal of Experimental & Clinical Cancer Research. 2011;30:9. doi: 10.1186/1756-9966-30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffith JQ, Farris EJ. lsquo;The Rat in Laboratory Investigations.’. London (Lippincott) 1942;460 [Google Scholar]
- 28.Wang BE, Wang HJ, Zhu JX, Liu EY. Experimental and clinical study of the therapeutic effect of composite salviae multiorrhizae on liver fibrosis. Ganzangbing Zazhi. 1993;1:69–72. [Google Scholar]
- 29.Schreiber E, Matthias P, Müller M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts', prepared from a small number of cells. Nuc Acid Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 31.Burnette N. Western blotting: electrphretic transfer of proteins from sodium dodecyl sulfate polyacrylamide gel to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A Ana Biochem USAP. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 32.Ohkawa H, Ohishi N, Yagi K. Assay of peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 33.Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 34.Rotruck J, Pope A, Ganther H, Swanson A, Hafeman D, Hoekstra W. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 35.Habig W, Pabst M, Jacoby W. Glutathione-S-Transferase the first enzymatic step in mer-capturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 36.Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247–255. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- 38.Riordan S, Williams R. The intestinal flora and bacterial infection in cirrhosis. J Hepatol. 2006;45:744–57. doi: 10.1016/j.jhep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Schuppan D, Krebs A, Bauer M, Hahn E. Hepatitis C and liver fibrosis. Cell Death Differ. 2003;10:59–67. doi: 10.1038/sj.cdd.4401163. [DOI] [PubMed] [Google Scholar]
- 40.Stanevsky A, Goldstein G, Nagler A. Umbilical cord blood transplantation: Pros, cons and beyond. Blood Rev. 2009;23:199–204. doi: 10.1016/j.blre.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Van de Ven C, Collins D, Bradley MB, Morris E, Cairo MS. The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp He-matol. 2007;35:1753–1765. doi: 10.1016/j.exphem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Moreira E, Fontana L, Periago JL, Sa'nchez de Medina F, Gil A. Changes in fatty acid composition of plasma, liver microsomes and erythrocytes in liver cirrhosis induced by oral intake of thioacetamide in rats. Hepatology. 1995;21:199. [PubMed] [Google Scholar]
- 43.Fontana L, Moreira E, Torres MI, Fernandez MI, Rios A, Sanchez de Medina F, Gil A. Serum amino acid changes in thioacetamide-induced liver cirrhosis in rats. Toxicology. 1996;106:197. doi: 10.1016/0300-483x(95)03177-h. [DOI] [PubMed] [Google Scholar]
- 44.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Petersen K, Olesen O, Mikkelsen J. Developmental expression of alpha-synuclein in rat hippocampus and cerebral cortex. Neuroscience. 1999;91:651–9. doi: 10.1016/s0306-4522(98)00596-x. [DOI] [PubMed] [Google Scholar]
- 46.Arthur M, Iredale J, Mann D. Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease, Alcohol. Clin Exp Res. 1999;23:940–943. doi: 10.1111/j.1530-0277.1999.tb04208.x. [DOI] [PubMed] [Google Scholar]
- 47.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 48.Kaplowitz N. Mechanisms of liver cell injury. J Hepatol. 2000;32:39–47. doi: 10.1016/s0168-8278(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 49.Waris G, Tardif KD, Siddiqui A. Endoplasmic reticulum (ER) stress: hepatitis C virus induces an ER-nucleus signal transduction pathway and activates NF-kappaB and STAT-3. Biochem Pharmacol. 2002;64:1425–1430. doi: 10.1016/s0006-2952(02)01300-x. [DOI] [PubMed] [Google Scholar]
- 50.Liu P, Kimmoun E, Legrand A, Sauvanet A, Degott C, Lardeux B, Bernuau D. Activation of NF-kappa B, AP-1 and STAT transcription factors is a frequent and early event in human hepatocellular carcinomas. J Hepatol. 2002;37:63–71. doi: 10.1016/s0168-8278(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 51.Jüngst C, Cheng B, Gehrke R, Schmitz V, Nischalke HD, Ramakers J, Schramel P, Schirmacher P, Sauerbruch T, Caselmann WH. Oxidative damage is increased in human liver tissue adjacent to hepatocellular carcinoma. Hepatology. 2004;39:1663–1672. doi: 10.1002/hep.20241. [DOI] [PubMed] [Google Scholar]
- 52.Bassiouny AR, Zaky A, Neenaa HM. synergistic effect of celecoxib on 5-flurouracil-induced apoptosis in hepatocellular carcinoma patients. Annals of hepatol. 2010;9:410–418. [PubMed] [Google Scholar]
- 53.Maso V, Avellini C, Crocè L, Rosso N, Quadrifoglio F, Cesaratto L, Codarin E, Bedogni G, Beltrami C, Tell G, Tiribelli C. Subcellular Localization of APE1/Ref-1 in Human Hepatocellular Carcinoma: Possible Prognostic Significance. Mol Med. 2007;13:89–96. doi: 10.2119/2006-00084.DiMaso. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsukada S, Parsons C, Rippe R. Mechanisms of liver fibrosis. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2006;364:33–60. doi: 10.1016/j.cca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Arthur M. Fibrogenesis II Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol. 2000;279:245–249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 56.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850–860. doi: 10.1053/jhep.2002.35625. [DOI] [PubMed] [Google Scholar]
- 57.Cao X, Kambe F, Ohmori S, Seo H. Oxidoreductive modification of two cysteine residues in paired domain by Ref-1 regulates DNA-binding activity of Pax-8. Biochem Biophys Res Commun. 2002;297:288–293. doi: 10.1016/s0006-291x(02)02196-4. [DOI] [PubMed] [Google Scholar]
- 58.Friedman S. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 59.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 60.Tilg H, Moschen AR. Adipocytokines mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 61.Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, Gambhir SS, Zern MA Differentiation and enrichment of hepatocytes-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- 62.Venugopal S, Chen J, Zhang Y, Follenzi A, Clemens D, Wu J, Zern M. Role of MAP kinase phosphatase-1 in sustained activation of c-Jun N-terminal Kinase (JNK) during ethanol-induced apoptosis in hepatocytes. J Biol Chem. 2007;282:31900–31908. doi: 10.1074/jbc.M703729200. [DOI] [PubMed] [Google Scholar]