Abstract
The author herein reports a very rare case of pure small cell carcinoma of the esophagus with an emphasis on KIT and PDGFRA. A 72-year-old man was admitted to our hospital because of dysphagia, and endoscopy showed a tumor in the esophagus. A biopsy of the esophageal tumor showed a small cell carcinoma consisting of malignant small cells with very hyperchromatic nuclei and inconspicuous nucleoli and without any differentiations. An immuno-histochemical study revealed positive reaction for cytokeratin (Dako, Glostrup, Denmark), KIT, PDGFRA, synapto-physin, p53 protein, and CD56, and negative reaction for chromogranin, CD45, CD20, CD3, and CD30. The Ki-67 labeling was 95%. A molecular genetic analysis showed no mutations of KIT and PDGFRA genes. The patient underwent radiation (50 Gray) and chemotherapy (cisplatin, 5 courses), but he developed liver and bone metastases and died of systemic carcinomatosis five months after the initial presentation.
Keywords: Esophagus, small cell carcinoma, KIT, PDGFRA
Introduction
Pure small cell carcinoma of the esophagus is very rare [1]. The author reports herein primary small cell carcinoma of the esophagus with an emphasis on KIT and PDFRRA genes and their products (KIT and PDGFRA), which are trans-membranous tyrosine kinases involved in tumorigenesis of several neoplasms including gastrointestinal stromal tumor (GIST) [2].
Case report
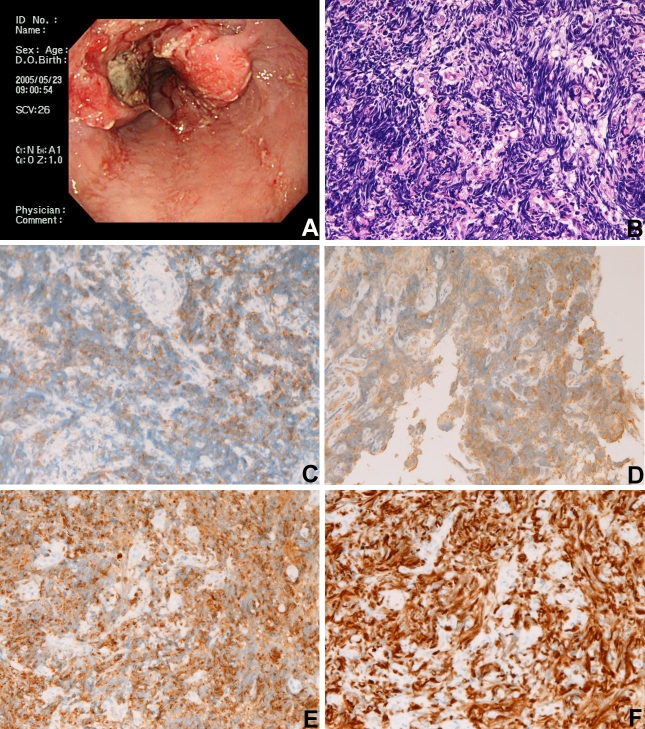
A 72-year-old man was admitted to our hospital because of dysphagia, and endoscopy showed a tumor in the esophagus (Figure 1A). A biopsy of the esophageal tumor showed a pure small cell carcinoma consisting of malignant small cells with very hyperchromatic nuclei and inconspicuous nucleoli and without any differentiations (Figure 1B).
Figure 1.
A: Endoscopic findings of an esophageal tumor; B: Biopsy histology of the esophageal tumor. It is small cell carcinoma. HE, x200; C: KIT protein is positive in the membrane. KIT immunostaining, x200; D: PDGFRA protein is positive in the membrane. PDGFRA immunostaining, x200; E: Synaptophysin is positive. Immunostaining, x200; F: KI-67 (MIB-1) labeling is 95%. Immunostaining, x200.
An immunohistochemical study was performed with the use of using Dako's EnVision method, as previously reported [3,4]. It revealed positive reaction for cytokeratin (Dako, Glostrup, Denmark), KIT (Dako) (Figure 1C), platelet derived growth factor receptor-α (PDGFRA) (Santa Cruz, CA, USA) (Figure 1D), synaptophysin (Figure 1E), p53 (Dako), and CD56 (Dako), and negative reaction for chromogranin (Dako), CD45 (Dako), CD20 (Dako), CD3 (Dako), and CD30 (Dako). The Ki-67 labeling (MIB-1, Dako) was 95% (Figure 1F).
A molecular genetic analysis was performed with the use of PCR-direct sequencing method, as previously reported [5-10]. In brief, genomic DNA was extracted from paraffin blocks with proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94°C for one minute, 52°C °C for one minute, 72°C for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The primers are shown in Table 1. The annealing temperature was 53°C. PCR products were extracted and subjected to a computed automatic DNA sequencer (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems, ABI, CA). Two cases of gastric GISTs were used as positive controls, and two uterine leiomyomas as negative controls. The analysis showed no mutations of KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12 and 18) genes.
Table 1.
Primer sequence
| Forward | Reverse |
|---|---|
| KIT exon 9 | |
| 5'-TCC TAG AGT AAG CCA GGG CTT-3' | 5'-TGG TAG ACA GAG CCT AAA CAT CC-3' |
| KIT exon 11 | |
| 5'-GAT CTA TTT TTC CCT TTC TC-3' | 5'AGC CCC TGT TTC ATA CTG AC-3' |
| KIT exon 13 | |
| 5'-GCT TGA CAT CAG TTT GCC AG -3' | 5'-AAA GGC AGC TTG GAC ACG GCT TTA-3' |
| KIT exon 17 | |
| 5'-CTC CTC CAA CCT AAT AGT GT-3' | 5'-GTC AAG CAG AGA ATG GGT AC-3' |
| PDGFRA exon 12 | |
| 5'-TTG GAT ATT CAC CAG TTA CCT GTC-3' | 5'-CAA GGG AAA AGC TCT TGG-3' |
| PDGFRA exon 18 | |
| 5'-ACC ATG GAT CAG CCA GTC TT-3' | 5'-TGA AGG AGG ATG AGC CTG ACC-3' |
The patient underwent radiation (50 Gray) and chemotherapy (cisplatin, 5 courses), but he developed liver and bone metastases and died of systemic carcinomatosis five months after the initial presentation. Autopsy was not performed.
Discussion
Small cell carcinoma can occur in any organ, but the vast majority develops in the lung. Small cell carcinoma is a very aggressive tumor and the prognosis is very poor, as in the present case. The present case is the first report of esophageal pure small cell carcinoma that examined KIT and PDGFRA proteins and KIT and PDGFRA genes. The present case showed KIT and PDGFRA expression. KIT has been reported to be expressed in 30-80% of small cell lung carcinoma [11,12]. The present case shows that esophageal small cell carcinoma also expresses KIT protein. No studies of PDGFRA protein has been reported in small cell carcinoma. The present study showed PDGFRA expression, suggesting that small cell carcinoma of the esophagus expresses this oncoprotein. The present case did not show mutations of KIT and PDFGRA genes. Most reports of small cell lung carcinoma have shown no mutations in KIT genes [11], except for Boldrini et al. [12] who found five mutations in 60 small cell lung carcinomas. On the other hand, Sihto et al. [11] showed no KIT mutations in 31 small cell lung carcinomas. More studies of KIT mutations remain to be performed in small cell carcinoma. With regard to PDFGRA mutations, Sihto et al. [11] showed no mutations in 31 small cell lung carcinomas. They insisted that KIT expression in small cell lung carcinoma is due not to KIT gene mutations but to KIT gene amplification [11].
Among many KIT-positive tumors, GIST is representative [1]. It is thought that GIST arises from interstitial cell of Cajal, a pacemaker neuronal cell that normally expresses KIT protein [1]. In contrast, small cell carcinoma is an undifferentiated carcinoma with neuroendocrine phenotypes. The original cells of small cell carcinoma is unknown. Recently, Blumming et al. [13] found that GIST expresses synaptic vesicle proteins, and suggested that GIST has endocrine features. Therefore, it is suggested that there may be an association between GIST and small cell carcinoma in that both have neuroendocrine features.
Several studies in GIST have revealed that there are minute subclinical microGISTs or “GIST tumorlets” in the gastrointestinal tract [14-16]. The incidence of these is about 20%, and these are considered as GIST precursors. Frequent KIT mutations (about 46%) and occasional PDGFRA mutations (about 4%) are present in these “GIST tumorlets” [14]. However, these “GIST tumorlets” do not always develop into clinical GIST. Other genetic events are necessary for the development of clinical GIST. In contrast, little is known about the precursor lesions in small cell carcinoma.
Recently, phosphorylation (activation) status of KIT and PDGFRA has been studies [17, 18]. This is particularly important in KIT mutation-negative tumors as in the present case. KIT kinase activation and downstream signaling proteins leading to tumorigenesis have been studied, but little is known as yet. Protein kinase C-theta and PI3-kinase/AKT are activated in imatinib-resistant GIST [17, 18, 19], and analyses of these KIT signaling molecules may be important in the treatment of GIST. Such studies are not performed in small cell carcinoma. In the present study, the author could not investigate these molecules, because no relevant antibodies were available. KIT tyrosine kinase activity and KIT signaling abnormalities in small cell carcinoma remain to be elucidated.
In summary, the author reported a very rare case of esophageal small cell carcinoma with KIT and PDGFRA expressions but without KIT and PDGFRA mutations.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Koide N, Saito H, Suzuki A, Sato T, Koiwai K, Nakamura N, Miyagawa S. Clinicopathologic features and histochemical analuses of proliferative activity and angiogenesis in small cell carcinoma of the esophagus. J Gastroenterol. 2007;42:932–938. doi: 10.1007/s00535-007-2114-0. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissue, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 3.Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Oncol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 4.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. Would J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Oncol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 6.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 7.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 9.Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol. 2010;15:453–456. doi: 10.1007/s10147-010-0087-0. [DOI] [PubMed] [Google Scholar]
- 10.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohistochem Mol Morphol. 2011 doi: 10.1097/PAI.0b013e31820d2872. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Sihto H, Sarlomo-Rikara M, Tynnienen O, Tanner M, Andersson LC, Franssila K. Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 12.Boldrini L, Ursino S, Gisfredi S, Faviana P, Donati V, Camcci T, Lucchi M, Mussi A, Basolo F, Pingitore R. Fontanini G. Expression and mutational status of c-kit in small-cell lung cancer: prognostic relevance. Clin Cancer Res. 2004;15:4101–4108. doi: 10.1158/1078-0432.CCR-03-0664. [DOI] [PubMed] [Google Scholar]
- 13.Blumming P, Nilsson O, Ahlman H, Welbencer A, Andersson MK, Sjolund K, Nilsson B. Gastrointestinal strumal tumor regularly express synaptic vesicle proteins: evidence of a neuroendocrine phenotype. Endocr Relat Cancer. 2007;14:853–863. doi: 10.1677/ERC-06-0014. [DOI] [PubMed] [Google Scholar]
- 14.Agaimy A, Wunsch PH, Hofstaedter F, Blaszyk H, Rummele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113–120. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]
- 15.Kawanoya K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Kanajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Agaimy A, Wunsch PH, Dirnhofer S, Bihl MP, Terracciano LM, Tornillo L. Microscopic gastrointestinal stromal tumors in esophageal and interstinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 cases. Am J Surg Pathol. 2008;32:867–873. doi: 10.1097/PAS.0b013e31815c0417. [DOI] [PubMed] [Google Scholar]
- 17.Ou WB, Zhu MJ, Demetri GD, Fletcher CD, Fletcher JA. Protein kinase C-theta regulates KIT expression and proliferation in gastrointestinal stromal tumor. Oncogene. 2008;27:5624–34. doi: 10.1038/onc.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in inatinib -resistant gastrointestinal stromal tumor: PI1-kinase/AKT is a crucial survival pathway. Oncogene. 2007;29:7560–7568. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]
- 19.Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int J Clin Exp Pathol. 2010;3:461–71. [PMC free article] [PubMed] [Google Scholar]