Abstract
A 60-year-old man presented with dysuria and elevated PSA (6.95 ng/ml). Needle biopsies of the prostate revealed well differentiated adenocarcinoma of Gleason's score 6. Prostatectomy and bilateral seminal vesiculotomy were performed. The material was totally cut into 16 preparations. The prostate showed well differentiated adenocarcinoma. The left seminal vesicle showed intraluminal monstrous large epithelial cells with acidophilic cytoplasm and hyperchromatic nuclei, simulating carcinoma cells. Lipochrome pigment was present in the monstrous cells, and some monstrous cells showed large bizarre nuclei. Such monstrous cells were also present in the mucosal seminal vesicle epithelium, and gradual merge between the intraluminal and mucosal monstorous epithelium. Immunohistochemically, the monstrous epithelial cells showed the following reactions: pancytokeratin (AE1/3, CAM5.2) +, cytokeratin (CK) 5/6 +, CK34βE12 -, CK7 +, CK8 -, CK14 -, CK18 +, CK19+, CK20 -, Ki-67 0%, p53 -, P63 -, NSE -, CEA -, EMA -, CA19-9 -, ER -, PgR -, HER2 -, HepPar1 -, CD34 -, CD10 +, PSA -, AMACR -, Desmin -, ASMA -, CD68 -, S100 -, CD45 -, synaptopysin -, TTF-1 -, CDX-2 -, MUC1 -, MUC2 -, MUC5AC - MUC6 +, CD56 -, PAS -, dPAS -, and alcian blue +. The immunoprofile of normal seminal vesicle epithelium was as follows: pancytokeratin (AE1/3, CAM5.2) +++, cy-tokeratin (CK) 5/6 +++, CK34βE12 -, CK7 +++, CK8 +, CK14 -, CK18 +++, CK19, +++, CK20 -, KI-67 1%, p53 -, P63 +++, NSE -, CEA - EMA -, CA19-9 -, ER -, PgR -, HER2 +, HepPar1 -, CD34 -, CD10 +, PSA -, AMACR -, Desmin -, ASMA -, CD68 -, S100 - , CD45 -, synaptopysin -, TTF-1 -, CDX-2 -, MUC1 -, MUC2 -, MUC5AC -, MUC6 +++, CD56 -, PAS -, dPAS -, and alcian blue +. That is, the immunophenotype was very similar but much weaker in monstrous cells than in normal seminal vesicle epithelium. These findings suggest that the monstrous seminal vesicle epithelial cells are degenerative changes. The monstrous epithelial cells should not be mistaken for carcinoma.
Keywords: Seminal vesicles, monstrous epithelial cells
Introduction
Monstrous (monster) epithelial cells (MEC) of the seminal vesicle are bizarre epithelial cells. They were first described by Peters and Frank [1] in 1952 in cytologic specimens of prostatic smears. Later in 1958, Arias-Stella and Takano-Moron [2] histologically identified peculiar atypical cells in the seminal vesicles. Kuo and Gomez [3] in 1981 named these cells “monstrous epithelial cells”, and stressed that these cell should not been mistaken for carcinoma cells. These MEC in the seminal vesicles had not been described thereafter in the English literature, to the best of the author's knowledge. MEC of the seminal vesicles is not written in Major Pathology textbooks including Robin's Pathology [4] and Rosai and Ackermann's Surgical Pathology [5], but MEC is briefly mentioned in Silver-berg's “Histology for Pathologists” [6]. The author recently encountered a patient with florid proliferation of MEC of the seminal vesicles. Herein, reported is this case.
Case report
A 60-year-old man was admitted to our hospital because of mild dysuria and elevated PSA (6.95 ng/ml). Needle biopsies of the prostate revealed well differentiated adenocarcinoma of Gleason's score 6. Prostatectomy and bilateral seminal vesiculotomy were performed. The specimen was totally cut into 16 preparations. The prostate showed well differentiated adenocarcinoma without lymph node invasion.
The left seminal vesicle showed a large amount of intraluminal monstrous large epithelial cells with acidophilic cytoplasm and hyperchromatic nuclei, simulating carcinoma cells (Figure 1A). Lipochrome pigment was present in the monstrous cells (Figure 1B), and some monstrous cells showed very large bizarre nuclei (Figure 1C). Such monstrous cells were also present in the mucosal seminal vesicle epithelium in single or clustered patterns (Figure 1D), and gradual merge between the intraluminal and mucosal monstrous epithelium (Figure 1E). The right seminal vesicle was normal.
Figure 1.
Histological features. A: Diffuse atypical epithelial cell proliferation is seen in the lumen of the seminal vesicle. HE, x5. B: The atypical cells show ample acidophilic cytoplasm and large nuclei. Lipochrome pigment is seen. HE, x200. C: Some monstorous cells show giant grotesque nuclei. D: The mucosa of the seminal vesicle shows mucosal monstrous epithelial cells (arrows). The right side is intraluminal monstrous epithelial cells. HE, x200. E: Transitions between mucosal mucosal epithelial cells to intraluminal monstrous cells are seen. HE, x200.
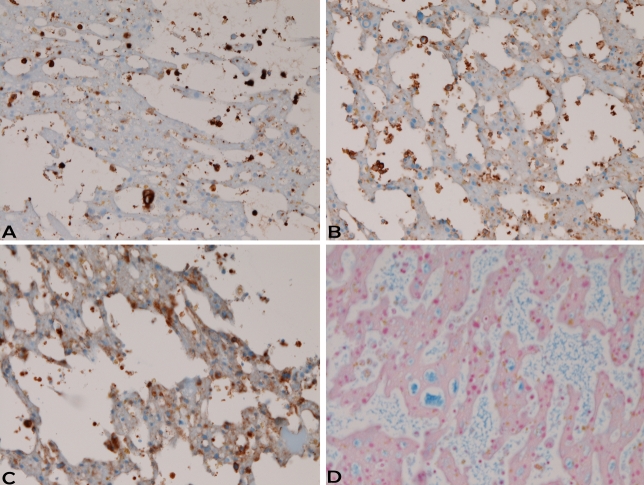
An immunohistochemical study was performed with the use of Dako's envision method, as previously described [7, 8]. Immunohistochemically, the MEC of both intraluminal and mucosal areas showed the following reactions: pancytokeratin (AE1/3, CAM5.2) + (Figure 2A), cytokeratin (CK) 5/6 +, CK34βE12 -, CK7 +, CK8 -, CK14 -, CK18 +, CK19+, CK20 -, Ki-67 -(labeling=0%), p53 -, P63 -, NSE -, CEA - EMA -, CA19-9 -, ER -, PgR -, HER2 -, HepPar1 -, CD34 -, CD10 + (Figure 2B), PSA -, AMACR -, Desmin -, ASMA-, CD68 -, S100 -, CD45 -, synaptophysin -, TTF-1 -, CDX-2 -, MUC1 -, MUC2 -, MUC5AC -MUC6 + (Figure 2C), CD56 -, PAS -, dPAS -, and alcian blue + (Figure 2D).
Figure 2.
Immunohistochemical features. Intraluminal monstrous cells were weakly positive for pancytokeratin AE1/3 (A), CD10 (B), MUC (6), and alcian blue (D). A,B,C,D: x200
The immunoprofile of normal (non-monstrous cells) seminal vesicle epithelium was as follows: pancytokeratin (AE1/3, CAM5.2) +++, cytokeratin (CK) 5/6 +++, CK34βE12 -, CK7 +++, CK8 +, CK14 -, CK18 +++, CK19 +++, CK20 -, Ki-67 + (Labeling= 1%), p53 -, P63 +++, NSE -, CEA - EMA -, CA19-9 -, ER -, PgR -, HER2 +, HepPar1 -, CD34 -, CD10 +, PSA -, AMACR -, Desmin -, ASMA -, CD68 -, S100 -, CD45 -, synaptophysin -, TTF-1 -, CDX-2 -, MUC1 -, MUC2 -, MUC5AC -, MUC6 +++, CD56 -, PAS -, dPAS -, and Alcian blue +. The patient is now free from tumor, and discharged.
Discussion
Diseases of seminal vesicles are small in number, and pathologists, like the author, are not familiar with the pathology of seminal vesicles. In the present study, the author presented a case of MEC in the seminal vesicles. The present study appears the first case examining immunohistochemistry of MEC, and disclosed CK profile and expression of other antigens. The immunophenotype of MEC was similar to normal seminal vesicle epithelium, but much weaker in MEC than in normal seminal vesicle epithelium. In the present case, the Ki-67 labeling of MEC was 0%, indicating that MEC has no proliferative activity. These findings suggest that MEC is a degenerative change. Previous studies have suggested that MEC is degenerative or hormone-related phenomenon, similar to Arias-Stella phenomenon of the endometrium [3, 6]. MEC is prevalent in old patients and relatively rare in young patients [3]. Kuo and Gomez [3] showed that the frequency of MEC in seminal vesicles was 75% (24 cases/ 32 cases). It is not related to prostatic cancer [3].
Unlike previously reported cases of MEC [3, 6], the present case showed large amount of intraluminal MEC forming a tumor-like lesions. Together with atypical features of MEC cells, MEC should not be mistaken for carcinoma. This possibility was also cautioned by Kuo and Gomez [3].
MEC in the present case showed CD10. This phenomenon appears new. Since normal seminal vesicle epithelium showed CD10, the MEC appears retain CD10 after degeneration. MEC in the present study expressed MUC6. Normal seminal vesicle epithelium also showed MUC6; this is already reported by Leroy et al. [9].
The present study showed CK profile and expression of other antigen in the normal seminal vesicle epithelium. This may provide the basic knowledge of seminal vesicle pathology.
Acknowledgments
The author has no conflict of interest.
References
- 1.Peters H, Frank IN. The cytologic interpretation of the prostatic smear. Surg Gynecol Obstet. 1952;94:69–76. [PubMed] [Google Scholar]
- 2.Arias-Stella J, Takano-Moron J. Atypical epithelial changes in the seminal vesicles. Arch Pathol. 1958;66:761–766. [PubMed] [Google Scholar]
- 3.Kuo TT, Gomez LG. Monstrous epithelial cells in human epididymis and seminal vesicles: a pseudomalignant change. Am J Surg Pathol. 1981;5:483–490. doi: 10.1097/00000478-198107000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI. The lower urinary tract and male genital system. In: Kumar V, Abras AK, Fausto N, editors. Robbins and Cortran Pathologic bases of disease. 7the Edition. Philadelphia: Elsevier Saunders; 2005. pp. 1023–1058. [Google Scholar]
- 5.Rosai J. Prostate and seminal vesicles. In: Rosai J, editor. Rosai and Ackermans Surgical Pathology. Ninth edition. Mosby, New York: 2004. pp. 1361–1411. Pp: 1361-1411. [Google Scholar]
- 6.Trainer TD. Testis and excretory duct system. In: Sternberg SS, editor. Histology for Pathologists. New York: Raven Press; 1992. pp. 731–748. [Google Scholar]
- 7.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 8.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 9.Leroy X, Ballereau C, Villers A, Saint F, Aubert S, Gosselin B, Porchet N, Copin MC. MUC6 is a marker of seminal vesicle-ejaculatory duct epithelium and is useful for the differential diagnosis with prostatic adenocarcinoma. Am J Surg Pathol. 2003;27:519–521. doi: 10.1097/00000478-200304000-00013. [DOI] [PubMed] [Google Scholar]