Abstract
Adeno-associated virus is a nonpathogenic human virus that has been developed into a gene-delivery vector due to its high efficiency of infection for many different cell types and its ability to persist and lead to long-term gene expression. This unit describes efficient methods to generate high-titer, research-grade, adenovirus-free recombinant single-stranded and self-complementary adeno-associated virus in various serotypes, along with methods to quantify the viral vectors. Two detailed methods are provided for viral vector delivery into the rodent brain and spinal cord, and for histological detection of transgene expression of GFP.
Keywords: Gene Therapy, Viral Vectors, Adeno Associated Virus, Purification, Serotypes, Transfection, Brain, Spinal Cord, Transduction, Gene Delivery
In this unit, several gene delivery systems for use in central nervous system (CNS) tissues are presented. Recombinant adeno-associated viral (rAAV) vectors are capable of delivering genes to both dividing and nondividing cells. This property, along with the physical stability of the virions, permits transduction of CNS cells both in tissue culture and in the whole animal. In this unit, protocols for the adenovirus-free production of rAAV vectors, their purification, and their quantitation are provided. First, the production and purification of the rAAV vectors are described (see Basic Protocol 1). Three assays for determining rAAV titers are presented: dot blot (see Support Protocol 1), quantitative PCR (qPCR, see Support Protocol 2) and in vitro infection with a transgene expression assay (see Support Protocol 3). Support Protocol 4 describes the production and purification of adenovirus helper stock, which is needed for rAAV titering. A method for administering virus directly to the brain in an intact animal is then detailed (see Basic Protocol 2), as well as a method to administer the vector diffusely to the spinal cord of an intact animal by intrathecal injection (see Basic Protocol 3). Finally, Support Protocol 5 describes methods for detecting green fluorescent protein expression in the brain or spinal cord.
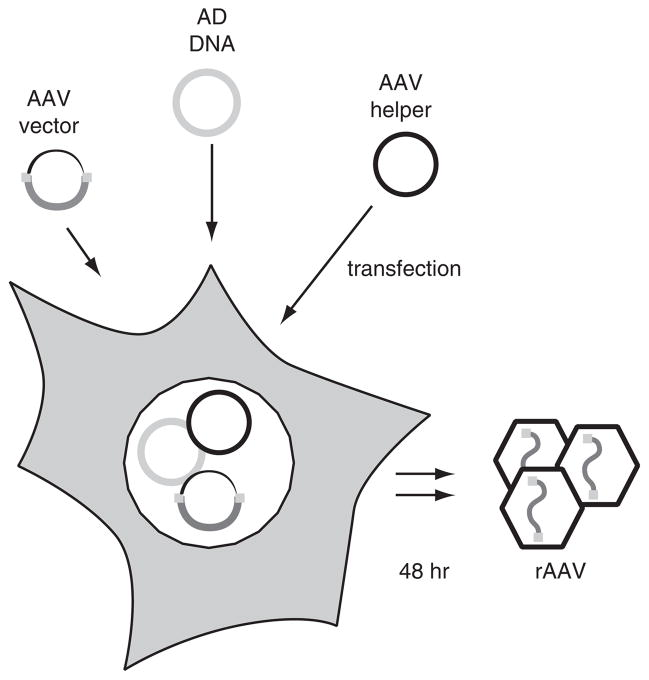
An overview of the rAAV production procedure is shown in Figure 4.17.1. The production of adenovirus-free rAAV particles requires four elements: an rAAV vector plasmid containing the transgene flanked by AAV inverted terminal repeats (ITRs), a plasmid that supplies the AAV viral proteins necessary for replicating and packaging the rAAV sequences (AAV helper plasmid), a plasmid supplying the adenoviral helper genes (Ad helper plasmid), and tissue culture cells. After co-transfection of the plasmids into the tissue culture cells, rAAV is produced and the cells are harvested. The rAAV is then purified by CsCl density gradient centrifugation and dialyzed prior to storage, use in tissue culture cells, or use in animals.
Figure 4.17.1.
Generation of adenovirus-free recombinant adeno-associated virus. 293 cells (which supply the Ad E1 gene) are transfected using three plasmids: the plasmid carrying the transgene (sub201-gene “X”, AAV vector), the plasmid supplying the replication and capsid genes of AAV2 without terminal repeats (pXX2, AAV helper), and the plasmid supplying the adenovirus helper genes E2, E4, and VA RNA genes (pXX6, AD DNA), thereby generating Ad-free rAAV.
This virus can then be used to infect tissue culture cells or can be infused into an intact animal as described in Basic Protocols 2 and 3. Basic Protocol 2 requires small amounts of high-titer rAAV that is slowly infused into the region of interest. This technique provides good transduction and virus spread with minimal cellular damage.
NOTE: All equipment and reagents coming in contact with tissue culture cells (uninfected and infected) and the virus following CsCl gradients should be sterile.
CAUTION: Virus work should be performed in a dedicated tissue culture hood and incubator separate from the hood and incubator used for the maintenance of laboratory cell lines. Proper disposal of virally contaminated materials should be performed.
BASIC PROTOCOL 1
PRODUCTION OF ADENOVIRUS-FREE RAAV BY TRANSIENT TRANSFECTION OF 293 CELLS
This protocol details the construction of rAAV vector plasmids, efficient transient transfection of tissue culture cells with rAAV vector and helper plasmids using a modified procedure, and purification of the rAAV by cesium chloride gradients. The efficient transfection procedure results in more rAAV virions being produced per cell and more productive cells being produced per plate. The CsCl gradient fractionation protocol described produces highly pure virus suitable for any CNS-directed applications, as long as the CsCl is sufficiently dialyzed away.
Materials
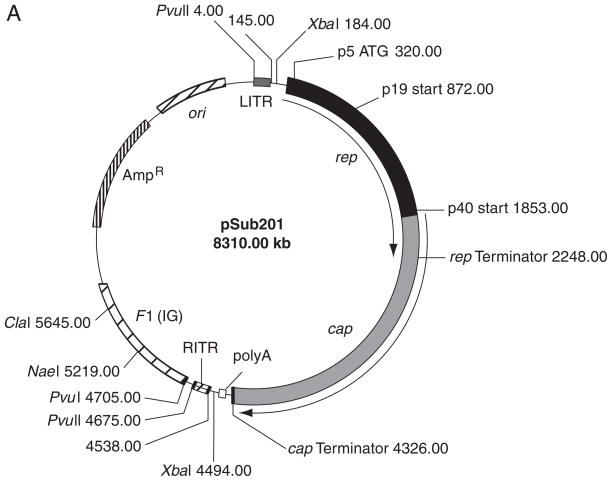
pSub201 plasmid: used to clone the transgene expression cassette between AAV termini to generate single-stranded rAAV vector (Fig. 4.17.2A; ATCC #68065; see Internet Resources for location of a map and the sequence)
XbaI, HindIII, Acc65I (or KpnI), and SalI restriction endonucleases, with appropriate buffers
Plasmid containing the transgene expression cassette with gene of interest
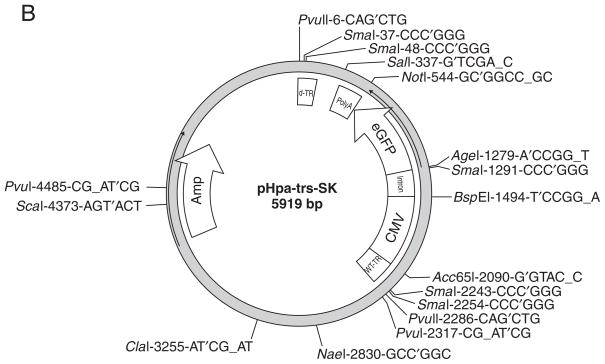
pHpa-trs-SK plasmid: used to clone the transgene expression cassette between AAV termini to generate self-complementary rAAV vector (Fig. 4.17.2B; see Internet Resources for location of a map and the sequence)
pXR series of plasmids (1–9 and others): the AAV serotypes helper plasmid (UNC
Vector Core Facility; see Internet Resources for location of a map and the sequence)
pXX6 plasmid: the adenoviral helper plasmid (UNC Vector Core Facility; see Internet Resources for location of a map and the sequence)
293 tissue culture cell line (ATCC #CRL 1573)
Complete DMEM/10% FBS (see recipe)
0.05% (w/v) trypsin/0.02% (w/v) EDTA
1.3 g/ml, 1.4 g/ml and 1.5 g/ml density CsCl (see recipe)
Phosphate-buffered saline (PBS; APPENDIX 2A)
15-cm tissue culture plates
50-ml disposable polystyrene and polypropylene centrifuge tubes
Cell scrapers
250-ml polypropylene centrifuge bottles
Sorvall centrifuge with GS-3 and SS-34 rotors or equivalents
Sonicator with a 3-mm diameter probe
Tabletop centrifuge
50-ml high-speed polypropylene centrifuge tubes
Ultracentrifuge
NVT65 rotor and corresponding 12-mL Beckman ultraclear tubes (or equivalent rotor and tubes)
Ti70 rotor and 32 mL Beckman Ultra-Clear tubes (or equivalent rotor and tubes)
70% ethanol
21-G needles
Pierce Slide-A-Lyzer dialysis cassettes (MWCO 10,000)
Additional reagents and equipment for restriction digestion of DNA (APPENDIX 1M), gel purification of DNA fragments (CPMB UNIT 2.6), subcloning DNA fragments (CPMB UNIT 3.16), plasmid preparation and CsCl purification (CPMB UNIT 1.7), tissue culture techniques including trypsinization of cells (APPENDIX 3B in this manual), and determination of rAAV titers by dot-blot assay (see Support Protocol 1); also see APPENDIX 1A in this manual
Figure 4.17.2.
(A) pSub201. The plasmid contains the terminal repeats and the replication (rep) and capsid (cap) genes of AAV Type 2. The rep-cap fragment can be replaced by the gene cassette of interest, as only the terminal repeats are needed for packaging. (B) Plasmid pHpa-trs-SK.
NOTE: All tissue culture incubations are performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
Construct rAAV vector plasmid
-
1a
To construct a single-stranded rAAV vector: Digest the plasmid pSub201 (Fig. 4.17.2A) with XbaI and HindIII restriction endonucleases (APPENDIX 1M) to remove the rep and cap fragments, and gel purify the 4000-bp plasmid backbone containing the AAV2 wt-ITRs (CPMB UNIT 2.6). Insert the desired transgene expression cassette between the XbaI sites to construct the single-stranded rAAV vector plasmid (CPMB UNIT 3.16).
For pSub201, HindIII is used in the digest to cut the rep and cap fragment in half for easy isolation of the plasmid backbone. The size of the rAAV construct (including two wild-type ITRs, which are each 145 bases for single-stranded rAAV genome) should be between 3.4 and 4.8 kb, with the optimum size being that of the wild-type AAV genome of 4681 bp. Therefore, the size of the transgene cassette in the cloning plasmid, which includes the gene of interest and the promoter sequences necessary for its expression, should span between 3400 and 4400 bp (for single-stranded rAAV). Filler sequence should be included if the transgene cassette is<3000 bp. -
1b
To construct a self-complementary rAAV vector: Digest the plasmid pHpa-trs-SK (Fig. 4.17.2B) with Acc65I (or KpnI) and SnaBI to remove the CMV-GFP-poly(A) gene expression cassette, gel purify the 4166-bp plasmid backbone containing the mutated AAV2 ITR (left ITR) and wt-ITR (right ITR), and replace it with the desired transgene expression cassette between Acc65I (or KpnI) and SnaBI.
The size of the rAAV self-complementary construct should be approximately half that of a single-stranded construct. The genome will therefore accommodate approximately 2100 bp of foreign DNA between the ITRs. -
2
Verification of ITR integrity: The AAV ITRs are unstable in E. coli, and plasmids that lose the ITRs have a replication advantage in transformed cells. For these reasons, bacteria containing ITR plasmids should not be grown longer than 12–14 hours, and any recovered plasmids should be assessed for retention of the ITRs. The ITRs contain SmaI (or XmaI) sites, and these sites can be used as diagnostics for ITR integrity. Digestion with SmaI (or XmaI) should give a bacterial backbone band corresponding to approximately 4 kb (for pSub201 or pHpa-trs-SK), plus bands that correspond to the size of the insert DNA. The secondary structure of the ITRs does not permit sequencing unless the plasmid is first digested with SmaI (or XmaI) to destroy the ITR secondary structure, and then sequenced from either direction.
DH10B competent cells (or other comparable high-efficiency strain) can be used to transform ligation reactions for ITR-containing plasmid cloning. After screening positive clones for ITR integrity, a good clone should then be transformed into SURE or SURE2 cells (Agilent Technologies) for production of plasmid and glycerol stocks. SURE cells are engineered to maintain irregular DNA structures, but have lower transformation efficiency compared to DH10B.Also see APPENDIX 1A in this manual for the cross-referenced molecular biological techniques. -
3
Purify a large-scale plasmid preparation (at least 1 mg) of the rAAV vector and the suitable AAV helper plasmid and pXX6 plasmids by double CsCl gradient fractionation, commercially-available chromatography-based kits, or other comparable method to recover pure plasmid DNA (CPMB UNIT 1.7).
AAV serotypes 1 to 9 can be generated by using different AAV helper plasmids of the pXR series (e.g., pXR1 for generating AAV serotype 1 capsids). The plasmid preparations must be free of contaminants (ethidium bromide, CsCl, endotoxins, and RNA) before use.
Transfect 293 cells
This procedure results in efficient transfection of 293 cells. A range of 25 to 800 kDa of polyethyleneimine (PEI) has been previously demonstrated to successfully transfect a wide range of different cell types. The 25-kDa linear PEI is employed here for efficient packaging of the plasmids. The transfection procedure described here is for five 15-cm dishes.
Additional Materials (also see Basic Protocol 1)
Serum-free DMEM (e.g., Invitrogen)
1 mg/ml polyethyleneimine (PEI; see recipe)
15-ml polystyrene tubes
-
1
Seed 2 × 107 293 cells in 15-cm dishes to achieve ~70–80% confluency the next morning. Maintain 293 cell line in complete DMEM/10% FBS and split cells before they reach confluency by trypsinizing with 0.05% trypsin/EDTA (APPENDIX 3B).
Cells should be transfected within 24 hr after seeding. This protocol is optimized for transfection of five 15-cm plates at a time. -
2
Prepare the plasmids as described in Basic Protocol 1, steps 1a or b, 2, and 3.
-
3
For transfection of five 15-cm dishes, combine the following in a 15-ml polystyrene tube:
60 μg pXX6 helper plasmid (adenoviral helper genes)
30 μg rAAV vector plasmid
50 μg AAV helper plasmid (AAV helper genes)
-
2.5 ml serum-free DMEM.
The total DNA is equal to 28 μg/plate and the molar ratio of AAV helper plasmid to Ad helper plasmid to rAAV vector plasmid is ~2:3:5. The amount of PEI per μg of DNA is roughly 2 μg.
-
4
Add 150 μl of 1 mg/ml PEI (pH 5). Vortex each tube 5 to 10 sec, then incubate at room temperature for 5 to 10 min.
The transfection solution turns yellowish after addition of PEI due to drop in pH, as indicated by phenol red. -
5
Add the transfection complex from step 4 dropwise to the medium in the plates from step 1 so as to cover the entire area in each plate. Swirl the medium to disperse evenly.
-
6
Repeat steps 3 to 5 to transfect groups of five dishes at a time until all dishes have been transfected.
-
7
Incubate the cells until 42 to 72 hr post-transfection.
Single-stranded AAV preparations can be incubated 48–72 hours. Self-complementary AAV vectors should be incubated 42–52 hours only. If scAAV preparations are incubated for longer time periods, there is increased risk that the genomes will revert to a single-stranded form.
Perform rAAV purification of fractionated cell lysates
In this procedure, incubation with deoxycholate, sonication of the cells, and centrifugation removes much of the cellular debris from the virus particules. This allows a greater number of cells to be processed and results in more concentrated stocks of rAAV following the CsCl gradient. These recipes are for ten 15-cm plates, but can be scaled up two to four times or more.
-
12
Collect the cells and the supernatant from the tissue culture plates by scraping the cells with a cell scraper to collect cells and medium. Alternatively, the cells should be only loosely adherent to the plate at harvest time and can be washed off with a pipette. Transfer the cell/media suspension to 250-ml or 500-mL polypropylene centrifuge bottles.
-
13
Centrifuge the cells at 1000 × g for 10 minutes. Remove the supernatant and discard appropriately.
-
14
Resuspend the pellet with approximately 25-mL of PBS, and transfer to a 50-mL conical centrifuge tube. Centrifuge the cells at 1000 × g for 10 min. Remove the supernatant and discard appropriately.
The pellet can be frozen indefinitely at −80°C at this point or processed immediately -
15
Resuspend the pellet (approximately 1 mL) with 6-mL of PBS and add 0.35-mL of a 10% DOC solution (0.5% final concentration). Mix well by inverting the tube, and incubate at room temperature for 10 min.
-
16
Add 1.4 μL of Benzonase (250 U/μL stock, 0.05 U/μL final concentration) and mix well by inverting the tube. Incubate at 37°C for 45 min.
DNaseI can be used in place of Benzonase as long as equivalent Units of activity are used. -
17
Sonicate the cell pellet (100 bursts, 50% duty, power level 5) in a tissue culture hood dedicated for virus work. The solution should be kept ice cold through sonication, either by keeping on ice during sonication or by sonicating in 25 pulse increments with incubation on ice for 1 minute in between increments.
Sonicating the cells liberates the virus particles, and a turbid cell lysate should be the result. Be sure to use ear protection when using the sonicator. -
18
Centrifuge the tubes for 30 min at 3000 × g in a tabletop centrifuge at room temperature to pellet insoluble debris.
-
19
Collect the clarified supernatant and transfer to a disposable 50-ml polypropylene tube.
Perform sequential CsCl gradient purification
-
20
Move the supernatant to a 32-mL ultraclear centrifuge tube (Beckman, for rotor Ti70). Layer 12-mL of 1.3 g/m3 CsCl from the bottom with a gradient pump, then layer with 1.5 g/m3 CsCl from the bottom until the tube is filled. Mark the border between the 1.3 and 1.5 g/m3 CsCl, then balance the tubes with PBS.
From top to bottom, the tube layers should be as follows: cell lysate, 1.3 g/m3 CsCl, 1.5 g/m3 CsCl. Following centrifugation, AAV should sediment to the interface between the 1.3 and 1.5 g/m3 CsCl. See Figure 4.17.3. -
21
Centrifuge at 360,000 × g (70,000 rpm in a Beckman Ti70 rotor) using a swing-bucket rotor for 1 hr. Remove the centrifuge tubes. Using a needle and syringe, insert the needle a little below the mark (1.3 and 1.5 g/m3 interface), and remove 5–10 mL of the solution. Avoid taking any debris from the top layer.
Caution: when the needle is removed the remaining gradient solution will come out of the needle hole in the centrifuge tube. CsCl is a chemical hazard and should be disposed of properly. -
22
Transfer the virus-containing solution to a 12-mL ultracentrifuge tube (for Sorvall rotor NVT65). Fill the remainder of the tube with 1.4 g/m3 CsCl. Balance and seal the tubes.
-
23
Centrifuge overnight at 366,000 × g (62,000 rpm in a Sorvall NVT65 rotor).
Figure 4.17.3.
CsCl step gradient before and after centrifugation. The step gradient is generated by underlying the virus-containing clarified cell lysate with 1.3 and 1.5 g/m3 CsCl solutions (see Basic Protocol 1 for instructions on forming the gradient). After centrifugation, the virus resides at the interface between the 1.3 and 1.5 g/m3 CsCl solutions. Care should be taken when removing this layer not to remove any of the less-dense cellular debris that remains above the 1.5 g/m3 solution.
Collect rAAV
-
24
Wipe the outside of the centrifuge tube with 70% ethanol and insert a 21-G needle ~1 cm from the bottom of the tube at a 90° angle and allow the gradient to drip into sterile microcentrifuge tubes. Collect 12 fractions of 1-mL each.
Typically, multiple gradients of the same rAAV are collected into one set of tubes (i.e., puncture the Ultra-Clear tubes at the same place and collect the same number of drops into the collection tubes). Collecting smaller fractions (e.g., 0.5-mL) will more sharply define the peak fractions and result in more concentrated virus. -
25
Assay 1 μl of each fraction by dot blot or qPCR using a rAAV-specific probe to find the peak (see Support Protocol 1 or 2).
The rAAV peak should band in the gradient with a density of 1.40 to 1.42 g/ml, and this can be checked with a refractometer (be sure to decontaminate the refractometer after use). -
26
Optional: Add 1.37 g/ml CsCl solution to the pooled peak fractions to attain a final volume of 12 ml. Add this to a new 12-mL ultracentrifuge tube and repeat steps 22–25.
Rebanding the fractions will increase the purity and concentration of the rAAV, but some loss will be incurred with the added manipulations. -
27
Dialyze the rAAV in MWCO 10,000 Slide-A-Lyzer dialysis cassettes against three 1-L changes of storage solution (1× PBS with 5% sorbitol and 212 mM additional NaCl – for a final NaCl concentration of 350 mM) for at least 3 hr each at 4°C.
Overnight dialysis will not result in any loss of titer and is recommended for one of the buffer changes. The rAAV can also be dialyzed against 1× PBS, but the sorbitol and additional NaCl greatly reduce the formation of virus aggregates. -
28
Divide the virus suspension into convenient aliquots (typically ~100 μl, depending on the anticipated applications) to avoid repeated freezing and thawing.
The virus can be stored at 4°C for up to 2 weeks or −80°C for more than a year.
SUPPORT PROTOCOL 1
DETERMINATION OF rAAV TITERS BY THE DOT-BLOT ASSAY
This assay detects the packaged rAAV genomes using DNA probes specific for the transgene cassette. A positive signal in this assay indicates that rAAV virions were produced, and quantitation yields a particle number in virions per ml. However, this assay will not indicate if the virus is infectious or if the expression cassette is functional. These properties can be deduced using Support Protocol 2 to determine a transducing titer. This assay can be used to determine the peak fraction or to obtain the finalized titer on dialyzed rAAV.
Materials
Virus fractions or final virus preparation (from Basic Protocol 1)
DNase digestion buffer (see recipe)
0.5 M EDTA (APPENDIX 2A)
Proteinase solution (see recipe)
Dot blot dilution solution (see recipe)
0.4 M NaOH
rAAV plasmid used to make recombinant virus (see Basic Protocol 1, step 1a or b)
0.4 M Tris·Cl, pH 7.5 (APPENDIX 2A)
Radiolabeled probe to transgene (made using Roche random-primed DNA labeling kit according to manufacturer’s instructions)
96-well plate
50°C water bath
Dot-blot apparatus
0.45-μm nylon membrane (Hybond N+ or XL, Amersham)
STORM phosphoimager and ImageQuant software (GE Healthcare)
Additional reagents and equipment for restriction digestion of DNA (APPENDIX 1M), hybridization of DNA to membranes (CPMB UNITS 2.9A or 2.9B &2.10 and APPENDIX 1A in this manual) and autoradiography (CPMB APPENDIX 3A and APPENDIX 1A in this manual)
Digest virus particles to release DNA
-
1
Prepare an experimental plan for the position of samples, controls, and DNA standards in duplicate in a 96-well format.
The samples are prepared in microcentrifuge tubes, but will be loaded onto a 96-well dot-blot apparatus at the end of the procedure. -
2
Place 1 or 10 μl of virus samples into microcentrifuge tubes. Add 100 μl DNase digestion buffer to each sample and incubate 1 hr at 37°C.
This step digests any DNA that may be present but has not been packaged into virions. Be aware that the CsCl salt in the samples collected from basic Protocol 1, step 24, can interfere with the DNase reaction and may affect the accuracy of titering the viral genomes. If non-dialyzed rAAV (still in CsCl) is assayed, use only 1 μL. For obtaining a final titer on dialyzed rAAV, start with 10 μL that has already been frozen once and re-thawed. -
3
Inactivate the DNase by adding 5 μl of 0.5 M EDTA. Mix well and incubate 10 minutes at 70°C. Release virion DNA by adding 120 μl of proteinase solution. Incubate a minimum of 2 hr (or up to overnight) at 50°C.
This step liberates the encapsidated DNA. Failure to mix well after the addition of EDTA and heat-inactivate the DNase will decrease the apparent titer. EDTA inactivates the DNase, which would otherwise degrade the virion DNA when it is released from the virus particle. -
4
Add Dot Blot Dilution Buffer (Appendix 2A) to bring the volume up to 500 μL.
For a quantitative dot blot (10 μL starting rAAV), the final digested solution will now contain 1 μL of starting rAAV genomes per 50 μL. -
5a
For quantitative dot blot (10 μL starting rAAV), perform 2 sets of five 2-fold serial dilutions of each tube of sample using Dot Blot Dilution Buffer. Two sets of 100 μL undiluted solution should be retained (corresponding to 2 μL of starting rAAV genomes in each).
Each diluted sample should yield a final volume of 100 μl. These should correspond to 2 μL, 1 μL, 0.5 μL, 0.25 μL, 0.125 μL, and 0.06 μL of starting rAAV. Two parallel sets of dilutions are done to increase the accuracy of the method. When working with multiple sets of samples, it can be helpful to do the dilutions and downstream steps in a 96-well plate before transferring to the dot blot apparatus -
5b
For assessing peak fractions from the CsCl gradient (1 μL starting rAAV), the entire digestion solution will be used undiluted.
-
6a
For quantitative dot blot (100 μL diluted rAAV solution), denature viral DNA by adding an equal volume (100 μl) of 0.4 M NaOH to each sample and incubate 30 min at room temperature.
-
6b
For assessing peak fractions from the CsCl gradient (500 μL of undiluted rAAV solution), denature viral DNA by adding an equal volume (500 μl) of 0.4 M NaOH to each sample and incubate 30 min at room temperature.
Prepare standards
-
7
Prepare 2 μg of linearized plasmid (the plasmid used for transfection of this virus or another plasmid that contains the same target sequence) by restriction digestion (APPENDIX 1M). Dilute the digested DNA with Dot Blot Dilution buffer (APPENDIX 2M), to a concentration of 5×108 single-stranded DNA molecules per μL. Retain 200 μL of plasmid standard at 5×108 single-stranded DNA molecules per μL for the highest standard dilution. Make 2 sets of 2-fold dilution series in Dot Blot Dilution buffer, to make 2 sets of 12 dilutions with final volume of each tube of 100 μL.
The plasmid standards should range from 5×1010 to 2.4×107 single-stranded molecules per tube. To calculate the number of single-stranded DNA molecules in 2 μg plasmid, use the conversion factors of 6.6×108 μg/mole·bp and 6.02×1023 molecules/mole. For example for pHpa-trs-SK (5.9 kb), 2 μg = 6.2×1011 single-stranded DNA molecules. [(5900 bp) × (6.6×108 μg/mole·bp) ÷ (6.02×1023 molecules/mole) = 6.5×10−12 μg per plasmid; so 1.55×1011 plasmids per μg. Two single-stranded DNA molecules per plasmid and 2 μg plasmid = 6.2×1011 single-stranded DNA molecules in 2 μg.] -
8
Denature DNA standards by adding 1 volume (100 μl) of 0.4 M NaOH to each tube and incubate at room temperature for 30 min.
Perform hybridization
-
9
Equilibrate nylon membrane by immersing for 10 min in 0.4 M Tris·Cl, pH 7.5.
-
10
Prepare a dot-blot manifold apparatus with the prewetted nylon membrane.
-
11
Add the denatured DNA standards and rAAV samples (200 μl volume; 1 mL volume if blotting fractions for peak determination from step 6b) in duplicate to the assembled dot blot apparatus. After the last sample is loaded onto the apparatus, apply the vacuum for 5–10 min.
-
12
Add 400 μl 0.5 M NaCl/0.5 M Tris-HCl, ph7.5 to each well of the membrane to wash. Apply the vacuum for 5–10 min.
-
13
Disassemble the apparatus and remove membrane.
The membrane can be cross-linked using a UV Stratalinker (Stratagene) or equivalent method and then stored indefinitely at this point. -
14
Probe the membrane with a radiolabeled probe specific for the rAAV sequences (CPMB UNIT 2.10 and APPENDIX 1A in this manual).
The probe should be limited to the transgene cassette and should not include the plasmid backbone or ITR sequences. -
15
Place the membrane against film and expose (autoradiography; CPMB APPENDIX 3A and APPENDIX 1A in this manual). After developing the film, align the spots, excise the regions of the filter, and quantify radioactivity in a scintillation counter. Alternatively, place filters into a PhosphorImager cassette for 30–60 min.
Quantify dot-blot analysis
-
16
Analyze the exposed cassette by densitometry using STORM and ImageQUANT software, or equivalent. Calculate how many molecules of the plasmid standards correspond to a given dilution of the rAAV stock.
Remember to take into consideration that plasmid standard quantities were calculated in terms of single-stranded DNA copies. This matches traditional rAAV virions, which harbor only a single strand. However, each single-stranded self-complementary AAV genome contains two copies of the transgene expression cassette (both the coding sequence and complementary sequence are packaged in the genome). Therefore, the amount of radioactive probe bound to a molecule of single-stranded self-complementary AAV genome is twice that of a single-stranded molecule of standard DNA. To compensate for this, the standard values should be halved, to be expressed as the number of double-stranded DNA molecules rather than the number of single-stranded DNA molecules.
SUPPORT PROTOCOL 2
DETERMINATION OF rAAV TITERS BY THE QUANTITATIVE PCR ASSAY
Quantitative PCR offers an alternative method to the dot blot assay (Support Protocol 1) to determine the yield of a rAAV preparation. It is faster than the dot blot and doesn’t require the use of radioactive materials, but it is more costly and requires a quantitative PCR instrument. This approach works very well to titer single-stranded rAAV, but in some cases underestimates the titer of self-complementary rAAV by as much as 5–10 fold. This is likely due to competition between the presence of 2 complementary halves of the target sequence on the same viral genome, which would anneal together and compete against primers to bind and amplify the DNA. Therefore, self-complementary rAAV preparations should be titered by dot blot in parallel with qPCR, at least initially, to determine the reliability of qPCR to accurately titer the self-complementary rAAV preparations. Just as with the dot blot, this method does not provide any information about the infectivity of the rAAV particles.
Materials
Virus fractions or final virus preparation (e.g., from Basic Protocol 1)
DNase digestion buffer (see recipe)
0.5 M EDTA (APPENDIX 2A)
Proteinase solution (see recipe)
DNase/RNase-free PCR-grade water
Primers corresponding to the target sequence
96-well qPCR reaction plate or individual reaction tubes (appropriate for your qPCR instrument)
Quantitative PCR instrument
Quantitative PCR reagents (follow the manufacturer’s instructions for reaction setup)
Digest particles to release DNA
-
1
Perform steps 2 and 3 from Support Protocol 1.
-
2
Boil the rAAV sample for 10 minutes to inactivate the Proteinase K.
This step is necessary to ensure that the Proteinase K doesn’t degrade the PCR polymerase during reaction setup. -
3
Dilute the sample with PCR-grade water to a final volume of 1 mL. Take 2 μL of this solution and add to 398 μL of PCR-grade water.
If the starting rAAV material was 1 μL, this gives a final dilution of 1:200,000. If the starting rAAV material was 10 μL, this gives a final dilution of 1:20,000. At these dilutions the rAAV genomic DNA copies should still be within the dynamic range for qPCR detection, but the components of the DNase and Proteinase K digestions will be sufficiently diluted so that they don’t interfere with the qPCR reaction.
Prepare the DNA Standards
-
4
Preferrably, the plasmid used for transfection of each virus should be used to make the standard. Alternatively, another plasmid that contains the same target sequence can be used. Dilute the plasmid DNA with PCR-grade water to a concentration of 5×108 single-stranded DNA molecules per μL. Make a set of (8) 1:10 dilutions in PCR-grade water. The final standard set should range from 5×108 to 5×101 single-stranded DNA molecules per μL.
To calculate the number of single-stranded DNA copies in a given quantity of plasmid, see Support Protocol 1, step 7. At these low concentrations of DNA in water, the DNA is subject to degradation and/or sticking to the walls of the tube, which can significantly affect the reliability of the standard quantification. For these reasons, standards should be prepared fresh daily.
Q-PCR Reaction Setup and Analysis
-
5
Set up the reactions using 2 μL of sample or standard per reaction tube. Add the primers and qPCR reagents specific for your instrument, following the manufacturer’s instructions.
For SyBR Green-based detection systems, the following primers can be used with GFP: Forward 5′-AGCAGCACGACTTCTTCAAGTCC; Reverse 5′-TGTAGTTGTACTCCAGCTTGTGC. In general, primers should amplify a target sequence of approximately 150–250 bp. -
6
Following the reaction, generate a standard curve based on the number of single-stranded molecules per tube and the crossing point. Using the crossing point of each rAAV sample, the vector genome copies can be determined. Most quantitative PCR instruments use software that will calculate these values automatically.
As 2 μL of each standard were used, the amount of standard in each tube ranged from 1×109 to 1×102 single-stranded DNA molecules per tube.Similarly, the dilution factor of rAAV DNA in each tube will be 1:10,000 (for 10 μL of starting rAAV) or 1:100,000 (for 1 μL of starting rAAV). Multiply the quantity of rAAV DNA in each tube by the appropriate dilution factor to get the titer of the original rAAV stock (in vector genomes per μL). Keep in mind that this value will need to be halved for self-complementary AAV, since there are 2 copies of the genome per AAV particle. -
7
Additional Considerations. When using a new primer set, the final PCR products following the qPCR reaction can be assayed by agarose gel electrophoresis to determine the purity of the PCR product. Non-specific PCR products can adversely affect the precision of the qPCR method. Also, the quantitation is based on the assumption that the standard and samples amplify with the same efficiency. This can be tested by making serial dilutions of the sample DNA (e.g., 1:1, 1:5, 1:25, 1:125) and running them as normal for qPCR. When accounting for the dilution factor, these should give the same value. If there is a clear trend of change for the values, this indicates that the PCR conditions and/or primers are not optimal.
SUPPORT PROTOCOL 3
INFECTION OF CELLS IN VITRO WITH rAAV AND DETERMINATION OF TITER BY TRANSGENE EXPRESSION
This protocol describes how to infect cells in tissue culture as a method for determining a transducing titer or for conducting other transgene-specific experiments. Assaying for transgene expression is the most stringent method of determining rAAV titer. However, the lack of standardization between the different expression assays used for each gene makes the yields of rAAV with various transgenes difficult to compare. A positive transduction signal indicates that the rAAV has successfully infected the cell, unpackaged, and expressed the transgene to a level sufficient to allow detection by the appropriate expression assay. Transducing titers can vary substantially with the cell type used, and the transducing titers obtained in vitro can’t necessarily be used to predict their utility for in vivo applications. However, with a standard cell line, transducing titers can be used to compare rAAV preps of the same transgene and capsid. This titer can be compared to the particle number to determine efficiency of a specific transgene in a particular cell type. The standard cell line for assessing rAAV transduction titers is the HeLa RC32 cell line, which was engineered to express AAV2 Rep and, in the presence of adenovirus co-infection, replicates any successfully-delivered transgene providing a clear positive signal for each transduced cell. This protocol describes the determination of infectious titer on HelaRC32 cells in the presence of adenovirus co-infection, but it can be easily adapted to other cell lines with or without adenovirus.
Materials
HeLaRC32 cells (ACCT catalog # CRL-2972)
Complete DMEM/10% FBS (see recipe, or use tissue culture medium for other target cells using the supplier’s instructions for those cells)
rAAV with appropriate transgene (see Basic Protocol 1)
Adenovirus (see Support Protocol 4)
Tissue culture plates (multiwell plates recommended for assaying transducing titer)
NOTE: All tissue culture incubations are performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified. Any manipulations using virus should be carried out in a tissue culture hood dedicated for virus work.
NOTE: To assay transducing titer, cells can be coinfected with adenovirus (see Support Protocol 4), which acts as a helper virus and increases the transduction efficiency. The addition of adenovirus gives a better indication of the number of particles that are competent to transduce a cell by inducing an optimal environment for AAV infection. However, the adenovirus has a cytopathic effect and should not be used in vivo. It is up to each investigator to establish a standard procedure for titering different rAAV preps.
-
For determination of transducing titer, seed 3×104 HeLaRC32 cells per well into a 48-well tissue culture plate with appropriate DMEM. Grow the cells overnight to achieve ~50% confluence with ~5×104 cells per well.
The appropriate target cell type, number, and density will depend on the expression assay. Cells must not be treated with DNA synthesis inhibitors at any time before infection. AAV is a single-stranded virus and requires second strand synthesis before gene expression is possible. -
Infect cells by adding rAAV directly to the medium of the cells or mix rAAV with fresh medium immediately before adding it to the cells. For assaying transducing titer with HeLa RC32 cells, the rAAV should be mixed with WT adenovirus at a multiplicity of infection (MOI) of 5 before adding to the cells (for ~5×104 cells this will be 2.5×105 infectious units of adenovirus). Cells should be infected with serial 5-fold dilutions of the rAAV stock in the presence of a constant amount of adenovirus.
Cells can also be infected at the time of plating. Incubation time depends on the assay. The appropriate amount of rAAV will depend upon the transgene, cells, and assay. -
For the determination of rAAV transducing titer in the presence of adenovirus, assay the cells 48 hours post-infection. Carry out the specific assay for expression of the transgene (e.g., immunofluorescence, histological staining, drug resistance).
CAUTION: Adenovirus is an infectious pathogen and will be produced in this assay. Samples and waste should be handled accordingly.
SUPPORT PROTOCOL 4
GROWING AN ADENOVIRUS HELPER STOCK
This protocol describes how to make, purify, and titer adenovirus stocks for their use in rAAV production. Adenovirus production is very efficient and results in high yields (wild-type virus yields can reach 10,000 infectious units per cell). Purification on a CsCl gradient results in a large white band that is easily recovered. During a productive infection, adenovirus kills the cells it infects and the dead cells release the newly made particles. To titer adenovirus, dilutions of the virus stock are made and used to infect monolayers of tissue culture cells. Agarose is then used to cover the cells so that the virus released from infected cells does not spread, and this results in the formation of a plaque.
Materials
Adenovirus type 5 (ATCC #VR5)
293 tissue culture cell line (ATCC #CRL 1573) growing in complete DMEM/10% FBS (see recipe) in 15-cm plates and in 60-mm plates
Complete DMEM/2% FBS (see recipe)
Tris-buffered saline (TBS; see recipe)
1.2 g/ml, 1.3 g/ml, 1.4 g/ml, and 1.5 g/ml density CsCl (see recipe)
2× adenovirus storage buffer (see recipe)
Serum-free DMEM
Plaque overlay solution (see recipe), 39°C
3.3 g/liter neutral red stain (Sigma; store up to 6 months at room temperature)
500-ml polypropylene centrifuge bottle
Sorvall centrifuge with GSA rotor or equivalent
50-ml disposable polypropylene centrifuge tubes
Dry ice/ethanol bath
Tabletop centrifuge
12.5-ml Beckman Ultra-Clear tubes for the SW-41 rotor
Beckman ultracentrifuge with SW-41 or equivalent ultracentrifuge and rotor
5-ml syringe
21-G needle
Econo Pump peristaltic pump (Bio-Rad)
50-μl borosilicate glass capillary pipets (Fisher)
NOTE: All tissue culture incubations are performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified. Any manipulations using virus should be carried out in a tissue culture hood dedicated for virus work.
Make the adenovirus stock
-
1
Add adenovirus dl309 to DMEM with 2% FBS at an MOI of 10. Replace the medium on ten 15-cm plates of 293 cells (70% to 80% confluent) with this Ad/DMEM solution. Incubate until full cytopathic effects (CPE) occur (36 to 56 hr).
CPE can be recognized by the presence of rounded cells that float instead of attach to the plate and by the medium’s turning an orange/yellow color. -
2
Harvest the cells and the culture supernatant and transfer to a 500-ml polypropylene centrifuge bottle. Centrifuge 5 min at 4000 × g (5000 rpm in a Sorvall GSA rotor), 4°C.
-
3
Decant supernatant and autoclave before discarding. Resuspend the pellet in 10 ml TBS. Transfer suspension to a disposable 50-ml polypropylene tube.
-
4
Freeze and thaw the cell suspension three times by transferring the tube between a dry ice/ethanol bath and a 37°C water bath.
-
5
Centrifuge for 5 min at 3000 × g in a tabletop centrifuge at 4°C and transfer the supernatant to a new tube.
-
6
Place 3.5 ml of 1.4 g/ml CsCl solution in each of two 12.5-ml Ultra-Clear centrifuge tubes. On top of these solutions, carefully layer 3.5 ml of 1.2 g/ml CsCl solution to produce CsCl step gradients. Finally, layer 5 to 6 ml of the supernatant from step 5 on top of each of the CsCl step gradients
-
7
Centrifuge 1 hr at 150,000 × g (30,000 rpm in a Beckman SW-41 rotor), 20°C.
-
8
Remove the lower virus band with a 21-G needle and 5-ml syringe (see basic Protocol 1, step 21/24), combine the bands from different tubes, then mix with 1.3 g/ml CsCl solution to a final volume of 12 ml. Place this into a 12.5-ml Ultra-Clear tube.
-
9
Using a glass capillary tube and setting the Econo Pump at a flow rate of 3 ml per minute, underlay the solution with 0.5 ml of 1.5 g/ml CsCl solution (see Alternate Protocol 2, steps 2 to 3). Centrifuge overnight at 150,000 × g in a SW-41 rotor, 20°C.
-
10
Remove the adenovirus band from the gradient with a 21-G needle and 5-ml syringe (see Basic Protocol 1, step 21/24).
-
11
Mix the adenovirus with an equal volume of 2× adenovirus storage buffer and store up to 1 year at −80°C.
Determine adenovirus titer by plaque assay
-
12
Make eight 10-fold serial dilutions of the adenovirus stock each in 1 ml of serum-free DMEM.
-
13
Infect 60-mm dishes of 293 cells (80% confluent) with 100 μl of the dilutions and incubate 2 hr.
Prepare the plaque overlay solution (see Reagents and Solutions) during this incubation. -
14
Aspirate the medium from the cells and slowly overlay with 5 ml of 39°C plaque overlay solution. Allow the agar to harden at room temperature.
-
15
Incubate the plates at 37°C in 5% CO2 for 5 days, then feed the plates by adding 2 ml of fresh 39°C plaque overlay solution.
Plaques can be detected in the microscope 3 to 4 days post infection. -
16
When plaques become visible to the naked eye (after day 7) feed the plates again with 2 ml of plaque overlay solution containing a 1:100 dilution of 3.3 g/liter neutral red stain.
When preparing the plaque assay solution, add the stain to 2× DMEM before combining with the agarose. The neutral red aids in the detection of the plaques, but kills the cells.
BASIC PROTOCOL 2
STEREOTACTIC MICROINJECTION OF rAAV INTO THE RAT BRAIN
Microinjection of rAAV allows one to assess transgene expression in the whole animal, which often does not mirror in vitro transduction patterns. Although rats are most commonly used, stereotaxic procedures can be adapted to most species, from mice to monkeys. In addition to rats, successful transduction with rAAV has been documented in mice, dogs, and monkeys (Samulski et al., 1999). For rats, single-site infusions usually consist of delivering 1 to 2 μl of rAAV over a period of 10 to 20 min using a syringe pump. This use of slow, uniformly delivered, small volumes results in minimal tissue damage, optimal delivery to the site, and excellent transduction. Faster infusion rates can lead to back-diffusion of the rAAV up the injector tract. If large areas need to be transduced, multiple injections can be performed. In contrast, infusion of larger volumes usually produces damage with little increase in the area of transduction. For maximal transduction, it is important to use high-titer virus (approximately 1012 vector genomes/ml).
NOTE: All animal surgery should be performed by experienced personnel, following procedures outlined in “Guide for the Care and Use of Laboratory Animals” [DHHS publication No. (NIH)85-23]. All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals.
Materials
Sprague-Dawley rats, 6 to 8 weeks old
Anesthetic: pentobarbital (50 mg/kg body weight)/atropine methyl nitrate (2 mg/kg body weight) in 0.9% NaCl or ketamine (150 mg/kg body weight)
Betadine scrub or 70% ethanol
1 mg/ml epinephrine
1% procaine·HCl
rAAV preparation (see Basic Protocol 1) diluted in PBS (APPENDIX 2A) to desired concentration
Rat brain atlas (e.g., Paxinos and Watson, 1986)
Dental drill or Dremel with #862 Dremel engraving cutter
Rat stereotaxic frame (Kopf) in sterile surgical area
Variable speed syringe pump
Surgical instruments: scalpel, forceps, needle holders, and small spatula
Sharp scissors or razor
30-G stainless steel for injector
PE-10 intramedic tubing, Clay Adams
0 to 10-μl syringe with Teflon-tipped plunger (e.g., Precision Sampling glass Teflon tip plunger, gas-tight 0 to 10-μl syringe; Valco Instruments)
1-ml syringes with 26-G and 30-G needles
Animal clippers
Sterile gauze
Bonewax
4-0 silk suture
Polysporin ointment
Heating pad, heated to 37°C
Clean cage without bedding
NOTE: Before beginning injections with virus, it is important to verify the placement coordinates. This can be done by infusing dye (e.g., fast green) into one or two animals using the following procedure and then slicing the brain to verify injector placement.
NOTE: A titer of at least 109 vector genomes (injected) is recommended. Using less than 108 vector genomes may not lead to a desired amount of transduced cells.
Prepare work area
-
1
Using the rat atlas, determine the injection coordinates.
-
2
Set up the stereotaxic frame, dental drill, and syringe pump in a sterile area appropriate for surgery.
-
3
Sterilize all surgical instruments (scalpel, forceps, needle holders, and spatula).
-
4
Determine setting on syringe pump needed to inject 1 μl over 10 min.
Construct injector
-
5
Using scissors, cut a 3- to 7-cm length of 30-G stainless steel. Cut a 12- to 18-inch length of PE-10 tubing.
32-G or 33-G tubing can be used if desired. This size causes less tissue damage but has a greater tendency to clog. -
6
Insert 1 to 2 cm of the 30-G stainless steel tubing into one end of the PE-10 tubing.
A water-tight seal must be formed. If 32- or 33-G tubing is used, place some Super Glue or contact cement on the stainless steel tubing, close to the end, and then slide it onto the PE-10 tubing. Allow glue to dry for 30 min. -
7
Test the integrity of the injector by filling a 1-ml syringe with water and fitting it with a 30-G needle. Insert the needle into the PE-10 tubing and slowly push the water through the injector.
There should be no leaks at the joint of the PE-10 and the stainless steel.
Fill injector
-
8
Fill the PE-10 tubing with sterile water.
-
9
Place the open end of the PE-10 tubing on the 10-μl syringe.
This seal should be airtight. -
10
Withdraw the syringe plunger 0.3 to 0.5 μl, introducing an air bubble into the injector tubing.
There should be no lag between the movement of the plunger and the movement of the air bubble, indicating an air-tight seal. Also, this air bubble will be used to monitor the microinfusion process. -
11
Submerge the injector tip into the rAAV solution and slowly withdraw the plunger. Place the syringe into the holder of the pump.
The air bubble should move back toward the syringe separating the water and the rAAV solution. During the infusion, movement of the air bubble should be monitored by marking the drug-air interface on the PE-10 tubing with a permanent marker before infusion. If the air bubble compresses instead of moving beyond the mark, then the injector tip is plugged.
Perform microinjection procedure
-
12
Using two 1-ml syringes equipped with 26-G needles, anesthetize rat with an intraperitoneal (i.p.) injections of 50 mg/kg pentobarbital and 2 mg/kg atropine methyl nitrate in 0.9% NaCl.
The atropine serves to prevent excess salivation. The rat is fully anesthetized when there is no toe pinch or eye blink response. -
13
Shave hair from top of the head, place the animal in the stereotaxic frame and clean the skin with betadine scrub or 70% ethanol.
UNIT 3.10 provides additional detail on how to position the animal in the stereotaxic apparatus. -
14
Make an incision no larger than 1.0 to 1.5 cm from the front of the skull to the back. Put several drops of 1 mg/ml epinephrine on the incision site, followed by a small amount of 1% procaine·HCl.
The epinephrine will constrict the capillary beds and reduce post-operative bleeding. -
15
Using blunt dissection, remove facia on the top of the skull wipe clean with sterile gauze and allow the skull to dry.
Suture lines should be obvious when the skull is completely dry. -
16
Plot the rostral/caudal and medial lateral coordinates. Use the rostral suture intersection (bregma) as the starting point. Mark the point with a permanent marker and replot to verify accuracy.
See Figure 3.10 for a photograph of the bregma. -
17
Drill a hole in the skull on the mark and lower injector the specified distance.
UNIT 3.10 (protocol on transplantation into the adult rodent brain) provides additional detail on determination of appropriate coordinates and performing the injection. -
18
When injector is in place, turn on the infusion pump.
Mark the air bubble at this point and monitor its progress. If the air bubble does not move, the injector tip is plugged. Sometimes the plug can be dislodged by moving the injector slightly up and then back down again. -
19
When specified volume (typically 1 to 2 μl) is infused, turn off pump and leave injector in place for 1 min before removing.
This time allows virus to diffuse away from point of injection. -
20
Remove injector, clean the skull, cover hole with a small amount of bone wax and suture the incision with 4–0 silk suture. Swab the incision with polysporin ointment to prevent infection. Allow the animal to recover in a clean cage lined with paper towels placed on a heating pad until fully mobile before returning to normal housing. Remove sutures in 5 to 7 days.
See Support Protocol for methods to detect expression from rAAV containing a green fluorescent protein reporter gene.
BASIC PROTOCOL 3
INTRATHECAL ADMINISTRATION OF RAAV IN MICE BY LUMBAR PUNCTURE
Intrathecal administration of rAAV is a method to deliver transgenes broadly to the dorsal root ganglia and spinal cord. The specific cells targeted, vector spread, and efficiency of gene delivery varies considerably based on the AAV serotype used, and it is highly recommended to consult the recent rAAV literature to determine the best rAAV capsid to use for a specific target or application. This protocol uses lidocaine with the injected solution to verify the injection, but the procedure could also be done in the absence of lidocaine and on anesthetized animals if the user was sufficiently comfortable with the technique. The technique can be practiced using a 12.5% lidocaine solution in PBS.
NOTE: All animal manipulations should be performed by experienced personnel, following procedures outlined in “Guide for the Care and Use of Laboratory Animals” [DHHS publication No. (NIH)85-23]. All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals.
Materials
Mice, at least 8 weeks old
Towel, at least 20 cm × 20 cm
60% (w/v) sterile lidocaine-HCl in PBS (MPI catalog #193917 or equivalent)
rAAV preparation (see Basic Protocol 1)
25 or 50 uL Hamilton syringe, with 30 gauge needle
Prepare the rAAV injection solution
-
1
The injection volume per animal will be 5 μL. Per 5 μL, add 1 μL 60% lidocaine (12.5% final concentration), rAAV vector to the desired concentration, and the remaining volume with PBS or rAAV dialysis solution (see Basic Protocol 1).
109 to 1010 total vector genomes is a typical range for this type of injection. The osmolarity of the solution can potentially affect the penetration of the vector into the spinal cord and dorsal root ganglia, so parallel experiments should maintain an equivalent final concentration of hyperosmolar sorbitol and NaCl, if these are present in the vector solution (see Basic Protocol 1 and recipe). -
2
Load the vector solution into the needle-and-syringe. Check the seal on the plunger by doing a test injection (on parafilm or in a microfuge tube) and verifying that the correct volume was ejected.
A 25 or 50 μL syringe is used so that the plunger won’t need to be withdrawn as far to inject 5 μL. This makes one-handed injection easier.
Handling and injecting the mouse
-
5
Place the unanesthetized mouse on the towel and fold the towel over its head and upper torso. The lower torso, hips, and hindlimbs should protrude out from beneath this fold. Firmly grip the mouse’s spine between the thumb and forefinger, just above the pelvis. Use the palm of the same hand to secure the upper torso of the mouse beneath the towel.
A firm grip of the mouse is critical, but this should not cause any pain to the animal. If the grip is correct, the mouse should have no possibility of moving its lower body. Ideally, the skin over the spine should be tight and the spine should be arched slightly upward to expose the joints between the vertebra. -
6
While holding the mouse with one hand, use the other to insert the needle (bevel facing up) at the midline between the hips at an angle of approximately 70–80° from the spine. When the needle contacts the spinal column, lower the syringe so the needle comes to a 30° angle and attempt to slide the needle in between the vertebra (between L5 and L6).
Penetration of the dura is indicated by a tail flick or “S” shape of the tail. If this is not observed, remove the needle and try again. Injection of lidocaine into the wrong location can kill the mouse. -
7
Inject the lidocaine/rAAV mixture over a period of 1–2 seconds. Leave the needle in place for a few seconds, then rotate the needle and slowly withdraw it.
If the injection is successful, the mice should experience bilateral hindlimb paralysis for 5–10 minutes. No paralysis indicates a failed injection, and the mouse should be discarded. Shorter paralysis or unilateral paralysis indicates a suboptimal injection. The mice should fully recover to normal ambulation within 20 min.NOTE: Occasionally the injection solution will be pushed upwards to the cervical region of the spinal cord. If this happens the mouse can experience partial or full paralysis of the diaphragm, leading to respiratory distress and possibly death. Increasing the injection volume and/or the lidocaine concentration will increase the probability of this occurring, and conversely this problem can be reduced by decreasing the injection volume to 2–3 μL. This would be recommended for mice less than approximately 15–18 g.
SUPPORT PROTOCOL 5
HISTOLOGICAL DETECTION OF GREEN FLUORESCENT PROTEIN EXPRESSION
Green fluorescent protein (GFP) is a common reporter gene used to assess viral transgene expression. It is relatively easy to detect in brain sections and thus provide an appropriate test of virus preparation and microinjection techniques. This protocol uses rodents that have been injected with AAV-GFP. A vibrating microtome is used to cut fixed brain or spinal cord tissue, a method that maintains tissue integrity and cellular morphology (see UNIT 1.1). As a consequence, however, tissue sections are rather thick (25 to 50 μm). In these sections, GFP expression can be detected directly by fluorescence microscopy or detected by histochemical staining. Depending on the strength of the promoter used, the native GFP signal may be weak and the fixation in paraformaldehyde results in a loss in GFP signal intensity. Thus, immunostaining of GFP may be necessary to view all transduced cells. The latter part of this protocol can be adapted for immunostaining of other proteins instead of GFP detection.
Materials
Rodents transduced with GFP (Basic Protocol 2 and 3; AAV-GFP virus available from UNC Vector Core Facility. pAAV-GFP plasmid constructs also available from UNC Vector Core Facility)
4% (w/v) paraformaldehyde in PBS
Phosphate-buffered saline (PBS; APPENDIX 2A), 4°C
2% agar (store at 4°C in solid form, heat to 70°C to liquefy before using)
Fluorescent mounting medium (see recipe)
Standard mounting medium, e.g., Permount or Accumount
Large forceps
Small forceps with hooks on the ends
50-ml conical centrifuge tubes
Single-edged razor blades
Super Glue
12-well and 24-well tissue culture plates
Vibrating microtome with blades
Fine-bristled paint brush
Anti-GFP antibody (Millipore AB3080 rabbit anti-GFP or equivalent)
VectaStain Elite Kit (rabbit): Vector Labs, # PK-6101
DAB (3,3′-diaminobenzidine tetracholide): Polysciences, Inc. # 04008
200 mM NaHPO4 (pH 7.4)
0.5% (w/v) CoCl2 in water
1% (w/v) (NH4)2Ni(SO4)2 in water
3% hydrogen peroxide
Glass petri dish, shallow
Dark work surface
Glass slides and coverslips
Drying rack
Fluorescence microscope with GFP filter
Light microscope
Additional reagents and equipment for perfusion fixation and sectioning of brains (UNIT 1.1)
Section brains (adapted to spinal cord as well)
-
1
Perform perfusion fixation of the brain or spinal cord (UNIT 1.1), except after removing brain or spinal cord, place in a 50-ml conical tube containing 15 to 20 ml 4% paraformaldehyde in PBS at 4°C overnight.
If the tissue will be used for immunohistochemical detection of GFP (or other transgene), it is critical that the animal be perfused completely to remove any red blood cells. These have high intrinsic peroxidase activity and they will be detected strongly and non-specifically in the tissue if they are not removed.The spinal cord may be easier to dissect intact if left in the spinal column during fixation. Once it is fixed, the vertebra can be removed with fine scissors to recover the spinal cord. -
2
Use large forceps to remove tissue from the 50-ml conical tubes. Using small forceps, remove as much of the meninges as possible. At this point, the tissue can be stored at least 1–2 weeks at 4°C in PBS or sectioned immediately.
If the tissue is to be processed to view native GFP fluorescence, keep the tissue in the dark. -
3
With a single-edge razor blade, block the brain by cutting in the coronal plane (parallel to the injection tract for brain, cross-sections for cord) ~5 mm both anterior and posterior to the infusion site. Make sure symmetry is maintained. Place on an absorbent paper towel to remove paraformaldehyde solution. Using a small amount of Super Glue, glue the brain to the mounting platform of the microtome. For the spinal cord, attach the end of the cord to the mounting platform, with the cord sticking up at a 90° angle. Press gently. Allow glue to dry.
Depending on the serotype of AAV and the dose, the GFP-positive cells will be mostly concentrated in the lumbar and sacral spinal cord. The cord can be divided into no longer than 1 cm segments for sectioning. -
4
Coat the brain or cord with a thin layer of melted (70°C) 2% agar and allow it to solidify. Place mounted brain in the vibrating microtome and set speed, vibration amplitude, and section thickness (40 to 60 μm).
Using agar to encase the tissue is critical for the success of spinal cord sectioning. The goal is to encase the upright spinal cord segment in a cone of agar to restrict any lateral movement. -
5
Set up the microtome using PBS to immerse the brain during sectioning. Fill several wells of the 24-well plate with PBS.
-
6
Begin sectioning, using a fine-bristled paint brush to guide sections during slicing. As the section is cut, use the paint brush to lift the section gently from the blade and into a PBS-filled well of the 24-well plate. Remove the agar as sections are placed into the 24-well plate. Tearing of the sections during slicing may indicate residual meninges, so if all the meninges are removed and tearing still occurs, increase the frequency of blade vibration and decrease the speed. Replace the blade after each brain for optimal results.
Consecutive sections can be placed in different wells if desired. This allows adjacent sections to be processed or stained differently and permits the investigator to view a representative portion of the brain without having to mount all the sections. Sections can be stored for several days at 4°C in PBS. If native GFP fluorescence will be viewed, keep the slices dark.
Process sections
For direct visualization of native GFP
-
7a
Mount sections by placing them in a shallow glass petri dish set on a dark surface and filled with 0.5× PBS solution. Using the paint brush, slide sections gently onto glass slides.
Sections will adhere when removed from the PBS. -
8a
Place slides upright to allow PBS to drain and allow sections to dry until they become translucent (~5 to 10 min depending upon thickness).
-
9a
Rinse sections by gently dipping the slide in distilled water several times. Repeat twice. Allow slides to dry another 10 to 30 min.
-
10a
Coverslip the tissue sections by placing a small amount of fluorescent mounting media on the slide and placing a glass coverslip carefully on top. Avoid producing bubbles. Remove excess mounting medium by draining briefly on a paper towel. Allow several minutes for medium to set (it will harden more firmly over the next 24 hr).
Slides can be stored in the dark at 4°C for ~2 weeks with minimal loss of fluorescence. Longer storage times result in progressive loss of fluorescence. Bubbles in the mounted slides will disrupt viewing and impair fluorescence after storage. -
11a
View GFP with a UV fluorescent microscope fitted with the correct filter.
For immunohistochemical (IHC) staining of GFP with nickel/cobalt intensification
-
7b
Use 12-well plates for the IHC steps. Block the sections by incubating in a solution of 1× PBS containing 3% goat serum and 0.1% Triton X-100 for 1 hr at room temperature with shaking.
-
8b
Transfer the sections to a new well containing 1× PBS containing 3% goat serum and 0.1% Triton X-100 with 1:500 dilution of anti-GFP antibody. Incubate with shaking for 48–72 hrs at 4° C.
Corning netwell inserts (Cat #3478) in 12-well plates make the transfer of samples easy from one well to another. Alternatively, a paintbrush can be used to do the transfer using any multiwall format. If too many sections are present in a well or the shaking is not vigorous enough, the antibody may not penetrate evenly to all areas of all the sections. -
9b
Wash the sections 3 times with 1× PBS by transferring them to new wells with PBS and shaking for 10 minutes at room temperature.
-
10b
Using the VectaStain ABC Elite Kit, add one (1) drop of stock anti-Rabbit (Blue Label) to 10 mL of PBS buffer in mixing bottle and mix. Then add three (3) drops of goat serum (Yellow Label) to the mixing bottle and mix. Shake at room temperature for 1 hour.
If a different antibody is used, make sure to use the appropriate secondary antibody kit (i.e. for rabbit & GFP, use Elite Rabbit IgG kit). -
11b
Wash the sections 3 times with 1× PBS by transferring them to new wells with PBS and shaking for 10 minutes at room temperature.
-
12b
Thirty minutes prior to use, add exactly two (2) drops of REAGENT A to 10mL of PBS buffer in the ABC-AP Reagent mixing bottle. Then add exactly two (2) drops of REAGENT B to the same mixing bottle and mix immediately. After incubating for 30 minutes, transfer the slices to a well containing this solution and shake for 1 hour at room temperature.
-
13b
Wash the sections 3 times with 1× PBS by transferring them to new wells with PBS and shaking for 10 minutes at room temperature.
-
14b
Prepare the DAB solution. Add 10 mL of distilled water to a single bottle of DAB and shake well. Add 10 mL of 200 mM NaHPO2 buffer (pH 7.4) and shake well. Add 200 μL of 0.5% CoCl2 and 150 μL of 1% (NH4)2Ni(SO4)2 and mix well. Just prior to use, add 4 μL of 3% hydrogen peroxide, mix well, and filter. Use the DAB solution immediately.
CAUTION: DAB is a carcinogen, and it should be handled carefully and appropriately. Wear gloves, eye protection, and a lab coat when handling. Clean up any spilled solution and dispose of materials coming in contact with DAB appropriately. -
15b
Transfer the sections to a well containing the DAB solution and incubate at room temperature with shaking. Incubation time will be approximately 2 minutes but this time should be optimized.
The longer the incubation, the more intense the DAB staining.Endogenous peroxidases in the tissue will precipitate the DAB and create background staining with prolonged incubation. The DAB reaction can be monitored by periodically removing sections and viewing them against a white background. When optimizing the incubation time, a negative control set of sections should be incubated in parallel to assess the background signal. -
16b
Transfer the sections to new wells containing PBS and wash 2 times with PBS to stop the DAB precipitation reaction, 2–5 minutes per wash at room temperature.
Dispose of the DAB solution properly. Neutralize the DAB by adding a ~1:1 ratio of 5% sodium hypochlorite (normal undiluted household bleach), incubate overnight, and dispose of the neutralized DAB solution properly. -
17b
Mount sections onto glass slides as in steps 7a to 9a (same as for GFP detection protocol, but use a white background instead of black).
-
11b
Place a small amount of standard mounting medium on sections and cover with glass coverslip, avoiding bubble formation as much as possible.
DAB staining appears brownish or black when viewed with a light microscope.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see SUPPLIERS APPENDIX.
Adenovirus storage buffer, 2×
10 mM Tris·Cl, pH 8.0 (APPENDIX 2A)
100 mM NaCl
1 mM MgCl2
50% (v/v) glycerol
0.1% (w/v) BSA
Filter sterilize
Store up to 1 year at 4°C
Complete DMEM/10% FBS
Dulbecco’s Modified Eagle Medium (DMEM; Life Technologies) containing:
4.5 g/liter glucose
10% fetal bovine serum (FBS)
100 U/ml penicillin
100 μg/ml streptomycin sulfate
Store up to 2 months at 4°C
CsCl gradient solutions
1.2 g/ml density:
27.5 g CsCl
PBS (APPENDIX 2A) to 100 ml
1.3 g/ml density:
40.5 g CsCl
PBS to 100 ml
1.37 g/ml density:
50 g CsCl
PBS to 100 ml
1.4 g/ml density:
54.5 g CsCl
PBS to 100 ml
1.5 g/ml density:
67.5 g CsCl
PBS to 100 ml
Check density of each solution by weighing 1 ml. Filter sterilize. Store the gradient solutions up to 1 year at room temperature.
Dialysis/storage solution for rAAV (1×PBS with 5% sorbitol and 350mM final NaCl, 1L)
100 mL 10× PBS (APPENDIX 2A)
50 g D-sorbitol
42.4 mL 5 M NaCl
H2O to 1L
Filter sterilize
Store up to 2 years at 4°C
DNase digestion buffer
10 mM Tris·Cl, pH 7.5 (APPENDIX 2A)
10 mM MgCl2
2 mM CaCl2
50 U/ml DNase I
Prepare fresh
Dot blot dilution buffer
4 mM Tris·Cl, pH 7.5 (APPENDIX 2A)
4 mM MgCl2
0.8 mM CaCl2
12 mM EDTA, pH 8.0
0.5% (w/v) N-lauroylsarcosine (Sarkosyl)
0.5 M NaCl
Fluorescent mounting medium
Dissolve 0.24 to 0.36 g n-propyl gallate (Sigma) in 12 g glycerol (this will take some time). Slowly add 4.8 g Mowiol (Calbiochem) to form a slurry. Slowly add 12 ml distilled deionized water while heating over low heat. Add 24 ml of 0.2 M Tris·Cl, pH 8.2 to 8.5. Continue stirring for several hours. Cover tightly with paraffin. Allow undissolved Mowiol to settle overnight at 4°C. Store in aliquots up to 6 months at −20°C.
The solution should have a slight tan color. If the color darkens significantly or turns yellow, discard solution.
Plaque overlay solution
Prepare 2% SeaPlaque low-melting/gelling temperature agarose (FMC Bioproducts) in distilled water. Autoclave and cool to 39°C. Also prepare 2× DMEM (without phenol red) from powder (Life Technologies) and supplement with 4% FBS, 25 mM MgCl2, 0.3% sodium bicarbonate, 40 mM HEPES pH 7.5, 100 U penicillin and 100 μg/ml streptomycin sulfate. Warm the medium to 37°C. Mix equal amounts of the agarose and cell culture medium solutions and promptly add to cells. Make fresh with each use.
Polyethyleneimine (PEI), 1 mg/ml
Dissolve 10 mg PEI (25 kDa, Polyscience Inc.) by adding 100 μl of 12 N HCl followed by 900 μl of PBS (without Ca2+/Mg2+; APPENDIX 2A) in a glass vial. Dilute to 1 mg/ml (working concentration) with PBS and adjust final pH to 5.0 using 12 N HCl. Filter sterilize and store up to 6 months at 4°C.
Proteinase solution
1 M NaCl
1% (w/v) N-lauroylsarcosine (Sarkosyl)
100 μg/ml proteinase K (Sigma)
Prepare fresh each time
Tris-buffered saline (TBS)
25 mM Tris·Cl pH 7.4 (APPENDIX 2A)
140 mM NaCl
30 mM KCl
7 mM Na2HPO4
6 mM dextrose
Filter sterilize
Store up to 1 year at room temperature
COMMENTARY
Background Information
Recombinant AAV (rAAV) vectors have been developed for stable delivery of genes to a variety of cell types. Based on the defective parvovirus, adeno-associated virus, these vectors have several advantages for use in the central nervous system. Most importantly rAAV vectors transduce both dividing and nondividing cells. Because of the physical stability of AAV, tissue culture and live-animal infections are readily accomplished. Secondly, long-lasting gene expression is produced with little or no cellular damage due to viral gene expression or immune infiltration (Mandel et al., 1998). Finally, both adult and neonatal animals have been successfully infected, with gene expression lasting for several months to over 1 year (McCown et al., 1996; Smith and McCown, 1997). The primary disadvantage of these vectors is the limited size capacity, allowing a gene cassette of only 4.4 kilobases (2.1 for self-complementary vectors).
rAAV vector plasmids can be easily constructed by cloning the desired gene cassette in place of the two open reading frames of an AAV plasmid construct. This results in a construct that contains the transgene cassette flanked by the AAV inverted terminal repeats (ITRs). Production of rAAV is possible because the replication, packaging, rescue, and integration signals are contained in the AAV ITRs (Cukor et al., 1984; Hermonat et al., 1984; McLaughlin et al., 1988; Samulski et al., 1989). A transgene can replace the AAV coding sequences because both the rep and cap gene products can be supplied in trans from another plasmid to make infectious rAAV virions. Several adenovirus genes are also required to complete the AAV life cycle. By providing these adenovirus helper functions via a plasmid, this protocol improves upon earlier protocols which resulted in rAAV preps contaminated with adenovirus. Thus adenovirus-free rAAV can be made by transfecting the rAAV plasmid, the AAV helper plasmid, and the adenovirus helper plasmid into 293 cells. The ITR flanked transgene is rescued and replicated and packaged as single strands of either polarity into AAV protein capsids. Cloning the transgene between the AAV ITRs of pSub201 or pHpa-trs-SK and using pXX2 or pXR helper plasmids as a helper plasmid (which provide rep and cap in trans) prevents recombination that would generate wild-type AAV (wtAAV), and thus contaminate the rAAV stocks (Samulski et al., 1989). For smaller gene-expression cassettes (small promoter and cDNA and siRNA expression cassette), self-complementary rAAV vector should be used because this is an improved gene-delivery vector. It bypasses rate-limiting second-strand synthesis so that the genome is transcriptionally competent upon nuclear entry (McCarty et al., 2003). In addition, the use of serotypes other than AAV2 as gene-delivery tools has tremendously increased due to the different tissue tropism of AAV serotype capsids. The pXR plasmid series allows high-titer production of each AAV serotype (serotypes 1 to 9 and others) by cross-packaging AAV2 ITR genomes into different serotype capsids (Rabinowitz et al., 2002). There are an increasing number of naturally isolated and genetically modified AAV capsid variants that can be used as gene-delivery vector. Reagents (helper plasmids) that allow the generation of these AAV serotypes or variants can be obtained from other investigators upon request as described in their publications.
Basic Protocol 1 allows for the isolation of adenovirus-free rAAV using an improved CsCl-based density gradient purification protocol. Although many protocols are now available to purify rAAV, this protocol provides highly pure rAAV and can be readily adopted by most laboratories. However, it is not ideal for large-scale production of rAAV (above ~1013 vg). It is also critical to remove as much CsCl as possible by dialysis, as the presence of small amounts of CsCl can affect the tropism and transduction efficiency of rAAV. Titering genome-containing viruses at the same time by dot-blot or qPCR is also critical when multiple serotypes are used.
The virus generated from Basic Protocol 1 can be used to infect cell lines or primary cell cultures or can be microinjected into intact animals. The efficiency of transduction is highly dependent upon cell type. In culture, a large range of cell types, including both neurons and glial cells from rat primary cultures, demonstrate moderate to high transduction efficiencies. In the intact animal, considerable variation between cell types and even brain regions can be found using the same rAAV preparation. Both virus entry into the cell and promoter choice play a part in this variability. In addition, since virions package only a single strand, conversion to a double-stranded template must occur within the cell before gene transcription and translation can occur. This requirement leads to a lag time in gene expression, often 1 to 3 weeks (less for self-complementary rAAV) before the maximum is reached. However, once maximal expression is reached, gene expression usually lasts several months to over a year with little apparent loss in expression.
Critical Parameters and Troubleshooting
Plasmid constructs
It is critical for success in all of the subsequent steps to begin with an appropriate transgene construct. The AAV capsid has a narrow packaging range which centers at the wild-type size of 4681 bases. Construct sizes between 3.4 to 4.8 kb can be packaged with reasonable efficiency, although the best packaging occurs at the wild-type size. It is important to increase or limit the size of the vector construct (including AAV ITRs) to within the range given.
Intact terminal repeats must also be maintained for efficient replication and packaging of the transgene. Because of the significant secondary structure of these sequences, deletion can occur during plasmid propagation in E. coli (Boissy and Astell 1985; Bi and Liu 1996). DH5Tα and DH10B cells are typically used, as well as SURE cells (Stratagene), which help maintain terminal repeat integrity. Integrity can be checked by digesting with SmaI (or XmaI) restriction endonuclease, which cuts within the terminal repeat sequence. The deletion percentage should not exceed 20% for best results.
Transfection parameters
Efficient production of rAAV depends on a good transient transfection. 293 cells are used because they transfect well. The PEI transfection method yields improved consistency and efficiency of transfection with a smaller total amount of DNA over previously described methods such as calcium phosphate (Boussif et al., 1995). Transfection efficiency can easily be scored by transfecting a CMV-GFP-containing plasmid, infecting with adenovirus, and visualizing GFP expression with a fluorescent microscope within 24 hr.
In vitro transduction
Transduction of any cell type requires viral entry, uncoating, and second-strand synthesis. If any of these cannot occur, no gene expression will occur. Viral entry is dependent upon the cell type expressing the appropriate receptor for virus attachment and entry, and different AAV serotypes utilize different receptors for cell entry. For example, AAV8 and AAV9 typically work very well in most in vivo applications, but typically perform very poorly in tissue culture. In tissue culture, most cell types have the necessary receptor for AAV2 including primary rat neuronal and glial cultures. However, in primary neuronal cultures, DNA synthesis inhibitors often are used to prevent cell division. Because AAV is single-stranded, no transduction will occur in the presence of these inhibitors. Cells must be infected at least 24 hr before any DNA synthesis inhibitors are applied. Longer periods of time between infection and inhibitor application may yield higher transduction efficiencies. For in vivo studies, it is important to consider several factors. Viral uptake varies between different brain areas, and the longevity of promoter activity also varies across different brain areas. Thus, it is important to determine the pattern and extent of transduction for each individual brain area to be studied. Storing virus inappropriately will also yield decreased transduction efficiencies. Virus can be stored for several weeks at 4°C or for several months at −80°C. Multiple freeze/thaw cycles should be avoided.
In vivo administration of rAAV – intracranial injection
The microinjection procedure described in Basic Protocol 2 should yield consistent injections in the desired location. If the injection site is incorrect or inconsistent, recheck coordinates with the atlas. Verify these coordinates with dye injections. Atlas coordinates are not exact, as structure positions may vary slightly with strain and age of the animal. Correct positioning of the animal in the stereotaxic frame is vital for consistent results as is the correct reading of coordinates from the stereotaxic frame.
Plugging of the injector tip can be a problem with the microinjections. A larger injector (30-G as opposed to a 32- or 33-G) will plug less often. If plugging still occurs with this larger tip, move the tip into position more slowly. The pump can also be turned on before lowering the tip into the brain. This produces a small flow of virus out of the tip to counteract the pressure of the material trying to move into the tip. Injector problems can also lead to poor spread of virus or virus leaking up the injector tract. These problems can also occur if virus is being infused too quickly or if the injector tip is not left in place long enough after infusion has stopped.
In vivo administration of rAAV – intracranial injection
The intrathecal injection procedure described in Basic Protocol 3 should provide widespread gene delivery to the lower spinal cord, particularly if AAV6, 8, or 9 are used. This injection is very difficult. To master this technique, extensive practice should be done on mice using a 12.5% lidocaine solution in PBS (without rAAV). More detail on this technique can be found in Fairbanks et al. (2003).
When performing the injection, you should be able to feel the needle slide in between the vertebra and see the mouse’s tail flick or curl into an “S” shape. Making sure the mouse’s spine is curved up slightly will aid in this. If bilateral paralysis is observed following injection but only for a short time (1–2 minutes), remake the lidocaine solution and also check to make sure the syringe is dispensing the correct volume. Also make sure the needle is held in place for a few seconds following injection so the solution doesn’t flow out through the needle hole in the dura.
Anticipated Results
In these protocols, twenty 15-cm tissue culture plates should yield ~1 × 1013 particles (less for self-complementary rAAV) as assayed by dot blot. The typical ratio of particles to transducing units should be anywhere from ~5:1 for rAAV2 to ~100:1 for rAAV9 (depending on the serotype) when assayed on a cell type that transduces well, such as HeLaRC32 cells in the presence of adenovirus. Cells that transduce less well will give a higher ratio, but this can often be related to the use of a less permissive cell rather than a less infectious virus. The particle preparations should be free of contaminating cellular and adenovirus proteins and free of infectious adenovirus.
The typical microinjection of 1 to 2 μl of high-titer virus (109 to 1010 vector genome-containing particles) yields a spread of ~1000 μm when injected intracranially. The number of cells transduced will depend on the area of the brain. (Fig. 4.17.4). Sections further away from the injection site will have fewer cells transduced. Tranduction variation depends both on the exact site of injection within a structure and the efficiency of the injection (i.e., whether the injector became plugged). Some structures in the brain may show more variability than others. Self-complementary rAAV vectors have a much stronger advantage when used with diffuse gene delivery approaches such as intrathecal or intravenous injection, where they are approximately 10 times more efficient than single-stranded AAV vectors (Fu et al., 2003; Gray et al., 2011). The use of different rAAV serotypes in the brain and CNS has shown great promise for region-specific delivery for specific disease models (Burger et al., 2004).
Figure 4.17.4.
Green fluorescent protein (GFP) expression in the inferior colliculus and the globus pallidus 2 weeks after infusion of 1 μl of AAV-GFP virus.
Time Considerations
Transfection of the cells requires 3-4 days. On day 1 the cells are split. On day 2 the cells are transfected. For self-complementary rAAV, cells are harvested on day 3. For single-stranded rAAV, they are harvested on day 4.
The purification described in Basic Protocol 1 requires at least 3 days. On day 1 the cells will be processed and the initial step CsCl gradient performed, then the overnight continuous CsCl gradient started. On day 2 the fractions are collected and the peak fractions determined by qPCR, then dialysis of the peak fractions are started overnight. On day 3 the dialysis is completed and the virus is divided into aliquots and frozen. An aliquot can be thawed and titered by qPCR on the 3rd day. An extra day would be required if an extra CsCl continuous gradient is used to concentrate the peak fractions, and if dot blots are used in place of qPCR this will add an extra 2 days (1 for determination of peak fractions and 1 for determination of final titer).
The dot-blot assay requires 2 days: 1 day to process the samples, bind to the filter, and hybridize, and another day to wash the filter and expose. The qPCR assay requires half a day.
Growing and purifying an adenovirus stock does not have to be done very often, but the procedure requires up to 18 days. On day 1 the 293 cells are seeded; on day 2 the cells are infected and incubated 48 to 60 hr for the infection to proceed. On day 5 the virus is purified on the step gradient and rebanded on the continuous gradient. On day 6 the virus is harvested and stored. The plaque assay requires 6 to 12 days.
Intracranial microinjections of animals (Basic Protocol 2) typically take 20 to 30 min per animal, not including setup and cleanup time. Length of time also depends on the amount of virus to be injected. Typically, animals are allowed to express the transgene for at least a week before sacrifice and detection. Longer time periods may also be desired.
Intrathecal injections of animals (Basic Protocol 3) typically takes 5 to 10 min per animal or less, not including setup and cleanup time. Animals can be injected sequentially, but they should be individually marked and monitored for the length of bilateral paralysis. Typically, animals are allowed to express the transgene for at least a week (preferably 3–4) before sacrifice and detection. Longer time periods may also be desired.
Animal perfusions should be accomplished on one day with the vibrating microtome sectioning occurring on the next day or the second day after perfusion, although fixed tissue can be stored at 4°C in PBS for several days before sectioning. GFP visualization can be done immediately after sections are mounted on slides. Immunohistochemical staining and mounting takes 2–3 days and can be done any time after sectioning, with the sections stored in 4°C for up to a few weeks after sectioning.
Footnotes
Key References
McCown et al., 1996. See above.
This paper uses the microinjection technique to demonstrates how several different brain areas can be transduced by rAAV. This is an early paper in which the virus titer is comparatively low and minimally contaminated with adenovirus. Higher titer adenovirus-free preparations give better transduction with less cellular damage and longer duration of gene expression.
Fairbanks et al., 2003. See above.
This paper details the intrathecal injection protocol and its applications.
Gray et al.,2010. See above.
This review provides an overview of AAV as a gene therapy vector, discusses different routes of delivery and serotype differences, and contains information on recent accomplishments using rAAV vectors in the CNS.
Grieger et al., 2006. See above.
This paper details the adenovirus-free rAAV production protocol and additional protocols related to rAAV production and validation.
Internet Resources
http://www.med.unc.edu/genether/
University of North Carolina Human Gene Therapy Center Web site. Contains map and sequence of the psub201, pHpa-trs-SK, pXX2, pXR1, pXR2, pXR3, pXR4, pXR5Bam, and pXX6 plasmids.
Updated (current) version provided by Steven J. Gray and Richard Jude Samulski
Original contributions by Vivian W. Choi, Aravind
Asokan, Rebecca A. Haberman,
Thomas J. McCown, and Richard Jude Samulski
University of North Carolina
Chapel Hill, North Carolina
Literature Cited
- Bi X, Liu LF. DNA rearrangement mediated by inverted repeats. Proc Natl Acad Sci USA. 1996;93:819–823. doi: 10.1073/pnas.93.2.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy R, Astell CR. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5′ terminal palindrome of minute virus of mice. Gene. 1985;35:179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Cukor G, Blacklow NR, et al. Biology of adeno-associated virus. In: Berns KI, editor. The Parvoviruses. Plenum; New York: 1984. pp. 33–66. [Google Scholar]
- Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;28;55(8):1007–41. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Fu H, Muenzer J, Samulski RJ, Breese G, Sifford J, Zeng X, McCarty DM. Self-complementary adeno-associated virus serotype 2 vector: Global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol Ther. 2003;8:911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda S, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and non-human primates. Mol Ther. 2011;19(6):1058–69. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Prot. 2006;1(3):1412–28. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Hermonat PL, Labow MA, et al. Genetics of adeno-associated virus: Isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984;51:329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel RJ, Rendahl KG, et al. Characterization of intrastriatal recombinant adeno-associated virus-mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydrolase I in a rat model of Parkinson’s disease. J Neurosci. 1998;18(11):4271–4284. doi: 10.1523/JNEUROSCI.18-11-04271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Xiao X, et al. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713(1–2):99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, Collis P, et al. Adeno-associated virus general transduction vectors: Analysis of proviral structures. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; London: 1986. [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-packaging of a single-adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Chang LS, et al. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J Virol. 1989;63(9):3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Woodard KT, Samulski RJ. Viral vectors and delivery strategies for CNS gene delivery. Therapeutic Delivery. 2010;1(4):517–534. doi: 10.4155/tde.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FE, McCown TJ. AAV vectors: General characteristics and potential use in the central nervous system. In: Chiocca EA, Breakefield XO, editors. Gene Transfer and Therapy for Neurological Disorders. Humana Press; Totowa, N.J: 1997. pp. 79–88. [Google Scholar]