Abstract
Complement factor H related protein 5 (CFHR5) nephropathy is a monogenic disorder of complement regulation that is endemic in Cyprus. The disease is characterised by haematuria, C3 glomerulonephritis and kidney failure. Its identification suggests a role for the CFHR5 protein in the regulation of complement in the kidney. In this review, we discuss how studying CFHR5 nephropathy can contribute to our understanding of the role of complement in kidney diseases such as dense deposit disease, C3 glomerulonephritis and atypical haemolytic uraemic syndrome.
Introduction
Complement factor H related protein 5 (CFHR5) nephropathy is a recently recognised kidney disease in which a heterozygous mutation in the CFHR5 gene is associated with autosomal dominant inheritance of glomerulonephritis and kidney failure (Gale et al., 2010). To date, the disease has only been reported in individuals from Cyprus, where it is an endemic cause of renal disease and accounts for a significant proportion of end-stage kidney failure on the island (Athanasiou et al., 2011). In this review, we first discuss how the features of CFHR5 nephropathy relate to our current understanding of complement regulation and its role in kidney disease. We next discuss the role of complement in membranoproliferative glomerulonephritis (MPGN), dense deposit disease (DDD), C3 glomerulonephritis and atypical haemolytic uraemic syndrome (aHUS). Finally, we outline potential avenues for clinical and basic research that could further expand our understanding of glomerular disorders.
CFHR5 nephropathy: clinical and laboratory features
CFHR5 nephropathy typically presents with haematuria (blood in the urine), usually in microscopic amounts, but 25–50% of patients report episodes in which there is visible blood in the urine (macroscopic haematuria), almost always at times of respiratory tract or other infections. These episodes can be associated with acute deterioration in renal function. In more than 80% of male (but only a small proportion of female) patients, there is progressive, stepwise deterioration in renal function leading to end-stage kidney failure in adulthood (usually between the ages of 30 and 70 years) (Athanasiou et al., 2011). Proteinuria is mild (usually less than 1 g/day) and is only present late in the disease when there is impaired renal function. Kidney biopsies show histological evidence of inflammation (in a pattern termed MPGN), and electron microscopy reveals electron-dense material deposited in the sub-endothelial glomerular basement membrane and in the mesangium. There is positive immunostaining for complement proteins C3, C5 and C9 in the glomeruli, but no evidence of immunoglobulin or C1q deposition. These histological features are referred to as C3 glomerulonephritis and imply that dysregulation of the complement alternative pathway (Fig. 1) is central to the pathophysiology of the disease.
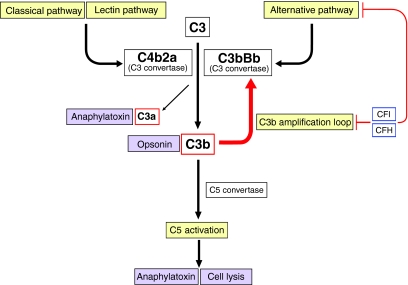
Fig. 1.
Simplified schematic of the complement pathways. The complement system is a network of proteins that is important in innate immunity. Effector functions of complement include opsonisation (leading to phagocytosis of pathogens), production of anaphylatoxins (which can cause inflammation, vasodilation etc.) and cell lysis (which can lead to tissue damage). At the centre of the complement system is a positive-feedback loop (known as the C3b amplification loop; red arrow) in which the production of activated C3 (termed C3b), through the generation of an enzyme complex termed a C3 convertase, can be rapidly increased. C3b can be generated by activation of three pathways, each with different modes of activation: the classical pathway, the lectin pathway and the alternative pathway. Runaway complement pathway activation and subsequent damage to host cells is prevented by tight regulation at many levels. This is achieved by several regulators that act either in the circulation, on host cell surfaces or both. Regulation of the alternative pathway is particularly important because it is continuously activated by spontaneous hydrolysis of plasma C3. Importantly, the regulators of the alternative pathway also downregulate the C3b amplification loop. The key regulators of these pathways are plasma proteins CFH and CFI. Dysregulation of the alternative pathway is associated with aHUS (renal thrombotic microangiopathy) and glomerulonephritis (C3 glomerulopathy). Schematic is adapted, with permission, from Pickering and Cook (Pickering and Cook, 2011).
Serum levels of complement proteins C3 and C4 are typically normal, even during acute episodes of macroscopic haematuria. Although outcomes following renal transplantation in patients with CFHR5 nephropathy have been generally good, the disease has been shown to recur following renal transplantation from an unrelated donor, proving that a circulating (as opposed to local) abnormality is responsible for pathology (Vernon et al., 2011).
The disease is inherited as an autosomal dominant trait with >90% penetrance, and it cosegregates with a 6.3 kbp genomic duplication that includes exons 2 and 3 of the CFHR5 gene (Gale et al., 2010). This mutation results in the production of an elongated version of the CFHR5 protein that is detectable in the circulation of patients. The mutation is present in ∼1 in 6500 Cypriots, and over 100 patients with the disease have been identified. All patients have recent Cypriot ancestry and share an extended haplotype flanking the mutation, proving that they share a common affected ancestor (Athanasiou et al., 2011). Why the disease is so common in the Cypriot population is yet to be determined –possibilities include genetic drift within the island population or positive selection, perhaps by the presence of another endemic disease. The mechanism by which this CFHR5 mutation causes the disease is not yet understood but might have relevance for more common diseases in which complement is deposited in the glomerulus; for example, similar glomerular pathology may be observed in patients with systemic lupus erythematosus (referred to as lupus nephritis).
At present, there is no treatment of proven efficacy for CFHR5 nephropathy. Disease progression seems to correlate with infectious episodes, and tonsillectomy has been used with apparently good long-term results in at least one case (Athanasiou et al., 2011). Responses to conventional immunosuppression (for instance, with corticosteroid, cytotoxic or antiproliferative therapies) have been inconsistent and are associated with exacerbation of renal injury in some patients. However, plasma exchange carried out during episodes of macroscopic haematuria and acute renal dysfunction has been associated with good short-term outcomes in some cases (D.P.G., unpublished observations). Whether any benefits observed following this treatment are a result of supplementation with wild-type CFHR5 protein from donor plasma, removal of the mutant CFHR5 protein or some other mechanism remains to be determined. It is not known whether eculizumab, a monoclonal antibody that prevents complement C5 activation, is of benefit in treating CFHR5 nephropathy. A better understanding of the functions of CFHR5 and the mechanism by which the mutation causes the disease might suggest a role for the CFHR5 protein itself in the treatment of the disease.
Clinical terms.
- Anaphylatoxin
a protein that causes stimulation and chemotaxis of immune cells
- Glomerulonephritis
inflammation of the glomeruli (the small blood vessels of the kidneys that are involved in filtering blood to form urine)
- Macroscopic haematuria
red discolouration of the urine owing to the presence of blood (in amounts greater than in microscopic haematuria)
- Microscopic haematuria
blood in the urine detectable using a microscope or dipstick test
- Nephrotic syndrome
clinical triad of proteinuria >3 g/day; swelling of limbs or body due to oedema; and low blood protein level
- Proteinuria
protein in the urine
- Synpharyngitic
occurring at the same time as an upper respiratory tract infection
The CFHR5 gene
The CFHR5 gene lies at the telomeric end of a gene cluster that also contains the complement factor H (CFH) gene and the four other CFHR genes (CFHR1-CFHR4). These genes are oriented head-to-tail on chromosome 1q32; this tandem arrangement of similar genes suggests that they are homologues that have arisen by successive genomic duplication events over time. This view is supported by the presence of frequent copy number variations (CNVs; duplications or deletions of genomic regions) reported within this gene cluster, some of which –including complete deletions of CFHR1, CFHR3 or CFHR4 – are common polymorphisms occurring with combined allele frequencies of over 20% in healthy controls (Venables et al., 2006; Schmid-Kubista et al., 2009; Raychaudhuri et al., 2010). The high frequency of CNVs in this region might be the result of the many nearby similar nucleotide sequences predisposing to non-allelic homologous recombination events during meiosis (Lupski, 1998). Importantly, even among closely related species (such as the human and chimpanzee), there is seldom one-to-one correspondence of orthologous CFHR genes. This has made it difficult to predict whether targeted deletion of CFHRs in the mouse (in which there are three Cfhr genes) would yield much useful information in understanding the function of the CFHRs in humans. This has probably contributed to the view (which prevailed over most of the ∼2 decades since CFHRs were first identified) that CFHRs were unlikely to perform significant functions.
The CFHR5 protein
CFH and the CFHR proteins are each made up of between 4 and 20 homologous short consensus repeat domains (SCRs). Each SCR comprises approximately 60 amino acids and folds into a globular structure with a hydrophobic core and two cysteine-cysteine disulphide bonds (Janatova et al., 1989; Norman et al., 1991). CFHR5 was first identified as a component of glomerular immune complexes and is consistently found colocalized with C3 and C5b-9 in glomerular deposits (McRae et al., 2001; Murphy et al., 2002). The wild-type CFHR5 protein is composed of nine SCRs, encoded by each of exons 2 to 10 of the gene. The mutation associated with CFHR5 nephropathy results in the duplication of SCRs 1 and 2 of the protein, with the first amino acid of the duplicated SCR1 changed from glycine to arginine by the splicing of its encoding exon (exon 2) downstream of exon 3 (which encodes SCR2) (Gale et al., 2010).
Case study.
A 17-year-old British male of Cypriot descent presented to his family doctor with headaches. He reported occasional episodes of red discolouration of the urine, which occurred at the same time as (or even 24 hours before) upper respiratory tract infections (termed synpharyngitic macroscopic haematuria). Routine clinical and neurological examination was normal with the exception of microscopic haematuria (blood +++ on urine dipstick test) and a blood pressure of 165/80 mmHg. Blood tests, including the blood count, biochemical and renal function tests, immunoglobulins, autoantibodies, viral serology, complement C3, complement C4 and C3NeF were all normal or negative. The headaches resolved on treatment of the high blood pressure with a single agent, and he proceeded to have a renal biopsy, which showed C3 glomerulonephritis: there was MPGN with deposition of complement C3 but not immunoglobulins or C1q in the glomerulus. Electron microscopy demonstrated mesangial and subendothelial glomerular basement membrane electron-dense deposits without dense transformation of the glomerular basement membrane. Subsequent molecular testing demonstrated the presence of a duplication of exons 2 and 3 of CFHR5. Over the following 10 years, there were further episodes of synpharyngitic macroscopic haematuria associated with acute stepwise deteriorations in renal function occurring approximately once each year. The mutation was also detected in DNA from his mother, who exhibited persistent microscopic haematuria without renal impairment, proteinuria or high blood pressure. A kidney biopsy performed in the mother some years previously had shown C3 glomerulonephritis.
The functions of CFHR5 are not well understood. Similar to CFH, it is produced in the liver and circulates in the blood, although at approximately 100-fold lower concentrations. It shares some of the biochemical properties of CFH, including affinity for C3b and for glycosaminoglycans on host cell surfaces (this can be estimated in the laboratory by measuring affinity for the glycosaminoglycan heparin). In addition, CFHR5 can inhibit the C3 convertase and can act as a cofactor for the cleavage of C3b. Although the affinity of CFHR5 for heparin seems greater than that of CFH, its cofactor and C3 convertase inhibitory activities are lower compared with CFH (McRae et al., 2005). Together with its much lower circulating abundance, this argues against a non-redundant role for CFHR5 in the regulation of complement activation in the circulation, because CFH would be predicted to be in massive excess – in both quantity and relative activity. The presence of CFHR5 within glomerular immune deposits and its high affinity for heparin suggests a particular role for CFHR5 in regulating complement within the glomerulus.
The mutant CFHR5 protein associated with CFHR5 nephropathy is detectable in the circulation of patients and, when compared with the wild-type protein, has reduced affinity for heparin and for complement-coated surfaces. This suggests a mechanism whereby the impaired ability of the mutant protein to localise to glomerular surfaces is important in the pathophysiology of the disease. The situation might be more complex than this, however, because the mutant protein also has enhanced factor I cofactor activity compared with the wild-type protein (although still substantially reduced compared with that of CFH) (Gale et al., 2010). Although the issue of whether CFHR5 nephropathy results from a loss-of-function or a gain-of-function change in the CFHR5 protein remains unresolved, the normal circulating C3 levels in patients with the disease suggest that CFHR5 nephropathy results from complement dysregulation specifically at glomerular surfaces, as opposed to systemic activation of complement in the fluid phase (see Fig. 2). This is in contrast to DDD, in which complement activation in the circulation causes glomerulonephritis. Understanding why, in CFHR5 nephropathy, complement dysregulation at a surface causes glomerulonephritis rather than thrombotic microangiopathy (the pattern of injury seen in aHUS; discussed below) requires a more detailed understanding of the biochemical properties and functions of CFHR5 itself.
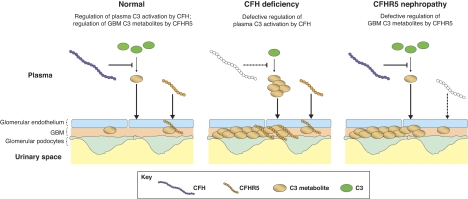
Fig. 2.
Proposed model whereby both CFH and CFHR5 are needed to prevent C3 accumulation along the glomerular basement membrane. CFH deficiency (represented by white CFH protein) leads to C3 consumption and the production of C3 metabolites in plasma; these C3 metabolites then accumulate along the glomerular basement membrane (GBM). In CFHR5 nephropathy, abnormal CFHR5 (represented by white CFHR5 protein) results in defective regulation of C3 metabolites within the GBM. In this hypothetical model, we assume that CFHR5 but not CFH interacts directly with C3 metabolites in the kidney. Consequently, where C3 metabolites accumulate in the setting of CFH deficiency, we depict increased binding of CFHR5 in the kidney.
Complement and membranoproliferative glomerulonephritis
The typical pathological lesion in the kidney of patients with CFHR5 nephropathy is MPGN. MPGN describes a histological pattern of renal injury in which there is expansion and hypercellularity of the glomerular mesangial regions, together with thickening of glomerular capillary walls. These features are most commonly observed in association with systemic diseases in which there is an increase in antibody production, such as monoclonal immunoglobulin production disorders, systemic lupus erythematosus and chronic infections; in these disorders, MPGN is usually associated with deposition of immunoglobulin and complement within the glomeruli. In many such cases, circulating levels of complement C3 and C4 are reduced, reflecting systemic activation of the classical and alternative complement pathways. It is not known why some individuals with these autoimmune or infectious diseases develop glomerulonephritis whereas other people with the same underlying diseases have no renal involvement. The invariable deposition of complement components at sites of renal injury has led to the view that complement plays a central role in the pathophysiology of MPGN (Alchi and Jayne, 2010). Moreover, the idea that variation in the complement pathway itself might be important in determining the severity of glomerulonephritis has been supported by recent evidence indicating that common genetic variation in the genes encoding complement regulators can affect susceptibility to these diseases in the general population (Gharavi et al., 2011; Zhao et al., 2011). Additional evidence of the role of complement in glomerular disease has come from the identification of rare disorders in which glomerulonephritis is associated with complement deposition in the absence of glomerular immunoglobulin. These rare but informative glomerular disorders include: DDD, C3 glomerulonephritis and CFHR5 nephropathy. Collectively these disorders are referred to as C3 glomerulopathies (Fakhouri et al., 2010), which we describe in the following sections. aHUS is also briefly discussed; although this condition is a thrombotic microangiopathy and not a glomerulonephritis, it is associated with complement dysregulation.
Dense deposit disease
DDD (formerly known as MPGN type 2) is a rare disorder affecting approximately two to three people per million in the population (Smith et al., 2007). It is most common in childhood and affects females and males at a ratio of 3:2 (Lu et al., 2007). Presentation is typically with nephrotic-range proteinuria with or without microscopic haematuria. There is gradual deterioration of renal function, with 50% of patients reaching end-stage renal failure 10 years from diagnosis. The disease is associated with ocular drusen (deposits of electron-dense material in Bruchs membrane, which lies beneath the retinal pigment epithelium of the eye), and visual impairment is a late complication (Colville et al., 2003). DDD is diagnosed by renal biopsy, which shows dense transformation of the glomerular basement membrane, often with MPGN. Immunostaining usually demonstrates C3 but not immunoglobulin deposition in the glomerulus, and approximately 80% of patients exhibit depletion of serum complement C3 (Schwertz et al., 2001). These clinicopathological features suggest that systemic activation of complement in the circulation underlies the disease.
DDD is most commonly associated with the presence of an antibody (termed C3 nephritic factor or C3NeF) that stabilises the alternative pathway C3 convertase (C3bBb) in the circulation, leading to runaway complement activation and depletion of C3. C4 levels are typically normal, reflecting the fact that the classical pathway is not activated (Schwertz et al., 2001). In addition to the presence of C3NeF, rare genetic defects of complement regulators have been reported that lead to uncontrolled activation of the alternative pathway in the circulation. Examples include the genetic deficiency of CFH and an activating mutation of C3 that causes defective fluid-phase regulation of C3 (Martinez-Barricarte et al., 2010; Smith et al., 2011). A spontaneously occurring breed of pigs that develops plasma C3 depletion and glomerulonephritis with histological features of MPGN was found to harbour genetic deficiency of circulating CFH (Hogasen et al., 1995). In addition, targeted deletion of the Cfh gene in mice leads to C3 depletion and glomerulonephritis with histological features of MPGN (Pickering et al., 2002). These animal studies illustrate the relationship between complement regulation and MPGN. However, the typical intramembranous changes seen in human DDD differ from those seen in CFH-deficient mice and pigs, suggesting that the full range of functions of CFH and its related proteins differ in different species.
C3 glomerulonephritis
Rarely, acquired C3NeF or genetic abnormalities of complement regulatory proteins are associated with glomerular C3 (but not immunoglobulin) deposition in the absence of dense transformation of the glomerular basement membrane. These features are referred to as C3 glomerulonephritis and are similar to the characteristic renal biopsy findings in CFHR5 nephropathy. The clinical spectrum of C3 glomerulonephritis is broad, with one series reporting haematuria in 63% of patients at diagnosis and proteinuria ranging from 0 to >5 g/day, with 11% of patients exhibiting nephrotic syndrome (Servais et al., 2007). Some of the genetic abnormalities found in this cohort of patients had previously been identified in patients with aHUS. These rare cases illustrate that abnormalities of complement regulation can lead to an overlapping spectrum of clinicopathological features.
Atypical haemolytic uraemic syndrome
Complement alternative pathway dysregulation can also occur at cell surfaces, resulting in aHUS. In patients with this disorder, haemolysis and complement-mediated disruption of endothelial surfaces occurs, leading to microthrombosis and organ damage. In many cases of aHUS, abnormalities in genes encoding complement regulators have been identified – most common are mutations in CFH that disrupt the ability of the protein to bind to glomerular endothelium (reviewed in Kavanagh and Goodship, 2010). Circulating C3 levels are frequently normal in these patients, reflecting the fact that regulation of the alternative pathway in the fluid phase need not be disrupted to cause the disease. Eculizumab seems to be a very potent therapy in this condition (e.g. Gruppo and Rother, 2009), and the final results of global trials in plasma-resistant and plasma-sensitive aHUS are awaited.
Clinical and basic research opportunities.
To determine why end-stage renal failure develops more frequently in males with CFHR5 nephropathy than in females with the disease.
To determine whether CFHR5 is important in the pathogenesis of IgA nephropathy.
To investigate the role of complement inhibitors in CFHR5 nephropathy.
To develop animal models of CFHR5 nephropathy.
Unanswered clinical questions
There are many outstanding questions regarding CFHR5 nephropathy that can be addressed through additional clinical studies and research in biological models. First, one of the most striking features of CFHR5 nephropathy is the different clinical course in men and women. In contrast to aHUS, in which the disease is generally more aggressive in females, almost all males with CFHR5 nephropathy require renal replacement therapy by the time they reach old age, whereas the majority of women are asymptomatic for their entire lives, with microscopic haematuria being the only clinical finding (Athanasiou et al., 2011). Possibilities to explain this sexual dimorphism include differences in expression of complement regulators or the known differences in the behaviour of the innate or adaptive immune systems between males and females: in general, females mount a more robust humoral and cell-mediated response to immunological stimulation, whereas, in males, innate inflammatory responses are more aggressive (for a review, see Marriott and Huet-Hudson, 2006). At least some of these differences can be attributed to the effects of sex hormones on lymphocytes (Weinstein et al., 1984), and there is epidemiological evidence suggesting that, although autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis are more common in women, men are more susceptible to severe bacterial infections (Schroder et al., 1998; Offner et al., 1999; Eaton et al., 2007). A further possibility is that there are intrinsic properties of the male kidney that render it more susceptible to complement-mediated injury. Understanding this phenomenon might yield insights that aid the development of a treatment for CFHR5 nephropathy.
Second, whereas patients with DDD and C3 glomerulonephritis not caused by CFHR5 mutation exhibit a wide variety of clinical features, often including nephrotic range proteinuria (Servais et al., 2007; Licht and Fremeaux-Bacchi, 2009), the clinical and histological features of CFHR5 nephropathy bear a remarkable and consistent similarity to those of IgA nephropathy. In fact, the only distinguishing features of CFHR5 nephropathy are its familial nature and the absence of glomerular immunoglobulin A deposition. That a mutation in the CFHR5 gene can reliably recapitulate so many clinical and pathological features of IgA nephropathy raises the question of whether the CFHR5 protein could be important in the pathophysiology of this disorder.
The exciting possibility exists that devising a successful treatment strategy for CFHR5 nephropathy will open up a new avenue for the treatment of not only C3 glomerulopathies but also more common glomerular disorders, such as IgA nephropathy and lupus nephritis, in which complement plays a significant role.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
FUNDING
M.C.P. is a Wellcome Trust Senior Fellow in Clinical Science [WT082291MA].
REFERENCES
- Alchi B., Jayne D. (2010). Membranoproliferative glomerulonephritis. Pediatr. Nephrol. 25, 1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou Y., Voskarides K., Gale D. P., Damianou L., Patsias C., Zavros M., Maxwell P. H., Cook H. T., Demosthenous P., Hadjisavvas A., et al. (2011). Familial C3 glomerulopathy associated with CFHR5 mutations: clinical characteristics of 91 patients in 16 pedigrees. Clin. J. Am. Soc. Nephrol. 6, 1436–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville D., Guymer R., Sinclair R. A., Savige J. (2003). Visual impairment caused by retinal abnormalities in mesangiocapillary (membranoproliferative) glomerulonephritis type II (“dense deposit disease”). Am. J. Kidney. Dis. 42, E2–E5 [DOI] [PubMed] [Google Scholar]
- Eaton W. W., Rose N. R., Kalaydjian A., Pedersen M. G., Mortensen P. B. (2007). Epidemiology of autoimmune diseases in Denmark. J. Autoimmun. 29, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri F., Fremeaux-Bacchi V., Noel L. H., Cook H. T., Pickering M. C. (2010). C3 glomerulopathy: a new classification. Nat. Rev. Nephrol. 6, 494–499 [DOI] [PubMed] [Google Scholar]
- Gale D. P., de Jorge E. G., Cook H. T., Martinez-Barricarte R., Hadjisavvas A., McLean A. G., Pusey C. D., Pierides A., Kyriacou K., Athanasiou Y., et al. (2010). Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376, 794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharavi A. G., Kiryluk K., Choi M., Li Y., Hou P., Xie J., Sanna-Cherchi S., Men C. J., Julian B. A., Wyatt R. J., et al. (2011). Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat. Genet. 43, 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppo R. A., Rother R. P. (2009). Eculizumab for congenital atypical hemolytic-uremic syndrome. N. Engl. J. Med. 360, 544–546 [DOI] [PubMed] [Google Scholar]
- Hogasen K., Jansen J. H., Mollnes T. E., Hovdenes J., Harboe M. (1995). Hereditary porcine membranoproliferative glomerulonephritis type II is caused by factor H deficiency. J. Clin. Invest. 95, 1054–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janatova J., Reid K. B., Willis A. C. (1989). Disulfide bonds are localized within the short consensus repeat units of complement regulatory proteins: C4b-binding protein. Biochemistry 28, 4754–4761 [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Goodship T. (2010). Genetics and complement in atypical HUS. Pediatr. Nephrol. 25, 2431–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht C., Fremeaux-Bacchi V. (2009). Hereditary and acquired complement dysregulation in membranoproliferative glomerulonephritis. Thromb. Haemost. 101, 271–278 [PubMed] [Google Scholar]
- Lu D. F., McCarthy A. M., Lanning L. D., Delaney C., Porter C. (2007). A descriptive study of individuals with membranoproliferative glomerulonephritis. Nephrol. Nurs. J. 34, 295–302; quiz 303 [PubMed] [Google Scholar]
- Lupski J. R. (1998). Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet. 14, 417–422 [DOI] [PubMed] [Google Scholar]
- Marriott I., Huet-Hudson Y. M. (2006). Sexual dimorphism in innate immune responses to infectious organisms. Immunol. Res. 34, 177–192 [DOI] [PubMed] [Google Scholar]
- Martinez-Barricarte R., Heurich M., Valdes-Canedo F., Vazquez-Martul E., Torreira E., Montes T., Tortajada A., Pinto S., Lopez-Trascasa M., Morgan B. P., et al. (2010). Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J. Clin. Invest. 120, 3702–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae J. L., Cowan P. J., Power D. A., Mitchelhill K. I., Kemp B. E., Morgan B. P., Murphy B. F. (2001). Human factor H-related protein 5 (FHR-5). A new complement-associated protein. J. Biol. Chem. 276, 6747–6754 [DOI] [PubMed] [Google Scholar]
- McRae J. L., Duthy T. G., Griggs K. M., Ormsby R. J., Cowan P. J., Cromer B. A., McKinstry W. J., Parker M. W., Murphy B. F., Gordon D. L. (2005). Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J. Immunol. 174, 6250–6256 [DOI] [PubMed] [Google Scholar]
- Murphy B., Georgiou T., Machet D., Hill P., McRae J. (2002). Factor H-related protein-5: a novel component of human glomerular immune deposits. Am. J. Kidney Dis. 39, 24–27 [DOI] [PubMed] [Google Scholar]
- Norman D. G., Barlow P. N., Baron M., Day A. J., Sim R. B., Campbell I. D. (1991). Three-dimensional structure of a complement control protein module in solution. J. Mol. Biol. 219, 717–725 [DOI] [PubMed] [Google Scholar]
- Offner P. J., Moore E. E., Biffl W. L. (1999). Male gender is a risk factor for major infections after surgery. Arch. Surg. 134, 935–938; discussion 938–940 [DOI] [PubMed] [Google Scholar]
- Pickering M., Cook H. T. (2011). Complement and glomerular disease: new insights. Curr. Opin. Nephrol. Hypertens. 20, 271–277 [DOI] [PubMed] [Google Scholar]
- Pickering M. C., Cook H. T., Warren J., Bygrave A. E., Moss J., Walport M. J., Botto M. (2002). Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 31, 424–428 [DOI] [PubMed] [Google Scholar]
- Raychaudhuri S., Ripke S., Li M., Neale B. M., Fagerness J., Reynolds R., Sobrin L., Swaroop A., Abecasis G., Seddon J. M., et al. (2010). Associations of CFHR1-CFHR3 deletion and a CFH SNP to age-related macular degeneration are not independent. Nat. Genet. 42, 553–555; author reply 555–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Kubista K. E., Tosakulwong N., Wu Y., Ryu E., Hecker L. A., Baratz K. H., Brown W. L., Edwards A. O. (2009). Contribution of copy number variation in the regulation of complement activation locus to development of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 50, 5070–5079 [DOI] [PubMed] [Google Scholar]
- Schroder J., Kahlke V., Staubach K. H., Zabel P., Stuber F. (1998). Gender differences in human sepsis. Arch. Surg. 133, 1200–1205 [DOI] [PubMed] [Google Scholar]
- Schwertz R., Rother U., Anders D., Gretz N., Scharer K., Kirschfink M. (2001). Complement analysis in children with idiopathic membranoproliferative glomerulonephritis: a long-term follow-up. Pediatr. Allergy Immunol. 12, 166–172 [DOI] [PubMed] [Google Scholar]
- Servais A., Fremeaux-Bacchi V., Lequintrec M., Salomon R., Blouin J., Knebelmann B., Grunfeld J. P., Lesavre P., Noel L. H., Fakhouri F. (2007). Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J. Med. Genet. 44, 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Alexander J., Barlow P. N., Botto M., Cassavant T. L., Cook H. T., de Cordoba S. R., Hageman G. S., Jokiranta T. S., Kimberling W. J., et al. (2007). New approaches to the treatment of dense deposit disease. J. Am. Soc. Nephrol. 18, 2447–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Harris C. L., Pickering M. C. (2011). Dense deposit disease. Mol. Immunol. 48, 1604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables J. P., Strain L., Routledge D., Bourn D., Powell H. M., Warwicker P., Diaz-Torres M. L., Sampson A., Mead P., Webb M., et al. (2006). Atypical haemolytic uraemic syndrome associated with a hybrid complement gene. PLoS Med. 3, e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon K. A., Gale D. P., de Jorge E. G., McLean A. G., Galliford J., Pierides A., Maxwell P. H., Taube D., Pickering M. C., Cook H. T. (2011). Recurrence of complement factor H-related protein 5 nephropathy in a renal transplant. Am. J. Transplant. 11, 152–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ran S., Segal S. (1984). Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 132, 656–661 [PubMed] [Google Scholar]
- Zhao J., Wu H., Khosravi M., Cui H., Qian X., Kelly J. A., Kaufman K. M., Langefeld C. D., Williams A. H., Comeau M. E., et al. (2011). Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet. 7, e1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]