Abstract
Hypoxia and oncogene expression both stimulate glycolytic metabolism in tumors, thereby leading to lactate production. However, lactate is more than merely a by-product of glycolysis: it can be used as a metabolic fuel by oxidative cancer cells. This phenomenon resembles processes that have been described for skeletal muscle and brain that involve what are known as cell-cell and intracellular lactate shuttles. Two control points regulate lactate shuttles: the lactate dehydrogenase (LDH)-dependent conversion of lactate into pyruvate (and back), and the transport of lactate into and out of cells through specific monocarboxylate transporters (MCTs). In tumors, MCT4 is largely involved in hypoxia-driven lactate release, whereas the uptake of lactate into both tumor cells and tumor endothelial cells occurs via MCT1. Translating knowledge of lactate shuttles to the cancer field offers new perspectives to therapeutically target the hypoxic tumor microenvironment and to tackle tumor angiogenesis.
Introduction
Research into tumor cell metabolism has recently entered a new age (Cairns et al., 2011; Kroemer and Pouyssegur, 2008). Although this field was actively explored in the pre-genomics era, the discovery of tumor suppressor genes and oncogenes dampened interest in metabolism as a potential source of cancer cell specificities and related therapeutic targets. However, although the study of tumor-associated genes has led to the identification of oncoproteins as new therapeutic targets, a cure for cancer is still far away. Scientists in the cancer field are therefore reconsidering earlier metabolic discoveries using the molecular tools that are now available. This has led to cancer metabolism’s comeback to business.
The altered metabolism of cancer cells compared with normal cells confers a selective advantage for their survival and proliferation. As the primary tumor expands, it outgrows the diffusion limits of its local blood supply, leading to hypoxia. Among other effects, hypoxia induces the expression of hypoxia-inducible factor (HIF), a transcription factor that initiates a range of responses, including angiogenesis and various pro-survival mechanisms (Denko, 2008). Cellular metabolism is consecutively shifted towards the glycolytic pathway (i.e. glucose to lactate) through the increased expression of glycolytic enzymes and glucose transporters, together with a decreased dependence on the oxidative pathway (i.e. pyruvate to lactate to acetyl-CoA). In parallel, stimulation of angiogenesis leads to chaotic development of the tumor vasculature, which only alleviates hypoxia to a limited temporal and spatial extent, further selecting for tumors that constitutively upregulate glycolysis. The Warburg effect describes this capacity of tumor cells to exploit glycolysis (i.e. without coupling to the Krebs cycle and mitochondrial respiratory chain) even in the presence of oxygen (Cairns et al., 2011). The expression of oncogenes such as those encoding for Myc and Ras, and/or loss of tumor suppressor genes such as p53, also promote the glycolytic pathway by acting on the same metabolic factors as those regulated by HIF (Levine and Puzio-Kuter, 2010).
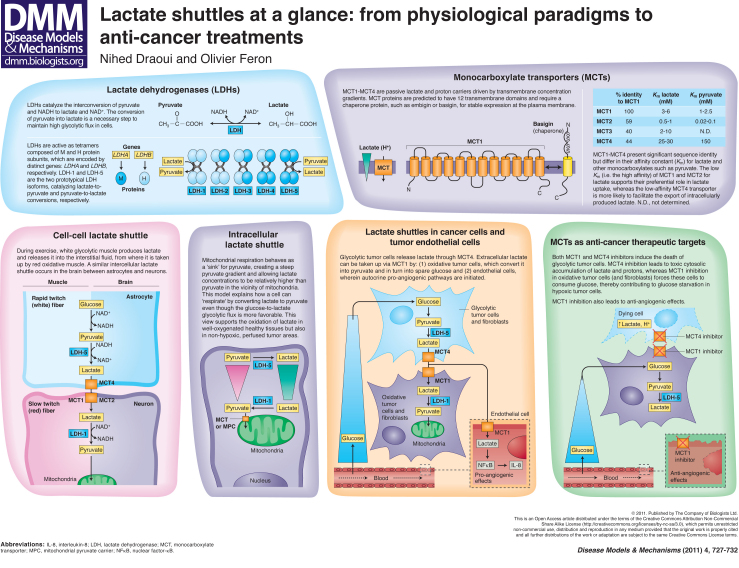
Lactate, the end product of glycolysis, is produced in large excess in tumors in response to the specific characteristics of the tumor microenvironment and the genetic features of tumor cells. Importantly, lactate also constitutes an alternative metabolic fuel for cancer cells (Sonveaux et al., 2008; Feron, 2009; Whitaker-Menezes et al., 2011), which is a phenomenon that has been well characterized in non-tumor tissues, including in skeletal muscle, brain and liver (Brooks, 2009; Gladden, 2004). Oxidative tumor cells can use lactate instead of (or in addition to) glucose, thereby sparing available glucose, which can, in turn, diffuse deeper into the tumor to fuel hypoxic cells located farther away from tumor blood vessels (Sonveaux et al., 2008). The use of lactate as an energy source requires the conversion of lactate into pyruvate (and back) as well as the transport of lactate into and out of tumor cells by way of specific transporters. Lactate dehydrogenase (LDH) isoforms and a family of monocarboxylate transporters (MCTs), respectively, regulate these processes. In this article and the accompanying poster, we describe lactate shuttles that involve these two lactate-related proteins in certain types of healthy tissues and tumor cells. Given their importance as controllers of tumor cell metabolism, the components of lactate shuttles might actually be promising therapeutic targets for cancer.
Lactate dehydrogenase: one enzyme, two functions
LDHs catalyze the interconversion of pyruvate and lactate, with concomitant interconversion of NADH and NAD+. These enzymes are homo- or hetero-tetramers composed of M and H protein subunits that are encoded by the LDHA and LDHB genes, respectively. Five isoforms are therefore possible: LDH-1 [four H subunits (4H)], LDH-2 (3H1M), LDH-3 (2H2M), LDH-4 (1H3M) and LDH-5 (4M) (see poster); a third gene, LDHC (also known as LDHX), is reportedly expressed in testes and sperm (Holmes and Goldberg, 2009).
LDH enzymes with a high M-subunit content (often referred to as LDHA proteins) are abundant in white skeletal muscle (rapid twitch glycolytic fibers), in which they reduce pyruvate into lactate. LDHA and pyruvate dehydrogenase kinase (PDK) are upregulated in solid tumors in response to hypoxia in a HIF-1α-dependent manner. PDK inactivates pyruvate dehydrogenase (PDH) and prevents the import of pyruvate into the mitochondrial matrix, whereas LDHA reduces the pyruvate into lactate and thereby regenerates the NAD+ stock necessary to maintain the glycolytic flux. In tumors, the glucose-to-lactate glycolytic pathway can also occur in the presence of oxygen, an observation described as the Warburg effect (Feron, 2009; Cairns et al., 2011). This is actually observed in most proliferating cells and occurs because dividing cells need to produce more biosynthetic intermediates to duplicate cell biomass and DNA (Feron, 2009). As long as glucose is available, NAD+ regeneration makes glycolysis self-sufficient and a crucial carbon source for protein, lipid and nucleotide biosynthesis. LDHA expression is also induced by a variety of oncogene products, including Myc (Shim et al., 1997), and might therefore contribute to the rapid consumption of pyruvate, the accumulation of which is potentially damaging. Lactate that is produced from hypoxia-induced or oncogene-driven LDHA expression also needs to be removed to avoid acidification of the intracellular compartment: this function is fulfilled by dedicated transporters called MCTs (see below).
LDH enzymes with high H-subunit content (often referred to as LDHB proteins) are mainly found in aerobic tissues (such as heart and brain), where they convert lactate into pyruvate. Transcriptional silencing of LDHB expression caused by aberrant methylation of the gene promoter region has been reported in gastric and prostate cancers (Maekawa et al., 2003; Leiblich et al., 2006), thereby reinforcing the idea that tumors preferentially express LDH isoenzymes with a high LDHA gene product content (LDH-5>LDH-4>LDH-3). In colorectal cancer, suppression of LDHB transcript in a more invasive phenotype was even proposed to account for a large part of the Warburg metabolic switch (i.e. independently of hypoxia-induced changes in LDHA expression) (Thorn et al., 2009).
Monocarboxylate transporters
MCTs constitute a family of 14 transporters [also known as solute carrier 16 (SLC16) proteins] that carry single-carboxylate molecules across biological membranes (Halestrap and Price, 1999; Halestrap and Meredith, 2004; Kennedy and Dewhirst, 2010). The MCT proteins are predicted to have 12 transmembrane domains, with the N- and C-termini facing the intracellular side of the membrane and a large cytosolic loop between domains 6 and 7 (see poster). Transmembrane domains are well conserved and MCTs differ mainly in their N- and C-termini and intermediary loop sequences.
Four members of the MCT family (MCT1-MCT4) have been described to be proton-linked MCTs. At physiological pH, lactic acid is actually more than 99% dissociated into lactate anions and protons. MCT1 is the most widely expressed MCT and plays an active role in the uptake of lactate in the heart, skeletal muscle and red blood cells, as well as in the liver (for gluconeogenesis) (Halestrap and Meredith, 2004). MCT2 is less ubiquitous and has been reported to play key roles in neurons at the postsynaptic density, and MCT3 expression is limited to the retinal pigment epithelium and choroid plexus epithelia (Halestrap and Meredith, 2004). MCT4 is primarily expressed in highly glycolytic cells, such as white (rapid twitch) muscle fibers, and is upregulated in response to hypoxia (Ullah et al., 2006). The affinity for lactate and pyruvate differs between MCTs, as reflected by the Michaelis constant (Km) value for each (i.e. the substrate concentration at which the transport rate is half of maximum) (see poster). In particular, the low Km value of MCT1 for lactate indicates that, when expressed in a cell, this transporter is the predominant regulator of lactate fluxes. As discussed below, MCT1 and MCT4 are the two major MCTs expressed in tumor cells and represent promising targets for therapy.
Ancillary proteins are required for MCTs to be properly expressed at the plasma membrane. MCT1, MCT3 and MCT4 associate with the chaperone protein basigin (also known as CD147, EMMPRIN or OX-47), whereas embigin (also known as gp70) is the preferred binding partner of MCT2 (Wilson et al., 2005; Kirk et al., 2000). Both basigin and embigin are single-pass transmembrane glycoproteins with extracellular immunoglobulin domains.
The current consensus on the mechanism of MCT1-MCT4 transporters is that monocarboxylate-proton symport occurs via a rapid equilibrium-ordered mechanism, with proton binding followed by monocarboxylate binding (Halestrap and Meredith, 2004; Halestrap and Price, 1999). Lactate export in response to high glycolytic flux (see above) is not the only function of MCTs: lactate can also be taken up by various specialized cells in a variety of tissues. Circulating lactate can be used by the liver for gluconeogenesis (Cori cycle) (Miller et al., 2002b; Roef et al., 2003) or directly consumed by oxidative cells located near to glycolytic lactate-producing cells. This symbiosis system – referred to as the cell-cell lactate shuttle (Brooks, 1985; Brooks, 1998) – has been extensively studied in skeletal muscle and brain, and was more recently documented in tumors (Sonveaux et al., 2008; Vegran et al., 2011). In addition to lactate exchange between cells, an intracellular lactate shuttle paradigm (Brooks, 1998; Brooks et al., 1999) was proposed to account for the apparently paradoxical conversion of exogenous lactate into pyruvate in glucose-fuelled cells.
Cell-cell lactate shuttle
Although lactate shuttles were identified only recently in tumors (see below), this process has been described in skeletal muscle for several years. Accumulation of lactate in contracting skeletal muscle was originally thought to be the consequence of anaerobic glycolysis (Wasserman, 1984). However, lately, numerous studies have documented that lactate can be produced and used continuously under fully aerobic conditions [for reviews and historical perspectives, see Gladden and Brooks (Gladden, 2004; Brooks, 2009)].
During exercise, white glycolytic (rapid twitch) muscle fibers produce lactate and release it into the blood (Skelton et al., 1995), mainly via the MCT4 transporter; a large part (∼one third) of plasma lactate is then transported into red blood cells via MCT1 (Garcia et al., 1994). When exercise is prolonged, but also during recovery from short-term exercise, lactate is taken up from the muscle interstitial fluid or back from the blood to red oxidative (slow twitch) muscle fibers (Brooks, 2000; Gladden, 2000; Miller et al., 2002a). Lactate oxidation thereby competes with glucose as a carbohydrate fuel source in skeletal muscle.
Similarly, the heart and brain are active consumers of lactate, which, under conditions of increased circulating lactate concentrations, can represent up to 60% and 25% of the metabolic fuel for these organs, respectively (Stanley, 1991; Ide and Secher, 2000; Gertz et al., 1988). Tracer studies indicate that essentially all of the lactate taken up by these organs is oxidized (Stanley, 1991; van Hall et al., 2009), suggesting that there is little carbon exchange from lactate to other metabolites.
Of note, whereas cardiac muscle mainly takes up lactate from the blood, in the brain, lactate exchange between neighboring cells has been documented (Bouzier-Sore et al., 2003). Pellerin and Magistretti originally reported that neurons can metabolize lactate originating from astrocytes in vitro (see poster) (Pellerin and Magistretti, 1994). The symbiosis in this case is further reinforced by the production and release of glutamate from neurons: glutamate is consecutively taken up by astrocytes together with Na+, thus requiring (Na+,K+)-ATPase activation to restore ionic homeostasis. Glycolysis is in turn stimulated in response to reduced ATP levels and glutamine synthesis from glutamate. As a consequence, lactate concentration rises in astrocytes and then lactate moves outward through MCT4 into the extracellular space, driving lactate influx into the surrounding neurons via MCT2. However, stoichiometric analyses do not support a strict lactate exchange between the two cell types, and it is generally accepted that glycolysis is also directly coupled to oxidative metabolism in astrocytes (Mangia et al., 2003).
A common requirement of the capacity of muscle and brain cells to use lactate is the presence of oxygen. In tumors, the temporal and/or local deficit in oxygen imposes an additional layer of regulation to the cell-cell lactate shuttle (see below).
Intracellular lactate shuttle
The concept of the intracellular lactate shuttle originates from the need to understand how oxidation of lactate to pyruvate can occur in well-oxygenated tissues, including in skeletal muscle, cardiac muscle and liver. This phenomenon is indeed paradoxical, considering that the Vmax of LDH is the highest of any enzyme in the glycolytic pathway and that the reaction equilibrium constant for the pyruvate-to-lactate conversion is more favorable than for the reverse reaction (Brooks, 2000; Brooks, 1998; Brooks et al., 1999).
Because of thermodynamic issues related to the conversion of lactate into pyruvate inside mitochondria, and the lack of undisputable evidence of significant mitochondrial LDH activity (Yoshida et al., 2007), a consensus is emerging that suggests that differential concentrations of lactate and pyruvate are present in different subcellular regions. If one considers that mitochondria can act as a ‘sink’ for pyruvate (into which it is taken up and used for the Krebs cycle), it can be proposed that there exists a steep pyruvate gradient between the plasma membrane and mitochondria. In other words, pyruvate concentrations are the lowest close to mitochondria, making lactate concentrations relatively high in this remote cytosolic location away from the plasma membrane. As a consequence, in the immediate vicinity of mitochondria, lactate and NAD+ can be converted back to pyruvate and NADH before they are taken up into mitochondria (Stainsby and Brooks, 1990; Gladden, 2004). The nature of the transporter that brings pyruvate into mitochondria is unclear: MCTs [in particular MCT2, which has a high affinity for pyruvate (Yoshida et al., 2007)] and a six transmembrane helix structure known as mitochondrial pyruvate carrier (MPC) (Kuan and Saier, Jr, 1993; Sugden and Holness, 2003) have been described.
The intracellular lactate shuttle model provides a framework to understand how (millimolar) lactate exchange and conversion into its more oxidized analog pyruvate can be used to maintain the redox balance in the cytosol and mitochondria. The presence of cell-cell and intracellular lactate shuttles gives rise to the notion that glycolytic (i.e. glucose to lactate) and oxidative (i.e. lactate to pyruvate to acetyl-CoA) pathways can simultaneously co-exist and even be linked in a given cell. In the following section, we comment on recent findings documenting that these different modes of lactate exchange, which are physiological in essence, can be usurped by tumors.
Lactate shuttles in tumors
We recently reported that both oxidative cancer cells and endothelial cells lining tumor blood vessels can take up lactate released by glycolytic tumor cells (Sonveaux et al., 2008; Vegran et al., 2011). The cell-cell and intracellular lactate shuttle concepts identified in non-cancer tissues can be applied in this context and open up exciting avenues, with concrete therapeutic perspectives.
Using cervix cancer cells as an in vitro model of oxidative cancer cells, we documented that lactate derived from glycolytic tumor cells could be used as a main source of metabolic fuel (Sonveaux et al., 2008). As a consequence, aerobic tumor cells (i.e. those located near to blood vessels) spare glucose, which can then diffuse a greater distance into the tumor to nourish glycolytic cells. MCT1 and MCT4 are key players in this process. Exogenous lactate uptake by oxidative tumor cells occurs through the high-affinity lactate transporter MCT1, whereas glycolysis-derived lactate is released through the low-affinity lactate transporter MCT4 (Sonveaux et al., 2008), recapitulating the cell-cell lactate shuttling processes observed in muscle and brain (see above). Interestingly, alteration in this symbion can have dramatic consequences (Sonveaux et al., 2008). Inhibition of MCT1 can shift the preference of oxidative tumor cells towards using glucose, thereby reducing the amount of glucose that can reach hypoxic tumor cells and altering their survival (see poster). Inhibition of MCT4 has instead the potential to directly target hypoxic tumor cells and promote their death by intracellular lactic acid accumulation.
Chatham and colleagues previously reported that, in rat heart, the production of lactate via glycolysis and the oxidation of exogenous lactate are functionally separate metabolic pathways (Chatham et al., 2001). This observation, which is in line with the intracellular lactate shuttle hypothesis, further supports the paradigm of preferential (or at least co-existing) fuelling of mitochondrial respiration in tumors by exogenous lactate (Sonveaux et al., 2008). This model might, in particular, apply in the context of cyclic hypoxia, in which tumor cells must switch from using glucose to an alternative source of energy to survive during periods of ischemia (Denko, 2008). Tumor-associated fibroblasts have also been documented to participate in lactate homeostasis in tumors (see poster). Koukourakis and colleagues reported that preferential expression of MCT1 and LDH-1 together with elevated PDH activity in tumor fibroblasts supports the metabolic use of lactate produced by tumor cells, and thereby prevents the development of a hostile acidic environment (Koukourakis et al., 2005; Koukourakis et al., 2006). By contrast, Lisanti and colleagues documented that tumor-associated fibroblasts can instead undergo aerobic glycolysis and ‘feed’ adjacent oxidative cancer cells with the released lactate (Bonuccelli et al., 2010; Whitaker-Menezes et al., 2011).
More recently, we reported that lactate can also enter tumor endothelial cells through MCT1 (Vegran et al., 2011). This study not only showed that lactate can favor the survival of serum-starved endothelial cells, but, more interestingly, that lactate can directly initiate pro-angiogenic signaling. We found that lactate stimulates an autocrine pathway involving nuclear factor-κB (NFκB) and interleukin-8 [IL-8; also known as C-X-C chemokine 8 (CXCL8)], which drives endothelial cell migration and tube formation. IL-8-specific blocking antibodies and IL-8-targeted siRNA both prevented lactate-induced angiogenesis. Using human colorectal and breast cancer models in mice, we further documented that lactate that is released from tumor cells through MCT4 (but not MCT1) is sufficient to stimulate IL-8-dependent angiogenesis and tumor growth (Vegran et al., 2011). Importantly, we also documented the anti-angiogenic potential of MCT1 and MCT4 inhibition through the blockade of endothelial cell lactate uptake and the reduction in extracellular lactate availability, respectively (see poster).
These findings establish a dual role for lactate in tumors: it acts as both a metabolic fuel and a signaling molecule, positioning lactate at the intersection of key processes in cancer progression, namely tumor metabolism and angiogenesis. Therefore, although lactate shuttles are physiologically active in some types of healthy tissue, the specific characteristics of some tumors make the regulators of cellular lactate handling, namely MCTs and LDHs, potential anti-cancer targets. Indeed, the exquisite ‘metabolic symbions’ that we and others have described between oxidative and glycolytic tumor cells – but also between tumor cells and stromal cells, including endothelial cells and fibroblasts – offer diverse rationales to support the development of MCT and LDH inhibitory strategies. However, as for all new anti-cancer therapies, the extent to which tumors are addicted (Feron, 2010) to these pathways (i.e. to lactate transport and conversion), and the capacity to therapeutically target these pathways without altering healthy tissues (Bouzin and Feron, 2007), will determine the success of these new therapeutic avenues in the anti-cancer drug armamentarium.
Footnotes
FUNDING
This work was supported by the Fonds de la Recherche Scientifique FRS-FNRS; the Fonds de la Recherche Scientifique Médicale; the Télévie; the Belgian Federation Against Cancer; the J. Maisin Foundation; and an Action de Recherche Concertée from the Communauté Française de Belgique [ARC 09/14-020].
COMPETING INTERESTS
N.D. is a Télévie fellow and O.F. an FRS-FNRS Research Director.
REFERENCES
- Bonuccelli G., Tsirigos A., Whitaker-Menezes D., Pavlides S., Pestell R. G., Chiavarina B., Frank P. G., Flomenberg N., Howell A., Martinez-Outschoorn U. E., et al. (2010). Ketones and lactate ‘fuel’ tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 9, 3506–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier-Sore A. K., Voisin P., Canioni P., Magistretti P. J., Pellerin L. (2003). Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J. Cereb. Blood Flow Metab. 23, 1298–1306 [DOI] [PubMed] [Google Scholar]
- Bouzin C., Feron O. (2007). Targeting tumor stroma and exploiting mature tumor vasculature to improve anti-cancer drug delivery. Drug Resist. Updat. 10, 109–120 [DOI] [PubMed] [Google Scholar]
- Brooks G. A. (1985). Anaerobic threshold: review of the concept and directions for future research. Med. Sci. Sports Exerc. 17, 22–34 [PubMed] [Google Scholar]
- Brooks G. A. (1998). Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. 120B, 89–107 [DOI] [PubMed] [Google Scholar]
- Brooks G. A. (2000). Intra- and extra-cellular lactate shuttles. Med. Sci. Sports Exerc. 32, 790–799 [DOI] [PubMed] [Google Scholar]
- Brooks G. A. (2009). Cell-cell and intracellular lactate shuttles. J. Physiol. 587, 5591–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G. A., Dubouchaud H., Brown M., Sicurello J. P., Butz C. E. (1999). Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl. Acad. Sci. USA 96, 1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns R. A., Harris I. S., Mak T. W. (2011). Regulation of cancer cell metabolism. Nat. Rev. Cancer 11, 85–95 [DOI] [PubMed] [Google Scholar]
- Chatham J. C., Des R. C., Forder J. R. (2001). Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am. J. Physiol. Endocrinol. Metab. 281, E794–E802 [DOI] [PubMed] [Google Scholar]
- Denko N. C. (2008). Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 8, 705–713 [DOI] [PubMed] [Google Scholar]
- Feron O. (2009). Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 92, 329–333 [DOI] [PubMed] [Google Scholar]
- Feron O. (2010). Challenge in pharmacology of anti-cancer dugs: the search for addictions. Front. Pharmacol. 1, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C. K., Goldstein J. L., Pathak R. K., Anderson R. G., Brown M. S. (1994). Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76, 865–873 [DOI] [PubMed] [Google Scholar]
- Gertz E. W., Wisneski J. A., Stanley W. C., Neese R. A. (1988). Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J. Clin. Invest. 82, 2017–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden L. B. (2000). Muscle as a consumer of lactate. Med. Sci. Sports Exerc. 32, 764–771 [DOI] [PubMed] [Google Scholar]
- Gladden L. B. (2004). Lactate metabolism: a new paradigm for the third millennium. J. Physiol. 558, 5–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Price N. T. (1999). The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 343, 281–299 [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Meredith D. (2004). The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 447, 619–628 [DOI] [PubMed] [Google Scholar]
- Holmes R. S., Goldberg E. (2009). Computational analyses of mammalian lactate dehydrogenases: human, mouse, opossum and platypus LDHs. Comput. Biol. Chem. 33, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide K., Secher N. H. (2000). Cerebral blood flow and metabolism during exercise. Prog. Neurobiol. 61, 397–414 [DOI] [PubMed] [Google Scholar]
- Kennedy K. M., Dewhirst M. W. (2010). Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 6, 127–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P., Wilson M. C., Heddle C., Brown M. H., Barclay A. N., Halestrap A. P. (2000). CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 19, 3896–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis M. I., Giatromanolaki A., Sivridis E., Gatter K. C., Harris A. L. (2005). Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia 7, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis M. I., Giatromanolaki A., Harris A. L., Sivridis E. (2006). Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 66, 632–637 [DOI] [PubMed] [Google Scholar]
- Kroemer G., Pouyssegur J. (2008). Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13, 472–482 [DOI] [PubMed] [Google Scholar]
- Kuan J., Saier M. H., Jr (1993). The mitochondrial carrier family of transport proteins: structural, functional, and evolutionary relationships. Crit. Rev. Biochem. Mol. Biol. 28, 209–233 [DOI] [PubMed] [Google Scholar]
- Leiblich A., Cross S. S., Catto J. W., Phillips J. T., Leung H. Y., Hamdy F. C., Rehman I. (2006). Lactate dehydrogenase-B is silenced by promoter hypermethylation in human prostate cancer. Oncogene 25, 2953–2960 [DOI] [PubMed] [Google Scholar]
- Levine A. J., Puzio-Kuter A. M. (2010). The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330, 1340–1344 [DOI] [PubMed] [Google Scholar]
- Maekawa M., Taniguchi T., Ishikawa J., Sugimura H., Sugano K., Kanno T. (2003). Promoter hypermethylation in cancer silences LDHB, eliminating lactate dehydrogenase isoenzymes 1–4. Clin. Chem. 49, 1518–1520 [DOI] [PubMed] [Google Scholar]
- Mangia S., Giove F., Bianciardi M., Di S. F., Garreffa G., Maraviglia B. (2003). Issues concerning the construction of a metabolic model for neuronal activation. J. Neurosci. Res. 71, 463–467 [DOI] [PubMed] [Google Scholar]
- Miller B. F., Fattor J. A., Jacobs K. A., Horning M. A., Navazio F., Lindinger M. I., Brooks G. A. (2002a). Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J. Physiol. 544, 963–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. F., Fattor J. A., Jacobs K. A., Horning M. A., Suh S. H., Navazio F., Brooks G. A. (2002b). Metabolic and cardiorespiratory responses to ‘the lactate clamp’. Am. J. Physiol. Endocrinol. Metab. 283, E889–E898 [DOI] [PubMed] [Google Scholar]
- Pellerin L., Magistretti P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 91, 10625–10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roef M. J., de Meer K., Kalhan S. C., Straver H., Berger R., Reijngoud D. J. (2003). Gluconeogenesis in humans with induced hyperlactatemia during low-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 284, E1162–E1171 [DOI] [PubMed] [Google Scholar]
- Shim H., Dolde C., Lewis B. C., Wu C. S., Dang G., Jungmann R. A., Dalla-Favera R., Dang C. V. (1997). c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA 94, 6658–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton M. S., Kremer D. E., Smith E. W., Gladden L. B. (1995). Lactate influx into red blood cells of athletic and nonathletic species. Am. J. Physiol. 268, R1121–R1128 [DOI] [PubMed] [Google Scholar]
- Sonveaux P., Vegran F., Schroeder T., Wergin M. C., Verrax J., Rabbani Z. N., De Saedeleer C. J., Kennedy K. M., Diepart C., Jordan B. F., et al. (2008). Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainsby W. N., Brooks G. A. (1990). Control of lactic acid metabolism in contracting muscles and during exercise. Exerc. Sport Sci. Rev. 18, 29–63 [PubMed] [Google Scholar]
- Stanley W. C. (1991). Myocardial lactate metabolism during exercise. Med. Sci. Sports Exerc. 23, 920–924 [PubMed] [Google Scholar]
- Sugden M. C., Holness M. J. (2003). Trials, tribulations and finally, a transporter: the identification of the mitochondrial pyruvate transporter. Biochem. J. 374, e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn C. C., Freeman T. C., Scott N., Guillou P. J., Jayne D. G. (2009). Laser microdissection expression profiling of marginal edges of colorectal tumours reveals evidence of increased lactate metabolism in the aggressive phenotype. Gut 58, 404–412 [DOI] [PubMed] [Google Scholar]
- Ullah M. S., Davies A. J., Halestrap A. P. (2006). The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J. Biol. Chem. 281, 9030–9037 [DOI] [PubMed] [Google Scholar]
- van Hall G., Stromstad M., Rasmussen P., Jans O., Zaar M., Gam C., Quistorff B., Secher N. H., Nielsen H. B. (2009). Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 29, 1121–1129 [DOI] [PubMed] [Google Scholar]
- Vegran F., Boidot R., Michiels C., Sonveaux P., Feron O. (2011). Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 71, 2550–2560 [DOI] [PubMed] [Google Scholar]
- Wasserman K. (1984). The anaerobic threshold measurement to evaluate exercise performance. Am. Rev. Respir. Dis. 129, S35–S40 [DOI] [PubMed] [Google Scholar]
- Whitaker-Menezes D., Martinez-Outschoorn U. E., Lin Z., Ertel A., Flomenberg N., Witkiewicz A. K., Birbe R. C., Howell A., Pavlides S., Gandara R., et al. (2011). Evidence for a stromal-epithelial ‘lactate shuttle’ in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 10, 1772–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Meredith D., Fox J. E., Manoharan C., Davies A. J., Halestrap A. P. (2005). Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4, the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J. Biol. Chem. 280, 27213–27221 [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Holloway G. P., Ljubicic V., Hatta H., Spriet L. L., Hood D. A., Bonen A. (2007). Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. J. Physiol. 582, 1317–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]