Abstract
The close correspondence between energy intake and expenditure over prolonged time periods, coupled with an apparent protection of the level of body adiposity in the face of perturbations of energy balance, has led to the idea that body fatness is regulated via mechanisms that control intake and energy expenditure. Two models have dominated the discussion of how this regulation might take place. The set point model is rooted in physiology, genetics and molecular biology, and suggests that there is an active feedback mechanism linking adipose tissue (stored energy) to intake and expenditure via a set point, presumably encoded in the brain. This model is consistent with many of the biological aspects of energy balance, but struggles to explain the many significant environmental and social influences on obesity, food intake and physical activity. More importantly, the set point model does not effectively explain the ‘obesity epidemic’ – the large increase in body weight and adiposity of a large proportion of individuals in many countries since the 1980s. An alternative model, called the settling point model, is based on the idea that there is passive feedback between the size of the body stores and aspects of expenditure. This model accommodates many of the social and environmental characteristics of energy balance, but struggles to explain some of the biological and genetic aspects. The shortcomings of these two models reflect their failure to address the gene-by-environment interactions that dominate the regulation of body weight. We discuss two additional models – the general intake model and the dual intervention point model – that address this issue and might offer better ways to understand how body fatness is controlled.
Introduction
Based on a sample of 592 measures of energy expenditure by doubly labelled water (Speakman and Westerterp, 2010), we can estimate that an average man aged 45 living in western Europe expends a total of 5180 MJ of energy during the course of a year. Similar to most people in the western world, our average man will end the year slightly heavier than when he started it – pointing to a discrepancy between intake and expenditure. If he gains the average 0.5 kg of weight per year that is typical of western societies (Van Wye et al., 2007), and if this weight gain was fat tissue, this additional tissue would contain about 350 g of lipids (Forbes, 1987). This would suggest that he ate 13.8 MJ more energy than he expended over the course of the year (i.e. 0.35 kg of fat multiplied by 39 MJ/kg). The discrepancy between the intake and expenditure amounts to only 0.27% of his total annual expenditure (13.8/5180). On a daily basis, this difference between intake and expenditure averages only 38 kJ – approximately equal to the cost of walking 150 metres, or drinking a regular cup of unsweetened coffee with milk. Refined computer models that also take into account the efficiency of energy transformations and the energy expenditure of the deposited tissue suggest a slightly higher but similarly small discrepancy of 74 kJ/day (Westerterp et al., 1995; Speakman et al., 2002; Hall, 2010a; Hall, 2010b). There are two perspectives on these suggested short-term (daily) implications of long-term (yearly) energy balance calculations that are worth noting. First, the matching of intake and expenditure on a daily basis may routinely be better than these calculations suggest. This is because the normal pattern of weight gain might not be to slowly accumulate very small amounts each day, but rather to be weight stable for protracted periods, interspersed with periods of gross imbalance during which most weight gain occurs. For example, weight gain during the holiday season in the United States (from Thanksgiving in November until the new year) is significantly higher than during the rest of the year (Yanovski et al., 2000) and is matched by seasonal variation in food intake (de Castro, 1991), although other studies have shown no change in overall weight but an increase in fatness over the same period (Hull et al., 2006). Conversely, matching of intake and expenditure over the time scale of a single day might actually be very poor, and highly variable, because the time scale over which a balance is struck is much longer. For example, short-duration experimental manipulations of either intake or expenditure (Levitsky and DeRosimo, 2010; Levitsky et al., 2005; King et al., 1997) tend not to be well compensated [for an exception, see Goldberg et al. (Goldberg et al., 1998)], consistent with the suggestion that energy balance occurs over much longer periods (Edholm et al., 1955). Therefore, extrapolating from an annual budget to explain what occurs during much shorter durations might be unjustified.
Similar estimations of a very small error in the precision to which energy intake of humans matches energy expenditure over long periods of time (years) have been made by many previous authors (e.g. Hill, 2009; Levitsky and Pacanowski, 2011). The UK Department of Health, for example, recently convened an expert working group to quantify the magnitude of weight change and energy imbalance in the UK population, concluding that the average weight gain was 6.7 kg over 10 years and that the daily energy imbalance necessary to generate this was about 25 kJ/day. The conclusion that is often drawn from these weight gain and energy balance calculations is that our bodies must therefore contain an exquisitely tuned system that controls our intake and expenditure with incredible precision to maintain our body mass at an almost constant level. From a treatment perspective, it is probably this tuning system that has made the pharmacotherapy of obesity such a challenge with regards to efficacy. Drugs aimed at single protein targets that affect intake, expenditure or both struggle to achieve significant weight loss to be sufficient to normalise body weight and fatness because they address only part of the system – that is, the molecular, genetic and physiological component. Obtaining a better understanding of the nature of this control system will ultimately lead to better therapies for obesity. In this Special Article, we review the two main ideas about the nature of this control system (the set point and settling point models), highlighting their strengths and weaknesses. We conclude by detailing alternative ideas that overcome many of the shortcomings of these two models.
The set point regulation model
Kennedy was among the first to suggest that body fat storage might be a regulated phenomenon involving a set point (Kennedy, 1953). He suggested that fat might produce a signal that was sensed by the brain, where it was compared with a target level of body fatness. Any discrepancy between the target and signal would subsequently trigger changes in intake or expenditure that would bring the actual levels of body fat (and its signal) back in line with the target. This has been termed the ‘lipostatic’ model of body fat regulation, and is based on the simple concept of a negative-feedback system around a target set point (Fig. 1). More than 40 years after the original proposition, leptin was discovered (Zhang et al., 1994), which is a hormone primarily produced by adipocytes that interacts with receptor populations in the brain in areas already known to be intimately linked to the regulation of energy balance, such as the arcuate nucleus in the hypothalamus (Mercer et al., 1996; Bellinger, 2001). This discovery provided strong molecular evidence for such a feedback system and prompted many reviews that resurrected Kennedy’s original set point model for the regulation of body fatness (e.g. Frederich et al., 1995; Keesey and Hirvonen, 1997; Friedman, 1998; Friedman and Halaas, 1998; Cowley et al., 1999; Cone, 1999; Schwartz et al., 2000). This model, and the role of leptin in it, has more recently been formalised mathematically (Tam et al., 2009). Moreover, in line with the model predictions, substantial work has shown that fluctuating leptin levels – either associated with weight gain or loss, or induced via central or peripheral administration in animal models – directly alter feeding behaviour and energy expenditure (Davis et al., 2011; Fam et al., 2007; Gautron and Elmquist, 2011; Hayes et al., 2010; Scott et al., 2011; Sousa et al., 2009). The discovery of individuals with loss-of-function mutations in the gene encoding leptin (O’Rahilly, 1998; Farooqi et al., 1999; Farooqi et al., 2001; Farooqi et al., 2002; Farooqi et al., 2007), who were extremely hyperphagic and obese, along with subsequent discoveries of other similarly obese individuals with mutations in other genes in the neural pathways downstream from leptin, provided strong support for the set point idea (Farooqi and O’Rahilly, 2008; O’Rahilly, 2009), with leptin as its central player. The high genetic contribution to the variation in body mass index (BMI; a commonly used surrogate of body fatness) (Allison et al., 1996; Luke et al., 2001; Zhu et al., 2002; Wu et al., 2002; Segal and Allison, 2002) is consistent with the set point theory, and with the important role of biology in the process of weight regulation. However, it is notable that obesity in most humans is not associated with mutations in the gene encoding leptin (Maffei et al., 1996; Speliotes et al., 2010).
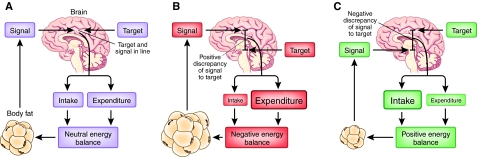
Fig. 1.
The lipostatic model of body fat regulation. This model was first suggested by Kennedy (Kennedy, 1953) and widely adopted in the 1990s following the discovery of leptin. In this model, fat tissue produces a signal (generally presumed to include leptin) that is passed to the brain, where it is compared with a target (the set point of the system) (A). Discrepancies between the level of the signal and the target are translated into effects on energy expenditure and energy intake to equalise the discrepancy and maintain homeostasis. That is, if the signal is too high (as in B, where body fatness is above the target level), expenditure is increased and intake decreased until fatness falls and the signal and target are brought back in line. Conversely, if the signal is low relative to the target (as in C, where the individual is too thin as determined by the set point), intake is increased and expenditure is reduced to drive the subject into a positive energy balance, resulting in an increase in fatness and bringing the target and signal back in line.
The set point model is bolstered by the observation that, when the system is perturbed – for example by a period of dieting (Luke and Schoeller, 1992; Dulloo and Jacquet, 1998; Hainer et al., 2000) or overfeeding (Leibel et al., 1995; Bouchard et al., 1988; Bouchard et al., 1990) – people lose or gain weight, respectively. However, once dieting or overfeeding ceases, they tend to regain any lost fat, or lose the accumulated fat, and return to a level approximating their original fatness (Bouchard et al., 1996; Anderson et al., 2001). Moreover, they modulate energy expenditure to resist the perturbation in intake (Deriaz et al., 1992; Tremblay et al., 2004; Rosenbaum et al., 2010; Rosenbaum et al., 2008; Rosenbaum et al., 2003; Rosenbaum et al., 1997; Leibel and Hirsch, 1984). This means that the amount of weight loss or gain is less, and the speed at which weight returns to baseline levels is more rapid, than would be predicted by only a passive system that was regulated by unchanging mean intake and expenditure levels. Indeed, this set point model in which the body defends a level of adiposity is often used to explain the common phenomenon of weight regain following acute weight loss and the failure of dieting as a strategy to promote prolonged weight loss (Anderson et al., 2001).
However, there are aspects of the set point model of regulation that are problematic, particularly its inability to explain the increasing prevalence of obesity that has been observed in many societies over the past 40 years (Flegal et al., 2010; Ogden et al., 1997; Troiano et al., 1995; Kuczmarski et al., 1994). That is, if such a strong biological feedback system regulating our body fatness exists, then why do most individuals in most western countries gain weight throughout the majority of their lives? Moreover, the set point model cannot explain why obesity tends to occur most frequently in the least affluent members of western populations (e.g. Dykes et al., 2004) but most frequently in the most affluent members of developing societies (e.g. Poskitt, 2009; Satia, 2010); why children who watch more TV are more obese (Epstein et al., 2008; Jordan and Robinson, 2008; Jackson et al., 2009; Matheson et al., 2004; Robinson, 2001; Robinson, 1999; Gortmaker et al., 1996); or why individuals gain weight in college (Cluskey and Grobe, 2009), after marrying (Sobal et al., 2009) or after moving from Asia to western countries. Although it has been suggested that obesity arises in such situations because of a shift in the set point (Mrosovsky and Powley, 1977; Stunkard, 1982), such notions effectively negate the utility of the set point concept. If the set point changes in response to our social class, our marital status, or whether or not we watch TV, then it is not a ‘set’ point. Nevertheless, it should be pointed out that there is also no indication that heritability estimates of BMI have changed over time (Maes et al., 1997). In addition to the environmental effects mentioned above, a large number of diseases and disorders can lead to more or less rapid weight gain or loss; examples include both somatic (e.g. infectious diseases, tumour cachexia, gastrointestinal disorders) and neuropsychiatric (e.g. anorexia nervosa, depression, dementia) disorders. The weight alterations observed in these disorders imply that the putative tight regulatory system implied by a set point can be perturbed substantially. Such disorders can also have long-term implications for body weight. For example, individuals with anorexia nervosa whose pre-morbid body weight is normally distributed (Coners et al., 1999) only infrequently become overweight or obese after recovery (Hebebrand et al., 1997).
Moreover, despite the popularity of the set point model among molecular biologists, a close look at the physiological and molecular data reveals discrepancies between the this model and reality [as proposed in various reviews (Keesey and Hirvonen, 1997; Friedman, 1998; Friedman and Halaas, 1998; Cowley et al., 1999; Cone, 1999; Schwartz et al., 2000) and illustrated in Fig. 1]. For example, obese individuals with large levels of stored lipids produce abundant amounts of leptin (Considine et al., 1996). Additionally, although daily injections of leptin reduce body mass in a dose-dependent manner, the extent of this effect is much smaller than would be anticipated if a set point system with leptin as the primary signal were in place (Heymsfield et al., 1999; Westerterp-Plantenga et al., 2001; Hukshorn et al., 2003; Lejeune et al., 2003). Also, it is difficult to imagine how such a set point system can operate when we know that the signals that we assume make up the regulatory system (including leptin, as well as multiple other signals such as glucose, fatty and amino acids, insulin, and gut or stress hormones) are not only chronically affected by the level of adiposity, but are also acutely responsive to changes in food intake (Saladin et al., 1995; Schoeller et al., 1997). In the short term (hours and days), food intake is extraordinarily variable (Edholm et al., 1955; Westerterp et al., 1995). Consequently, at the short time scales over which the signals presumed to reflect adiposity are fluctuating enormously, there is no balance between intake and expenditure (Donnelly et al., 2011). This might be partly due to the time that is necessary to fully adapt to changes in macronutrient balance, and hence for the respiratory quotient (RQ) to match the food quotient (FQ) (Schrauwen et al., 1997; Schrauwen et al., 1998; Schrauwen and Westerterp, 2000; Schrauwen et al., 2000). In other words, the time period over which regulation seems to occur (weeks and months) is at odds with the time period (hours and days) over which the regulatory signals are responsive to energy imbalance. A useful analogy is to imagine a thermostat controlling your house temperature against a background of someone periodically pouring hot and cold water over the temperature sensor. It is possible to imagine scenarios by which this system could work –for example, the long-term effects of time-averaged leptin levels might drive neuronal architecture, and leptin might therefore have a role in tuning the sensitivity of the system. However, whether the system works in this way is currently uncertain, and this explanation is not what was originally proposed in the papers mentioned earlier (Frederich et al., 1995; Keesey and Hirvonen, 1997; Friedman, 1998; Friedman and Halaas, 1998; Cowley et al., 1999; Cone, 1999; Schwartz et al., 2000).
Finally, it should be noted that the set point model mainly focuses on the importance of fat mass for the feedback loop – which is undoubtedly supported by the discovery of leptin and the associated pathways that provide the link between adipose tissue and the central nervous system (CNS). However, fat mass accounts for only a fraction of total body mass, ranging from as low as 5% to >45% (Romero-Corral et al., 2008). At any given BMI, percent fat mass varies substantially between individuals. Despite the fact that BMI and percent fat mass are correlated, the relatively constant body weight experienced by healthy individuals cannot solely be explained by the feedback loop between adipose tissue and the CNS. Instead, it seems that if body weight is closely regulated, then fat-free mass must also be under relatively tight control.
The settling point regulation model
Establishment of the set point of the system effectively denies a role for socioeconomic and environmental factors in the aetiology of obesity, subsuming everything into the physiology, which seems unlikely (Symonds et al., 2011). Thus, it is not surprising that the set point model is not well regarded among scientists involved in investigating the social and environmental factors that drive the obesity epidemic. This schism in the obesity research community was highlighted by Hirsch in 2004 in his acceptance speech following receipt of his lifetime achievement award from the North American Association for the Study of Obesity [NAASO; now called The Obesity Society (TOS)]. Hirsch pointed out that much of the obesity research field is effectively split into two groups – physiologists-molecular biologists-geneticists and behaviourists-psychologists-nutritionists – each functioning more or less independently of one another.
The behaviourists-psychologists-nutritionists community implicitly or explicitly hold a different position on the extraordinary match between intake and expenditure that we highlighted above – and hold views that are instead in line with the ‘settling point’ model of body fatness. Like the set point model, this idea is also based on engineering control systems. An analogy for the regulation of body energy stores as explained by the settling point model is the levels of water in a lake (Fig. 2). In any system in which there is a reservoir (such as body fat stores) with an input (food energy) and an output (energy expenditure), the reservoir of the system comes to a natural equilibrium if either the inputs are downregulated in proportion to the reservoir volume, or the outputs are upregulated in direct proportion to the reservoir volume. There is no regulated level of the volume in this system, and yet it behaves as if this is a parameter that is being regulated. This idea of a passive regulatory system that does not involve any set point is called a settling point system: the system ‘settles’ at a point defined by the level of the unregulated parameter (either inflow or outflow).
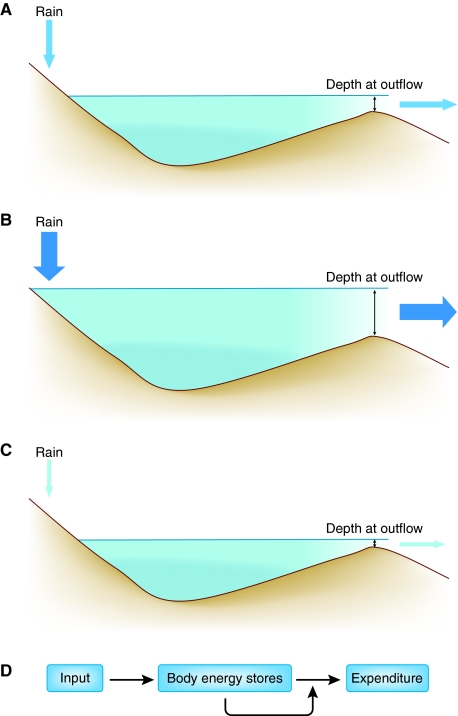
Fig. 2.
An example of a settling point system: levels of water in a lake. (A) In this schematic, the input to the lake is rain falling in the hills. The output of water from the lake is directly related to the depth of water at the outflow. The depth of the water in the lake reaches a settling point at which the outflow is equal to the inflow (indicated by the sizes of the arrows). (B) If the amount of rainfall increases (denoted by the larger arrow), the level of water in the lake increases until a new settling point is reached, at which the outflow is equal to the inflow. (C) Conversely, if the amount of rainfall decreases, the water level in the lake falls until a new settling point is reached, again where the outflow matches inflow. (D) The key characteristics of the settling point system are that a parameter of interest (e.g. body energy stores) has both inputs (energy intake) and outputs (energy expenditure). Importantly, for a settling point system to operate, one of these parameters must be independent of the size of the parameter of interest, and the other must vary in direct relation to the size of the given parameter (in this case the expenditure). The resulting settling point of the system varies in direct proportion to the unregulated flow.
It has been suggested for at least 35 years that such a settling point system might explain the apparent regulation of adiposity in humans (Wirtshafter and Davis, 1977; Payne and Dugdale, 1977a; Payne and Dugdale, 1977b; Garrow, 1988; Speakman et al., 2002). As Payne and Dugdale illustrated using a mathematical model for weight regulation (Payne and Dugdale, 1977a), any imbalance between energy intake and energy requirements would result in a change in body weight, which, in turn, would alter the maintenance energy requirements so as to counter the original imbalance and would hence be stabilising. That is, if body fatness increases owing to an increase in the rate of intake, the rate of energy expenditure also increases to offset it. The system thus exhibits dynamic equilibrium. To understand how such a system might operate, it is useful to consider, for example, an individual who eats 12 MJ per day, expends 12 MJ per day and weighs 70 kg, and is in energy balance. Imagine that the person is placed on a 9 MJ per day diet, resulting in an intake flow to the reservoir that is lower than the output. The discrepancy between input and output of 3 MJ is expected to result in weight loss, comprising some fat and some lean tissue that is burned to supply the shortfall between intake and expenditure. Now, owing to the lack of this fat and lean tissue, which previously required metabolic expenditure, the person’s daily expenditure will be less than 12 MJ, and the discrepancy between intake and expenditure will diminish. This passive response occurs owing to the inevitable reduction in expenditure caused by decreasing fat and lean body mass. Any discrepancy between intake and expenditure will therefore tend to disappear over time because of changes in storage that diminish the discrepancy. Once fat and lean tissue have decreased to a point where expenditure is 9 MJ per day, the individual will be back in energy balance and no further weight loss will take place. This condition of re-established balance occurs because of the link between the reservoir (fatness) and the output (expenditure).
Imagine that the same individual then goes back to eating 12 MJ per day. The discrepancy between intake and expenditure is now in the opposite direction, which leads to an increase in body mass. This slowly causes an increase in expenditure, which will eventually return to 12 MJ per day, and there will no longer be an imbalance or weight gain. Crucially, however, the body will reach this balance when the body composition has returned to the same state it was in before the diet started. To an outside observer who is unaware of the actual control system, this return to the original body composition could be misinterpreted as the individual defending a level of adiposity. That is, the discrepancy between the actual body composition and this defended level (or set point) at the end of the diet generated a signal that resulted in elevated intake once the diet was terminated to close the discrepancy and return the individual to the set point. Yet, clearly, in this situation there is neither an actual set point nor a feedback signal from the reservoir (see also Speakman et al., 2002).
In this non-regulated energy system, the level of the reservoir (fat stores) settles to an equilibrium that is determined by the inflow (food intake), which is matched to the outflow (energy expenditure) because the rate of outflow is passively related to the level of the reservoir. As body weight (fat) increases, so does the rate of energy expenditure, owing to the increase in lean body mass necessary to support the increased fat mass and to the physics of moving a larger body mass.
The settling point model provides cogent explanations for many phenomena that the set point model cannot explain. Hence, under the settling point model, the increasing prevalence of obesity is explained as a consequence of the elevated availability of food or greater exposure to food cues (i.e. elevated food intake) or a downward shift in the need to engage in physical activity – the so-called ‘obesogenic’ environment. Energy intake can be increased by one or more of the following environmental factors: an increase in portion sizes (Rolls et al., 2007), increased exposure to high energy density foods (Hetherington and Rolls, 2008; Rolls, 2010), an increase in the variety of foods offered (Rolls and Hetherington, 1989), a greater tendency to eat outside the home (Thornton et al., 2010) where portion sizes are larger (Piernas and Popkin, 2011; Duffey and Popkin, 2011) and where eating behaviour is increased by eating with others (Hetherington et al., 2006), or other concurrent activities such as eating while watching television (Epstein et al., 1992; Epstein et al., 1997; Wansink, 2004; Temple et al., 2007). These factors interact with psychological (and probably genetic) factors in given individuals (Westerterp-Plantenga et al., 1996; Vogels and Westerterp-Plantenga, 2005; Vogels et al., 2005).
Note that the settling point model requires at least one parameter on the inflow or outflow of the reservoir that is not regulated by the reservoir and at least one parameter that is regulated by the reservoir for this system to work. In the example given above, we assumed that the unregulated parameter was food intake, because we are familiar with the passive link between body composition and resting metabolic rate. However, the unregulated parameter could also be physical activity, both activity and food intake, or all these factors, but to different extents in different individuals. For example, an interesting interaction between food intake and energy expenditure, especially physical activity, was found in men but not in women (Westerterp-Plantenga, 2004b; Westerterp-Plantenga, 2004a). In men with a medium fat-free mass (the older men), meal frequency was positively related to resting energy expenditure and inversely related to activity-induced energy expenditure. In men with a high fat-free mass (the younger men), meal frequency was inversely related to resting energy expenditure and positively related to activity-induced energy expenditure. So, a higher habitual meal frequency implied a lower energy intake in the younger men with a high fat-free mass and activity-induced energy expenditure, and a higher energy intake in the older men with a medium fat-free mass and a lower activity-induced energy expenditure.
However, there are many data that conflict with the settling point model. The semi-starvation study of Keys et al. is a classic example (Keys et al., 1950). During that study, widely known as the Minnesota Experiment, individuals of normal weight were placed on a very low calorie diet and lost a large amount (25%) of body weight (both fat and lean tissue). As predicted by the settling point model, the weight loss under conditions of semi-starvation reached a plateau. On release from the restriction, however, the test subjects did not simply return to their old habits and gradually settle back to their old body weights, but rather they increased body and fat mass rapidly –suggesting that they were over-eating and were under some form of active regulation that was attempting to drive up their body mass or adiposity (or lean mass). In a re-analysis of Key’s Minnesota Experiment, the hyperphagic response to food deprivation was shown to be dictated as much by the psychobiological responses to dietary restraint as by the extent to which body fat, and to a lesser extent lean mass, were depleted (Dulloo et al., 1997). This result strongly suggests that there is some active control over intake that is related to changes in body composition (more specifically, the discrepancy between lean mass or adiposity and a set point target).
Moreover, during weight loss, there is evidence that resting energy expenditure does not simply decrease in relation to the falling body weight, but rather that it is driven down actively at a greater rate to oppose the body and fat mass loss (Luke and Schoeller, 1992; Dulloo and Jacquet, 1998), while powerful biological signals produce feelings of hunger that compel individuals to ‘break’ their dietary restriction (as revealed by the Minnesota Experiment detailed above). In addition, all of the elements of the energy balance equation seem to be strongly linked to body mass, as is revealed by doubly labelled water and hood respirometry (measuring gas exchange of subjects under a ventilated hood) measurements in individuals that are in approximate energy balance. Which of these parameters is independent of the reservoir size is unclear, but at least one of them must be because, as mentioned above, at least one independent parameter is essential in the settling point model. Finally, an environmentally determined settling point cannot adequately explain the inter-individual susceptibility to weight gain in a common environment. Genetic studies strongly suggest that the reason we do not all get fat has something to do with our genetic make-up, because there is a genetic contribution to the variation in BMI (Maes et al., 1997; Allison et al., 1996; Segal and Allison, 2002). How this fact fits into the settling point idea is unclear.
Some alternative ideas
The set point and settling point models for the regulation of body weight and adiposity are a reflection of a broader divide in our conceptualisation of the obesity problem. The set point model is rooted firmly in the domain of physiological and genetic determinism, whereas the settling point model is more grounded in the effects of social, nutritional and environmental factors. However, we know that this distinction is artificial, because genotypes can only work in the context of an environment, and environments have effects that are dependent on genotypes (e.g. Li et al., 2010). Understanding the gene-by-environment interaction is therefore of paramount importance if we are to reach a complete understanding of this (and many other) phenomena (Speakman, 2004). The failings of the set point and settling point models are therefore primarily a reflection of their failure to accommodate the gene-by-environment nature of the problem. This gene-by-environment interaction can readily be demonstrated in individuals who take drugs that either increase or reduce body weight. Furthermore, monozygotic twin pairs react quite similarly with respect to the dynamics of the weight change and the achieved plateau (Gebhardt et al., 2010). Another example is the effect on body weight of the interaction between smoking tobacco and genotype (Freathy et al., 2011). Furthermore, the potential that obesity in adults is influenced by environmental factors experienced during development must be accounted for (e.g. Symonds et al., 2009; Symonds et al., 2011; Budge et al., 2005).
In the last part of this paper, we present two alternative views of the regulation of body weight that attempt to overcome this artificial separation with more integrated models. We then conclude with a molecular-genetic and a psychobiological perspective on these models and the obesity problem.
The general model of intake regulation
The ‘general model of intake regulation’ (de Castro and Plunkett, 2002) combines components of the set point and settling point models into a comprehensive model of food intake and body weight regulation (Fig. 3). The model asserts that food intake is affected by a wide range of physiological, environmental, social, psychological and dietary factors. The model sorts factors into two sets, referred to as uncompensated (primarily environmental) and compensated (primarily physiological) factors. A key difference between these types of factors is that compensated factors have negative feedback loops with intake, simultaneously affecting and being affected by intake, whereas uncompensated factors affect but are not affected by intake. Each factor is assumed to account for only a small portion of the total variance in intake. In addition, the level and impact of these factors can vary from individual to individual, and these individual differences are affected by heredity. A twin study of food intake supported the notion that environmental and physiological factors have individual preferred levels that are affected by the genes and have different impacts on intake, and these impacts are also affected by the genes (de Castro, 2010).
Fig. 3.
The general model of intake regulation. This model is from de Castro and Plunkett (de Castro and Plunkett, 2002). In the model, intake (I) is controlled by two sets of factors, labelled as uncompensated (Ui; primarily environmental) and compensated (Ci; primarily physiological) factors. A key difference between these types of factors is that compensated factors have negative feedback loops with intake, simultaneously affecting and being affected by intake, whereas uncompensated factors affect intake, but are not affected by intake. Inheritance affects the system by determining: the preferred level for intake and compensated and uncompensated factors; the level of impact of the compensated (WCi) and uncompensated (WUi) factors on intake; and also the level of impact of intake minus expenditure (I–E) on compensated factors (i.e. WFi; the weighting factor). The model combines the concepts of negative feedback inherent in the set point model and uncompensated factors inherent in the settling point model.
The model hypothesises that intake results from the net sum of the activity of all of the compensated and uncompensated factors acting simultaneously. It is very general and works well not only with food intake but also when applied to other regulatory systems such as fluid or salt intake. It should be noted that the model does not assume that there are any set points for intake or body weight. Rather, it suggests that the level that is defended is quite malleable. A change in one or more other factors would result in a new defended level. If the internal and external milieu are relatively stable, then the system would act much like there was a set point. After a deviation from that level, the model would predict that the system would tend to promote the restoration of the set point level. However, if the internal and/or external milieu were to change, that level might not be defended, and a new defended level would be established.
To ascertain whether the general model of intake regulation can produce predicted outcomes that parallel observed changes in intake and body weight, a computer simulation was implemented. The simulation was designed to test the model’s response to changes that are similar to those that occur in the natural environment, as well as individual differences in responsiveness to environmental changes (de Castro, 2006). The model’s response to a simulated change in the environment was investigated by doubling the level of one uncompensated factor. In response to the change, the body weight initially became unstable and oscillated at a markedly higher level before stabilizing and settling at a 7% higher body weight (Fig. 4). The model then maintained this new body weight provided that no further changes occurred. Subsequently, the model’s response to differences in individual responsiveness was investigated. The weighting factor was manipulated in conjunction with the doubling of the uncompensated factor, as above. When the weighting factor was small, the doubling of the uncompensated factor produced only a small increase in body weight but, when the weighting factor was large, the model’s output reflected a large increase in body weight (Fig. 4). The output body weight was found to depend on both the amount that the uncompensated factor level increased and the magnitude of the weighting factor. Hence, the model predicted that a sustained change in the environment would trigger a sustained change in body weight; the magnitude of the change would depend on the individual’s inherited responsiveness to the factor.
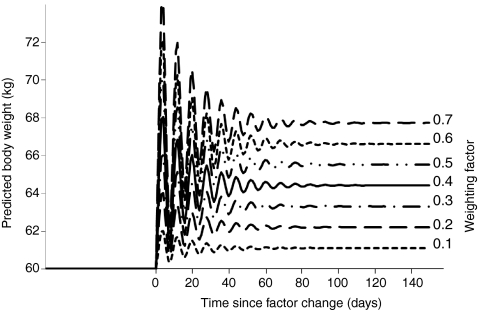
Fig. 4.
Simulated responses of the general intake model. This figure was reproduced, with permission, from de Castro and Plunkett (de Castro and Plunkett, 2002); see also Fig. 3 and main text. The model’s response to a simulated change in the environment was investigated by doubling the level of one uncompensated factor. In response to the change, the body weight became unstable and oscillated before stabilising at a higher body weight. When the weighting factor was low, the doubling of the uncompensated factor produced only a small increase in body weight. But when the weighting factor was large, the model’s output reflected a large increase in body weight.
The model predicts that a chronic change in the environment would result in a maintained and defended change in body weight. It further predicts that, after a loss of body weight, compensated factors would drive intake above former levels until the prior body weight is re-acquired. Given the large recent changes in the environment, the model can provide a possible explanation of the recent epidemic of obesity. The model also can explain changes in body weight that occur throughout the lifespan of an individual through known changes in intake and expenditure with age. Overall, this model provides an integrated and comprehensive view of how environmental, physiological and genetic influences might fit together to control intake. A potential weakness of the model, however, is that it focuses only on the regulation of intake, subsuming expenditure as one of the compensated factors.
The dual intervention point model
An attractive alternative to the set point and settling point models to explain how body weight and fatness are regulated is the dual intervention point model (Herman and Polivy, 1984; Levitsky, 2002; Speakman, 2007). In this model there is not a single set point. Instead, there are upper and lower boundaries that define the points at which active physiological regulation becomes dominant, and between which there is only weak or no physiological regulation of weight and/or fatness (although there could still be physiological control of some of the components of energy balance such as food intake and/or energy expenditure) (Fig. 5). One might argue that this is simply a more realistic version of the set point model. In reality, most set point systems do not have an absolutely defined point above or below which opposing control measures are enabled, because the system would then be constantly flipping between conflicting mechanisms. Rather, control in a set point system is activated when the target value falls outside some narrow tolerance range on either side of the control point. However, the dual intervention point model differs from this explanation in that, first, there is no defined target and, second, the two intervention points are suggested to be regulated independently. Hence, the range between the two intervention points could be quite wide, and its width could vary considerably between individuals. This aspect of the model is useful in that it allows for the inter-individual susceptibility to weight gain in a common environment, and is consistent with the results of studies showing a genetic contribution to the variance in BMI. Such a model is effectively a hybrid that combines the set point model involving active regulation based on fatness, which would operate outside of the upper and lower intervention points, with the settling point model of passive regulation operating in between them. However, the nature of the intervention points is unclear, and might be determined by a combination of genetic and environmental factors acting in concert.
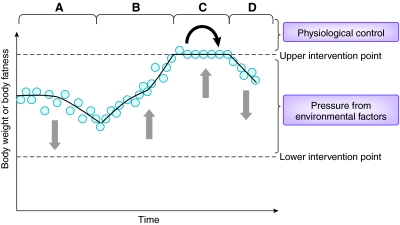
Fig. 5.
The dual intervention point model. This model is illustrated here by changes in body weight over time. The body weight varies depending on the prevailing direction of the environmental pressures. In period A, these pressures largely favour weight loss, and the body weight or adiposity declines. In period B, these factors largely favour weight gain and body mass increases. At these times weight is largely dictated by environmental factors. However, at C, the pressure to gain weight has resulted in weight increasing to the upper intervention point. Further weight gain is resisted by physiological (genetic) factors (depicted by black arrow). The weight therefore remains in balance: declines are prevented by the upward environmental pressures, and increases are prevented by physiological factors. Weight will only start to decline again (D) when the environmental pressure to increase weight is reversed (or an intervention is started). In any situation in which there is a constant environmental pressure favouring weight gain, individuals will increase to their upper intervention points, which vary among individuals and are hypothesised to be genetically determined. (Similarly, weight loss becomes resisted at the lower intervention point by other physiological mechanisms: not illustrated here.) This model also combines the ideas of settling points and uncompensated factors, which dominate between the intervention points, and physiological feedback controls that operate when the intervention points are reached.
Unlike the other models discussed, there is a strong evolutionary rationale to explain why such a system might evolve, with the lower intervention point defined by the risk of starvation and the upper intervention point defined by the risk of predation (Speakman, 2007; Speakman, 2008). This model has the additional benefit of providing a context of understanding the asymmetry of weight control. The lower intervention point explains why we are generally resistant to weight loss: as weight is lost, energy expenditure is reduced, thereby preventing further weight loss. By contrast, variation in the upper intervention point explains why some individuals are rather poor at defending against weight gain and therefore prone to becoming obese when food is readily available, whereas others can resist weight gain in the face of the same environmental stimuli. The source of individual variation in the upper intervention point has been a matter of debate (Speakman, 2007; Speakman, 2008; Prentice et al., 2008). It has been suggested, based on numerous small animal studies, that the upper intervention point in most animals is probably regulated by the risk of predation. In humans who developed tools and weapons, discovered fire and became social animals about 2 million years ago (Homo erectus), the risk of predation was effectively eliminated. It is suggested that this release from predation might have created the conditions for allele frequencies of the genes coding for the upper intervention point to drift over time, and what we now experience is the consequence of that drift. Some individuals have been lucky in the ‘mutation lottery’ and can still regulate their weight and adiposity because their upper intervention point has not moved, but, for others, the intervention point has drifted upwards and the strong control preventing weight increases is no longer present. This suggested individual variability in the distance between the upper and lower intervention boundaries is a key aspect of the model.
The dual intervention point model can explain many aspects of the obesity phenomenon that one or other of the set point and settling point models cannot (reviewed above). A major benefit of the model is that it accommodates both the socioeconomic-environmental views and the molecular-physiological views of energy balance within a single framework. Within the gap between upper and lower intervention points is the space where environmental effects on energy balance hold sway. So, even a person with widely separated intervention points will only gain excess weight in certain environmental conditions. More broadly, the model can explain the obesity epidemic as a consequence of increased food supplies driving up food intake, while also explaining why only some people become overweight and obese in this obesogenic environment. The idea that genetics determines the distance between the upper and lower bounds might explain why there is a genetic contribution to variation in BMI. Interestingly, the results of genome-wide association studies (GWAS) for BMI have identified several targets that are close to some genes that are components of the well-established leptin-brain neuropeptide system that is believed to underpin the set point model (reviewed in Schwartz et al., 2000), such as the melanocortin-4 receptor (MC4R) and pro-opiomelanocortin (POMC). There are also many other targets identified by GWAS that are not part of this leptin-brain neuropeptide system, but that are expressed in areas of the brain believed to be linked to food intake regulation [such as the fat-mass- and obesity-related gene FTO, brain-derived neurotrophic factor (BDNF) and SH2B1]. Yet other targets seem to be involved in adipocyte metabolism. Faced with these surprising new targets, a common question has been: “Does gene X affect BMI via a functional effect on food intake or energy expenditure?”. A classic example is the FTO gene, which was the first genetic variant identified by GWAS approaches that was unequivocally shown to be associated with obesity (Frayling et al., 2007). This spawned a plethora of papers designed to establish whether the variant was associated with either intake or expenditure (Speakman et al., 2008; Timpson et al., 2008; Wardle et al., 2009; Haupt et al., 2009; Cecil et al., 2008; Hetherington and Cecil, 2010; Den Hoed et al., 2009). In this instance, the answer seems to be that the variant mainly affects food intake [see above references but also see the following (Johnson et al., 2009; Fischer et al., 2009; Ruiz et al., 2010)], which might be tempered by physical activity differences (Li et al., 2010). Additionally, the effect of the variant might reflect developmental factors (Sebert et al., 2010). Despite the tremendous increase in our knowledge of the many genetic variants that differentiate the obese from the non-obese, we still do not understand how these genotypes translate into phenotypes in terms of eating behaviour or energy expenditure. This probably reflects the challenges that have been encountered in the pharmacotherapy of obesity. Loss of greater than 10% of total body weight is rarely seen with monotherapy that targets a single gene or mechanism that might affect intake, expenditure or both.
Perhaps we are limited by the technology to unobtrusively measure energy intake accurately for sufficient periods of time to discover how genes influence intake and expenditure. At the same time, we might have been measuring the wrong markers. For example, we now know that brown adipose tissue (BAT) is present throughout life, rather than only in neonates (Cannon and Nedergaard, 2004; Symonds et al., 2011); thus, markers relevant to BAT metabolism or maintenance were not previously assessed and might have been ‘missed’. Alternatively, the mechanisms through which genes cause an increase in energy intake might act very subtly – for example, by changing the sensitivity of certain individuals to react more to environmental food cues than others – meaning that their influence on energy intake is difficult to uncover. However, it is also possible that posing the question “does gene X affect intake or expenditure?” is the problem. That is, the answer might be “neither” in some cases, because gene X contributes to encoding the upper or lower intervention point, and not directly to differences in food intake or expenditure. Thus, searching for a functional effect of gene X on either intake or expenditure might be futile and argues against the value of many so-called endophenotypes (i.e. one gene for one phenotype) in gene-finding exercises. It is important to recognise that this statement does not imply that people can become obese without an energy imbalance – clearly, an energy imbalance is a pre-requisite for weight gain. Rather, we propose that some genes might influence obesity not by directly affecting food intake or expenditure, but because they affect the level at which physiological control mechanisms become activated (the upper intervention point).
A molecular genetic perspective
Classical genetic studies indicate that about 50–70% of the variance (i.e. the broad sense heritability or h2) in BMI is genetic. However, heritability estimates vary according the study design (twin studies vs family studies vs adoption studies) and the method used to assess heritability. In general, heritability estimates tend to be higher when derived from twin studies compared with family and adoption studies. As explained in more detail in several papers and reviews (Allison, 1995; Segal and Allison, 2002; Segal et al., 2009), classic twin studies will overestimate h2 if the so-called equal environments assumption is violated. By contrast, classic family and adoption studies underestimate h2 if there is substantial non-additive genetic variance, including that due to dominance effects at individual loci, epistasis (i.e. gene-by-gene interaction) and gene-by-age interactions. Substantial evidence from both model organisms and from humans indicates that all of these sources of non-additive genetic variance are present and are quite substantial. Furthermore, special human twin studies (such as those of monozygotic twins reared apart), which do not rely on the equal environments assumption, yield results that largely confirm the classical twin studies, suggesting that the classical twin studies are not biased. Thus, at present, the best estimate of h2 for BMI is roughly 0.65 (Segal and Allison, 2002). Notably, heritability also varies according to the phenotype used to assess obesity, tending to be higher for phenotypes indexing fat distribution (e.g. waist circumference or abdominal fat) than for phenotypes indexing total body mass or total body fatness. Overall, these heritability studies tell us how much of the within-population variance in BMI or adiposity is genetic, but they do not tell us which genes are involved.
The Genetic Investigation of ANthropometric Traits (GIANT) consortium has performed the largest meta-analysis of GWAS for BMI thus far, which in total included 123,865 individuals of European ancestry (Speliotes et al., 2010). The follow-up analysis of the best independent loci in up to 125,931 additional individuals resulted in the identification of 32 variants with P-values <5×10−8. These variants explained a mere 1.5% of the BMI variance; this roughly corresponds to 3% of the genetic variance based on an estimated BMI heritability of 0.5. Speliotes et al. estimated that there are approximately an additional 200 loci (95% CI: 98–350) with similar effect sizes as the detected 32, which together would account for roughly 3.5% of the variation in BMI or 7% of the genetic variation (Speliotes et al., 2010). The average BMI increment per risk allele was estimated at 0.17 kg/m2. The per allele change in BMI ranged from 0.06 to 0.39 kg/m2; a total of ten single nucleotide polymorphisms (SNPs) showed per allele changes <0.1, which is equivalent to less than 324 g and 273 g in males and females of average heights (1.8 m and 1.65, respectively).
We can now definitely conclude that common alleles with effect sizes of >0.5 kg are very unusual. Infrequent variants with stronger effect sizes in many different genes might in part explain the missing heritability. Alternatively, the effect sizes of most of the polygenes involved in weight regulation are well below 150 g/allele (Hebebrand et al., 2010); in this scenario, obese individuals would harbour hundreds to thousands of such alleles, and the variance they explain in combination is not well estimated by standard single gene GWAS analyses (de los Campos et al., 2010; Makowsky et al., 2011). Similar to highly heritable psychiatric phenotypes, the molecular elucidation of body weight regulation based on data from GWAS has proven more difficult than, for instance, for body height, inflammatory bowel disease or specific complex neurological disorders.
This complexity of the genetic mechanisms underlying body weight regulation needs to be taken into account for the discussion of any hypothesis about the nature of this regulation. It seems that many different genes are involved in food selection, food intake, absorption, metabolism and energy expenditure, including physical activity – we might be looking at a puzzle of well over 1000 pieces. If gene-by-gene or gene(s)-by-environment(s) interactions are also considered in such a scenario, the complexity increases further still. How these relationships map into any of the models discussed above is currently uncertain. However, if we consider the integrated models, it seems reasonable to assume that at least some (and perhaps many) of the genes associated with regulating body weight define the intervention points in the dual intervention point model. It is perhaps also worth noting that the genetic architecture revealed by the GWAS approach – indicating a role for many genes of very small effect, or alternatively a few high penetrance alleles that have large effects but in relatively small populations – is inconsistent with the ‘thrifty gene’ perspective (Neel, 1962) on causality of the genetic contribution to obesity, which invokes strong natural selection as a causative agent (see also Prentice, 2001; Prentice et al., 2005; Prentice, 2008; Chakravarthy and Booth, 2004; Eknoyan, 2006; Wells, 2006). Rather, the genetic architecture revealed by GWAS is more consistent with a model of genetic drift [i.e. the ‘drifty gene’ hypothesis (Speakman, 2007, Speakman, 2008)], which has been invoked previously as an underlying cause of the individual variation in positioning of the upper intervention points (see above).
A psycho-biological perspective
We ingest food to meet the energy and nutrient demands of living, but food is also rewarding and therefore meets reward needs as well (Berthoud, 2007). Food reward has classically been analysed in terms of ‘liking’ and ‘wanting’. These are represented in the brain in distinct but overlapping areas. In the fasted state, wanting is signalled in the hypothalamus and striatum, and coincides with hunger signalling in the hypothalamus. By contrast, liking is signalled in the nucleus accumbens, in anticipation of food intake. Post-prandially, in the absence of hunger, wanting signalling in the pallidum and liking signalling in the striatum, anterior insula and cingulate cortex both predict food intake (Born et al., 2011), suggesting that these behaviours are reward rather than homeostatically regulated. Post-prandial food choice and food intake in the absence of hunger are exaggerated under stress, especially in overweight individuals with visceral adiposity (Born et al., 2010; Lemmens et al., 2010; Lemmens et al., 2011). Stress-induced eating is not only related to enhanced post-prandial wanting but also to reduced post-prandial liking (Martens et al., 2010). Reward deficiency is most apparent in the absence of hunger, in agreement with the notion that reward deficiency leads to reward seeking that can result in overconsumption (Born et al., 2010). A recent hypothesis proposes that, to avoid reward deficiency, it might be beneficial for an individual to eat what he or she likes, as long as this happens in the appropriate time relative to homeostatic demands (i.e. when hungry) (Lemmens et al., 2009; Lemmens et al., 2010). As long as meal-time food intake meets energy as well as reward homeostasis, this could prevent overeating between meals. Taken together, these studies suggest that to tune energy intake to energy requirements (determined by energy expenditure), food intake regulation consists partly of energy homeostasis and partly of reward homeostasis. In the fasted state, in the presence of hunger, wanting- and liking-related brain signalling coincide and facilitate food intake in agreement with both energy and reward needs (Van Gemert et al., 2000; Westerterp-Plantenga et al., 2002; Westerterp-Plantenga et al., 2003). Post-prandially, consumption of food in the absence of hunger might be caused by previously failing to achieve reward homeostasis.
How this psychological perspective bears on the nature of intervention points in the dual-intervention point model is currently unclear. It is possible that the upper intervention point is influenced, for example, by changes in the reward features of food as body mass increases. Supporting this idea, it has been shown that obese-resistant individuals respond to periods of positive energy balance by downregulating appetitive responses to the sight of food, whereas individuals prone to weight gain do not show reductions in the salience of food cues during periods of overfeeding and hence lack strong control over food intake and weight increases (Cornier et al., 2009). Furthermore, it has been reported that lean participants show reduced neuronal responsiveness, as measured by functional magnetic resonance imaging (MRI), to visual food stimuli in the insula and hypothalamus after a period of overfeeding, whereas obese participants who have achieved weight loss do not show attenuated responsiveness in these brain regions in the same setting (Cornier et al., 2009).
Final thought
We mentioned earlier Hirsch’s speech in which he commented on the two communities of scientists that make up the obesity research field (physiologists-molecular biologists-geneticists and behaviourists-psychologists-nutritionists), and that the set point and settling point models might, in part, be a reflection of a divided scientific culture. Here, we suggest that the general intake model and the dual intervention point models each offer conceptual frameworks for understanding obesity that are compatible with the approaches and beliefs of both groups. Indeed, these models reinforce the idea that genes and environments cannot be considered as separate domains and, as such, we hope that they will facilitate interactions across the cultural divide that is in danger of becoming ingrained in the field of obesity research.
Acknowledgments
We are grateful to The Company of Biologists for funding the workshop that led to this paper. We also thank Nicky Le Blond for her exceptional organisational skills displayed before, during and after the meeting, and ‘Bo’ Bogardus and Emily Dhurandhar for their perceptive comments on the manuscript.
Footnotes
This paper was written as a direct consequence of discussions held at The Company of Biologists workshop entitled “Obesity: the gene-by-environment interaction”, organised by John Speakman and held at Melville Castle in Edinburgh, Scotland in May 2010. All the authors were attendees of the workshop and contributed to this manuscript. Workshops held by The Company of Biologists aim to bring together scientists with diverse views to debate hot topics of current interest. For more information, visit http://workshops.biologists.com/.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
REFERENCES
- Allison D. B. (1995). Methodological Issues in Obesity Research: Examples from Biometrical Genetics. In Obesity: New Directions in Assessment and Management (ed. Vanitallie T. B., Simopoulos A. P.), pp. 122–132 Philadelphia: The Charles Press [Google Scholar]
- Allison D. B., Kaprio J., Korkeila M., Koskenvuo M., Neale M. C., Hayakawa K. (1996). The heritability of BMI among an international sample of monozygotic twins reared apart. Int. J. Obes. Relat. Metab. Disord. 20, 501–506 [PubMed] [Google Scholar]
- Anderson J. W., Konz E. C., Frederich R. C., Wood C. L. (2001). Long-term weight-loss maintenance: a meta-analysis of US studies. Am. J. Clin. Nutr. 74, 579–584 [DOI] [PubMed] [Google Scholar]
- Bellinger L. L. (2001). The dorsomedial hypothalamic nucleus and its role in ingestive behaviour and body weight regulation: lessons learned from lesioning studies. Physiol. Behav. 76, 431–442 [DOI] [PubMed] [Google Scholar]
- Berthoud H. R. (2007). Interactions between the ‘cognitive’ and ‘metabolic’ brain in the control of food intake. Physiol. Behav. 91, 486–498 [DOI] [PubMed] [Google Scholar]
- Born J. M., Lemmens S. G., Rutters F., Nieuwenhuizen A. G., Formisano E., Goebel R., Westerterp-Plantenga M. S. (2010). Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int. J. Obes. 34, 172–181 [DOI] [PubMed] [Google Scholar]
- Born J. M., Lemmens S. G., Martens M. J., Formisano E., Goebel R., Westerterp-Plantenga M. S. (2011). Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. Am. J. Clin. Nutr. 94, 392–403 [DOI] [PubMed] [Google Scholar]
- Bouchard C., Tremblay A., Despres J. P., Poehlman E. T., Theriault G., Nadeau A., Lupien P. J., Moorjani S., Dussault J. (1988). Sensitivity to over-feeding – the Quebec experiment with identical twins. Prog. Food Nutr. Sci. 12, 45–72 [PubMed] [Google Scholar]
- Bouchard C., Tremblay A., Despres J. P., Nadeau A., Lupien P. J., Theriault G., Dussault J., Moorjani S., Pinault S., Fournier G. (1990). The response to long term overfeeding in identical twins. N. Engl. J. Med. 322, 1477–1482 [DOI] [PubMed] [Google Scholar]
- Bouchard C., Tremblay A., Despres J. P., Nadeau A., Lupien P. J., Moorjani S., Theriault G., Kim S. Y. (1996). Overfeeding in identical twins: 5-year post overfeeding results. Metab. Clin. Exp. 45, 1042–1050 [DOI] [PubMed] [Google Scholar]
- Budge H., Gnanalingham M. G., Gardner D. S., Mostyn A., Stephenson T., Symonds M. E. (2005). Maternal nutritional programming of fetal adipose tissue development: long-term consequences for later obesity. Birth Defects Res. C 75, 193–199 [DOI] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Phys. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- Cecil J. E., Tavendale R., Watt P., Hetherington M. M., Palmer C. A. N. (2008). An obesity-associated variant in the FTO gene is associated with increased food intake in young children. N. Engl. J. Med. 359, 2558–2566 [DOI] [PubMed] [Google Scholar]
- Chakravarthy M. V., Booth F. W. (2004). Eating, exercise, and ‘thrifty’ genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J. Appl. Physiol. 96, 3–10 [DOI] [PubMed] [Google Scholar]
- Cluskey M., Grobe D. (2009). College weight gain and behavior transitions: male and female differences. J. Am. Diet. Assoc. 109, 325–329 [DOI] [PubMed] [Google Scholar]
- Cone R. D. (1999). The central melanocortin system and energy homeostasis. Trends Endocrinol. Metab. 10, 211–216 [DOI] [PubMed] [Google Scholar]
- Coners H., Remschmidt H., Hebebrand J. (1999). The relationship between premorbid body weight, weight loss, and weight at referral in adolescent patients with anorexia nervosa. Int. J. Eat. Disord. 26, 171–178 [DOI] [PubMed] [Google Scholar]
- Considine R. V., Sinha M. K., Heiman M. L., Kriauciunas A., Stephens T. W., Nyce M. R., Ohannesian J. P., Marco C. C., McKee L. J., Bauer T. L., Caro J. F. (1996). Serum immunoreactive leptin concentrations in normal-weight and obese humans. New Engl. J. Med. 334, 292–295 [DOI] [PubMed] [Google Scholar]
- Cornier M. A., Salzberg A. K., Endly D. C., Bessesen D. H., Rojas D. C., Tregellas J. R. (2009). The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE 4, e6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley M. A., Pronchuk N., Fan W., Dinulescu D. M., Colmers W. F., Cone R. D. (1999). Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163 [DOI] [PubMed] [Google Scholar]
- Davis J. F., Choi D. L., Schurdak J. D., Fitzgerald M. F., Clegg D. J., Lipton J. W., Figlewicz J. P., Benoit S. C. (2011). Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol. Psychiatry 69, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro J. M. (1991). Seasonal rhythms of human nutrient intake and meal patterns. Physiol. Behav. 50, 243–248 [DOI] [PubMed] [Google Scholar]
- de Castro J. M. (2006). Macronutrient and dietary energy density influences on the intake of free-living humans. Appetite 46, 1–5 [DOI] [PubMed] [Google Scholar]
- de Castro J. M. (2010). The control of food intake of free-living humans: putting the pieces back together. Physiol. Behav. 100, 446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro J. M., Plunkett S. (2002). A general model of intake regulation. Neurosci. Biobehav. Rev. 26, 581–595 [DOI] [PubMed] [Google Scholar]
- de los Campos G., Gianola D., Allison D. B. (2010). Predicting genetic predisposition in humans: the promise of whole-genome markers. Nat. Rev. Genet. 11, 880–886 [DOI] [PubMed] [Google Scholar]
- Den Hoed M., Westerterp-Plantenga M. S., Bouwman F. G., Mariman E. C., Westerterp K. R. (2009). Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am. J. Clin. Nutr. 90, 1426–1432 [DOI] [PubMed] [Google Scholar]
- Deriaz O., Fournier G., Tremblay A., Despres J. P., Bouchard C. (1992). Lean body mass composition and resting energy expenditure before and after long-term overfeeding. Am. J. Clin. Nutr. 56, 840–847 [DOI] [PubMed] [Google Scholar]
- Donnelly J., Hall K. H., Heymsfield S., Kemnitz J., Klein S., Schoeller D. A., Speakman J. R. (2011). Energy balance and body weight regulation: a useful concept for understanding the obesity epidemic. Am. J. Clin. Nutr. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey K. J., Popkin B. (2011). Energy density, portion size, and eating occasions: contributions to increased energy intake in the United States, 1977–2006. PloS Med. 8, e10010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo A. G., Jacquet J. (1998). Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am. J. Clin. Nutr. 68, 599–606 [DOI] [PubMed] [Google Scholar]
- Dulloo A. G., Jacquet J., Girardier L. (1997). Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am. J. Clin. Nutr. 65, 717–723 [DOI] [PubMed] [Google Scholar]
- Dykes J., Brunner E. J., Martikainen P. T., Wardle J. (2004). Socioeconomic gradient in body size and obesity among women: the role of dietary restraint, disinhibition and hunger in the Whitehall II study. Int. J. Obes. 28, 262–268 [DOI] [PubMed] [Google Scholar]
- Edholm O. G., Fletcher J. M., Widdowson E. M., McCance R. A. (1955). The energy expenditure and food intake of individual men. Br. J. Nutr. 9, 286–300 [DOI] [PubMed] [Google Scholar]
- Eknoyan G. (2006). A history of obesity, or how what was good became ugly and then bad. Adv. Chronic Kidney Dis. 13, 421–427 [DOI] [PubMed] [Google Scholar]
- Epstein L. H., Rodefer J. S., Wisniewski L., Caggiula A. R. (1992). Habituation and dishabituation of human salivary response. Physiol. Behav. 51, 945–950 [DOI] [PubMed] [Google Scholar]
- Epstein L. H., Paluch R., Smith J. D., Sayette M. (1997). Allocation of attentional resources during habituation to food cues. Psychophysiology 34, 59–64 [DOI] [PubMed] [Google Scholar]
- Epstein L. H., Roemmich J. N., Robinson J. L., Paluch R. A., Winiewicz D. D., Fuerch J. H., Robinson T. N. (2008). A randomized trial of the effects of reducing television viewing and computer use on body mass index in young children. Arch. Paediatr. Adolesc. Med. 162, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam B. C., Morris M. J., Hansen M. J., Kebede M., Andrikopoulos S., Proietto J., Thorburn A. W. (2007). Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diabetes Obes. Metab. 9, 840–852 [DOI] [PubMed] [Google Scholar]
- Farooqi I. S., O’Rahilly S. (2008). Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat. Clin. Pract. Endocrinol. Metab. 4, 569–577 [DOI] [PubMed] [Google Scholar]
- Farooqi I. S., Jebb S. A., Langmack G., Lawrence E., Cheetham C. H., Prentice A. M., Hughes I. A., McCamish M. A., O’Rahilly S. (1999). Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 [DOI] [PubMed] [Google Scholar]
- Farooqi I. S., Keogh J. M., Kamath S., Jones S., Gibson W. T., Trussell R., Jebb S. A., Lip G. Y., O’Rahilly S. (2001). Partial leptin deficiency and human adiposity. Nature 414, 34–35 [DOI] [PubMed] [Google Scholar]
- Farooqi I. S., Matarese G., Lord G. M., Keogh J. M., Lawrence E., Agwu C., Sanna V., Jebb S. A., Perna F., Fontana S., et al. (2002). Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi I. S., Bullmore E., Keogh J., Gillard J., O’Rahilly S., Fletcher P. C. (2007). Leptin regulates striatal regions and human eating behavior. Science 317, 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J., Koch L., Emmerling C., Vierkotten J., Peters T., Brüning J. C., Rüther U. (2009). Inactivation of the Fto gene protects from obesity. Nature 458, 894–898 [DOI] [PubMed] [Google Scholar]
- Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. (2010). Prevalence and trends in obesity among US adults, 1999–2008. J. Am. Med. Assoc. 303, 235–241 [DOI] [PubMed] [Google Scholar]
- Forbes G. B. (1987). Human Body Composition. New York: Springer [Google Scholar]
- Frayling T. M., Timpson N. J., Weedon M. N., Zeggini E., Freathy R. M., Lindgren C. M., Perry J. R. B., Elliott K. S., Lango H., Rayner N. W., et al. (2007). A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freathy R. M., Kazeem G. R., Morris R. W., Johnson P. C. D., Paternoster L., Ebrahim S., Hattersley A. T., Hill A., Hingorani A. D., Holst C., et al. (2011). Genetic vatiation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence body mass index. Int. J. Epidemiol. [E-pub ahead of print] 10.1093/ije/dyr077 [DOI] [PMC free article] [PubMed]
- Frederich R. C., Lollmann B., Hamann A., Napolitanorosen A., Kahn B. B., Lowell B. B., Flier J. S. (1995). Expresson of ob messenger-RNA and its encoded protein in rodents – impact of nutrition and obesity. J. Clin. Invest. 96, 1658–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M. (1998). Leptin, leptin receptors, and the control of body weight. Nutr. Rev. 56, S38–S46 [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Halaas J. L. (1998). Leptin and the regulation of body weight in mammals. Nature 395, 763–770 [DOI] [PubMed] [Google Scholar]
- Garrow J. S. (1988). Obesity and Related Diseases. London: Churchill-Livingstone [Google Scholar]
- Gautron L., Elmquist J. K. (2011). Sixteen years and counting: an update on leptin in energy balance. J. Clin. Invest. 121, 2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt S., Theisen F. M., Haberhausen M., Heinzel-Gutenbrunner M., Wehmeier P. M., Krieg J. C., Kühnau W., Schmidtke J., Remschmidt H., Hebebrand J. (2010). Body weight gain induced by atypical antipsychotics: an extension of the monozygotic twin and sib pair study. J. Clin. Pharm. Ther. 35, 207–211 [DOI] [PubMed] [Google Scholar]
- Goldberg G. R., Murgatroyd P. R., McKenna A. P. M., Heavey P. M., Prentice A. M. (1998). Dietary compensation in response to covert imposition of negative energy balance by removal of fat or carbohydrate. Br. J. Nutr. 80, 141–147 [PubMed] [Google Scholar]
- Gortmaker S. L., Must A., Sobol A. M., Peterson K., Colditz G. A., Dietz W. H. (1996). Television viewing as a cause of increasing obesity among children in the United States, 1986–1990. Arch. Pediatr. Adolesc. Med. 150, 356–362 [DOI] [PubMed] [Google Scholar]
- Hainer V., Stunkard A. J., Kunesová M., Parízková J., Stich V., Allison D. B. (2000). Intrapair resemblance in very low calorie diet-induced weight loss in female obese identical twins. Int. J. Obes. 24, 1051–1057 [DOI] [PubMed] [Google Scholar]
- Hall K. D. (2010a). Mathematical modelling of energy expenditure during tissue disposition. Br. J. Nutr. 104, 4–7 [DOI] [PubMed] [Google Scholar]
- Hall K. D. (2010b). Predicting metabolic adaptation, body weight change and energy intake in humans. Am. J. Physiol. 298, E449–E466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt A., Thamer C., Staiger H., Tschritter O., Kirchhoff K., Machicao F., Haering H.-U., Stefan N., Fritsche A. (2009). Variation in the FTO gene influences food intake but not energy expenditure. Exp. Clin. Endocrinol. Diabetes 117, 194–197 [DOI] [PubMed] [Google Scholar]
- Hayes M. R., Skibicka K. P., Leichner T. M., Guarnieri D. J., DiLeone R. J., Bence K. K., Grill H. J. (2010). Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 11, 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebebrand J., Himmelmann G. W., Herzog W., Herpertz-Dahlmann B. M., Steinhausen H. C., Amstein M., Seidel R., Deter H. C., Remschmidt H., Schäfer H. (1997). Prediction of low body weight at long-term follow-up in acute anorexia nervosa by low body weight at referral. Am. J. Psychiatry 154, 566–569 [DOI] [PubMed] [Google Scholar]
- Hebebrand J., Volckmar A. L., Knoll N., Hinney A. (2010). Chipping away the ‘missing heritability’: GIANT steps forward in the molecular elucidation of obesity – but still lots to go. Obes. Facts 3, 294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C. P., Polivy J. (1984). A boundary model for the regulation of eating. In Eating and Its Disorders (eds Stunkard A. J., Stellar E.), pp. 141–156 New York: Raven Press; [PubMed] [Google Scholar]
- Hetherington M. M., Rolls B. J. (2008). From protocols to populations: establishing a role for the energy density of food in the obesity epidemic. In Obesity: Causes, Mechanisms and Prevention (ed. Blass E.), pp. 301–318 Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- Hetherington M. M., Cecil J. E. (2010). Gene-environment interactions in obesity. Forum Nutr. 63, 195–203 [DOI] [PubMed] [Google Scholar]
- Hetherington M. M., Anderson A. S., Norton G. N. M., Newson L. (2006). Situational effects on meal intake: a comparison of eating alone and eating with others. Physiol. Behav. 88, 498–505 [DOI] [PubMed] [Google Scholar]
- Heymsfield S. B., Greenberg A. S., Fujioka K., Dixon R. M., Kushner R., Hunt T., Lubina J. A., Patane J., Self B., Hunt P., et al. (1999). Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575 [DOI] [PubMed] [Google Scholar]
- Hill J. O. (2009). Can a small-changes approach help address the obesity epidemic? A report of the joint task force of the American Society of Nutrition, Institute of Food Technologists and International Food Information Council. Am. J. Clin. Nutr. 89, 477–484 [DOI] [PubMed] [Google Scholar]
- Hukshorn C. J., Westerterp-Plantenga M. S., Saris W. H. (2003). Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted, overweight men. Am. J. Clin. Nutr. 77, 771–776 [DOI] [PubMed] [Google Scholar]
- Hull H. R., Hester C. N., Fields D. A. (2006). The effect of the holiday season on body weight and composition in college students. Nutr. Metab. 3, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. M., Djafarian K., Stewart J., Speakman J. R. (2009). Increased television viewing is associated with elevated body fatness but not with lower total energy expenditure in children. Am. J. Clin. Nutr. 89, 1031–1036 [DOI] [PubMed] [Google Scholar]
- Johnson L., van Jaarsveld C. H., Emmett P. M., Rogers I. S., Ness A. R., Hattersley A. T., Timpson N. J., Smith G. D., Jebb S. A. (2009). Dietary energy density affects fat mass in early adolescence and is not modified by FTO variants. PLoS ONE 4, e4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A. B., Robinson T. N. (2008). Children, television viewing, and weight status: summary and recommendations from an expert panel meeting. Ann. Am. Acad. Polit. Soc. Sci. 615, 119–132 [Google Scholar]
- Keesey R. E., Hirvonen M. D. (1997). Body weight set-points: determination and adjustment. J. Nutr. 127, 1875S–1883S [DOI] [PubMed] [Google Scholar]
- Kennedy G. C. (1953). The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. B 140, 578–592 [DOI] [PubMed] [Google Scholar]
- Keys A., Brozek J., Henschel A., Mickelsen O., Taylor H. L. (1950). The biology of human starvation (2 vols). Minneapolis: University of Minnesota Press [Google Scholar]
- King N. A., Lluch A., Stubbs R. J., Blundell J. E. (1997). High dose exercise does not increase hunger or energy intake in free-living males. Eur. J. Clin. Nutr. 51, 478–483 [DOI] [PubMed] [Google Scholar]
- Kuczmarski R. J., Flegal K. M., Campbell S. M., Johnson C. L. (1994). Increasing prevalence of overweight among US adults – the national health and nutrition examination surveys 1960 to 1991. J. Am. Med. Assoc. 272, 205–211 [DOI] [PubMed] [Google Scholar]
- Leibel R. L., Hirsch J. (1984). Diminished energy requirements in reduced obese patients. Metabolism 33, 164–170 [DOI] [PubMed] [Google Scholar]
- Leibel R. L., Rosenbaum M., Hirsch J. (1995). Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621–628 [DOI] [PubMed] [Google Scholar]
- Lejeune M. P., Hukshorn C. J., Saris W. H., Westerterp-Plantenga M. S. (2003). Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. Int. J. Obes. 27, 1494–1499 [DOI] [PubMed] [Google Scholar]
- Lemmens S. G., Schoffelen P. F., Wouters L., Born J. M., Martens M. J., Rutters F., Westerterp-Plantenga M. S. (2009). Eating what you like induces a stronger decrease of ‘wanting’ to eat. Physiol. Behav. 98, 318–325 [DOI] [PubMed] [Google Scholar]
- Lemmens S. G., Born J. M., Rutters F., Schoffelen P. F., Wouters L., Westerterp-Plantenga M. S. (2010). Dietary restraint and control over ‘wanting’ following consumption of ‘forbidden’ food. Obesity 18, 1926–1931 [DOI] [PubMed] [Google Scholar]
- Lemmens S. G., Rutters F., Born J. M., Westerterp-Plantenga M. S. (2011). Stress augments food ‘wanting’ and energy intake in visceral overweight subjects in the absence of hunger. Physiol. Behav. 103, 157–163 [DOI] [PubMed] [Google Scholar]
- Levitsky D. A. (2002). Putting behavior back into feeding behavior: a tribute to George Collier. Appetite 38, 143–148 [DOI] [PubMed] [Google Scholar]
- Levitsky D. A., DeRosimo L. (2010). One day of food restriction does not result in an increase in subsequent daily food intake in humans. Physiol. Behav. 99, 495–499 [DOI] [PubMed] [Google Scholar]
- Levitsky D. A., Pacanowski C. R. (2011). Free will and the obesity epidemic. Public Health Nutr. 19, 1–16 [DOI] [PubMed] [Google Scholar]
- Levitsky D. A., Obarzanek E., Mrdjenovic G., Strupp B. J. (2005). Imprecise control of energy intake: absence of a reduction in food intake following overfeeding in young adults. Physiol. Behav. 84, 669–675 [DOI] [PubMed] [Google Scholar]
- Li S., Zhao J. H., Luan J., Ekelund U., Luben R. N., Khaw K. T., Wareham N. J., Loos R. J. (2010). Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med. 7, e1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A., Schoeller D. (1992). Basal metabolic rate, fat-free mass, and body cell mass during energy restriction. Metabolism 41, 450–456 [DOI] [PubMed] [Google Scholar]
- Luke A., Guo X., Rotimi C. N., Adeyemo A. A., Wilks R., Forrester T., Lowe W., Comuzzie A., Martin L. J., Zhu X., et al. (2001). Heritability of obesity-related traits among Nigerians, Jamaicans and US black people. Int. J. Obes. 25, 1034–1041 [DOI] [PubMed] [Google Scholar]
- Maes H. H., Neale M. C., Eaves L. J. (1997). Genetic and environmental factors in relative body weight and human obesity. Behav. Genet. 27, 325–351 [DOI] [PubMed] [Google Scholar]
- Maffei M., Stoffel M., Barone M., Moon B., Dammerman M., Ravussin E., Bogardus C., Ludwig D. S., Flier J. S., Talley M., et al. (1996). Absence of mutations in the human OB gene in obese diabetic subjects. Diabetes 45, 679–682 [DOI] [PubMed] [Google Scholar]
- Makowsky R., Pajewski N. M., Klimentidis Y. C., Vazquez A. I., Duarte C. W., Allison D. B., de los Campos G. (2011). Beyond missing heritability: prediction of complex traits. PLoS Genet. 7, e1002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens M. J. I., Lemmens S. G. T., Born J. M., Westerterp-Plantenga M. S. (2010). Changes in liking of foods during stress as a function of body weight. Obes. Rev. 11, 313 [Google Scholar]
- Matheson D. M., Killen J. D., Wang Y., Varady A., Robinson T. N. (2004). Children’s food consumption during television viewing. Am. J. Clin. Nutr. 79, 1088–1094 [DOI] [PubMed] [Google Scholar]
- Mercer J. G., Hoggard N., Williams L. M., Lawrence C. B., Hannah L. T., Trayhurn P. (1996). Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 387, 113–116 [DOI] [PubMed] [Google Scholar]
- Mrosovsky N., Powley T. L. (1977). Set points for body-weight and fat. Behav. Biol. 20, 205–223 [DOI] [PubMed] [Google Scholar]
- Neel J. V. (1962). Diabetes mellitus a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am. J. Hum. Genet. 14, 352–353 [PMC free article] [PubMed] [Google Scholar]
- Ogden C. L., Troiano R. P., Briefel R. R., Kuczmarski R. J., Flegal M., Johnson C. L. (1997). Prevalence of overweight among preschool children in the United States, 1971 through 1994. Pediatrics 99, E1. [DOI] [PubMed] [Google Scholar]
- O’Rahilly S. (1998). Life without leptin. Nature 392, 330–331 [DOI] [PubMed] [Google Scholar]
- O’Rahilly S. (2009). Human genetics illuminates the paths to metabolic disease. Nature 462, 307–314 [DOI] [PubMed] [Google Scholar]
- Payne P. R., Dugdale A. E. (1977a). Mechanisms for the control of body weight. Lancet 8011, 583–586 [DOI] [PubMed] [Google Scholar]
- Payne P. R., Dugdale A. E. (1977b). A model for the prediction of energy balance and body weight. Ann. Hum. Biol. 4, 525–535 [DOI] [PubMed] [Google Scholar]
- Piernas C., Popkin B. M. (2011). Food portion patterns and trends among U.S. children and the relationship to total eating occasion size, 1977–2006. J. Nutr. 141, 1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskitt E. M. (2009). Countries in transition: underweight to obesity non-stop? Ann. Trop. Paediatr. 29, 1–11 [DOI] [PubMed] [Google Scholar]
- Prentice A. M. (2001). Obesity and its potential mechanistic basis. Br. Med. Bull. 60, 51–67 [DOI] [PubMed] [Google Scholar]
- Prentice A. M. (2008). Obesity in emerging nations: evolutionary origins and the impact of a rapid nutrition transition emerging societies – coexistence of childhood malnutrition and obesity. In Emerging Societies – Coexistence of Childhood Malnutrition (ed. Kalhan S. C., Prentice A. M., Yajnik C. S.), Nestle Nutr, Workshop Ser. Pediatr. Program 63, 47–57 [DOI] [PubMed] [Google Scholar]
- Prentice A. M., Rayco-Solon P., Moore S. E. (2005). Insights from the developing world: thrifty genotypes and thrifty phenotypes. Proc. Nutr. Soc. 64, 153–161 [DOI] [PubMed] [Google Scholar]
- Prentice A. M., Hennig B. J., Fulford A. J. (2008). Evolutionary origins of the obesity epidemic: natural selection of thrifty genes or genetic drift following predation release? Int. J. Obes. 32, 1607–1610 [DOI] [PubMed] [Google Scholar]
- Robinson T. N. (1999). Reducing children’s television viewing to prevent obesity: a randomized controlled trial. J. Am. Med. Assoc. 282, 1561–1567 [DOI] [PubMed] [Google Scholar]
- Robinson T. N. (2001). Television viewing and childhood obesity. Pediatr. Clin. North Am. 48, 1017–1022 [DOI] [PubMed] [Google Scholar]
- Rolls B. J. (2010). Dietary strategies for the prevention and treatment of obesity. Proc. Nutr. Soc. 69, 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls B. J., Hetherington M. M. (1989). The role of variety in eating and body weight. In Psychobiology of Human Eating and Nutritional Behavior (ed. Shepherd R.), pp. 58–84 Sussex: John Wiley and Sons [Google Scholar]
- Rolls B. J., Roe L. S., Meengs J. S. (2007). The effect of large portion sizes on energy intake is sustained for 11 days. Obesity 15, 1535–1543 [DOI] [PubMed] [Google Scholar]
- Romero-Corral A., Somers V. K., Sierra-Johnson J., Thomas R. J., Collazo-Clavell M. L., Korinek J., Allison T. G., Batsis J. A. (2008). Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 32, 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M., Nicolson M., Hirsch J., Murphy E., Chu F., Leibel R. L. (1997). Effects of weight change on plasma leptin concentrations and energy expenditure. J. Clin. Endocrinol. Metab. 82, 3647–3654 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Vandenborne K., Goldsmith R., Simoneau J. A., Heymsfield S., Joanisse D. R., Hirsch J., Murphy E., Matthews D., Segal K. R., et al. (2003). Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am. J. Physiol. 285, R183–R192 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Hirsch J., Gallagher D. A., Leibel R. L. (2008). Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am. J. Clin. Nutr. 88, 906–912 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Kissileff H. R., Mayer L. E. S., Hirsch J., Leibel R. L. (2010). Energy intake in weight-reduced humans. Brain Res. 1350, 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. R., Labayen I., Ortega F. B., Legry V., Moreno L. A., Dallongeville J., Martínez-Gómez D., Bokor S., Manios Y., Ciarapica D., et al. (2010). Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents: the HELENA study. Arch. Pediatr. Adolesc. Med. 164, 328–333 [DOI] [PubMed] [Google Scholar]
- Saladin R., Devos P., Guerremillo M., Leturque A., Girard J., Staels B., Auwerx J. (1995). Transient increase in obese gene expression after food intake or insulin administration. Nature 377, 527–529 [DOI] [PubMed] [Google Scholar]
- Satia J. A. (2010). Dietary acculturation and the nutrition transition: an overview. Appl. Physiol. Nutr. Metab. 35, 219–223 [DOI] [PubMed] [Google Scholar]
- Schoeller D. A., Cella L. K., Sinha M. K., Caro J. F. (1997). Entrainment of the diurnal rhythm of plasma leptin to meal timing. J. Clin. Invest. 100, 1882–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P., Westerterp K. R. (2000). The role of high-fat diets and physical activity in the regulation of body weight. Br. J. Nutr. 84, 417–427 [DOI] [PubMed] [Google Scholar]
- Schrauwen P., van Marken Lichtenbelt W. D., Saris W. H., Westerterp K. R. (1997). The adaptation of nutrient oxidation to nutrient intake on a high-fat diet. Z. Ernahrungswiss. 36, 306–309 [DOI] [PubMed] [Google Scholar]
- Schrauwen P., Lichtenbelt W. D., Saris W. H., Westerterp K. R. (1998). Fat balance in obese subjects: role of glycogen stores. Am. J. Physiol. 274, E1027–E1033 [DOI] [PubMed] [Google Scholar]
- Schrauwen P., van Marken Lichtenbelt W. D., Westerterp K. R. (2000). Fat and carbohydrate balances during adaptation to a high-fat diet. Am. J. Clin. Nutr. 72, 1239–1241 [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Woods S. C., Porte D., Seeley R. J., Baskin D. G. (2000). Central nervous system control of food intake. Nature 404, 661–671 [DOI] [PubMed] [Google Scholar]
- Scott M. M., Williams K. W., Rossi J., Lee C. E., Elmquist J. K. (2011). Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 121, 2413–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebert S. P., Hyatt M. A., Chan L. L., Yiallourides M., Fainberg H. P., Patel N., Sharkey D., Stephenson T., Rhind S. M., Bell R. C., et al. (2010). Influence of prenatal nutrition and obesity on tissue specific fat mass and obesity-associated (FTO) gene expression. Reproduction 139, 265–274 [DOI] [PubMed] [Google Scholar]
- Segal N., Allison D. B. (2002). Twins and virtual twins: bases of relative body weight revisited. Int. J. Obes. 26, 437–441 [DOI] [PubMed] [Google Scholar]
- Segal N. L., Feng R., McGuire S. A., Allison D. B., Miller S. (2009). Genetic and environmental contributions to body mass index: comparative analysis of monozygotic twins, dizygotic twins and same-age unrelated siblings. Int. J. Obes. 33, 37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobal J., Hanson K. L., Frongillo E. A. (2009). Gender, ethnicity, marital status, and body weight in the United States. Obesity 17, 2223–2231 [DOI] [PubMed] [Google Scholar]
- Sousa M., Bras-Silva C., Leite-Moreira A. (2009). The role of leptin in the regulation of energy balance. Acta Med. Port. 22, 291–298 [PubMed] [Google Scholar]
- Speakman J. R. (2004). Obesity: the integrated roles of environment and genetics. J. Nutr. 134, 2090S–2105S [DOI] [PubMed] [Google Scholar]
- Speakman J. R. (2007). A nonadaptive scenario explaining the genetic predisposition to obesity: the ‘Predation release’ hypothesis. Cell Metab. 6, 5–12 [DOI] [PubMed] [Google Scholar]
- Speakman J. R. (2008). Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the ‘drifty gene’ hypothesis. Int. J. Obes. 32, 1611–1617 [DOI] [PubMed] [Google Scholar]
- Speakman J. R., Westerterp K. R. (2010). Associations between energy demands, physical activity and body composition in adult humans between 18 and 96 years of age. Am. J. Clin. Nutr. 92, 826–834 [DOI] [PubMed] [Google Scholar]
- Speakman J. R., Stubbs R. J., Mercer J. G. (2002). Does body weight play a role in the regulation of food intake? Proc. Nutr. Soc. 61, 473–487 [DOI] [PubMed] [Google Scholar]
- Speakman J. R., Rance K. A., Johnstone A. M. (2008). Polymorphisms of the FTO gene are associated with variation in energy intake but not energy expenditure. Obesity 16, 1961–1965 [DOI] [PubMed] [Google Scholar]
- Speliotes E. K., Willer C. J., Berndt S. I., Monda K. L., Thorleifsson G., Jackson A. U., Allen H. L., Lindgren C. M., Luan J., Mägi R., et al. (2010). Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard A. J. (1982). Anorectic agents lower a bodyweight set point. Life Sci. 30, 2043–2055 [DOI] [PubMed] [Google Scholar]
- Symonds M. E., Sebert S. P., Hyatt M. A., Budge H. (2009). Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 5, 604–610 [DOI] [PubMed] [Google Scholar]
- Symonds M. E., Budge H., Perkins A. C., Lomax M. A. (2011). Adipose tissue development – impact of the early life environment. Prog. Biophys. Mol. Biol. 106, 300–306 [DOI] [PubMed] [Google Scholar]
- Tam J., Fukumura D., Jain R. K. (2009). A mathematical model of murine metabolic regulation by leptin: energy balance and defense of a stable body weight. Cell Metab. 9, 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple J. L., Giacomelli A. M., Kent K. M., Roemmich J., Epstein L. H. (2007). Television watching increases motivated responding for food and energy intake in children. Am. J. Clin. Nutr. 85, 355–361 [DOI] [PubMed] [Google Scholar]
- Thornton L. E., Crawford D. A., Ball K. (2010). Who is eating where? Findings from the SocioEconomic Status and Activity in Women (SESAW) study. Public Health Nutr. 10, 1–9 [DOI] [PubMed] [Google Scholar]
- Timpson N. J., Emmett P. M., Frayling T. M., Rogers I., Hattersley A. T., McCarthy M. I., Davey Smith G. (2008). The fat mass- and obesity-associated locus and dietary intake in children. Am J. Clin. Nutr. 88, 971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A., Perusse L., Bouchard C. (2004). Energy balance and body-weight stability: impact of gene-environment interactions. Br. J. Nutr. 92, S63–S66 [DOI] [PubMed] [Google Scholar]
- Troiano R. P., Flegal K. M., Kuczmarski R. J., Campbell S. M., Johnson C. L. (1995). Overweight prevalence and trends for children and adolescents – the national health and nutrition examination surveys 1963 to 1991. Arch. Paediatr. Adolesc. Med. 149, 1085–1091 [DOI] [PubMed] [Google Scholar]
- Van Gemert W. G., Westerterp K. R., van Acker B. A., Wagenmakers A. J., Halliday D., Greve J. M., Soeters P. B. (2000). Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int. J. Obes. 24, 711–718 [DOI] [PubMed] [Google Scholar]
- Van Wye G., Dublin J. A., Blair S. N., DiPietro L. (2007). Adult obesity does not predict 6-year weight gain in men: the aerobics center longitudinal study. Obesity 15, 1571–1577 [DOI] [PubMed] [Google Scholar]
- Vogels N., Westerterp-Plantenga M. S. (2005). Categorical strategies based on subject characteristics of dietary restraint and physical activity, for weight maintenance. Int. J. Obes. 29, 849–857 [DOI] [PubMed] [Google Scholar]
- Vogels N., Mariman E. C., Bouwman F. G., Kester A. D., Diepvens K., Westerterp-Plantenga M. S. (2005). Relation of weight maintenance and dietary restraint to peroxisome proliferator-activated receptor gamma2, glucocorticoid receptor, and ciliary neurotrophic factor polymorphisms. Am. J. Clin. Nutr. 82, 740–746 [DOI] [PubMed] [Google Scholar]
- Wansink B. (2004). Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu. Rev. Nutr. 24, 455–479 [DOI] [PubMed] [Google Scholar]
- Wardle J., Llewellyn C., Sanderson S., Plomin R. (2009). The FTO gene and measured food intake in children. Int. J. Obes. 33, 42–45 [DOI] [PubMed] [Google Scholar]
- Wells J. C. K. (2006). The evolution of human fatness and susceptibility to obesity: an ethological approach. Biol. Rev. 81, 183–205 [DOI] [PubMed] [Google Scholar]
- Westerterp K. R., Donkers J. H., Fredrix E. W., Boekhoudt P. (1995). Energy intake, physical activity and body weight: a simulation model. Br. J. Nutr. 73, 337–347 [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga M. S. (2004a). Effects of energy density of daily food intake on long-term energy intake. Physiol. Behav. 81, 765–771 [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga M. S. (2004b). Modulatory factors in the effect of energy density on energy intake. Br. J. Nutr. 92, S35–S39 [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga M. S., Pasman W. J., Yedema M. J., Wijckmans-Duijsens N. E. (1996). Energy intake adaptation of food intake to extreme energy densities of food by obese and non-obese women. Eur. J. Clin. Nutr. 50, 401–407 [PubMed] [Google Scholar]
- Westerterp-Plantenga M. S., Saris W. H., Hukshorn C. J., Campfield L. A. (2001). Effects of weekly administration of pegylated recombinant human OB protein on appetite profile and energy metabolism in obese men. Am. J. Clin. Nutr. 74, 426–434 [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga M. S., Kovacs E. M., Melanson K. J. (2002). Habitual meal frequency and energy intake regulation in partially temporally isolated men. Int. J. Obes. 26, 102–110 [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga M. S., Goris A. H., Meijer E. P., Westerterp K. R. (2003). Habitual meal frequency in relation to resting and activity-induced energy expenditure in human subjects: the role of fat-free mass. Br. J. Nutr. 90, 643–649 [DOI] [PubMed] [Google Scholar]
- Wirtshafter D., Davis J. D. (1977). Set points, settling points and the control of body weight. Physiol. Behav. 19, 75–78 [DOI] [PubMed] [Google Scholar]
- Wu X., Cooper R. S., Borecki I., Hanis C., Bray M., Lewis C., Zhu X., Kan D., Luke A., Curb D. (2002). A combined analysis of genome-wide linkage scans for BMI from the NHLBI Family Blood Pressure Program. Am. J. Human Genet. 70, 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski J. A., Yanovski S. Z., Sovik K. N., Nguyen T. T., O’Neill P. M., Sebring N. G. (2000). A prospective study of holiday weight gain. N. Engl. J. Med. 342, 861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. (1994). Positional cloning of the mouse obese gene and its human homolog. Nature 372, 425–432 [DOI] [PubMed] [Google Scholar]
- Zhu X., Cooper R. S., Luke A., Chen G., Wu X., Chakravarti A., Weder A. (2002). A genome-wide scan for obesity in African Americans. Diabetes 51, 541–544 [DOI] [PubMed] [Google Scholar]