Abstract
Conservation of major signaling pathways between humans and flies has made Drosophila a useful model organism for cancer research. Our understanding of the mechanisms regulating cell growth, differentiation and development has been considerably advanced by studies in Drosophila. Several recent high profile studies have examined the processes constraining the metastatic growth of tumor cells in fruit fly models. Cell invasion can be studied in the context of an in vivo setting in flies, enabling the genetic requirements of the microenvironment of tumor cells undergoing metastasis to be analyzed. This Perspective discusses the strengths and limitations of Drosophila models of cancer invasion and the unique tools that have enabled these studies. It also highlights several recent reports that together make a strong case for Drosophila as a system with the potential for both testing novel concepts in tumor progression and cell invasion, and for uncovering players in metastasis.
Introduction
Cancer is a leading cause of death worldwide and, according to the World Health Organization, was responsible for the death of 7.9 million people in 2007 (www.who.int). This disease is characterized by the uncontrolled malignant growth of cells; however, the vast majority of human fatalities arise from secondary metastatic tumors. These secondary tumors generally spread from the original site via the blood or lymphatic system and are highly invasive and aggressive. The metastatic process involves several discrete biological steps: loss of cellular adhesion, increased motility and invasiveness, entry and survival of tumor cells in the circulation, their exit into new tissue, and their eventual colonization of a distant site (Chambers et al., 2002; Gupta and Massague, 2006). Understanding the mechanisms that promote tumor invasion and the role of the microenvironment is important for developing therapeutic strategies to treat metastatic cancers (Yang and Weinberg, 2008; Nguyen et al., 2009; Hanahan and Weinberg, 2011).
Over the last decade, the fruit fly Drosophila melanogaster has become an important model system for cancer studies. Reduced redundancy in the Drosophila genome compared with that of humans, coupled with the ability to conduct large-scale genetic screens in this organism, has enabled its use to determine the molecular characterization of important signaling cascades, developmental processes and growth control. For example, our understanding of the Hippo, Notch, Dpp and JAK-STAT signaling pathways, all of which are involved in tumor formation, has been enhanced by research in Drosophila (for reviews, see Brumby and Richardson, 2005; Vidal and Cagan, 2006; Januschke and Gonzalez, 2008). Drosophila genetics has revealed many genes that, when mutated or dysregulated, result in or contribute to tumorigenesis. Hyperplastic tumor suppressors, including components of the Hippo pathway, promote increased proliferation or survival, but do not disrupt tissue structure or differentiation. By contrast, Drosophila neoplastic tumor suppressors, such as the apical-basal cell polarity regulators (e.g. Lgl), lead to loss of tissue architecture, defects in differentiation and failure to exit the cell cycle. Elegant genetic and cell biology techniques have enabled the effects of tumor suppressors and oncogenes to be examined in the context of the whole animal. The capacity to generate patches (clones) of mutant tissue for specified genes during fly development has facilitated investigations into the role of the microenvironment in tumor development. Similarly, studies using clonal analysis in Drosophila have begun to elucidate cell competition mechanisms, which could potentially confer malignant cells with a growth advantage over their neighbors.
In conjunction with studies using other model organisms, flies have contributed greatly to our understanding of the mechanisms involved in cancer initiation and progression, revealing previously unknown molecular components and concepts. In turn, these have served to guide researchers who use mammalian systems to study cancer. This Perspective focuses on recent developments using fly tumor models that have been generated by tumor suppressor mutations or oncogene overexpression to induce neoplastic tumors of neuronal and epithelial origin as a means to probe the mechanisms involved in cellular invasion and metastasis.
Insights from metastatic neuronal tumors in the developing larval brain
Single gene mutations in a unique subset of genes {lethal (3) malignant brain tumor [l(3)mbt], brain tumor (brat), discs large (dlg), scribble (scrib), lethal giant larvae (lgl), miranda (mira), prospero (pros), partner of inscuteable (pins) (for reviews see Januschke and Gonzalez, 2008; Froldi et al., 2008)} cause malignant neoplastic tumors in the Drosophila larval brain. These genes have crucial roles in regulating proliferation and development [l(3)mbt] (Gateff et al., 1993) and apical-basal cell polarity (dlg and scrib) (Woods et al., 1989; Bilder et al., 2000).
The human homologs of Dlg, Scrib and Lgl are important regulators of cell polarity, and mutations or splice variants have been linked to poor prognosis for colorectal (Schimanski et al., 2005) and hepatocarcinoma (Lu et al., 2009) patients. Importantly, all three complex components are targets for E6 papillomavirus-mediated degradation (Nakagawa and Huibregtse, 2000; Humbert et al., 2003; Handa et al., 2007). L(3)mbt has also been linked to various myeloid haemopoietic disorders (Boccuni et al., 2003) and is important for DNA replication and genomic stability (Gurvich et al., 2010).
Of these fly neoplastic tumor suppressors, lgl and brat are the best characterized. Lgl is localized to the cellular cortex, and functions in the same genetic pathway as dlg and scrib to maintain cellular polarity. Neuroblasts from lgl mutant larvae undergo aberrant symmetrical cell divisions, rather than the normal asymmetric divisions that are required for neuroblast and ganglion mother cell production (Mechler et al., 1985). Brat regulates ribosomal RNA (rRNA) synthesis and is a translational repressor. Brat is also asymmetrically localized to the ganglion mother cell after neuroblast division and is necessary for neuronal differentiation and proliferation control (Arama et al., 2000). Malignant tumors resulting from mutations in these fly neoplastic tumor suppressors cause late larval lethality but can be propagated and will metastasize when allografted into a recipient adult host (Gateff, 1978; Januschke and Gonzalez, 2008).
Metastasis of lgl and brat tumors
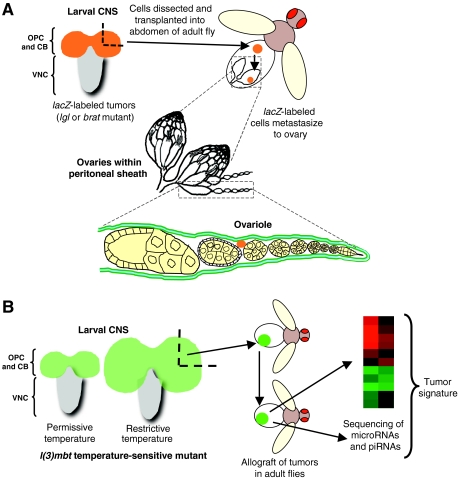
The Shearn group has utilized allograft experiments to investigate the metastatic behavior of lgl and brat mutant cells (Fig. 1A). Neoplastic brain tumors labeled with β-galactosidase (encoded by lacZ) from mutant larvae were dissected and allografted into the abdomens of wild-type adult flies. These tumors grew rapidly and resulted in host lethality within 12 days. Ovaries from allograft recipients were dissected and assayed for cells expressing lacZ. Because the ovary is contained in a non-porous epithelial sheet and muscle layers surround the ovarioles, only cells that are capable of metastasizing can invade into this organ (Fig. 1A). lacZ-positive cells were detected in recipient ovarioles from lgl and brat mutant tumors but not from control brains. These lacZ-positive cells in the ovary were shown to retain their neuronal and glial cell markers. This work provided the first evidence that Drosophila cells can metastasize through epithelial tissue and colonize new sites distant from the initial tumor (Beaucher et al., 2007a).
Fig. 1.
Drosophila models of tumor metastasis caused by loss-of-function mutations in lgl or brat and l(3)mbt. The Drosophila larval brain is composed of two hemispheres and the ventral nerve cord (VNC). Mutant tumorous tissue in these models is restricted to the outer proliferative center (OPC) and central brain (CB) regions. (A) Neoplastic brain tumors caused by mutations in either lgl or brat can be engineered to express a reporter gene (lacZ; orange). Larval brains from these lgl or brat mutant animals are quartered and transplanted into the abdomens of adult female flies. The transplanted tissue continues to proliferate in the abdomen and, after several days, the ovaries of the host can be dissected and examined by immunofluorescence to detect labeled tumor cells that have metastasized. Drosophila ovaries are encased within a peritoneal sheath. Each ovary consists of 15–20 ovarioles, which are surrounded by an epithelial sheath of cells in addition to a muscle layer (blue) between two layers of extracellular matrix (green). Any lacZ-expressing cells found within the ovarioles must have traversed these layers and are therefore considered to have undergone metastasis. (B) Modeling metastasis using tumors caused by loss-of-function mutations in l(3)mbt. Temperature-sensitive mutations in l(3)mbt result in tissue overgrowth in the developing larval CNS when grown at restrictive temperatures (29°C). Tumor tissue (engineered to express GFP) can be dissected and transplanted into the abdomens of wild-type adult flies (allografts). These can be maintained and multiple rounds of allografts can be performed by transplantation into other flies, thereby generating sufficient material for biochemical analysis (e.g. microarray analysis, sequencing and western blotting). Comparison of the expression profiles of l(3)mbt and brat mutant tumors enabled an l(3)mbt-specific tumor expression signature to be obtained (Janic et al., 2010).
To understand the processes regulating this metastasis, a candidate approach was used to identify genes that are required for invasion by lgl and brat cells. Matrix metalloproteases (MMPs) have long been linked to metastasis in human cancer and were therefore excellent candidates. The Drosophila genome encodes two MMPs (MMP1 and MMP2). MMP1 expression is strongly upregulated in lgl mutant cells and is necessary for their metastatic behavior (Beaucher et al., 2007b). By contrast, MMP1 expression is not required in brat mutant cells but is required in the host tissue, suggesting that MMP1 co-operates non-cell-autonomously with brat mutant cells to enable their metastasis.
Molecular profiling of l(3)mbt neuronal tumors
Recent studies from the Gonzalez group have taken a fresh approach to identifying genes required for l(3)mbt tumor growth (Janic et al., 2010). l(3)mbt is a substoichiometric member of the Drosophila dREAM-Myb complex, which is required for gene silencing, and functions in establishing and maintaining differentiation states (Lewis et al., 2004). Similar to lgl and brat tumors, those arising from l(3)mbt mutations result in larval lethality and can be allografted (Fig. 1B).
By conducting extensive expression arrays on larval neuronal and allograft cultured tumors, gene expression profiles were identified for tumors that result from single gene mutations [in l(3)mbt, brat, lgl, mira, pros and pins] (Janic et al., 2010). By comparing profiles, Janic and colleagues characterized an l(3)mbt-specific tumor signature. The concatenate sequencing of Piwi-interacting RNAs (piRNAs) and microRNAs from these tumors identified small RNA changes that are characteristic of the l(3)mbt tumors. Together with immunohistochemical analyses, these approaches provide a basis for understanding the gene expression changes that are specific to l(3)mbt tumors. The l(3)mbt tumor signature surprisingly contains a disproportionately high number of germline-specific genes and microRNAs. This result suggests that a germline fate is associated with l(3)mbt tumor growth, and is in agreement with studies from Caenorhabditis elegans that linked soma-to-germline transition with increased fitness and longevity (Curran et al., 2009; Wang et al., 2005).
Genetic studies then tested the capacity of mutations in upregulated germline-specific genes to modify l(3)mbt tumor formation and allograft metastasis (Janic et al., 2010). Interestingly, only mutations in a subset of germline-specific genes (piwi, vasa, aub and nos) could suppress l(3)mbt tumor formation. By contrast, other upregulated germline-specific transcripts (zpg, Pxt and AGO) were unable to prevent tumor formation or metastatic growth. This result suggests that only a limited number of germline-specific genes are capable of restricting tumor growth. These findings provide a detailed understanding of the gene expression and small RNA changes in l(3)mbt neoplastic tumors, and highlight the possibility that a soma-to-germline transition accompanies metastasis. However, this approach has not yet identified genes that are specifically required for invasion, because all metastatic suppressors also prevented l(3)mbt tumor growth. Further analysis of the l(3)mbt tumor signature will hopefully identify other metastasis-promoting factors.
Using mutations to generate neoplastic tumors has significant advantages. First, only a single mutational event is required to initiate tumor growth in an isogenic background. Second, the l(3)mbt alleles are particularly advantageous because they are temperature sensitive, permitting gene expression arrays to be conducted from a single stock at both permissive and restrictive temperatures. Finally, these tumors develop rapidly and metastasize when allografted. These experiments permit expansion of the tumor mass for biochemical analysis and metastasis modeling.
Insights from neuronal metastatic tumors induced by oncogene overexpression
Modeling RasV12 tumors in the larval eye neural epithelium
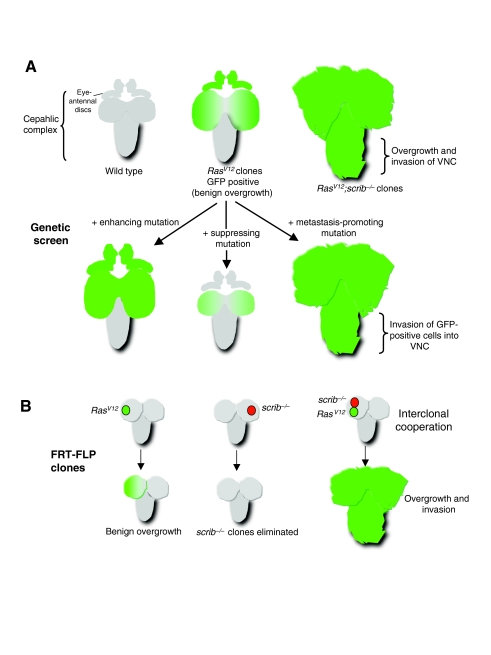
Overexpression of oncogenic Ras protein (RasV12) causes benign tumor growth in Drosophila. This model has been used to identify pathways that are required for tumor development and metastasis. The mosaic analysis with a repressible cell marker (MARCM) technique can be used to generate clones expressing activated RasV12 by FLP-FRT-mediated mitotic recombination; clones are concomitantly labeled with a visible marker (UAS-GFP) (Theodosiou and Xu, 1998; Lee and Luo, 2001; Elliott and Brand, 2008). Using an eye-specific flippase (ey-FLP) to convert an inactive GAL4 driver (Act>y+>GAL4) to an active conformation (Act>GAL4) enables the ectopic expression of UAS transgenes to be stimulated in the larval neural tissue, in an otherwise wild-type animal (Pagliarini and Xu, 2003). Expression of activated Ras (UAS-RasV12) causes benign tumor growth in the larval midbrain, which can be visualized through imaging living larvae or pupae directly. These benign tumors can then be used to identify genetic modifiers that are required for either initial tumor development and/or metastasis (Fig. 2A).
Fig. 2.
Modeling tumor invasion and metastasis in the Drosophila larval brain. (A) RasV12 overexpression clones generated by the MARCM system in eye-antennal discs. These clones (marked with GFP, and shown in green) produce a benign overgrowth throughout the cephalic complex. When RasV12 clones are also mutant for scrib, they become tumorigenic and metastasize, as shown by invasion of GFP-labeled cells into the ventral nerve cord (VNC). Genetic screens have identified modifiers of the RasV12 phenotype [suppressor, JNK; enhancer, large giant lethal (lgl); metastatic enhancer, deep orange (dor)] (Chi et al., 2010; Wu et al., 2010). (B) Interclonal cooperation of RasV12 and scrib−/−clones. As described in A, RasV12 overexpression produces benign overgrowth. scrib−/− clones grow at a reduced rate and are excluded via cell competition. Adjacent RasV12 and scrib−/− clones cooperate to overproliferate and undergo metastasis. The large tumor and metastatic growth consists almost exclusively of RasV12 cells, suggesting that scrib−/− cells actively cooperate with RasV12 cells during the early stages of tumor growth and metastasis (Wu et al., 2010).
This system identified the cell polarity genes scrib, lgl and dlg as being crucial for constraining metastatic growth (Pagliarini and Xu, 2003). All three of them genetically interact and cause overgrowth of RasV12 tissue when mutated. These studies also identified bazooka, stardust and cdc42 as factors that do not induce overgrowth when mutated singly but can strongly stimulate oncogenic Ras-mediated tumor growth and metastasis (Pagliarini and Xu, 2003). Each of these genes can also regulate cell polarity and E-cadherin expression. Crucially, although downregulation of E-cadherin is necessary for RasV12;scrib−/−-induced metastasis, it is not sufficient, implicating other contributing factors in metastatic growth.
The relationship between oncogenic Ras and JNK signaling
Several Drosophila laboratories have highlighted the importance of the oncogenic cooperation between Ras and JNK signaling. Constitutive activation of the Ras signaling pathway prevents fly cells from undergoing JNK-mediated apoptosis in response to cellular stresses (Brumby and Richardson, 2003; Brumby and Richardson, 2005). The Xu group has linked JNK upregulation to cell polarity and changes in E-cadherin expression in RasV12;scrib−/−clones (Igaki et al., 2006). They also demonstrated that overexpression of negative regulators of the JNK pathway [using a transgene to express a dominant-negative form of Drosophila JNK (encoded by the bsk gene)] prevents tumor formation and metastasis (Igaki et al., 2006). The Bohmann laboratory has shown that the invasion potential of RasV12;scrib−/− clones is dependent on the Fos-mediated transcriptional activation of mmp1 downstream of JNK (Uhlirova and Bohmann, 2006). Expression of the MMP inhibitor, TIMP, or mmp1 RNA interference (RNAi) knockdown, was able to suppress cell invasiveness (Uhlirova and Bohmann, 2006).
Recent studies of the sds22 gene in Drosophila have strengthened the idea that epithelial integrity and JNK signaling cooperate to drive the metastatic growth of RasV12;scrib−/− clones (Jiang et al., 2011). Sds22 is a conserved regulatory subunit of protein phosphatase 1 (PP1), and acts as a regulator of epithelial polarity and as a neoplastic tumor suppressor in Drosophila (Grusche et al., 2009). Loss of sds22 in RasV12 clones results in reduced epithelial integrity, and the clones become invasive. Overexpression of Sds22 in RasV12;scrib−/− cells largely suppresses their tumorigenic growth, and mechanistic studies suggest that Sds22-PP1 inhibits non-muscle myosin II and JNK activity (Jiang et al., 2011). The authors of this study also showed that human SDS22 is deleted or downregulated in multiple types of carcinoma.
A recent study by the Richardson group identified key regulators of the actin cytoskeleton and cell morphology, including Rho1-family GTPases and RhoGEFs, as Ras-cooperating proteins (Brumby et al., 2011). The hyperplastic eye phenotype produced by driving UAS-RasV12 with ey-GAL4 was screened for modifiers using a collection of P-element enhancer insertion lines bearing UAS promoter sequences. These lines result in overexpression of the gene adjacent to the insertion element in RasV12 cells. JNK pathway activation is crucial for the cooperation of these actin cytoskeletal regulators with RasV12. The relevance to human cancer of the collaboration between oncogenic Ras and JNK was demonstrated in this study by correlating JNK signaling with the upregulation of Ras in breast cancers (Brumby et al., 2011).
The role of JNK signaling in promoting metastatic behavior seems to be context specific. In contrast to the promotion of Ras-induced metastasis by JNK, analysis of lgl−/− clones suggests that the activity of Diap1 (a caspase inhibitor) and JNK loss are essential for invasion of mutant cells out of the clone (Grzeschik et al., 2010a; Grzeschik et al., 2010b). Although Diap1 expression is sufficient to inhibit widespread apoptosis, cells at the boundary of lgl−/− clones undergo cell death in a JNK-dependent manner. By blocking the JNK signaling cascade, apoptosis of these cells is specifically inhibited, stimulating de-differentiation and cellular invasion.
Lysosome dysfunction in RasV12 tumor growth and invasion
Recent studies from the Xu laboratory have used genetic screens to identify mutations that promote RasV12 cell metastasis (Fig. 2A). The authors tested 3119 ethyl-methanesulfonate-mutagenized lines (on the X chromosome) for their capacity to modify RasV12 larval tumors. This screen identified 516 suppressors and 351 enhancers of tumor growth, of which 23 enhanced tumor growth as well as metastasis (Chi et al., 2010). Two of these metastasis-promoting mutations occurred within the class C vacuolar protein sorting (VPS) complex member deep orange (dor). By testing mutations in genes encoding other components of this lysosome complex, namely carnation and vps16A, they confirmed that loss of lysosome activity stimulates metastatic growth of the RasV12 tissue (Chi et al., 2010). Characterization of other modifiers from this screen might provide a clearer understanding of the mechanisms underlying Ras-mediated tumor growth in Drosophila. This study also confirmed their genetic findings: feeding chemical inhibitors of lysosomal function to larvae with RasV12 overgrowths promoted tumor development and metastasis. This work illustrates the potential of Drosophila to screen for therapeutic cancer drugs in vivo, a system that might one day provide a cheap and rapid means for drug discovery.
Interclonal cooperation between RasV12 and scrib mutant cells
Exciting recent work using the RasV12-scrib−/− system has demonstrated a non-cell-autonomous effect between neighboring clones (called interclonal cooperation; Fig. 2B). Adjacent RasV12 clones and scrib−/− clones actively cooperate to form tumors and can metastasize (Wu et al., 2010). Interclonal tumors and metastatic growths are smaller than those produced from a single RasV12;scrib−/− clone but still represent a significant tumor burden to the hosts (Fig. 2B). During later stages of tumor development, these interclonal tumors are made up almost exclusively of RasV12-expressing cells, suggesting that the scrib−/− mutant cells are only required for the initial stages of tumor formation and metastasis (Wu et al., 2010). These studies also identified the JAK-STAT signaling pathway as being a crucial downstream target of JNK activity, implicating JNK and JAK-STAT as important oncogenic drivers for Ras-mediated tumor growth (Wu et al., 2010). In agreement with these findings, the authors went on to demonstrate that JNK activation after wounding is also able to promote the overgrowth of RasV12 cells. This study potentially forges a link between tissue damage and Ras-stimulated JNK activity – a situation resembling the chronic inflammation that has been reported to contribute to tumorigenesis in humans (Mantovani, 2010). Other recent Drosophila studies have also highlighted the role of the immune response in tumor growth (Box 1).
Box 1. The role of the immune system in tumor growth in Drosophila.
Several recent reports suggest that the immune system plays a crucial role in Drosophila tumor models, as is the case in mammalian tumors. Circulating blood cells, known in Drosophila as hemocytes, are part of the fly immune system and have been found to associate with RasV12;scrib−/− tumors and to reduce tumor growth in scrib−/− animals (Pastor-Pareja et al., 2008). The Drosophila genome encodes a single member of the tumor necrosis factor (TNF) family, named Eiger (Egr). Egr has been shown to be required for the JNK-dependent cell death of scrib or dlg clonal tissue (Igaki et al., 2009). In the absence of egr, these mutant clones grow aggressively and develop into tumors (Igaki et al., 2009). By contrast, another study showed that loss of egr in RasV12;scrib−/− tumors prevented invasive overgrowth, which was correlated with reduced JNK activation and a failure to express MMP1 (Cordero et al., 2010). Therefore, in the presence of oncogenic Ras, Egr seems to play a role as a tumor promoter. This study also revealed that Egr is produced in the hemocytes associated with RasV12;scrib−/− tumors (Cordero et al., 2010). Together, these Drosophila models might provide an excellent parallel to mammalian tumors, in which TNF is produced in both tumor cells and associated immune cells, where it has been shown to have both oncogenic and tumor-suppressive roles (Balkwill, 2009).
Screening for regulators of invasive growth caused by activation of the Notch pathway
The Notch signaling cascade was originally identified as an important regulator of proliferation and differentiation in flies. Extensive genetic and biochemical studies have identified and characterized the components and regulators of this pathway (Artavanis-Tsakonas and Muskavitch, 2010). Drosophila studies have revealed that, similar to oncogenic Ras, Notch signaling cooperates with JNK to promote dysregulation of epithelial integrity (Brumby and Richardson, 2003; Leong et al., 2009). Aberrant Notch signaling is associated with several human cancers, including skin, breast, lung and ovarian cancer (for a review, see Allenspach et al., 2002). Tumors containing amplifications of genes encoding Notch signaling components (e.g. Notch and Jagged) tend to be highly aggressive and metastatic.
Overexpression of the Notch activator Delta (Dl) using the eyeless-GAL4 driver (ey-GAL4>UAS-Dl) stimulates a non-metastatic overproliferation of eye tissue. Flies treated with a γ-secretase inhibitor to prevent Notch receptor proteolysis showed complete rescue of the ey-GAL4>UAS-Dl phenotype (Palomero et al., 2007). This model has been employed by the Dominguez group to screen for modifiers of this phenotype using a library of P-element UAS insertion lines that result in gene overexpression(Ferres-Marco et al., 2006). One of these lines, eyeful, strongly promoted the metastatic growth of ey-GAL4>UAS-Dl eye tissue, resulting in secondary eye growths throughout the body. Using various tissue-specific GAL4 drivers, the authors demonstrated that co-expression of Dl and eyeful could stimulate massive overgrowth and metastatic invasion in multiple tissue settings. The eyeful construct was mapped within the divergently transcribed genes of longitudinals lacking (lola) and pipsqueak (psq), which produce a myriad of alternatively spliced BTB (BR-C, ttk and bab) proteins, which are required for the recruitment of histone deacetylases and Polycomb complexes to promoter regions. Further biochemical studies demonstrated that silencing of Retinoblastoma (Rbf) expression via promoter hypermethylation strongly contributed to the metastatic phenotype (Ferres-Marco et al., 2006).
This screen also identified the P-element insertion GS1D233C as an enhancer of the ey-GAL4>UAS-Dl phenotype. This insertion was mapped to the Akt1 locus, which encodes an important serine/tyrosine kinase linked to phosphatase and tensin homolog (PTEN) and Notch signaling (Palomero et al., 2007). These results suggest that flies will provide a useful system to test new pharmacological reagents targeted against the Notch pathway.
The Hassan group used the ey-GAL4>UAS-Dl/eyeful phenotype to screen for mutations that could further enhance metastatic growth (Bossuyt et al., 2009). This screen identified atonal (ato), a transcription factor required for retinal terminal differentiation, as a crucial tumor suppressor. Mutations affecting ato dramatically enhanced tumor burden and metastasis rates, and tumors displayed elevated levels of proliferation markers (such as phosphorylated histone H3). Conversely, overexpression of atonal upregulated both Decapo (also known as p21 cell-cycle inhibitor) and phosphorylated-JNK levels, inhibiting proliferation and inducing apoptosis. Furthermore, overexpressing dominant-negative JNK (bsk) partially mimics ato downregulation in the eyeful (ey-GAL4>UAS-Dl/eyeful) model, indicating that JNK signaling is downstream of atonal and that atonal requires active JNK signaling to inhibit overgrowth (Bossuyt et al., 2009).
Modeling glioma in Drosophila
Developing Drosophila models of specific human tumor types is limited in many cases owing to the lack of directly homologous organs (e.g. pancreas, liver and lung). However, recent studies have capitalized on the similarity of mammalian and Drosophila glial cells to model glioblastoma in flies (Read et al., 2009; Witte et al., 2009). Glioblastomas are the most common tumors of the central nervous system (CNS), and their rapid proliferation and malignancy result in poor patient prognosis.
Mutation or amplification of the gene encoding epidermal growth factor receptor (EGFR) tyrosine kinase, loss of PTEN [which antagonizes the growth promoting effects of phosphoinositide 3-kinase (PI3K) signaling] or activating mutations in PI3K (Furnari et al., 2007) are genetic lesions that are commonly associated with gliomas. Consequently, the glial-specific Repo-GAL4 driver (incorporating the promoter reversed polarity) was used to simultaneously express transgenes encoding constitutively active EGFR and PI3K in larval glia. Co-activation of EGFR (or Ras) and PI3K in larval glia results in neoplasia, neurological defects and lethality (Read et al., 2009; Witte et al., 2009). The overproliferation and neoplastic transformation seems to be specific for glia, because overexpression of EGFR and PI3K in neurons, neuroblasts or other glial cells (such as oligodendrocyte-like neuropil glia and astrocyte-like cortex glia) failed to transform them.
The neoplastic glia induced in this Drosophila model mimic the highly proliferative anaplastic glia from high-grade human gliomas. They ectopically express Cyclin-B (CycB), Cyclin-E (CycE) and MMP1, promoting cell cycle entry and invasive growth. These studies demonstrated that glia expressing activated EGFR or Ras and PI3K invade into inappropriate areas of the brain, such as along Bolwig nerves, which are not normally accompanied by glial cells (Read et al., 2009; Witte et al., 2009).
Genetic experiments have revealed that the malignant neoplastic transformation of larval glia induced by EGFR and PI3K occurs via a complex network of genetic factors that are commonly mutated or activated in human gliomas. These downstream effectors include Tor, Myc, G1 cyclin-Cdk complexes (such as those including Cyclin B or E) and the Rb-E2F pathway. Interestingly, pharmacological inhibitors, including compounds that are used to treat patients, rescued these phenotypes in larvae. An EGFR inhibitor (gefitinib) partially reduced the migration of EGFR- and PI3K-transformed glia, whereas a PI3K inhibitor (wortmannin) and an Akt inhibitor (triciribine) completely prevented invasion (Witte et al., 2009). Together, these studies suggest that this Drosophila model is useful for deciphering the signaling cascades underlying the abnormal behavior of glioma cells, including their metastatic potential.
Modeling tumor invasion in the epithelia of the Drosophila wing disc
Roles for both receptor and non-receptor tyrosine kinases in cell transformation and progression towards malignant phenotypes are well established (Blume-Jensen and Hunter, 2001). The SRC family kinases (SFKs) are membrane-linked non-receptor tyrosine kinases that are required for regulating adhesion and cytoskeleton reorganization, cell cycle progression, and migration (Guarino, 2010; Thomas and Brugge, 1997). Elevated SRC levels have been reported in a wide variety of human cancers, including those of the colon, liver, lung, breast and pancreas (Ishizawar and Parsons, 2004; Summy and Gallick, 2003). SFK activity is inhibited by the C-terminal SRC kinase (CSK) family of tyrosine kinases [CSK and CSK homologous kinase (CHK)] (Chong et al., 2005); both CSK and CHK phosphorylate and inactivate SFKs, and mutations disrupting this activity have been implicated in a plethora of cancers. Elevated SRC levels (either by amplification of SRC or loss of CSK function) promote anchorage-independent cell growth, tumor cell invasion and metastasis (Guarino, 2010). Although SRC is a crucial regulator of epithelial-mesenchymal transition (EMT), the exact mechanism of how SRC promotes metastatic growth remains elusive.
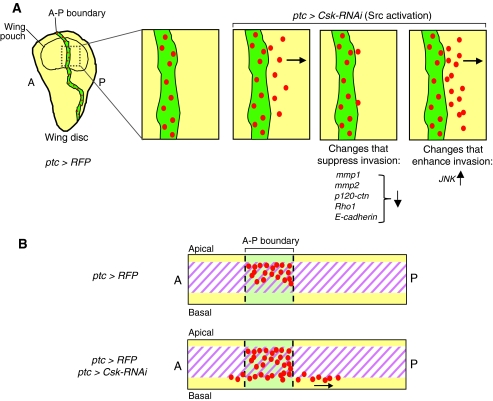
Recent studies from the Cagan group have utilized the pseudostratified epithelia of the Drosophila larval wing imaginal disc to model invasive cell growth (Vidal et al., 2006). Global depletion of the sole Drosophila CSK/CHK ortholog, Csk, using either RNAi or Csk mutations, elevates active levels of Src, leading to significant overgrowth in developing larvae. By contrast, targeting Csk depletion to a discrete stripe along the anterior-posterior (A-P) boundary of the larval wing disc using a patched-GAL4 driver (ptc-GAL4) to express a Csk RNAi transgene (UAS-Csk-RNAi) produces a metastatic phenotype (Vidal et al., 2006). Src-transformed cells lose their apical profile and are excluded from the epithelium. These cells invasively migrate through the basal extracellular matrix, and eventually apoptose (Fig. 3). This invasive migration has been used to model tumor metastasis. Invasion occurs only at the boundaries between Csk mutant cells and the adjacent wild-type cells, suggesting that the microenvironment is crucial in determining the outcome of the Src-activated cells (Vidal et al., 2006).
Fig. 3.
Modeling tumor invasion in the Drosophila larval wing disc. (A) The larval wing disc consists of a sheet of epithelial cells (yellow). A ptc-GAL4 driver is used to knock down Csk, a negative regulator of Src, specifically in a stripe along the A-P boundary, using an RNAi transgene (UAS-Csk-RNAi). The Src-transformed cells are labeled with red fluorescent protein (RFP). The ptc domain is shown in green. The Csk-RNAi cells at the boundary of the ptc domain have the potential to invade into the surrounding tissue. Activation of the JNK pathway or knockdown of regulators of the cytoskeleton (mmp1, mmp2, p120-ctn, Rho1 and E-cadherin) using transgenic overexpression, different genetic backgrounds or RNAi, can either enhance or suppress the invasion of the Csk-RNAi cells, highlighting the importance of the tumor microenvironment (Vidal et al., 2006). (B) Horizontal cross-section of the boxed area of the wing disc shown in A. On silencing of Csk, labeled cells (red) are basally excluded and migrate from the boundary through the extracellular matrix (purple hatch).
By utilizing this model of metastasis, Vidal and co-authors examined the capacity of GFP-labeled Csk-RNAi cells to invasively migrate in different mutant backgrounds. These studies implicate JNK signaling in the apoptotic response of Csk-RNAi cells. Mutations in puckered (puc), a Drosophila JNK-specific phosphatase, cause an upregulation in JNK signaling and enhance both the apoptotic and the invasion phenotype of ptc>Csk-RNAi cells. Conversely, puc overexpression (UAS-puc) using ptc-GAL4 prevents apoptosis within the stripe. Similar experiments revealed that the small GTPase Rho1 is a positive mediator of the JNK signal in Csk-RNAi boundary cells, similar to that seen in activated RasV12 tumors in the eye epithelia (discussed above) (Brumby et al., 2011).
Cadherin-containing complexes are required for maintaining adherens junctions, which are important for cell adhesion and tissue structure. Src modulates the integrity of these adhesion sites: E-cadherin-dependent adhesion is reduced in ptc>Csk-RNAi tissue. These results implicated E-cadherin in the recognition and elimination of Csk-RNAi cells. Depletion of E-cadherin suppresses both the migratory and apoptotic phenotypes of ptc>Csk-RNAi boundary cells. Using candidate approaches, p120-catenin and both MMPs (MMP1 and MMP2) were found to be important for the Csk-RNAi invasive phenotype (Vidal et al., 2006; Vidal et al., 2010). mmp1 transcript levels were specifically upregulated at the leading edge of Csk-RNAi migrating cells (Singh et al., 2010; Vidal et al., 2010), suggesting that rearrangement of the extracellular matrix is crucial for this invasive growth.
In a separate study, the capacity of Src to contribute to RasV12-induced tumor growth was tested (Vidal et al., 2007), and malignant overgrowth of RasV12 tumors was found to correlate with elevated Src levels. These studies suggest a progressive role for Src, whereby low levels promote proliferation during early tumorigenesis and high levels are required for the later stages of invasive migration and metastasis (Vidal et al., 2007).
Invasive migration phenotypes similar to those of ptc>Csk-RNAi cells are produced by the overexpression of the oncogene Abl in the A-P boundary of the wing disc (Singh et al., 2010). Co-expression of ptc>Csk-RNAi and UAS-Abl results in synergistic enhancement of the invasive phenotype. Conversely, the migration phenotype induced by UAS-Csk-RNAi is suppressed by reducing Abl function using RNAi. Together, these findings suggest that Abl functions downstream of Csk and Src to mediate cell invasion. In addition to activating JNK (required for cell invasion and apoptosis), Abl overexpression also stimulates ERK signaling, further promoting cellular proliferation. Interestingly, the authors of this study defined a positive feedback loop whereby Abl increases the activity of Src, resulting in signal amplification (Singh et al., 2010).
This system provides an excellent model in which to study the function of genes in cell proliferation, survival and invasive behavior in the context of cell polarity. Although these reports only tested candidate genes for their ability to modify the invasion phenotype induced by Csk RNAi, the system should prove amenable to genetic screening using UAS-RNAi fly lines to identify genes involved in metastasis. In addition, the identification of the factors that collaborate to control cell migration suggests possible approaches for dual therapeutic targeting in combating metastasis in various cancers.
Conclusions, limitations and perspectives
This review has highlighted the many advantages of Drosophila as a model for studying tumor progression. A streamlined genome, coupled with powerful genetic tools, provides a unique system in which to explore the mechanisms regulating the metastatic growth of tumor cells. However, as with any model organism, it has several limitations that should be considered. In mammals, malignant cells undergoing metastasis enter a local blood or lymph vessel before colonizing a distant tissue and forming secondary tumors. This is difficult to model in Drosophila because flies have rudimentary hematopoietic systems and a dramatically different lymphatic system compared with mammals. In addition, the metastatic potential of tumors induced in Drosophila is greatly reduced compared with their mammalian counterparts; tumor cells tend to invade the local surrounding tissue distally (e.g. in the cephalic complex in the case of RasV12 overexpression).
Tumor formation is generally stimulated by a single mutation or activated oncogene expression in Drosophila. However, as the examples outlined here demonstrate, Drosophila has also proved to be a powerful experimental system for examining ‘two-hit’ models of tumor overgrowth and invasion. Although the Drosophila models discussed here are largely limited to tumors that develop in the larval stage, adult flies can be employed to examine the metastatic potential of these tumors using allografts, providing an ideal way to propagate tumors for extended studies.
The systems of modeling tumor development in Drosophila capitalize on the unique advantages of this model organism coupled with recent innovative technologies (see Box 2). Undoubtedly, the major strength of Drosophila is the ease of conducting large-scale genetic screens, which can now make use of the large publicly available collections of isogenic deficiencies and RNAi lines that cover the entire genome (Dietzl et al., 2007; Parks et al., 2004). Elegant targeting techniques – including the UAS/GAL4 system, FLP-FRT-mediated recombination and MARCM clonal analysis –enable gene knockdown in specific tissues or patches of cells, bypassing issues of organism lethality. Because these cancer models involve an in vivo setting, they are also ideally suited for studies that examine the role of the microenvironment. In addition, Drosophila offers the potential for drug screening. Together, these approaches make Drosophila an excellent model organism to elucidate the basic mechanisms governing tumorigenesis and tumor progression. Validation of this research in mammalian cancer models and human cancer cell lines could lead to new insights into tumor invasion. Metastasis modeling in Drosophila is in its infancy but, as better tools and models of specific tumor types are developed, it offers the potential to probe the basic mechanisms regulating cancer cell invasion.
Box 2. Advantages of Drosophila for modeling tumor invasion and metastasis.
The signaling pathways controlling growth, differentiation and development that are involved in tumorigenesis and tumor progression are largely conserved between Drosophila and humans.
Models of tumor formation and cell invasion have been created in Drosophila using a wide variety of gene targeting strategies, such as loss-of-function mutations and tissue-specific RNAi knockdown, as well as transgenic overexpression of activated oncogenes found in human cancers.
The ability to generate clones that are mutant for specific genes juxtaposed with wild-type cells using the FLP-FRT and MARCM systems allows the genetics of the tumor microenvironment required for invasion and metastasis to be examined.
Genome-wide screens using either de novo mutagenesis or tissue-specific knockdown by RNAi in Drosophila can identify genes with previously unidentified roles in cancer progression.
Drosophila tumor models can be used for pharmacological screening.
Footnotes
FUNDING
Work in the authors’ laboratories is supported by the National Institutes of Health (NIH) [R01GM53202] to N.J.D.; and the Department of Defense (DOD) [W81XWH-09-1-0487] to J.A.W.
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
REFERENCES
- Allenspach E. J., Maillard I., Aster J. C., Pear W. S. (2002). Notch signaling in cancer. Cancer Biol. Ther. 1, 466–476 [DOI] [PubMed] [Google Scholar]
- Arama E., Dickman D., Kimchie Z., Shearn A., Lev Z. (2000). Mutations in the beta-propeller domain of the Drosophila brain tumor (brat) protein induce neoplasm in the larval brain. Oncogene 19, 3706–3716 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Muskavitch M. A. (2010). Notch: the past, the present, and the future. Curr. Top. Dev. Biol. 92, 1–29 [DOI] [PubMed] [Google Scholar]
- Balkwill F. (2009). Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371 [DOI] [PubMed] [Google Scholar]
- Beaucher M., Goodliffe J., Hersperger E., Trunova S., Frydman H., Shearn A. (2007a). Drosophila brain tumor metastases express both neuronal and glial cell type markers. Dev. Biol. 301, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucher M., Hersperger E., Page-McCaw A., Shearn A. (2007b). Metastatic ability of Drosophila tumors depends on MMP activity. Dev. Biol. 303, 625–634 [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P., Hunter T. (2001). Oncogenic kinase signalling. Nature 411, 355–365 [DOI] [PubMed] [Google Scholar]
- Boccuni P., MacGrogan D., Scandura J. M., Nimer S. D. (2003). The human L(3)MBT polycomb group protein is a transcriptional repressor and interacts physically and functionally with TEL (ETV6). J. Biol. Chem. 278, 5412–5420 [DOI] [PubMed] [Google Scholar]
- Bossuyt W., De Geest N., Aerts S., Leenaerts I., Marynen P., Hassan B. A. (2009). The atonal proneural transcription factor links differentiation and tumor formation in Drosophila. PLoS Biol. 7, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby A. M., Richardson H. E. (2003). scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769–5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby A. M., Richardson H. E. (2005). Using Drosophila melanogaster to map human cancer pathways. Nat. Rev. Cancer 5, 626–639 [DOI] [PubMed] [Google Scholar]
- Brumby A. M., Goulding K. R., Schlosser T., Loi S., Galea R., Khoo P., Bolden J. E., Aigaki T., Humbert P. O., Richardson H. E. (2011). Identification of novel ras-cooperating oncogenes in Drosophila melanogaster: a RhoGEF/Rho-family/JNK pathway is a central driver of tumorigenesis. Genetics 188, 105–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. F., Groom A. C., MacDonald I. C. (2002). Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 [DOI] [PubMed] [Google Scholar]
- Chi C., Zhu H., Han M., Zhuang Y., Wu X., Xu T. (2010). Disruption of lysosome function promotes tumor growth and metastasis in Drosophila. J. Biol. Chem. 285, 21817–21823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y. P., Mulhern T. D., Cheng H. C. (2005). C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)-endogenous negative regulators of Src-family protein kinases. Growth Factors 23, 233–244 [DOI] [PubMed] [Google Scholar]
- Cordero J. B., Macagno J. P., Stefanatos R. K., Strathdee K. E., Cagan R. L., Vidal M. (2010). Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev. Cell 18, 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S. P., Wu X., Riedel C. G., Ruvkun G. (2009). A soma-to-germline transformation in long-lived Caenorhabditis elegans mutants. Nature 459, 1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 [DOI] [PubMed] [Google Scholar]
- Elliott D. A., Brand A. H. (2008). The GAL4 system: a versatile system for the expression of genes. Methods Mol. Biol. 420, 79–95 [DOI] [PubMed] [Google Scholar]
- Ferres-Marco D., Gutierrez-Garcia I., Vallejo D. M., Bolivar J., Gutierrez-Avino F. J., Dominguez M. (2006). Epigenetic silencers and Notch collaborate to promote malignant tumours by Rb silencing. Nature 439, 430–436 [DOI] [PubMed] [Google Scholar]
- Froldi F., Ziosi M., Tomba G., Parisi F., Garoia F., Pession A., Grifoni D. (2008). Drosophila lethal giant larvae neoplastic mutant as a genetic tool for cancer modeling. Curr. Genomics 9, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari F. B., Fenton T., Bachoo R. M., Mukasa A., Stommel J. M., Stegh A., Hahn W. C., Ligon K. L., Louis D. N., Brennan C., et al. (2007). Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 [DOI] [PubMed] [Google Scholar]
- Gateff E. (1978). Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200, 1448–1459 [DOI] [PubMed] [Google Scholar]
- Gateff E., Loffler T., Wismar J. (1993). A temperature-sensitive brain tumor suppressor mutation of Drosophila melanogaster: developmental studies and molecular localization of the gene. Mech. Dev. 41, 15–31 [DOI] [PubMed] [Google Scholar]
- Grusche F. A., Hidalgo C., Fletcher G., Sung H. H., Sahai E., Thompson B. J. (2009). Sds22, a PP1 phosphatase regulatory subunit, regulates epithelial cell polarity and shape [Sds22 in epithelial morphology]. BMC Dev. Biol. 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Allott M. L., Harvey K. F., Richardson H. E. (2010a). Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Richardson H. E. (2010b). Lgl, the SWH pathway and tumorigenesis: it’s a matter of context & competition! Cell Cycle 9, 3202–3212 [DOI] [PubMed] [Google Scholar]
- Guarino M. (2010). Src signaling in cancer invasion. J. Cell. Physiol. 223, 14–26 [DOI] [PubMed] [Google Scholar]
- Gupta G. P., Massague J. (2006). Cancer metastasis: building a framework. Cell 127, 679–695 [DOI] [PubMed] [Google Scholar]
- Gurvich N., Perna F., Farina A., Voza F., Menendez S., Hurwitz J., Nimer S. D. (2010). L3MBTL1 polycomb protein, a candidate tumor suppressor in del(20q12) myeloid disorders, is essential for genome stability. Proc. Natl. Acad. Sci. USA 107, 22552–22557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- Handa K., Yugawa T., Narisawa-Saito M., Ohno S., Fujita M., Kiyono T. (2007). E6AP-dependent degradation of DLG4/PSD95 by high-risk human papillomavirus type 18 E6 protein. J. Virol. 81, 1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert P., Russell S., Richardson H. (2003). Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. BioEssays 25, 542–553 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pagliarini R. A., Xu T. (2006). Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16, 1139–1146 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pastor-Pareja J. C., Aonuma H., Miura M., Xu T. (2009). Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16, 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawar R., Parsons S. J. (2004). c-Src and cooperating partners in human cancer. Cancer Cell 6, 209–214 [DOI] [PubMed] [Google Scholar]
- Janic A., Mendizabal L., Llamazares S., Rossell D., Gonzalez C. (2010). Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science 330, 1824–1827 [DOI] [PubMed] [Google Scholar]
- Januschke J., Gonzalez C. (2008). Drosophila asymmetric division, polarity and cancer. Oncogene 27, 6994–7002 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Scott K. L., Kwak S. J., Chen R., Mardon G. (2011). Sds22/PP1 links epithelial integrity and tumor suppression via regulation of myosin II and JNK signaling. Oncogene 30, 3248–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254 [DOI] [PubMed] [Google Scholar]
- Leong G. R., Goulding K. R., Amin N., Richardson H. E., Brumby A. M. (2009). Scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs. BMC Biol. 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. W., Beall E. L., Fleischer T. C., Georlette D., Link A. J., Botchan M. R. (2004). Identification of a Drosophila Myb-E2F2/RBF transcriptional repressor complex. Genes Dev. 18, 2929–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Feng X., Man X., Yang G., Tang L., Du D., Zhang F., Yuan H., Huang Q., Zhang Z., et al. (2009). Aberrant splicing of Hugl-1 is associated with hepatocellular carcinoma progression. Clin. Cancer Res. 15, 3287–3296 [DOI] [PubMed] [Google Scholar]
- Mantovani A. (2010). Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 10, 369–373 [DOI] [PubMed] [Google Scholar]
- Mechler B. M., McGinnis W., Gehring W. J. (1985). Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 4, 1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Huibregtse J. M. (2000). Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 20, 8244–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D. X., Bos P. D., Massague J. (2009). Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 [DOI] [PubMed] [Google Scholar]
- Pagliarini R. A., Xu T. (2003). A genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 [DOI] [PubMed] [Google Scholar]
- Palomero T., Sulis M. L., Cortina M., Real P. J., Barnes K., Ciofani M., Caparros E., Buteau J., Brown K., Perkins S. L., et al. (2007). Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med. 13, 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., Huppert K., Tan L. R., Winter C. G., Bogart K. P., Deal J. E., et al. (2004). Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36, 288–292 [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja J. C., Wu M., Xu T. (2008). An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis. Model Mech. 1, 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read R. D., Cavenee W. K., Furnari F. B., Thomas J. B. (2009). A Drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 5, e1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski C. C., Schmitz G., Kashyap A., Bosserhoff A. K., Bataille F., Schafer S. C., Lehr H. A., Berger M. R., Galle P. R., Strand S., et al. (2005). Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene 24, 3100–3109 [DOI] [PubMed] [Google Scholar]
- Singh J., Aaronson S. A., Mlodzik M. (2010). Drosophila Abelson kinase mediates cell invasion and proliferation through two distinct MAPK pathways. Oncogene 29, 4033–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summy J. M., Gallick G. E. (2003). Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 22, 337–358 [DOI] [PubMed] [Google Scholar]
- Theodosiou N. A., Xu T. (1998). Use of FLP/FRT system to study Drosophila development. Methods 14, 355–365 [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. (1997). Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 [DOI] [PubMed] [Google Scholar]
- Uhlirova M., Bohmann D. (2006). JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25, 5294–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Cagan R. L. (2006). Drosophila models for cancer research. Curr. Opin. Genet. Dev. 16, 10–16 [DOI] [PubMed] [Google Scholar]
- Vidal M., Larson D. E., Cagan R. L. (2006). Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev. Cell 10, 33–44 [DOI] [PubMed] [Google Scholar]
- Vidal M., Warner S., Read R., Cagan R. L. (2007). Differing Src signaling levels have distinct outcomes in Drosophila. Cancer Res. 67, 10278–10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M., Salavaggione L., Ylagan L., Wilkins M., Watson M., Weilbaecher K., Cagan R. (2010). A role for the epithelial microenvironment at tumor boundaries: evidence from Drosophila and human squamous cell carcinomas. Am. J. Pathol. 176, 3007–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Kennedy S., Conte D., Jr, Kim J. K., Gabel H. W., Kamath R. S., Mello C. C., Ruvkun G. (2005). Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436, 593–597 [DOI] [PubMed] [Google Scholar]
- Witte H. T., Jeibmann A., Klambt C., Paulus W. (2009). Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia 11, 882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. F., Bryant P. J. (1989). Molecular cloning of the lethal(1)discs large-1 oncogene of Drosophila. Dev. Biol. 134, 222–235 [DOI] [PubMed] [Google Scholar]
- Wu M., Pastor-Pareja J. C., Xu T. (2010). Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature 463, 545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Weinberg R. A. (2008). Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 [DOI] [PubMed] [Google Scholar]