SUMMARY
Usher syndrome is the most prevalent cause of hereditary deaf-blindness, characterized by congenital sensorineural hearing impairment and progressive photoreceptor degeneration beginning in childhood or adolescence. Diagnosis and management of this disease are complex, and the molecular changes underlying sensory cell impairment remain poorly understood. Here we characterize two zebrafish models for a severe form of Usher syndrome, Usher syndrome type 1C (USH1C): one model is a mutant with a newly identified ush1c nonsense mutation, and the other is a morpholino knockdown of ush1c. Both have defects in hearing, balance and visual function from the first week of life. Histological analyses reveal specific defects in sensory cell structure that are consistent with these behavioral phenotypes and could implicate Müller glia in the retinal pathology of Usher syndrome. This study shows that visual defects associated with loss of ush1c function in zebrafish can be detected from the onset of vision, and thus could be applicable to early diagnosis for USH1C patients.
INTRODUCTION
Usher syndrome (USH) is characterized by recessively inherited deaf-blindness. Previous estimates of the worldwide prevalence of USH ranged from 1/17,000 to 1/25,000 (Boughman et al., 1983; Rosenberg et al., 1997). However, a r-ecent study of deaf and hard-of-hearing children suggested a prevalence of 1/6000 (Kimberling et al., 2010). USH1 is the most severe of the three clinical subtypes, with profound congenital deafness and onset of vision loss usually before age 10. Vestibular dysfunction is also typical in USH1 cases. USH2, the most common clinical subtype worldwide, is characterized by moderate to severe congenital hearing impairment and the onset of visual defects usually in the second decade of life. No balance problems are associated with USH2. USH3 is rare in most populations, and distinct from the other clinical subtypes in that both hearing and vision impairments are progressive, and vestibular dysfunction is variable (for reviews, see Saihan et al., 2009; Yan and Liu, 2010). Cochlear implants are indicated in cases of profound deafness seen in USH1, and hearing aids can compensate for some of the hearing loss in USH2 and USH3, but there is currently no treatment to counter the vision loss resulting from retinitis pigmentosa. Early diagnosis is important for genetic counseling and preparation for late-onset blindness.
At least 11 different loci are implicated in USH. The nine identified USH genes encode structurally and functionally heterogeneous proteins (Keats and Savas, 2004; Reiners et al., 2006; Ebermann et al., 2007), but most possess protein interaction domains that suggest the ability to form molecular complexes with one another. Biochemical studies have confirmed these binding propensities (Reiners et al., 2005; Pan et al., 2009). Colocalization of the USH proteins in subcellular domains of auditory hair cells and retinal cells, along with evidence of mislocalization of USH proteins in various USH mutant backgrounds, suggest that at least some USH proteins directly interact with one another (Adato et al., 2005; Reiners et al., 2005; Lefevre et al., 2008; Bahloul et al., 2010; Yang et al., 2010; Caberlotto et al., 2011).
Previous studies of USH proteins have concentrated on their potential functions in the region of the connecting cilium of the photoreceptor (Liu et al., 2007; van Wijk et al., 2006; Maerker et al., 2008; Yang et al., 2010) and the stereocilia of hair cells (Adato et al., 2005; Delprat et al., 2005; Seiler et al., 2005; Söllner et al., 2004; Kikkawa et al., 2005; Prosser et al., 2008; Lefevre et al., 2008). In the ear, several studies have provided evidence for colocalization of USH proteins in the tip and ankle-linking regions of the stereocilia and at hair cell ribbon synapses (Mburu et al., 2003; Siemens et al., 2004; Reiners et al., 2005; Michalski et al., 2007; Kazmierczak et al., 2007; Lefevre et al., 2008). Most of the USH proteins have been shown to colocalize in the retina at the connecting cilium and/or at the photoreceptor synapse (Liu et al., 1997; Reiners et al., 2006; Liu et al., 2007; Overlack et al., 2008; Yang et al., 2010). In addition to these regions of colocalization, many of the USH proteins are found individually at discrete subcellular locations (Hasson et al., 1995; Reiners et al., 2006; Overlack et al., 2008; Williams et al., 2009), suggesting that at least some have functions independent of the proposed complex. In hair cells, USH proteins are thought to stabilize the growth and orientation of stereocilia during development (for reviews, see Brown et al., 2008; Yan and Liu, 2010), whereas USH proteins in the region of the connecting cilium are proposed to contribute to the trafficking of molecular cargo from the photoreceptor inner segment to the outer segment (Maerker et al., 2008; Liu et al., 2007). USH proteins have also been immunolocalized at the ribbon synapses of these sensory cells, but their potential functions in this region are unknown.
The USH1C gene encodes the PDZ-domain-containing protein harmonin (Verpy et al., 2000), which has been proposed to function as a key scaffolding molecule to which most other USH proteins can bind (Reiners et al., 2005). Patients with mutations in USH1C exhibit the classic USH1 pathology, with profound congenital hearing impairment, vestibular dysfunction, and clinically appreciable retinal degeneration in childhood or early adolescence. USH1C mutations resulting in non-syndromic deafness have also been reported (Ouyang et al., 2002). The deaf circler mouse (Johnson et al., 2003), harboring an in-frame deletion in the murine Ush1c gene, has provided a valuable model for the mechanosensory hair cell defects that cause deafness in USH1C patients, but does not exhibit a retinal phenotype (Williams et al., 2009). Similarly, the Ush1c knockout mouse exhibits profound deafness without notable retinal defects (Tian et al., 2010). Interestingly, the c.216G>A mouse model of USH1C (Ush1c216AA), a knock-in of the human exon 3 USH1C c.216G>A mutation (plus surrounding sequence) cloned from an Acadian USH1C patient (Lentz et al., 2007), shows both the well-characterized auditory defects of the other USH1C mouse models, as well as visual defects comparable to the human disease. In addition to the disorganized stereocilia and hair cell degeneration observed in the cochleae of USH1C c.216G>A mice, reduced visual function was noted after 1 month of age and progressive loss of rod photoreceptors was observable after 6.5 months of age (Lentz et al., 2010). These intriguing differences in retinal pathology between the Ush1c knockout and the USH1C c.216G>A knock-in could indicate differences between the functions of endogenous mouse and human harmonin in the retina.
In vitro assays have demonstrated that many of the known USH proteins bind preferentially to one or more of the three PDZ domains in harmonin, hence its depiction as a central scaffold upon which an USH protein complex can form (Adato et al., 2005). Although the subcellular localization of harmonin and other USH proteins in hair cells is compatible with this hypothesis, there have been conflicting reports of localization of other USH proteins at the photoreceptor synapse, where harmonin localization is undisputed (Reiners et al., 2005; Liu et al., 2007; Yang et al., 2010). Moreover, harmonin is notably absent from the connecting cilium region (Reiners et al., 2005; Williams et al., 2009), where the presence of most other USH proteins has been well documented (Liu et al., 2007; Maerker et al., 2008; Yang et al., 2010). Thus, the normal cellular functions of harmonin, the types of complexes it forms with other USH proteins in vivo, and the early, pre-degenerative manifestations of the retinal pathology in USH1C remain mysterious.
Previously, zebrafish mutants for myo7a (ush1b), cdh23 (ush1d) and pcdh15 (ush1f) have been described as exhibiting severe swimming and balance defects consistent with the auditory and vestibular component of the human disease (Ernest et al., 2000; Söllner et al., 2004; Seiler et al., 2005). Although expression in the retina has been reported for each of these genes, only the morpholino knockdown of pcdh15b has been reported to have an effect on visual function (Seiler et al., 2005). Photoreceptor organization is severely affected in pcdh15b morphants, and electroretinogram (ERG) traces are notably attenuated. Here, we describe two zebrafish models for USH1C that exhibit a strong USH-like phenotype at early developmental stages. Young fish with depleted ush1c function have penetrant and specific visual defects as well as compromised hair cell development, resulting in hearing and balance impairment. Detailed analyses of these phenotypes have confirmed a requirement for harmonin in zebrafish hair cell morphogenesis, and have implicated Müller glial cells in supporting harmonin-mediated ribbon synapse stability and function. USH has traditionally not been considered a developmental disease of vision. The results of this study, however, show that visual defects can be detected from the onset of vision in zebrafish with depleted ush1c function, thus providing a rationale for examination of Müller cell function in USH1C patients.
RESULTS
ush1c expression in zebrafish inner ear sensory patches, the lateral line and retina
We used the human harmonin protein sequence in tBLASTn searches to identify a single ortholog of USH1C in the zebrafish genome (Fig. 1A). We then used a radiation hybrid panel (Hukriede et al., 1999) to position this gene on chromosome 25. Previous studies of the mouse and human genes have indicated that the mammalian genes express three splice forms, which vary slightly in structure and subcellular localization (Reiners et al., 2003; Reiners et al., 2005; Williams et al., 2009). The most abundant zebrafish transcript found in whole larvae or adult retinas corresponded to isoform A, containing 21 exons that encode a 548 amino acid protein with three PDZ domains and a coiled-coil domain (Fig. 1A). Zebrafish harmonin isoform A was found to be structurally similar to the corresponding human isoform, with 70% amino acid identity overall and highest similarity in the PDZ domains and the N-terminal region (Pan et al., 2009). A hydrophobicity plot of the human and zebrafish proteins revealed a high level of conservation, even where residue substitutions occur (Fig. 1A). Using reverse transcriptase (RT)-PCR and RACE primers within and between regions encoding the second and third PDZ domains, we identified unique transcripts that might correspond to the B or C isoforms of the gene, although no variant encoding a second coiled-coil or PST domain, as would be predicted for an isoform B transcript, was identified. All transcripts other than that of isoform A were in low abundance such that we were unable to obtain sequence definitively identifying them as belonging to a particular isoform class. The probes generated from these partial sequences showed weak, generalized signal in all tissues and time points analyzed (data not shown).
Fig. 1.
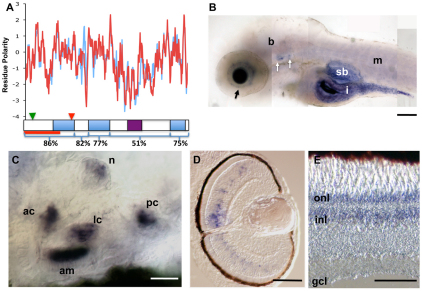
Zebrafish ush1c is expressed in retina and the inner ear. (A) The predominant splice form of ush1c corresponds to mammalian isoform A, with 21 exons that produce a 1.6 kb mRNA. A hydrophobicity plot of zebrafish (red) and human (blue) isoform A protein sequence shows conserved residue polarity. % amino acid identity with human protein is indicated below the plot. The positions of the ush1cfh293 nonsense mutation (red arrowhead), the MO target (green arrowhead) and the region recognized by the zebrafish harmonin antibody (red bar) are indicated. (B) At 5 dpf, ush1c transcripts are abundant in the inner retina (black arrow), the sensory patches of the ear (white arrows), the intestinal tract (i) and the swim bladder (sb). Fainter expression in the brain (b) and trunk muscles (m) is also observed. (C) High-magnification view of a 5 dpf whole-mount zebrafish ear showing ush1c expression in all sensory patches (ac, lc, pc: anterior, lateral and posterior cristae, respectively; am: anterior macula) and an adjacent head neuromast (n). (D) ush1c transcripts are restricted to cells in the inner nuclear layer in 5 dpf sectioned retina. (E) Central section of an adult zebrafish retina showing continued strong expression in the inner retina; expression is detected in the outer nuclear layer as well. onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer. Scale bars: 100 μm (B); 20 μm (C); 50 μm (D,E).
cRNA probes directed against either the unique sequence of isoform A or the 5′ region predicted to be common to all splice forms yielded identical expression patterns in larval tissues. RNA expression was first detected in the ear at 27 hours post-fertilization (hpf), when the first hair cells are specified (data not shown), and hair cell expression persisted through all later larval stages examined [5 days post-fertilization (dpf) shown in Fig. 1B,C]. We also observed strong expression in the neuromasts of the lateral line (Fig. 1C). This neuromast expression was consistent with previous reports of the zebrafish USH gene orthologs myo7a, cdh23 and pcdh15a (Ernest et al., 2000; Söllner et al., 2004; Seiler et al., 2005). Retinal expression was present beginning at 48 hpf, when retinal lamination is complete and cell specification is underway. By 72 hpf, strong expression was observed in a subset of cells in the inner nuclear layer, which persisted through all larval stages examined (5 dpf, shown in Fig. 1D). In situ hybridization of adult retinal tissue using all described probes showed persistent, strong expression in the inner nuclear layer as well as expression in the outer nuclear layer (Fig. 1E). We also observed strong expression of ush1c in the gut and swim bladder, and weaker expression in the brain and trunk muscles (Fig. 1B), consistent with reported expression in human tissues (Verpy et al., 2000; Hirai et al., 2004).
Mutation or morpholino knockdown of ush1c depletes harmonin protein in zebrafish larvae
We obtained a nonsense mutation in ush1c from the Zebrafish TILLING Consortium by screening for mutations in the first PDZ-domain-encoding region of the gene (PDZ1). We recovered a line, ush1cfh293, that harbors a nonsense mutation in the fifth exon, which encodes the C-terminal portion of the PDZ1 domain (red arrowhead, Fig. 1A; supplementary material Fig. S1).
We also designed morpholino oligonucleotides to knock down the function of ush1c: one to block splicing at the exon-2–intron-2 boundary (MO1; green arrowhead, Fig. 1A) and one that recognizes the translation initiation site (MO2). We obtained identical phenotypes with both morpholinos, but found that the exon-2-targeting morpholino (MO1) provided more consistent results; thus, MO1 was used for all morpholino experiments and all uses of ‘morpholino’, ‘MO’ or ‘morphant’ refer to MO1. Using RT-PCR analysis of MO-injected embryos and larvae, we identified an aberrant splice form in which exon 2 is skipped, producing out-of-frame splicing of exons 1 and 3 that results in an early termination codon (supplementary material Fig. S1). This altered splice form predominates for the first 4 dpf, and persists through 6 dpf, although recovery of the normal splice form is evident by 5 dpf (Fig. 2A).
Fig. 2.
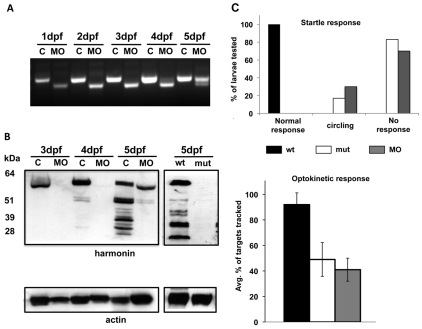
Mutation and morpholino knockdown of ush1c produce behavioral defects. (A) RT-PCR of control and splice-blocking morpholino-injected embryos from 1 to 5 dpf reveals a smaller splice form lacking exon 2 that predominates for the first 4 days of development and persists through the fifth day. (B) Western blots from extracts of control + ush1c MO-injected embryos from 3 to 5 dpf (left) and ush1cfh293 homozygous mutant embryos alongside wild-type siblings (right) confirm morpholino efficacy and antibody specificity. Samples from 3 dpf to 5 dpf control larvae probed with the zebrafish harmonin antibody show a 60 kDa band consistent with the predicted size of isoform A. Additional smaller bands are visible faintly in the 4 dpf control and are more pronounced and abundant in the 5 dpf control. The band corresponding to the isoform A variant is undetectable in 3 dpf and 4 dpf morphant samples. The major band reappears at 5 dpf at a slightly smaller weight, and there is a reduced number of smaller bands in this sample. In extracts from 5 dpf ush1cfh293 homozygous mutants, no bands are observed. Extracts from wild-type siblings from the same clutch show the characteristic banding pattern of the 5 dpf uninjected controls, allowing for the slightly longer running time for this gel. A pan-actin antibody was used as a loading control. (C) Behavioral assays of control, mutant and morpholino-injected larvae show (top) impaired startle response and balance, and (bottom) reduced optokinetic response, tracking an average of 50% or fewer of the dark stripes (average targets tracked for wild type: 11.5±1.27; ush1c mutants: 6.13±2.78, P<0.0001; ush1c MO: 5.13±2.85, P<0.0001; n=30 for each group).
We obtained a polyclonal rabbit antibody raised against the first 100 amino acids of zebrafish harmonin (red bar, Fig. 1A), a region common to all predicted isoforms, and probed protein extracts on a western blot to test antibody specificity and to determine the extent of protein depletion in morphant and mutant larvae (Fig. 2B). In wild-type controls, we observed a single band in the 3 dpf sample, which was slightly smaller than the corresponding bands in the 4 dpf and 5 dpf samples. In addition to this band, which, at approximately 60 kDa, corresponded to the predicted size of zebrafish harmonin isoform A, we noted progressively increasing numbers of smaller bands in the 4 dpf and 5 dpf controls. Consistent with the time course of morpholino efficacy revealed by RT-PCR (Fig. 2A), strong knockdown of harmonin protein levels was observed at 3–4 dpf in ush1c morphants. Moreover, although the upper band was restored nearly to wild-type levels in the 5 dpf sample, the size was slightly reduced, consistent with the control band from the 3 dpf time point (Fig. 2B). These temporal changes in size and abundance of the major band, recapitulated by the recovering morphant protein extracts, might reflect a period of post-translational modification.
We performed RT-PCR experiments on zebrafish cDNA from multiple developmental stages and discovered five distinct expressed sequence tags (ESTs), but no complete sequences of splice variants other than isoform A. Thus, we cannot definitively identify the smaller bands on the western blots, but postulate that they might be additional isoforms or products of protein degradation of isoform A, the largest running band. Similar, smaller bands were detected by western analysis of mouse harmonin (Williams et al., 2009). We did not observe any bands larger than ∼60 kDa at any larval time point examined. This, combined with the RT-PCR and RACE results described above, suggests that isoform B splice variants are not active through 5 dpf.
No bands were detected in protein samples from ush1c mutant larvae at any time point examined (5 dpf shown alongside wild-type sibling in Fig. 2B). However, the ush1c nonsense mutation in the fifth exon could potentially produce a truncated protein that would be recognized by the antibody. We investigated RNA levels in ush1c mutants using a riboprobe against the first six exons of ush1c. We observed reduced probe signal in all ush1c-expressing tissues examined (ear and gut tissue shown in supplementary material Fig. S2), suggesting that this mutant transcript is unstable.
Reduced harmonin function produces defects in vestibular, auditory and visual function
At 5 dpf, wild-type fish exhibited rapid evasive swimming behavior when startled by a tap on the Petri dish. We tested the startle reflex of 5 dpf offspring from natural crosses between ush1c−/+ heterozygous carriers (n=952 larvae) and found that 25% (n=236) of these larvae had abnormal responses (Fig. 2C, top). Within this group, 83% did not exhibit any evasive swimming behavior, and those that did respond swam in circles. Subsequent genotyping revealed that 100% of the larvae exhibiting these defects were homozygous ush1cfh293 mutants.
Free-swimming 5 dpf ush1c morphant larvae exhibited vestibular defects identical to ush1c mutants, with only 30% of the larvae responding to tapping on the Petri dish, and all responders swimming in circles (n=400). These defects are similar to those reported for other zebrafish USH gene mutations or knockdowns (Ernest et al., 2000; Söllner et al., 2004; Seiler et al., 2005) and indicate reduced function of both the auditory and vestibular systems.
To assess visual function in mutant and morphant animals, we used the optokinetic response (OKR) assay. At 5 dpf, ush1c mutants and MO-treated larvae produced significantly fewer saccades, tracking only 40–50% of the targets compared with wild type (Fig. 2C, bottom).
Loss of harmonin results in morphological defects in mechanosensory hair cells
To gain insights into the cause of the hearing and balance defects in harmonin-deficient larvae, we used immunofluorescence to examine the mechanosensory hair cells of the otic sensory patches. Harmonin localized to sensory patches and neuromasts by 80 hpf (Fig. 3A). We detected no harmonin signal at 80 hpf in ush1c mutants (Fig. 3B) or morphants (Fig. 3C), confirming the strong knockdown or depletion indicated by the western blot results. Using phalloidin to visualize stereocilia, we examined individual sensory patches. At 5 dpf, wild-type hair bundles had a characteristic shape and orientation, projecting more or less orthogonally from the plane of each cuticular plate and appearing tightly tapered at their tips (Fig. 3D–F). All otic sensory patches in the mutant larvae displayed reduced numbers of hair bundles, as well as defects in stereocilia organization at 5 dpf (Fig. 3G–I). We observed 40% fewer hair bundles than in wild-type siblings in the anterior and posterior maculae, and existing stereocilia were splayed and bent, with a notable lack of tapering of the hair bundles. In morpholino-injected animals, we found a reduced number of hair bundles, with 48% fewer bundles in the anterior maculae and 33% fewer hair bundles in the posterior maculae of 5 dpf morphants compared with controls. Stereocilia were also disorganized, although to a lesser extent than we observed in the mutant. Bundles appeared bent and disoriented relative to the plane of the cuticular plate, but the splaying or absence of tapering at the tips was less pronounced than in ush1c mutants (Fig. 3J–L).
Fig. 3.
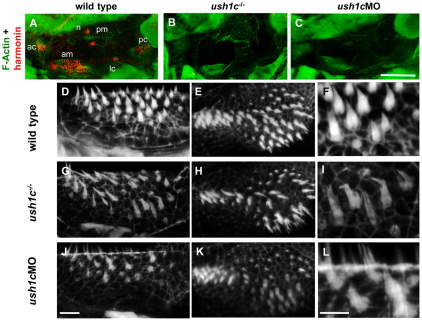
Harmonin is required in otic sensory patches for normal bundle development. (A–C) Phalloidin-stained F-actin (green) and anti-zebrafish harmonin antibody labeling (red) in 80-hpf wild-type ears show localization of harmonin relative to the stereocilia in the five otic sensory patches (ac, anterior crista; am, anterior macula; lc, lateral crista; pc, posterior crista; pm, posterior macula) and in the neuromasts (n). The anti-harmonin antibody signal is reduced in sensory patches and in neuromasts in ush1c mutants (B) and ush1c-MO-injected larvae (C). (D–L) High-magnification view of phalloidin-stained hair bundles in the anterior and posterior maculae of 5 dpf larvae. Hair bundle number is reduced and organization of the existing bundles is disrupted in mutant (G–I) and morphant (J–L) anterior (G,J) and posterior (H,K) maculae, compared with the anterior and posterior maculae of wild-type controls (D and E, respectively). Closeup images of hair bundles of the anterior maculae (F,I,L) show bent and splayed bundles in mutants and morphants. For mutant analysis, anterior macular wild-type average=54.6±3.5 bundles; n=8; ush1c mutant average=33±3.5; n=7; P<0.0001. Posterior macula wild-type average=68.2±2.6 bundles; n=9; ush1c mutant average=40.5±4.2; n=10; P<0.0001. For the morpholino experiment, anterior macula control average=41.8±4.8 bundles; MO average=21.8±3.8; n=5 for each group; P<0.0001. Posterior macula control average=71.8±4.6 bundles; MO average=48.2±6.9; n=5 for each; P<0.0001, 5 dpf. Scale bars: 50 μm (A–C); 10 μm (D–L).
We next investigated the subcellular localization of harmonin in the anterior maculae of 5 dpf larvae (Fig. 4). In wild-type hair cells, harmonin localized to both stereocilia and kinocilia as well as within cell bodies and at synapses (Fig. 4A–G). In ush1c mutants (Fig. 4H–J), we detected reduced levels of harmonin in stereocilia and kinocilia. Protein was also detected within cell bodies, but the distribution appeared abnormal compared with controls. Harmonin levels in hair cells of morphant larvae (Fig. 4K–M) were markedly diminished at 5 dpf, although some signal could be detected apical to the hair bundle in a position consistent with kinocilia localization. When we injected embryos from ush1c+/− heterozygous parents with the ush1c morpholino, raised the larvae to 5 dpf and analyzed harmonin staining in sensory patches, residual staining was undetectable in injected genotypically ush1c−/− animals, suggesting that a truncated form of harmonin is produced in the mutants. This truncated protein might be too low in abundance or too unstable to be detected in a western blot, but was detectable in fixed tissue using enhanced signal detection protocols.
Fig. 4.
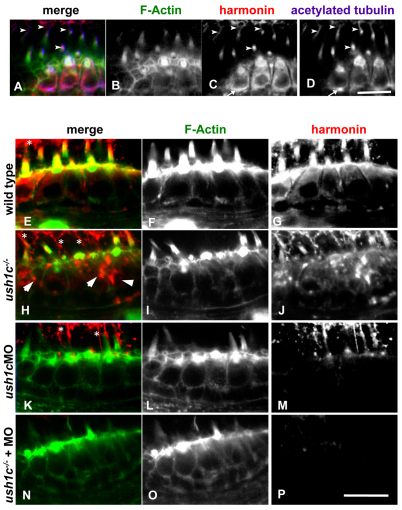
Subcellular localization of harmonin in mechanosensory hair cells. Co-labeling of wild-type 5 dpf anterior macula hair cells (A–D) shows anti-zebrafish harmonin localization (red) in the hair bundles (green), kinocilia (arrowheads), cell bodies and at synaptic boutons (arrow in C and D). Incubation conditions favoring hair bundle visualization (E–P) show strong colocalization of harmonin within the bundle structure as well as in the cuticular plate in 5 dpf wild types (E–G). Kinocilia labeling can be seen above the bundle region (asterisks). Enhanced signal detection in mutant hair cells (H–J) shows localization persisting in the kinocilia and in existing bundle structures. Kinocilia are more visible in these panels owing to the reduced bundle architecture in the mutant. Protein distribution seems globular and disorganized in the cell bodies (arrowheads). Hair cells of ush1c MO larvae (K–M) show strong knockdown of harmonin, with localization persisting only in kinocilia at this stage, and no signal observed within hair bundles. Injecting the morpholino into the ush1c−/− mutants reduces subcellular localization of harmonin to undetectable levels in hair cells (N–P). Scale bars: 10 μm.
To investigate cell degeneration or reduced cell proliferation as possible causes for the observed reduction in hair bundle number, we stained larvae at stages between 1 and 5 dpf with TUNEL to label dying cells or BrdU to label dividing cells. The results in both cases were indistinguishable between morphants and controls (not shown), and were consistent with previously reported normal cell death levels in the ear (Bever and Fekete, 1999). Mutant cell proliferation was also unaffected at these stages, as were cell death levels through 4 dpf. In 5 dpf mutants, we noted a small but significant increase in TUNEL-labeled cells for both the anterior macula (0.1±0.32 for wild type vs 1.2±1.03 for mutant; n=10 for each group; P=0.008) and posterior macula (0.1±0.32 for wild type vs 1.3±0.95 for mutant; n=10 for each; P=0.003). Although statistically significant, the numbers of dead cells were insufficient to account for the extent of the reduction in bundles. To confirm that hair cells were being specified properly in mutant larvae, we used a probe to the hair-cell-specification factor atoh1b and noted no differences in labeling of sensory patches in wild type compared with mutant (data not shown). Thus, we conclude that the defects in hair bundle formation occur post-mitotically, consistent with previously reported studies in mammals (Lefevre et al., 2008), and that increased cell death, reduced cell proliferation, or impaired cell fate specification cannot be the primary causes of bundle loss. Together, these results confirm a requirement for harmonin in hair bundle development. Additionally, the milder stereocilia disorganization phenotype in ush1c morphants observed at 5 dpf, in conjunction with the partial recovery of harmonin localization at 5 dpf observed in the region of the kinocilia, suggests that harmonin also plays a role in maintaining hair bundle integrity.
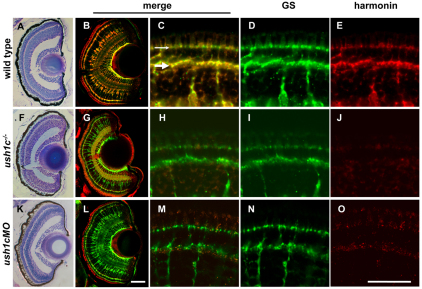
Harmonin localizes to Müller glial cells in the retina
Retinal architecture appeared unaffected in ush1c mutants and morphants compared with wild type (Fig. 5A,F,K; supplementary material Fig. S3); however, because visual function defects were observed, we also examined harmonin protein localization in retinas. We observed abundant protein levels specifically in the cell bodies and processes of Müller glia at all larval stages examined (Fig. 5B–E; supplementary material Fig. S4), consistent with the restricted inner retinal expression noted by in situ hybridization with our ush1c riboprobes at 5 dpf (Fig. 1D). We did not observe specific photoreceptor labeling with this antibody between 5 and 8 dpf.
Fig. 5.
Harmonin localization in the larval retina is restricted to Müller glia. Semi-thin plastic sections stained with toluidine blue show consistent size, lamination and organization of 5 dpf wild-type, mutant and morphant retinas (A,F,K). 16-μm larval retina sections (B,G,L) show colocalization of the Müller cell marker glutamine synthetase (GS; green) and anti-zebrafish harmonin (red) in wild type (B). Both antibodies label Müller cell processes throughout the retina. Anti-zebrafish harmonin labeling is reduced in mutant (G) and morphant (L) Müller cells. Müller cell number and morphology is unaffected. Nonspecific signal associated with photoreceptor outer segments is observed in all panels at this magnification. See supplementary material Fig. S4 for comparison. High-magnification images of anti-zebrafish harmonin and GS labeling in Müller cell processes at the OPL (large arrow) and OLM (small arrow) of the 5 dpf retina are shown (wild type, C–E; mutant, H–J; morphant, M–O). Scale bars: 50 μm (A,B,F,G,K,L); 20 μm (C–E,H–J,M–O).
We examined harmonin antibody labeling in wild-type, mutant and morphant retinas at 5 dpf. Harmonin protein levels in the retinas of ush1c−/− homozygous mutants were markedly reduced, although Müller cells were present and appeared morphologically normal (Fig. 5G–J). Similarly, after morpholino injection, we observed a strong reduction of harmonin labeling in Müller cells through 5 dpf (Fig. 5L–O). We noted that the duration of protein depletion was prolonged in the eye, compared with our results from morphant hair cells, in which we observed rebounding harmonin localization in some kinocilia by 5 dpf (Fig. 3F). We therefore examined harmonin labeling in 6 dpf morphants, at which time we observed increased harmonin labeling in Müller cells of the peripheral retina, adjacent to the ciliary marginal zone (supplementary material Fig. S4).
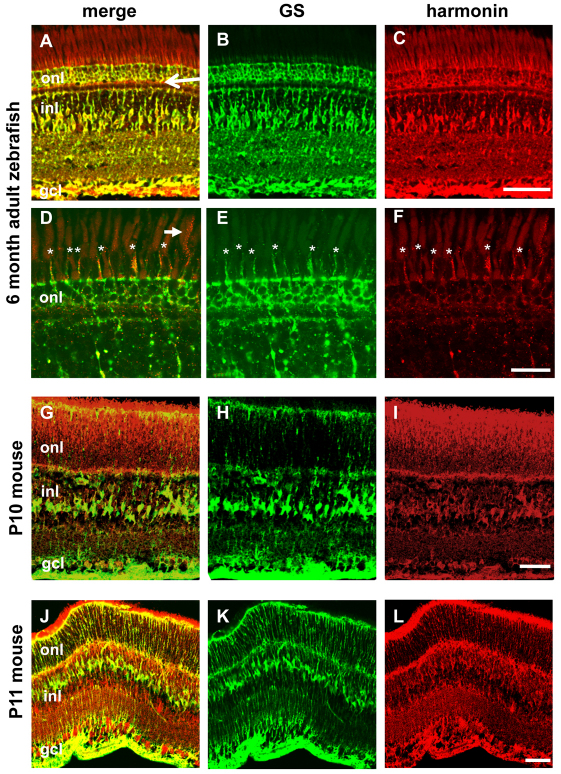
Owing to the apparent presence of ush1c transcript in adult photoreceptors (Fig. 1E), we evaluated the subcellular localization of harmonin in 6 month adult retinas (Fig. 6A–F). We continued to see strong harmonin localization within Müller cells in the adult retina, extending past the outer limiting membrane (OLM) and into the glial processes in the subapical region between photoreceptor cell bodies (Fig. 6, asterisks). In some adult retinas we also observed what might be a low level of protein localization at the photoreceptor synapses (Fig. 6A, arrow). Occasionally, signal was detected in photoreceptor inner or outer segments at a lower level and with inconsistent distribution compared with the robust staining observed in Müller glia (Fig. 6D, arrow), suggesting that this signal was due to nonspecific labeling or autofluorescence, although we were unable to confirm this with our current immunofluorescence methods.
Fig. 6.
Harmonin localizes in Müller cells through all zebrafish life stages and is evolutionarily conserved. (A–F) Localization of anti-zebrafish harmonin (red) in Müller cells persists in adult zebrafish retinas. Some low level signal is sometimes observed in photoreceptor synapses (arrow in A) and in photoreceptor outer segments (arrow in D). The strongest signal within the photoreceptor layer is associated with Müller cell processes projecting into the subapical region (asterisks). Nonspecific fluorescence of photoreceptor outer segments at this stage can be compared with panel H in supplementary material Fig. S4. (G–L) Anti-mouse harmonin (SDI; G,I) and anti-human harmonin (Santa Cruz; J,L) detect localization in photoreceptors and Müller cells [glutamine synthetase (GS) is labeled in green; harmonin antibodies in red] in retinas from P10–P11 mouse pups. The outer nuclear layer (onl), inner nuclear layer (inl) and ganglion cell layer (gcl) are labeled in merged panels. Scale bars: 50 μm (A–C,G–L); 20 μm (D–F).
Because Müller cell localization of harmonin has not been reported previously, we used antibodies against murine (Fig. 6G–I) and human (Fig. 6J–L) harmonin to label retinas from postnatal day 10–11 mouse pups. In addition to the previously described labeling in photoreceptors, we also observed harmonin immunoreactivity in Müller glial cell bodies and processes extending throughout the retina. The anti-human harmonin antibody (Fig. 6J–L) also cross-reacted in 5 dpf zebrafish retinas, labeling Müller cells, but not photoreceptors (data not shown). Given that the observed localization of harmonin in murine Müller cells differs from published work (Reiners et al., 2005), further investigation is needed, but our results are consistent with the possibility of a phylogenetically conserved role for harmonin in retinal glia.
Harmonin was recently detected in the postsynaptic processes of murine bipolar cells and horizontal cells, using immunoelectron microscopy (Williams et al., 2009). With our immunofluorescence protocols, we found no evidence for harmonin localization within cell bodies of bipolar or horizontal cells at any time points examined in zebrafish (Fig. 5; Fig. 6A,C). Owing to the strong harmonin labeling in Müller glial processes, we cannot determine whether synaptic processes of horizontal or bipolar cells might also be positive for harmonin in the outer plexiform layer (OPL).
Given the retinal degeneration experienced by USH1C patients, we examined retinal cell death in zebrafish ush1c mutants and morphants at 5 and 8 dpf, using an anti-active caspase-3 antibody. Death rates by retinal cell layer were as follows (n=10 for all groups; see also supplementary material Fig. S4): in the photoreceptor layer, wild-type animals at 5 dpf had 0.20±0.41 labeled cells compared with 0.31±0.60 in morphant retinas (P=0.5) and 3.09±1.3 in mutants (P<0.0001); in the inner nuclear layer, no labeled cells were observed in wild type, compared with 1.86±1.23 in morphants (P<0.0001) and 1.88±0.73 in mutants (P<0.0001); in the ganglion cell layer, 0.062±0.25 cells were labeled in controls, compared with 0.25±0.45 in morphants (P=0.16) and 0.09±0.30 in mutants (P=0.79). Noting the small but significant elevation of inner nuclear layer (INL) cell death in 5 dpf mutants and morphants, we co-labeled sectioned 5 dpf retinas with caspase-3 and glutamine synthetase to determine what proportion of the dying cells were Müller glia (n=10 for each group). In ush1c−/− animals, exhibiting an average of 1.88 labeled cells per eye, 67% of the cells labeled with caspase-3 were also positive for glutamine synthetase. Similarly, 61% of caspase-labeled cells were co-labeled with glutamine synthetase in ush1c morphant retinas, in which an average of 1.85 cells in the INL were labeled. In both cases, the remaining labeled cells could not be positively identified as Müller cells, based on either their position within the nuclear layer or by labeling with the Müller cell marker. Although our current experiments do not rule out the possibility that a small subset (<0.7 cells per eye) of caspase-positive cells in the INL might be second order neurons, it is also possible that these cells were apoptotic Müller glia that no longer produced glutamine synthetase.
At 8 dpf, we noted increased photoreceptor cell death in the ush1c mutant retinas, compared with wild-type animals (wild type: 0 labeled photoreceptors; mutant: 5.33±1.32 labeled photoreceptors; P<0.0001; see supplementary material Fig. S4). No statistically significant differences were observed between mutant and wild-type siblings in either the inner nuclear or ganglion cell layers at this stage, nor were there significant differences seen in the labeling of morpholino and control animals in any cell layers at 8 dpf.
Harmonin is required for photoreceptor synaptic organization and function
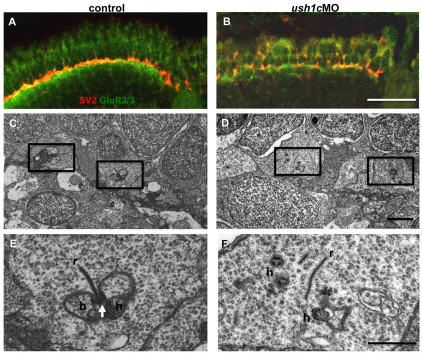
The specific depletion of harmonin from Müller cells in larval mutant and morphant retinas provided an opportunity to study the role of harmonin in this cell type. Because the morphants presented no significant photoreceptor cell death at 5 dpf despite strong protein knockdown, we used morphants for the following analyses. We began by examining synaptic integrity in morphant larvae at 5 dpf, using antibodies against the pre- and postsynaptic proteins SV2 and GluR2/3, respectively. In control retinas, these markers exhibited an organized, parallel and largely colocalized alignment within the OPL (Fig. 7A). In ush1c morphants, both proteins were present in the OPL, but they were remarkably misaligned and less colocalized, indicating a disruption in synaptic organization (Fig. 7B).
Fig. 7.
Synaptic maturation is impaired in ush1c morphants. (A,B) Anti-SV2 (red) and -GluR2/3 (green) antibodies mark the pre-and postsynaptic regions of the OPL, respectively. Tightly associated localization of these factors is apparent in the control retina (A), and is notably disrupted in the morphant retina (B) at 5 dpf. (C–F) Electron micrographs of cone pedicles in 6 dpf control and morphant larvae. In control retinas (C,E), the presence of multiple triads (boxed areas), consisting of a synaptic ribbon (r), arciform density (arrow) and postsynaptic processes from bipolar (b) and horizontal (h) cells, indicate normal synaptic maturation. An enlargement of a triad is shown in E. In morphant retinas (D,F), fewer triads are present (boxed areas in D; enlarged in panel F), and floating ribbons (r) and distant postsynaptic processes (h) are observed in cone pedicles. Scale bars: 20 μm (A,B); 1 μm (C,D); 500 nm (E,F).
To investigate this phenotype further, we examined the ultrastructure of cone pedicles in morphant larvae via transmission electron microscopy. We examined cone pedicles from retinas of two uninjected controls at 6 dpf in a quantitative comparison with cone pedicle ultrastructure from retinas of four stage-matched ush1c MO larvae. We found that cone pedicles in uninjected controls (Fig. 7C,E) contained an average of 1.667±0.299 ribbons per pedicle (n=36 pedicles) and a large number of mature-appearing ribbon synapses, with 55% of the synaptic ribbon profiles anchored by an arciform density (ribbons with arciform density per pedicle=1.08±0.238); 85% of these membrane-anchored ribbons were associated with postsynaptic processes from horizontal and bipolar cells in a triad (triads per pedicle=0.97±0.128). In morphant retinas (Fig. 7D,F), the average number of ribbons per pedicle was consistent with controls (1.659±0.607; n=44 pedicles), but only 38% of the ribbon profiles appeared anchored by arciform densities (ribbons with arciform densities per pedicle=0.636±0.647; P=0.0002). Synaptic ribbons unanchored by an arciform density seemed to ‘float’ in the pedicle, and postsynaptic processes, when present, frequently appeared distant from these floating ribbons (Fig. 7D,F). As in the controls, most (83%) anchored ribbons in morphants were incorporated into triads (triads per pedicle=0.523±0.412), suggesting a specific defect in ribbon association with the membrane. To investigate whether the observed defects could be attributed to a developmental delay, we also examined sections of ribbon synapses in cone pedicles of two wild-type and three morphant retinas at 8 dpf. The number of ribbons observed per pedicle (1.34±0.56; n=41) was significantly reduced in 8 dpf morphants compared with controls (1.70±0.22; n=43; P=0.0002). As seen in the 5 dpf analysis, significantly fewer ribbons in the 8 dpf morphants (52%; n=55 ribbons) compared with controls (82%; n=73 ribbons; P<0.0001) appeared to be anchored to the membrane. The percentage of anchored ribbons associated with triads was comparable between morphants and controls (68% and 65%, respectively), again suggesting a primary defect in the formation of arciform densities. Retinal morphology was otherwise unaffected, and significant retinal cell death was not observed in 8 dpf morphants in any retinal cell layers by anti-active caspase-3 labeling. Thus, we conclude that temporary depletion of harmonin from Müller glia during retinal development has a lasting, adverse impact on maturation of cone ribbon synapses.
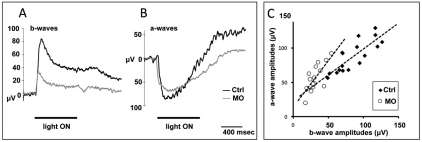
To determine the effect of the morphological defect on synaptic transmission, we recorded ERGs from 5 dpf morphant larvae (n=6) and compared them with uninjected larvae (n=7). Rod photoreceptors are detectable molecularly by 3 dpf and morphologically by 5 dpf. However, physiological analyses demonstrate that rods are not functional until 15 dpf (Morris and Fadool, 2005). ERGs performed at 5 dpf show a cone dominant response in both light-adapted and dark-adapted conditions (Bilotta et al., 2001; Seeliger at el., 2002), but dark-adapted retinas at this stage are less susceptible to bleaching and exhibit a more sensitive ON response than in light-adapted conditions (Makhankov et al., 2004). Thus, we chose to perform our experiments under scotopic conditions, in which we observed that the b-wave amplitude, an indicator of synaptic transmission, was markedly reduced in the MO-injected animals (Fig. 8A) compared with controls. The a-wave, which is primarily a read out of photoreceptor membrane depolarization as a result of phototransduction, is often quenched by conditions conducive to generating b-wave amplitudes and is therefore not observable on the same trace. Thus, we isolated the a-wave pharmacologically with the synaptic transmission inhibitors L-AP4 and TBOA (Trombley and Westbrook, 1992; Huang and Bordey, 2004; Wong et al., 2005), whereupon we observed a slight reduction in isolated a-wave amplitude compared with controls (P=0.01), but less of an effect overall than that observed from the b-wave experiments (P=0.0007; Fig. 8B). Plotting a-wave and b-wave amplitudes against each other shows that the b-wave to a-wave ratio is significantly diminished in the morphant group (P<0.0007; Fig. 8C). These data, taken together with the disorganization of synaptic proteins in the OPL (Fig. 7B), indicate that postsynaptic activity is reduced relative to the level of the phototransduction response to light stimulus (Takada et al., 2008). Although we observed a very small increase in the number of caspase-positive cells in the INL, the majority of which were identified as Müller glia, we would not expect the loss of so few cells to account for such a large reduction in the b-wave amplitude. Thus, our data suggest that loss of harmonin in Müller cells results in decreased function of the photoreceptor ribbon synapses.
Fig. 8.
Deficits in synaptic function of photoreceptors in ush1c morphants. (A,B) Representative larval zebrafish ERG recordings showing b-wave amplitudes (A) or a-wave amplitudes (B) of uninjected control (black) and MO-injected (gray) larvae at unattenuated light intensity. (A) b-wave amplitudes are significantly reduced in morphant animals. (B) The a-wave of these same subjects was isolated by treatment with L-AP4 and TBOA to block synaptic transmission. a-waves are only slightly attenuated in morphant animals compared with controls. Light stimulus duration is 800 mseconds. (C) Comparison of the relative changes of b- and a-wave amplitudes in morphant and control larvae obtained at 0, −1 and −2 log units of light intensity relative to unattenuated light intensity. The decreased b-wave:a-wave ratio indicates a specific synaptic defect. Steeper slopes of the trend lines (dashed lines) represent reduced synaptic efficacy.
DISCUSSION
In this study, we describe the requirement for harmonin in zebrafish mechanosensory hair cell and retinal development, providing the first evidence that USH proteins function in photoreceptor synaptic transmission and ribbon synapse development. Our studies also implicate Müller glial cells as contributors to the retinal pathology of USH.
The localization of harmonin in hair cells and lateral line neuromasts indicates a phylogenetically conserved role in mechanosensory cells, and our studies of the zebrafish inner ear reveal defects in hair bundle organization and development, consistent with the stereocilia elongation and organization defects described in Ush1c mutant mice (Lefevre et al., 2008). Our results indicate that, in zebrafish, the predominant splice variant and protein isoform present during larval development is harmonin isoform A. The severe disruption of hair bundle development seen in ush1c mutant and morphant zebrafish provides an intriguing contrast to the absence of hair bundle defects reported in some colonies of dfcr mice, which harbor a deletion affecting harmonin isoform B (Grillet et al., 2009).
The presence of the USH protein harmonin in Müller glia from an early time point in retinal development and the specific defects in synaptic structure and function in ush1c morphants prior to detectable ush1c transcript or protein synthesis in photoreceptors suggest a non-cell-autonomous role for harmonin in facilitating synaptic maturation in cone pedicles. Müller glial cells have established roles in the maintenance of retinal neurons via their regulation of glutamate neurotransmitter levels and their expression of neurotrophic factors (Fields and Stevens-Graham, 2002; Newman, 2004; de Melo Reis et al., 2008). Although a role for Müller glia in retinal synaptogenesis has been suggested by several studies (Georges et al., 2006; Diaz et al., 2007), little is known about how this putative function might be accomplished. In the brain, glutamate receptor function has been shown to be important for synaptogenesis (Han and Stevens, 2009; Kakegawa et al., 2009), so glutamate uptake by Müller cells might similarly be important for synaptic development of photoreceptors. Interestingly, a recent zebrafish study (Williams et al., 2010) used a Müller-cell-specific line to ablate individual glia and observe effects on synaptogenesis and the behavior of neighboring glia. Their data indicated that localized loss of Müller cells did not affect the maturation of cone synapses at 5 dpf. These data are consistent with our findings, because they argue against the small and transient levels of Müller cell death being the cause of the reduced synaptic function and structural defects observed, and suggest instead that harmonin in Müller cells has a paracrine effect on cone synapse stability, such that the sustained absence of this protein results in the observed dysfunction and progressive photoreceptor cell death.
The cone synapse phenotype of ush1c morphants is similar to that of the zebrafish nrc mutant, in which the synaptojanin gene is disrupted (Allwardt et al., 2001; Van Epps et al., 2004). The nrc mutant has a diminished b-wave and defects in photoreceptor synapse formation, with floating ribbons and unassociated postsynaptic processes in cone pedicles. Synaptojanin has been shown to be required for cytoskeletal organization, endocytosis, Ca2+ signaling and post-synaptic glutamate transport (Sakisaka et al., 1997; Schuske et al., 2003; Gong and DeCamilli, 2008). Although no link between synaptojanin and USH has been proposed previously, nrc mutants have swimming and balance defects in addition to blindness (Van Epps et al., 2004), similar to the USH-like phenotype we observe in ush1c mutants and morphants. More recently, a report describing three new mutant alleles of zebrafish synaptojanin, isolated by screening for vestibular defects, illustrates synaptic transmission defects in the hair cells (Trapani et al., 2009).
Given the distinct, penetrant defects in visual function and photoreceptor synapse formation in harmonin-depleted larvae, we were surprised by the lack of harmonin localization in larval photoreceptors. It is puzzling that there would be no requirement for harmonin function in larval zebrafish photoreceptors, especially in light of the abundant antibody signal detected in the photoreceptors of young mice. An as-yet-undetected duplicate ush1c gene in zebrafish could account for the apparent protein localization discrepancies between mammals and zebrafish. However, our searches of the zebrafish genome reveal only one locus for ush1c, and we find only one copy of this gene in the Tetraodon, Takifugu and Threespine stickleback genome assemblies, suggesting that only a single copy of the ush1c gene has been retained in the teleost lineage.
Another possible explanation for the absence of harmonin in larval photoreceptors is the presence of ohnologous genes for two other PDZ-domain-containing proteins: Cip98 and Pdzd7. Alignments with the human CIP98 (whirlin) protein sequence, causative of USH2D, uncovered two orthologs in the zebrafish genome assembly. Both of these transcripts, cip98a and cip98b, are expressed in photoreceptors from early larval time points (J.B.P., unpublished); thus, the presence of an additional PDZ protein with similar binding affinities to harmonin (van Wijk et al., 2006) might be able to compensate for the absence of ush1c expression in these cells. However, given that the N-terminal regions of harmonin and Cip98 are not functionally interchangeable (Pan et al., 2009), any functional substitution of harmonin by Cip98 in the photoreceptor must not rely on protein interactions in the N-terminal region. Although PDZD7 is not currently recognized as a monogenic cause of human USH, it has been shown to act as a modifier of USH gene function and to interact physically with USH proteins. Both zebrafish genes, pdzd7a and pdzd7b, are expressed in photoreceptors at larval stages (Ebermann et al., 2010).
Our ERG recordings from ush1c morphant larvae reveal a specific reduction in b-wave amplitude in the absence of significant retinal degeneration, which might provide an explanation for the early cellular dysfunctions that precede retinitis pigmentosa in USH1C patients. A retrospective ERG analysis of USH1 patients as young as 17 months revealed abnormal waveforms prior to clinically detectable retinitis pigmentosa (Flores-Guevara et al., 2009), although other studies of USH1 patients in which photoreceptor loss was already clinically evident revealed no synaptic dysfunction in the remaining viable photoreceptors (Jacobson et al., 2008; Williams et al., 2009). Notably, the Ush1c216AA knock-in mouse (Lentz et al., 2010) exhibits an attenuated ERG as early as 1 month of age, prior to any histologically appreciable cell loss. However, in contrast to our results with larval zebrafish, both a- and b-waves were attenuated in Ush1c216AA mice. It could be that, in human manifestations of USH1C, the later consequence of diminished harmonin levels in photoreceptors is so severe that it obscures comparatively subtle early defects that might result from impaired synaptic activity.
Our data also indicate that sustained absence of harmonin in Müller cells, as seen in ush1c mutants, results in progressive retinal degeneration. It is not clear whether this cell death results from synaptic defects or from some other impairment of Müller cell function, but the non-cell-autonomous nature of this degeneration is notable. Clinical analyses of the retinal pathology of USH patients might provide insight into the link between Müller cell function and retinal integrity. Ophthalmic examinations of USH1B patients (who have mutations in the MYO7A gene) using high-resolution optical coherence tomography (Jacobson et al., 2009) showed that morphological changes in the OLM (the apical terminus of Müller cell processes) marked a transitional zone between regions in which photoreceptors were diseased or depleted and morphologically normal regions, suggesting that such alterations in OLM integrity might precede photoreceptor degeneration. Our understanding of the role that the OLM plays in maintaining photoreceptor integrity is incomplete; however, numerous junction proteins, including members of the Crumbs complex, have been shown to localize to the OLM (for reviews, see Gosens et al., 2008; Omri et al., 2010) and, in particular, to the subapical region, perhaps contributing to junctions within a semipermeable barrier. Mutations in Crumbs orthologs in mammals cause progressive retinal degeneration and the retinal pathology reveals diminished contacts between photoreceptors and the OLM (Mehalow et al., 2003; van de Pavert, 2004). USH proteins have not been reported in the OLM, or in Müller cells in general, before this study, although whirlin (USH2D) has been linked biochemically to the Crumbs pathway (Gosens et al., 2007).
Although the extent to which Müller cells might contribute to the USH retinal pathology remains to be investigated, our histological analyses of both zebrafish and mouse retinas, and our functional studies in ush1c morphants and mutants, suggest that additional investigation of Müller cell function is required to understand the complex etiology of USH. As gene replacement therapies are developed for USH patients, it will be important to know whether retinal cell types in addition to photoreceptors should be targeted for optimal rescue of the vision defects. The ability to isolate Müller-cell-associated pathology in our zebrafish USH1C model will allow further investigation of the roles of Müller cells as well as investigations of USH protein function at the OLM.
In summary, we have characterized the hearing and vision defects in a zebrafish ush1c mutant and morpholino knockdown. Our findings support a phylogenetically conserved developmental role for harmonin isoform A in hair bundle organization, implicate Müller cells as contributors to retinal dysfunction and photoreceptor cell death, and demonstrate that visual defects can be detected from the onset of vision in these zebrafish models.
METHODS
Animal strains and maintenance
All zebrafish studies were conducted with Oregon AB wild-type larvae or ush1cfh293 mutants in the AB background. Animals were raised in a 10-hour dark and 14-hour light cycle, and maintained as described (Westerfield, 2007). Owing to the swimming and balance defects exhibited by ush1c mutants and morphants, all experimental and control larvae for experiments occurring up to 8 dpf were raised without food so as not to give normal swimmers a nutritional advantage over their swimming-impaired siblings. Animals were staged according to the standard series (Kimmel et al., 1995), or by hpf or dpf. All animal-use protocols were IACUC approved.
Cloning of zebrafish ush1c cDNA and sequencing
tBLASTn queries with the human harmonin protein sequence were used to identify the zebrafish sequence, and primers based on this sequence were used to amplify the full-length open reading frame of isoform A and partial sequence from additional isoforms from whole larvae or adult retinal cDNA (see supplementary material Table S1 for primer sequences). PCR products were purified using gel electrophoresis and sequenced on an ABI 3100 using the fluorescent dideoxy terminator method. The gene is cataloged in NCBI under accession number NC_007136. All novel transcripts were submitted to the NCBI dbEST, with accession numbers HO243997-HO244001 and HS587020. Gene and protein names follow the zebrafish nomenclature guidelines (https://wiki.zfin.org/display/general/ZFIN+Zebrafish+Nomenclature+Guidelines).
Genetic mapping of zebrafish ush1c
The zebrafish ush1c locus was mapped by PCR using the LN54 collection of radiation hybrids (obtained from Marc Ekker, University of Ottawa), which contain the zebrafish AB9 cell DNA and mouse B78 cell DNA (Hukriede et al., 1999).
ush1c mutant discovery
Labeled primers (supplementary material Table S1) to PCR amplify a 500 bp region surrounding exon 5 of ush1c were generated and used to screen 8448 genomes in a TILLING screen as described (Moens et al., 2008). Sperm from the identified carrier sample were thawed and used to fertilize wild-type AB eggs. Adult F1 carriers of the mutation were identified by fin-clip genotyping, and identified heterozygotes were crossed to produce homozygous offspring.
Morpholino injections
Antisense morpholinos (GeneTools, Philomath, OR) were injected into one-cell-stage embryos as described (Nasciveus and Ekker, 2000). Embryos were injected with 1.8–2.0 nM of ush1c MO1 targeted to the splice donor site of exon 2 (5′-TAGAATTG-AGACTTACTGATGGTAC-3′), or 0.6–1.0 nM of ush1c MO2 targeted to the translation start site (5′-TTTTCAGTCCGT-TGGTCGTCTTGCT-3′).
RNA isolation and RT-PCR
Total RNA was isolated from euthanized larvae at 24 hpf to 7 dpf using Trizol (Invitrogen, Carlsbad, CA). Reverse transcription was performed according to manufacturer instructions using the Superscript Reverse Transcriptase II kit (Invitrogen).
Western blot
Embryos were homogenized in 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, 0.1% SDS and 5 mg/ml protease inhibitor cocktail (Pierce, Rockford, IL). Samples were run on an 8% acrylamide gel and transferred to nitrocellulose membranes with Nupage reagents (Invitrogen). Membranes were washed in PBS twice, each for 15 minutes, incubated in blocking solution (3% NFDM in PBS) for 1 hour at room temperature, then in primary antibodies [rabbit anti-zebrafish harmonin (SDIX, Newark, DE) 1:300; mouse monoclonal pan-actin (Millipore, Billerica, MD) 1:5000] in blocking solution overnight at 4°C. Membranes were rinsed three times for 5 minutes each in PBS, incubated in donkey anti-rabbit HRP (Jackson, West Grove, PA) for 1 hour at room temperature, then rinsed three times for 5 minutes each in PBS. HRP was detected using the ECL plus detection system (Pierce). To detect actin, membranes were rehydrated and incubated in donkey anti-mouse HRP for 1 hour, rinsed three times for 5 minutes each in PBS, and labeled with the ECL plus detection system (Pierce).
In situ hybridization and immunohistochemistry
In situ hybridization on whole and sectioned tissue and antibody labeling on sectioned tissue was performed as described (Jensen et al., 2001; Ebermann et al., 2010). For imaging of subcellular protein localization in sectioned retinas, incubation with primary antibodies and a goat anti-mouse Alexa-Fluor-488 to visualize the glutamine synthetase antibody was followed by incubation with an anti-rabbit biotinylated antibody diluted in block (10% NGS, 2% BSA in PBS-T) overnight at 4°C. Sections were then rinsed four times for 5 minutes each in PBS and signal was detected with the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) and TSA kit (Perkin-Elmer, Waltham, MA). For whole-mount imaging of stereocilia, 5 dpf larvae were fixed in ‘BT’ fix (4% PFA, 0.15 mM CaCl2, 4% sucrose in PBS) for 48 hours, rinsed four times for 5 minutes each in PBS + 0.01% Triton X-100, and then permeabilized with 2.5% Triton X-100 in PBS at room temperature until otoliths dissolved. Larvae were rinsed four times for 5 minutes each in PBS + 0.01% Triton X-100, blocked for 2 hours at room temperature in 10% NGS, 10% BSA in PBS + 0.01% Triton X-100, then incubated with Alexa-Fluor-488–phalloidin diluted in block for 24 hours at 4°C. Following four 5-minute PBS + 0.01% Triton X-100 washes, larvae were blocked using the Avidin/Biotin blocking kit (Molecular Probes, Carlsbad, CA), and incubated in antibody solutions overnight at 4°C. Larvae were rinsed four times for 30 minutes each in PBS-Triton, followed by signal detection with the Vectastain ABC Elite kit (Vector) and TSA (Perkin-Elmer) kit. After rinsing four times for 5 minutes each in PBS-Triton, larvae were immersed in Vectashield (Vector) and mounted in a lateral orientation on chambered slides. Images were captured on a Zeiss LSM5 confocal or BioRad Radiance 2100 confocal microscope. The following antibodies and concentrations were used in this study: rabbit anti-harmonin (SDIX) antibody generated against aa1-100 of zebrafish harmonin, 1:200 for sections, 1:1000 for whole mount; rabbit anti-harmonin (SDIX) antibody generated against aa306–363 of mouse harmonin, 1:200; goat anti-harmonin (Santa Cruz Biotechnology, Santa Cruz, CA) against the N-terminus of the human protein, 1:40; rabbit anti-harmonin (Sigma-Aldrich, St Louis, MO) against aa42–118 of human harmonin, 1:40; mouse anti-glutamine synthetase (Millipore), 1:500; mouse zpr1 (Zebrafish International Resource Center, Eugene, OR), 1:200; mouse anti-SV2 (DSHB), 1:2000; rabbit anti-GluR2/3 (Millipore), 1:50; rabbit anti-active caspase-3 (BD Biosciences, San Jose, CA), 1:200; mouse anti-acetylated tubulin (Sigma), 1:500. Goat anti-mouse, chicken anti-goat and goat anti-rabbit Alexa-Fluor-488, −568 and −633 secondary antibodies (Molecular Probes) were used at 1:300 and the anti-rabbit biotinylated antibody was used at 1:500. Alexa-Fluor-488–phalloidin (Invitrogen) was diluted to a final concentration of 2.5 μM.
Electron microscopy
Tissue was prepared as described previously (Schmitt and Dowling, 1999). Ultrathin sections were obtained from Epon-embedded tissue, stained with uranyl acetate and lead citrate, and examined by conventional transmission electron microscopy.
Optokinetic response
To measure optokinetic response, we used a modification of the method of Brokerhoff (Brokerhoff, 2006). Larvae were placed in a small Petri dish containing 3% methylcellulose and mounted on a stationary platform surrounded by a rotating drum of 8 cm in diameter. The drum interior displays ten alternating black and white stripes, each subtending an angle of 28°. The larvae were oriented in an upright position, illuminated with fiber optic lights and viewed through a dissecting microscope positioned over the drum, which rotated at 5 rpm clockwise and then counter clockwise, for 30 seconds in each direction. Eye movements were recorded during this time period, after which the larvae were washed out of the methylcellulose and returned to embryo medium.
ERG recordings
ERG recordings (Makhankov et al., 2004) were made to examine retinal function of morphants (morpholino-injected larvae) compared with control larvae. 5 dpf larvae were incubated in Ringer’s solution for at least 1 hour prior to the experiment and dark-adapted for a minimum of 30 minutes. Under dim red illumination, larvae anesthetized with tricaine (Sigma Aldrich) and paralyzed with 300 μM pancuronium bromide (Sigma Aldrich) were placed on a piece of sponge so that the pupil of one eye was on axis with the stimulus light beam. A recording electrode was placed on the cornea to measure the change in voltage induced by stimulation. The reference electrode was a AgCl-coated silver wire inserted into the sponge. The recording electrode was connected via a AgCl-coated silver wire to a low noise recording amplifier (Axopatch 200A with a CV201A head stage), and data were acquired and stored digitally (Digidata 1200 interface and pClamp7 and pClamp8 software; recording hardware and software from Axon Instruments). Signals were filtered at 2 kHz.
Stimulation
Recordings were performed in a Faraday cage housing a Zeiss UEM microscope, with a 4× objective lens used for visualizing the eye and delivering light stimuli. An ultra bright white LED array (Atlas series high brightness module NT-42D0-0426 from Lamina Lighting, with an LED driver circuit locally designed and constructed by the technical support group) was used to present the stimulus. Stimulus durations were 800 mseconds. The light intensity of the unattenuated beam was 1500 μW/cm2. Appropriate neutral density filters were inserted into the light beam to achieve accurate light stimulus intensities over a 5 log10 unit range. Three to five responses per intensity level were averaged and filtered for 60 Hz noise.
Isolation of the a-wave component
Directly after recording the b-wave (in Ringer’s solution), pharmacological compounds were applied externally to the eye using an Eppendorf pipette. The compounds used in this study were L-(+)-2-amino-4-phosphonobutyric acid (L-AP4; 30–300 μM), DL-threo-b-Benzyloxyaspartic acid (DL-TBOA; 15 μM) and Ringer’s solution with 5% DMSO as control. After 15–30 minutes, the a-wave traces were recorded.
Data analysis
The amplitudes of the b-waves were measured from the bottom of the a-wave to the peak of the b-wave; the a-wave was measured from the baseline to the peak of the a-wave. We used larvae that had a consistent response to a test flash delivered (±10%) at the beginning and the end of the session. Unhealthy or damaged wild-type larvae that showed a decreased ERG or changed waveform at the end of the experiment compared with the beginning were not included in the analysis. For statistics, we used a linear mixed model approach and a Student’s t-test.
TRANSLATIONAL IMPACT.
Clinical issue
Usher syndrome (USH) is a recessively inherited disease of combined deafness and blindness. Hearing impairment is usually apparent from birth, whereas vision loss is slow and progressive, beginning in the first or second decade of life. Cochlear implants and hearing aids can compensate for some of the hearing deficits, but there is currently no treatment to counter the vision loss, which is a form of retinitis pigmentosa. Early diagnosis is important for genetic counseling and preparation for late onset blindness.
There is a great deal of clinical variability among patients with USH, and nine genes that can cause this disease when mutated have been identified to date. These genes encode a wide variety of proteins that have complex and dynamic localization patterns within the cells of the inner ear and the retina that are affected in USH. Discovering more about how these mutations cause deafness and blindness at the cellular and molecular level will enhance our understanding of the etiology of this disease, and contribute to the development of effective treatments.
Results
In this paper, the authors describe a zebrafish model for Usher type 1C (USH1C), a severe form of USH in humans that is characterized by profound congenital deafness, balance problems and a relatively early onset of progressive vision loss. The authors disrupt the normal function of the ush1c gene by using morpholino oligonucleotides to interfere with mRNA splicing. In addition, they identify an ush1c zebrafish mutant by screening DNA from individual mutagenized fish to find a lesion in the ush1c gene. Both morpholino-injected animals and ush1c mutants have distinct defects in hearing, balance and vision that are apparent in the first days of life. The authors examine the sensory cells involved in these behaviors –mechanosensory hair cells and retinal cells – and discover defects in their development and function. In the ear, the structural portion of the mechanosensory hair cells that are important for intercepting sound waves does not form properly. Surprisingly, the authors discover that, in the retina, the ush1c gene functions in Müller glia rather than in photoreceptors, the cell type that has been reported to express Ush1c in mammals. Subsequently, they show that this gene is also expressed in mammalian Müller glia. Importantly, although zebrafish ush1c is not active in photoreceptors, loss of ush1c function in Müller cells leads to compromised photoreceptor function and cell death.
Implications and future directions
Further insights into the role of ush1c in the development and function of sensory cells are vital to providing early diagnosis and intervention to forestall the devastating effects of this disease. This work contributes to the understanding of USH1C pathology by providing information about the specific molecules involved in the formation of stereocilia in mechanosensory hair cells, and by identifying a new retinal cell type through which ush1c functions to facilitate and maintain synaptic connections between photoreceptor cells and interneurons of the retina, namely Müller glia. USH has traditionally not been considered a developmental disorder of vision. The results of this study, however, show that visual defects in the zebrafish model of USH1C can be detected from the onset of vision, and thus might have clinical relevance for the early diagnosis of USH1C in humans.
Supplementary Material
Acknowledgments
The authors thank David Raible, Kelly Owens and John Constable for help with experimental design and reagents, the Cecilia Moens laboratory for their work on the TILLING screen, and Jeremy Wegner for technical assistance. Support was provided by NIH EY07042, DC004186, DC010447, RR020833 and HD22486, and by the Foundation Fighting Blindness. J.B.P. was supported by an American Heart Association Postdoctoral Fellowship. D.S.W. is a Jules and Doris Stein RPB Professor.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
J.B.P. designed and performed experiments, analyzed data and wrote the manuscript with input from the other authors. B.B.-S. designed and performed experiments and analyzed data. J.J.L. designed and performed experiments and contributed experimental reagents. A.T., K.K. and M.M. performed experiments and analyzed data. S.S., Z.G.J. and P.F.H. performed experiments. D.S.W., P.W., B.J.K. and M.W. conceived and designed the experiments.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.006429/-/DC1
REFERENCES
- Adato A., Michel V., Kikkawa Y., Reiners J., Alagramam K. N., Weil D., Yonekawa H., Wolfrum U., El-Amraoui A., Petit C. (2005). Interactions in the network of Usher syndrome type 1 proteins. Hum. Mol. Genet. 14, 347–356 [DOI] [PubMed] [Google Scholar]
- Allwardt B. A., Lall A. B., Brockerhoff S. E., Dowling J. E. (2001). Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J. Neurosci. 21, 2330–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahloul A., Michel V., Hardelin J.-P., Nouaille S., Hoos S., Houdusse A., England P., Petit C. (2010). Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type 1 genes, form a ternary complex and interact with membrane phospholipids. Hum. Mol. Genet. 19, 3557–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever M. M., Fekete D. M. (1999). Ventromedial focus of cell death is absent during development of Xenopus and zebrafish inner ears. J. Neurocytol. 28, 81–93 [DOI] [PubMed] [Google Scholar]
- Bilotta J., Saszik S., Sutherland S. E. (2001). Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Dev. Dyn. 222, 564–570 [DOI] [PubMed] [Google Scholar]
- Boughman J. A., Vernon M., Shaver K. A. (1983). Usher syndrome: definition and estimate of prevalence from two high-risk populations. J. Chronic Dis. 36, 595–603 [DOI] [PubMed] [Google Scholar]
- Brockerhoff S. E. (2006). Measuring the optokinetic response of zebrafish larvae. Nat. Protoc. 1, 2448–2451 [DOI] [PubMed] [Google Scholar]
- Brown S. D., Hardisty-Hughes R. E., Mburu P. (2008). Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat. Rev. Genet. 9, 277–290 [DOI] [PubMed] [Google Scholar]
- Caberlotto E., Michel V., Foucher I., Bahloul A., Goodyear R. J., Pepermans E., Michalski N., Perfettini I., Alegria-Prévot O., Chardenoux S., et al. (2011). Usher type 1G protein sans is a critical omponent of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc. Natl. Acad. Sci. USA 108, 5825–5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo Reis R. A., Ventura A. L., Schitine C. S., de Mello M. C., de Mello F. G. (2008). Muller glia as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochem. Res. 33, 1466–1474 [DOI] [PubMed] [Google Scholar]
- Delprat B., Michel V., Goodyear R., Yamasaki Y., Michalski N., El-Amraoui A., Perfettini I., Legrain P., Richardson G., Hardelin J. P., et al. (2005). Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly. Hum. Mol. Genet. 14, 401–410 [DOI] [PubMed] [Google Scholar]
- Diaz C. M., Macnab L. T., Williams S. M., Sullivan R. K., Pow D. V. (2007). EAAT1 and D-serine expression are early features of human retinal development. Exp. Eye Res. 84, 876–885 [DOI] [PubMed] [Google Scholar]
- Ebermann I., Scholl H. P., Charbel Issa P., Becirovic E., Lamprecht J., Jurklies B., Millan J. M., Aller E., Mitter D., Bolz H. (2007). A novel gene for Usher syndrome type 2, mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum. Genet. 121, 203–211 [DOI] [PubMed] [Google Scholar]
- Ebermann I., Phillips J. B., Liebau M. C., Koenekoop R. K., Schermer B., Lopez I., Schäfer E., Roux A. F., Dafinger C., Bernd A., et al. (2010). PDZD7 is a modifier of retinal disease and contributer to digenic Usher syndrome. J. Clin. Invest. 120, 1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest S., Rauch G. J., Haffter P., Geisler R., Petit C., Nicolson T. (2000). Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum. Mol. Genet. 9, 2189–2196 [DOI] [PubMed] [Google Scholar]
- Fields R. D., Stevens-Graham B. (2002). New insights into neuron-glia communication. Science 298, 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Guevara R., Renault F., Loundon N., Marlin S., Pelosse B., Momtchilova M., Auzoux-Cheve M., Vermersch A. I., Richard P. (2009). Usher syndrome type 1, Early detection of electroretinographic changes. Eur. J. Paediatr. Neurol. 13, 505–507 [DOI] [PubMed] [Google Scholar]
- Georges P., Cornish E. E., Provis J. M., Madigan M. C. (2006). Müller cell expression of glutamate cycle related proteins and anti-apoptotic proteins in early human retinal development. Br. J. Ophthalmol. 90, 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L.-W., De Camilli P. (2008). Regulation of postsynaptic AMPA responses by synaptojanin 1. Proc. Natl. Acad. Sci. USA 105,17561–17566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens I., van Wijk E., Kersten F. F. J., Krieger E., van der Zwaag B., Märker T., Letteboer S. J. F., Dusseljee S., Peters T., Spierenburg H. A., et al. (2007). MPP1 links the Usher protein network and the Crumbs protein complex in the retina. Hum. Mol. Genet. 16, 1993–2003 [DOI] [PubMed] [Google Scholar]
- Gosens I., den Hollander A. I., Cremers F. P. M., Roepman R. (2008). Composition and function of the Crumbs protein complex in the mammalian retina. Exp. Eye Res. 86, 713–726 [DOI] [PubMed] [Google Scholar]
- Grillet N., Xiong W., Reynolds A., Kazmierczak P., Sato T., Lillo C., Dumont R. A., Hintermann E., Sczaniecka A., Schwander M., et al. (2009). Harmonin mutations cause mechanotransduction defects in cochlear hair cells. Neuron 62, 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E. B., Stevens C. F. (2009). Development regulates a switch between post-and presynaptic strengthening in response to activity deprivation. Proc. Natl. Acad. Sci. USA 106, 10817–10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T., Heintzelman M. B., Santos-Sacchi J., Corey D. P., Mooseker M. S. (1995). Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc. Natl. Acad. Sci. USA 92, 9815–9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai A., Tada M., Furuuchi K., Ishikawa S., Makiyama K., Hamada J., Okada F., Kbayashi I., Fukuda H., Moriuchi T. (2004). Expression of AIE-75 PDZ-domain protein induces G2/M cell cycle arrest in human colorectal adenocarcinoma SW480 cells. Cancer Lett. 211, 209–218 [DOI] [PubMed] [Google Scholar]
- Huang H., Bordey A. (2004). Glial glutamate transporters limit spillover activation of presynaptic NMDA receptors and influence synaptic inhibition of Purkinje neurons. J. Neurosci. 24, 5659–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukriede N. A., Joly L., Tsang M., Miles J., Tellis P., Epstein J. A., Barbazuk W. B., Li F. N., Paw B., Postlethwait J. H., et al. (1999). Radiation hybrid mapping of the zebrafish genome. Proc. Natl. Acad. Sci. USA 96, 9745–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S. G., Cideciyan A. V., Aleman T. S., Sumaroka A., Roman A. J., Gardner L. M., Prosser H. M., Mishra M., Bech-Hansen N. T., Herrera W., et al. (2008). Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum. Mol. Genet. 17, 2405–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S. G., Aleman T. S., Sumaroka A., Cideciyan A. V., Roman A. J., Windsor E. A. M., Schwartz S. B., Rehm H. L., Kimberling W. J. (2009). Disease boundaries in the retina of patients with Usher syndrome caused by MYO7A gene mutations. Invest. Ophthalmol. Vis. Sci. 50, 1886–1894 [DOI] [PubMed] [Google Scholar]
- Jensen A. M., Walker C., Westerfield M. (2001). mosaic eyes: a zebrafish gene required in pigmented epithelium for apical localization of retinal cell division and lamination. Development 128, 95–105 [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Gagnon L. H., Webb L. S., Peters L. L., Hawes N. L., Chang B., Zheng Q. Y. (2003). Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum. Mol. Genet. 12, 3075–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W., Miyazaki T., Kohda K., Matsuda K., Emi K., Motohashi J., Watanabe M., Yuzaki M. (2009). The N-terminal domain of GluD2 (GluRdelta2) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivo. J. Neurosci. 29, 5738–5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P., Sakaguchi H., Tokita J., Wilson-Kubalek E. M., Milligan R. A., Muller U., Kachar B. (2007). Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91 [DOI] [PubMed] [Google Scholar]
- Keats B. J., Savas S. (2004). Genetic heterogeneity in Usher syndrome. Am. J. Med. Genet. A 130, 13–16 [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Mburu P., Morse S., Kominami R., Townsend S., Brown S. D. (2005). Mutant analysis reveals whirlin as a dynamic organizer in the growing hair cell stereocilium. Hum. Mol. Genet. 14, 391–400 [DOI] [PubMed] [Google Scholar]
- Kimberling W. J., Hildebrand M. S., Shearer A. E., Jensen M. L., Halder J. A., Trzupek K., Cohn E. S., Weleber R. G., Stone E. M., Smith R. J. H. (2010). Frequency of Usher syndrome in two pediatric populations: Implications for genetic screening of deaf and hard of hearing children. Genet. Med. 12, 512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Lefevre G., Michel V., Weil D., Lepelletier L., Bizard E., Wolfrum U., Hardelin J. P., Petit C. (2008). A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development 135, 1427–1437 [DOI] [PubMed] [Google Scholar]
- Lentz J., Pan F., Ng S., Deininger P., Keats B. (2007). Ush1c 216A knock-in mouse survives Katrina. Mutat. Res. 616, 139–144 [DOI] [PubMed] [Google Scholar]
- Lentz J. J., Gordon W. C., Farris H. E., MacDonald G. H., Cunningham D. E., Robbins C. A., Tempel B. L., Bazan N. G., Rubel E. W., Oesterle E. C., et al. (2010). Deafness and retinal degeneration in a novel USH1C knock-in mouse model. Dev. Neurobiol. 70, 253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Vansant G., Udovichenko I. P., Wolfrum U., Williams D. S. (1997). Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil. Cytoskeleton 37, 240–252 [DOI] [PubMed] [Google Scholar]
- Liu X., Bulgakov O. V., Darrow K. N., Pawlyk B., Adamian M., Liberman M. C., Li T. (2007). Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc. Natl. Acad. Sci. USA 104, 4413–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerker T., van Wijk E., Overlack N., Kersten F. F., McGee J., Goldmann T., Sehn E., Roepman R., Walsh E. J., Kremer H., et al. (2008). A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum. Mol. Genet. 17, 71–86 [DOI] [PubMed] [Google Scholar]
- Makhankov Y. V., Rinner O., Neuhauss S. C. F. (2004). An inexpensive device for non-invasive electroretinograms in small aquatic vertebrates. J. Neurosci. Methods 135, 205–210 [DOI] [PubMed] [Google Scholar]
- Mburu P., Mustapha M., Varela A., Weil D., El-Amraoui A., Holme R. H., Rump A., Hardisty R. E., Blanchard S., Coimbra R. S., et al. (2003). Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat. Genet. 34, 421–428 [DOI] [PubMed] [Google Scholar]
- Mehalow A. K., Kameya S., Smith R. S., Hawes N. L., Denegre J. M., Young J. A., Bechtold L., Haider N. B., Tepass U., Heckenlively J. R., et al. (2003). CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum. Mol. Genet. 12, 2179–2189 [DOI] [PubMed] [Google Scholar]
- Michalski N., Michel V., Bahloul A., Lefevre G., Barral J., Yagi H., Chardenoux S., Weil D., Martin P., Hardelin J. P., et al. (2007). Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J. Neurosci. 27, 6478–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens C. B., Donn T. M., Wolf-Saxon E. R., Ma T. P. (2008). Reverse genetics in zebrafish by TILLING. Brief. Funct. Genomic. Proteomic. 7, 454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. C., Fadool J. M. (2005). Studying rod photoreceptor development in zebrafish. Physiol. Behav. 86, 306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A., Ekker S. C. (2000). Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26, 216–220 [DOI] [PubMed] [Google Scholar]
- Newman E. A. (2004). A dialogue between glia and neurons in the retina: modulation of neuronal excitability. Neuron Glia Biol. 1, 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri S., Omri B., Savoldelli M., Jonet L., Thillaye-Goldenberg B., Thuret G., Gain P., Jeanny J. C., Cristanti P., Behar-Cohen F. (2010). The outer limiting membrane (OLM) revisited: clinical implications. Clin. Ophthalmol. 4, 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X. M., Xia X. J., Verpy E., Du L. L., Pandya A., Petit C., Balkany T., Nance W. E., Liu X. Z. (2002). Mutations in the alternatively spliced exons of USH1C cause non-syndromic recessive deafness. Hum. Genet. 111, 26–30 [DOI] [PubMed] [Google Scholar]
- Overlack N., Maerker T., Latz M., Nagel-Wolfrum K., Wolfrum U. (2008). SANS (USH1G) expression in developing and mature mammalian retina. Vision Res. 48, 400–412 [DOI] [PubMed] [Google Scholar]
- Pan L., Yan J., Wu L., Zhang M. (2009). Assembling stable hair cell tip link complex via multidentate interactions between harmonin and cadherin 23. Proc. Natl. Acad. Sci. USA 106, 5575–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser H. M., Rzadzinska A. K., Steel K. P., Bradley A. (2008). Mosaic complementation demonstrates a regulatory role for myosin VIIa in actin dynamics of stereocilia. Mol. Cell. Biol. 28, 1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners J., Reidel B., El-Amraoui A., Boëda B., Huber I., Petit C., Wolfrum U. (2003). Differential distrubution of harmonin isoforms and their possible role in Usher-1 protein complexes in mammalian photoreceptor cells. Invest. Ophthalmol. Vis. Sci. 44, 5006–5015 [DOI] [PubMed] [Google Scholar]
- Reiners J., van Wijk E., Märker T., Zimmermann U., Jurgens K., te Brinke H., Overlack N., Roepman R., Knipper M., Kremer H., et al. (2005). Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Hum. Mol. Genet. 14, 3933–3943 [DOI] [PubMed] [Google Scholar]
- Reiners J., Nagel-Wolfrum K., Jurgens K., Märker T., Wolfrum U. (2006). Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp. Eye Res. 83, 97–119 [DOI] [PubMed] [Google Scholar]
- Rosenberg T., Haim M., Hauch A.-M., Parving A. (1997). The prevalence of Usher syndrome and other retinal dystrophy-hearing impairment associations. Clin. Genet. 51, 314–321 [DOI] [PubMed] [Google Scholar]
- Saihan Z., Webster A. R., Luxon L., Bitner-Glindzicz M. (2009). Update on Usher syndrome. Curr. Opin. Neurol. 22, 19–27 [DOI] [PubMed] [Google Scholar]
- Sakisaka T., Itoh T., Miura K., Takenawa T. (1997). Phosphatidylinositol 4,5-bisphosphate phosphatase regulates the rearrangement of actin filaments. Mol. Cell. Biol. 17, 3841–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E. A., Dowling J. E. (1999). Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analysis. J. Comp. Neurol. 404, 515–536 [PubMed] [Google Scholar]
- Schuske K. R., Richmond J. E., Matthies D. S., Davis W. S., Runz S., Rube D. A., van der Bliek A. M., Jorgensen E. M. (2003). Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron 40, 749–762 [DOI] [PubMed] [Google Scholar]
- Seeliger M. W., Rilk A., Neuhauss S. C. F. (2002). Ganzfeld ERG in zebrafish larvae. Doc. Ophthalmol. 104, 57–68 [DOI] [PubMed] [Google Scholar]
- Seiler C., Finger-Baier K. C., Rinner O., Makhankov Y. V., Schwarz H., Neuhauss S. C., Nicolson T. (2005). Duplicated genes with split functions: independent roles of protocadherin15 orthologues in zebrafish hearing and vision. Development 132, 615–623 [DOI] [PubMed] [Google Scholar]
- Siemens J., Lillo C., Dumont R. A., Reynolds A., Williams D. S., Gillespie P. G., Müller U. (2004). Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428, 950–955 [DOI] [PubMed] [Google Scholar]
- Söllner C., Rauch G. J., Siemens J., Geisler R., Schuster S. C., Muller U., Nicolson T. (2004). Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428, 955–959 [DOI] [PubMed] [Google Scholar]
- Takada Y., Vijayasarathy C., Zeng Y., Kjellstron S., Bush R. A., Sieving P. A. (2008). Synaptic pathology in Retinoschisis knockout (Rs1−/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest. Ophthalmol. Vis. Sci. 49, 3677–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C., Liu X. Z., Han F., Yu H., Longo-Guess C., Yang B., Lu C., Yan D., Zheng Q. Y. (2010). Ush1c gene expression levels in the ear and eye suggest different roles for Ush1c in neurosensory organs in a new Ush1c knockout mouse. Brain Res. 1328, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani J. G., Obholzer N., Mo W., Brockerhoff S. E., Nicolson T. (2009). synaptojanin1 is required for temporal fidelity of synaptic transmission in hair cells. PLoS Genet. 5, e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley P. Q., Westbrook G. L. (1992). L-AP4 inhibits calcium currents and synaptic transmission via a G-protein-coupled glutamate receptor. J. Neurosci. 12, 2043–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert S. A., Kantardzhieva A., Malysheva A., Meuleman J., Versteeg I., Levelt C., Klooster J., Geiger S., Seeliger M. W., Rashbass P., et al. (2004). Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J. Cell Sci. 117, 4169–4177 [DOI] [PubMed] [Google Scholar]
- Van Epps H. A., Hayashi M., Lucast L., Stearns G. W., Hurley J. B., De Camilli P., Brockerhoff S. E. (2004). The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J. Neurosci. 24, 8641–8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk E., van der Zwaag B., Peters T., Zimmermann U., Te Brinke H., Kersten F. F., Marker T., Aller E., Hoefsloot L. H., Cremers C. W., et al. (2006). The DFNB31 gene product whirlin connects to the Usher protein network in the cochlea and retina by direct association with USH2A and VLGR1. Hum. Mol. Genet. 15, 751–765 [DOI] [PubMed] [Google Scholar]
- Verpy E., Leibovici M., Zwaenepoel I., Liu X. Z., Gal A., Salem N., Mansour A., Blanchard S., Kobayashi I., Keats B. J., et al. (2000). A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat. Genet. 26, 51–55 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2007). The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene: University of Oregon Press [Google Scholar]
- Williams D., Aleman T., Lillo C., Lopes V. S., Hughes L. C., Stone E. M., Jacobson S. G. (2009). Harmonin in the murine retina and the retinal phenotypes of Ush1c-mutant mice and human USH1C. Invest. Ophthalmol. Vis. Sci. 50, 3381–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. R., Suzuki S. C., Yoshimatsu T., Lawrence O. T., Waldron S. J., Parsons M. J., Nonet M. L., Wong R. O. (2010). In vivo development of outer retinal synapses in the absence of glial contact. J. Neurosci. 30, 11951–11961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. Y., Adolph A. R., Dowling J. E. (2005). Retinal bipolar input mechanisms in giant Danio. I. Electroretinographic analysis. J. Neurophysiol. 93, 84–93 [DOI] [PubMed] [Google Scholar]
- Yan D., Liu X. Z. (2010). Genetics and pathological mechanisms of Usher syndrome. J. Hum. Genet. 55, 327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X., Zhao Y., Adamian M., Pawlyk B., Sun X., McMillan D. R., Liberman M. C., Li T. (2010). Ablation of Whirlin long isoform disrupts the USH2 protien complex and causes vision and hearing loss. PLoS Genet. 6, e1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.