Abstract
The Cryptosporidium parvum acidic ribosomal protein P2 (CpP2) is an important immunodominant marker in C. parvum infection. In this study, the CpP2 antigen was evaluated as a vaccine candidate using a DNA vaccine model in adult C57BL/6 IL-12 knockout (KO) mice, which are susceptible to C. parvum infection. Our data show that subcutaneous immunization in the ear with DNA encoding CpP2 (CpP2-DNA) cloned into the pUMVC4b vector induced a significant anti-CpP2 IgG antibody response that was predominantly of the IgG1 isotype. Compared to control KO mice immunized with plasmid alone, CpP2-immunized mice demonstrated specific in vitro spleen cell proliferation as well as enhanced IFN-γ production to recombinant CpP2. Further, parasite loads in CpP2 DNA-immunized mice were compared to control mice challenged with C. parvum oocysts. Although a trend in reduction of infection was observed in the CpP2 DNA-immunized mice, differences between groups were not statistically significant. These results suggest that a DNA vaccine encoding the C. parvum P2 antigen is able to provide an effective means of eliciting humoral and cellular responses and has the potential to generate protective immunity against C. parvum infection but may require using alternative vectors or adjuvant to generate a more potent and balanced response.
Keywords: Cryptosporidium, DNA vaccine, antigen, P2, acidic ribosomal proteins
Introduction
Cryptosporidiosis in humans is primarily an enteric disease caused by the parasitic protozoa Cryptosporidium hominus and by Cryptosporidium parvum, the latter zoonotic species affecting a variety of mammalian species, including rodents, livestock and humans. Cryptosporidia are obligate intracellular parasites that infect epithelial cells and have been identified as a significant cause of diarrhea and are associated with morbidity and mortality in individuals with compromised immune systems (1, 2, 3). Presently, there is no effective chemotherapeutic agent for the treatment of the infection in immunodeficient individuals. Thus, there have been increasing efforts directed toward the development of alternative therapeutic strategies, such as vaccines, to control the disease.
The acidic ribosomal proteins have been described as prominent antigens during Chagas’ disease (4, 5, 6), Brucella abortus infection (7), Babesia bovis infection (8), and systemic lupus erythematosus (SLE) (9, 10, 11). In particular, ribosomal proteins P0 and P2 of a number of protozoa, including Leishmania sp., Plasmodium falciparum, and Toxoplasma gondii, have been reported to be immunostimulatory, as sera from infected animals and humans recognize these antigens (12, 13, 14, 15). In addition, it has been suggested that these antigens may be possible vaccine candidates. In recent reports, immunization with the P-domain peptide of ribosomal protein P0 provided protection against P. falciparum challenge (16). Furthermore, an L. infantum ribosomal protein DNA vaccine conferred protective immunity to L. major infection in mice (17). Although these antigens are associated with cytosolic ribosomes, they are also reported to be translocated and expressed on the surface of the parasite and, thus, may play an additional role in parasite infection. For example, PfP0 blocked the invasion of P. falciparum merozoites into red blood cells, and inhibition of invasion and protection has been demonstrated in L. major mice (17, 12).
The newly characterized CpP2 antigen has a molecular mass in the range of 17-kDa but is distinct from the C. parvum 17-kDa antigen family (18). All three acidic ribosomal proteins (P1, P0, and P2) from C. parvum react with sera from infected individuals; CpP2 in particular, is highly immunogenic, reacting with ~70% of sera from infected individuals from developing countries (18). In highly endemic areas such as Haiti, individuals who had a strong anti-CpP2 antibody response were also antibody positive for the 27-kDa antigen, suggesting a role for the antigen in the generation of immune responses against C. parvum. Therefore, we evaluated the immunological responses in C57BL/6 interleukin-12p40 (IL-12p40) knock out mice to a DNA vaccine vector encoding the CpP2 antigen. Mice immunized with CpP2 DNA generated an antibody titer and specific cellular response as evident by T cell proliferation to the rCpP2 antigen. We also examined the protective properties of the CpP2 DNA vaccine against challenge infection with C. parvum. This is the first report of humoral and cellular immune responses elicited by the CpP2 antigen delivered as a DNA vaccine.
Materials and methods
Animals
Six-to-eight week-old female C57BL/6 interleukin-12p40 (IL-12p40) knockout (KO) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed at the Veterans Affairs Medical Center (Decatur, GA) animal facility. Animals were fed sterile food and water and kept in HEPA filtered barrier-isolated facilities. Mice were anaesthetized with ketamine and xylazine before immunizing and bleeding procedures. All manipulations were performed in HEPA-filtered biological containment hoods. All experiments were conducted under the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee approvals in accordance with all of the applicable federal, state, and local regulations.
DNA Extraction
The C. parvum isolate used for DNA extraction was the Iowa bovine isolate. Oocysts were collected, purified through discontinuous sucrose and cesium chloride gradients, and stored as previously described (19). Total genomic DNA was isolated from purified oocysts by several freeze-thaw cycles in the presence of RNase A and proteinase K, followed by phenol-chloroform extraction. Finally, DNA was ethanol precipitated in the presence of 0.3 M sodium acetate (pH 5.2) and resuspended in nuclease-free water.
CpP2 vaccine construction and DNA immunization protocol
Synthesis of the DNA coding region of the CpP2 gene (GenBank accession no. XP625382) was performed by polymerase chain reaction (PCR). C. parvum genomic DNA was used as a PCR template since CpP2 is predicted to be an intronless gene, as reported by the CryptoDB database (http://www.cryptodb.org). The CpP2 antigen coding sequence was amplified using CpP2 sense primer (5′-CGCGAATTCATGGGTATGAAATACGTTGC-3′) and CpP2 antisense primer (5′-GCGGCGGCCGCTTAGTCAAACAATGAGAAAC-3′). The primers allowed the introduction of EcoRI and NotI restriction sites (underlined above). The fragment was ligated into the EcoRI and NotI restriction enzyme sites of the pUMVC4b expression vector (Adevron, Fargo, ND). This vector has a CMV promoter and immunoadjuvant site that enhances immune responses. The ligation mix was then transformed into UltraMAX™ DH5a-FT™ Competent Cells (Invitrogen Corporation, Carlsbad, CA) and transformants were selected on LB agar containing kanamycin (50 μg/ml). Positive clones were confirmed by restriction digest and sequencing. Endotoxin-free plasmid DNA was isolated using the EndoFree Plasmid Giga Kit (Qiagen, Valencia, CA). Mice (10 per group) were injected subcutaneously in the ear with 100 μg plasmid pUMVC4b-CpP2 in TBE on days 0, 14, and 29. For prime-boost regime, mice were immunized in the ear with 100 μg plasmid pUMVC4b-CpP2 in TBE on days 0, 14, and 29, followed by 3 μg of rP2 in Hunter’s TiterMax:PBS on days 43 and 57. The control group mice were injected with the empty pUMVC4b vector. Blood samples were collected from the retro-orbital plexus or submandibular vein at baseline, 29 and 46 days post-immunization. Sera were obtained from each blood sample by standard centrifugation, aliquoted and stored at −20°C until analyzed for antibody levels.
Production of recombinant CpP2
The rCpP2 protein fused to a Schistosoma japonicum glutathione-S-transferase (GST) tag was expressed from plasmid pGex4T-CpP2 in E. coli BL21 cells following manufacturer’s instructions. The GST fusion tag was cleaved with thrombin (GE Healthcare, Piscataway, NJ) and then thrombin was removed using pAminoBenzamidine-Agarose (SIGMA # A7155). Endotoxin was removed using Detoxi-Gel Endotoxin Removing Columns (Thermo Scientific, Rockford, IL). rCpP2 was also expressed as a 6xHis fusion protein in pQE81 vector (Qiagen, Valencia, CA) using E. coli DH5α cells (Invitrogen, Carlsbad, CA) and purified as previously described (18).
Measurement of antibody response by ELISA
Serum immunoglobulin A (IgA), IgG, IgG1, and IgG2a antibody responses specific to a highly purified rCpP2 were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (20, 21). Briefly, flat-bottom 96-well Immulon 2 ELISA plates were coated with 2 μg/ml of rCpP2 in 0.1 M carbonate buffer (pH 9.6) using 50 μl per well and incubated overnight at 4°C. The plates were blocked with PBS containing 0.3% Tween-20 for 1 h at 4°C. Individual serum samples were diluted 1:100 (IgA and IgG) and 1:50 (IgG1 and IgG2a), respectively, in 0.05% Tween 20-PBS, applied to the wells in duplicate, and incubated for 2 h at room temperature. After the plates were washed, bound antibodies were detected by incubation for 1 h at room temperature with biotin-labeled conjugate goat anti-mouse IgG and IgA diluted 1:1000 in PBS-T, and IgG1, IgG2a (Southern Biotech, Birmingham, AL) diluted 1:800. The plates were washed and incubated for 30 min with a 1:500 dilution of peroxidase labeled streptavidin (KPL, Gaithersburg, MD) and developed using the TMB microwell system (KPL). The optical density (OD) was measured in an ELISA reader at 450 nm.
Measurement of antibody response by multiplex bead assay
A multiplex bead assay was designed to detect IgG antibodies directed against the carboxy-terminal sequence of the CpP2 protein as well as those directed against epitopes present on the full-length P2 protein. A peptide with the sequence SGSGKKKEEEEEEEGDLGFSLFD was dissolved in PBS buffer (10 mg/ml) and adjusted to a pH of approximately 7. Purified GST protein (1 mg) and P2 peptide (1 mg) were incubated in a final volume of 1 ml of PBS buffer for 1 hour at room temperature in the presence of 10 μmoles of glutaraldehyde. Unbound peptide and low-molecular-weight byproducts were removed by multiple PBS washes in a Centricon-30 centrifugal filter device (Millipore, Billerica, MA). GST, P2 peptide-GST, and rCpP2 were covalently cross-linked to carboxylated polystyrene microspheres (SeroMap beads, Luminex Corp., Austin, TX) as previously described (22). Mouse sera were diluted 1:200 in PBS buffer containing 0.5% BSA, 0.05% Tween-20, 0.02% sodium azide, 0.5% polyvinyl alcohol, and 0.8% polyvinylpyrrolidone, treated as previously described (22), and assayed at a final dilution of 1:400. A biotinylated monoclonal rat anti-mouse IgG antibody (1:500 dilution, Southern Biotech) and R-phycoerythrin-labeled streptavidin (1:200 dilution, Invitrogen) were used to detect antibodies bound to the beads. Assays were run in duplicate using a Luminex instrument (Luminex Corp.) with Bio-Plex 4.1 software (BioRad, Hercules, CA), and the median fluorescent intensity (MFI) for each analyte was calculated. The mean values for the duplicate wells are reported. Human sera that react with the carboxy-terminal 15 amino acids of the CpP2 protein (18) exhibited strong IgG responses to both the full-length protein and to the P2 peptide-GST construct by multiplex assay (data not shown).
Cytokine assays
For the detection of interferon gamma, splenocytes from immunized mice were cultured with different stimuli as described for the lymphocyte proliferation assay. Cell-free supernatants were assayed in triplicate according to the manufacturer’s direction (R&D Systems, Minneapolis, MN).
C. parvum isolation and challenge infection
The C. parvum isolate used for this study was the IOWA bovine isolate. Oocysts were collected, purified through discontinuous sucrose and cesium chloride gradients and stored as previously described (19). Oocyst inocula were prepared by washing purified oocysts (stored < 6 months) with PBS (pH 7.2) to remove potassium dichromate. The different treatment groups of IL-12p40KO mice were challenged with a dose of 1 × 103 C. parvum oocysts by oral gavage 2 wk after the last immunization. Parasite burden was assessed by flow cytometry as previously described (23). Briefly, fecal samples were collected from the mice at 3-day intervals and processed through microscale sucrose gradients in 2.0 ml microcentrifuge tubes. The partially purified stool concentrate containing oocysts was incubated for 30 min at 37°C with 5 μl of an oocyst-specific monoclonal antibody conjugated with fluorescein isothiocyanate (OW50-FITC) and then analyzed by flow cytometry as previously described (23). Absolute counts were calculated from the data files for oocysts per 100 μl of sample suspension.
Proliferation assays
Spleen cells were harvested from CpP2-DNA-immunized or control (vector-immunized) mice 14 days after the last immunization. Cells were seeded in triplicate in 96 well flat-bottomed microtiter plates (Nalge Nunc Inc, Denmark) at 2 × 105 cells per well in a total volume of 200 μl culture medium. Each well was stimulated with or without different concentrations of recombinant CpP2 protein. Con A and LPS were used as measures of overall responsiveness (positive controls). Plates were incubated for 72 h in 5% CO2 at 37°C, followed by an additional 18 h after the cells were pulsed with 0.5 mCi/ml [3H]-thymidine (ICN Radiochemicals, Aurora, OH). Cells were harvested onto glass fiber mats using a cell harvester (Skatron Instrument Inc, Sterling, VA). The proliferative response of the cells was assessed by measuring [3H]-thymidine incorporation with a scintillation counter (Beckman Coulter, Indianapolis IN). The results are expressed as counts per minute (cpm) ± standard deviation (SD) in each triplicate.
Statistical analysis
Data are expressed as means ± standard deviations. Proliferation data, cytokine expression, antibody levels and parasite load were analyzed by Student’s t-test and by using analysis of variance. Parasite load data were analyzed with SPSS 17.0. Statistical significance was assessed using an alpha level of 0.05 unless otherwise noted. Normality of the samples of parasite was assessed using the Shapiro-Wilk test. A P value of <0.05 was considered to be significant.
Results
Serum antibody responses in CpP2-vaccinated mice
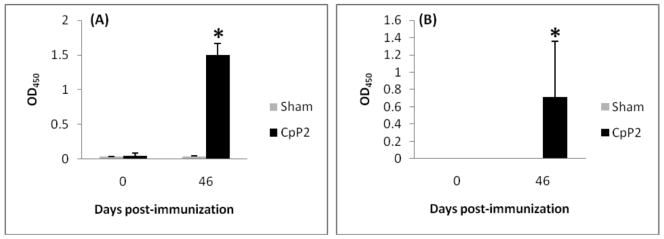
Immunization of C57BL/6 IL-12p40KO mice three times with 100 μg of the recombinant plasmid pUMVC4b-CpP2 at two week intervals resulted in the generation of a specific anti-P2 IgG antibody response (Fig. 1, panel A). Antibody response was detected four weeks after the primary dose (but two weeks after the first booster dose) of the vaccine was administered (data not shown). The anti-P2 antibody response was further enhanced after the third dose of the vaccine was administered. The anti-CpP2 antibody response was not observed in control mice (C57BL/6 IL-12p40KO) that were injected with the empty plasmid vector. We saw poor responses to the recombinant plasmid in wildtype mice (data not shown). In addition to the whole IgG antibody levels, IgG isotypes in the serum of immunized C57BL/6 IL-12p40KO mice were also assessed. pUMVCb4-CpP2 DNA vaccine induced only antibodies of the IgG1 isotype (Fig. 1, panel B); an IgG2a response was not observed (data not shown). Again, no anti-CpP2 antibody response was detected in control animals injected with the vector plasmid. Sera were also tested for IgA responses to CpP2, but no specific antibody of this class was detected in any of the experimental groups (data not shown).
FIG. 1.
CpP2-specific serum immunoglobulin response in IL-12p40KO mice immunized three times with 100 μg of the recombinant plasmid containing CpP2-DNA. (A) Specific-IgG and (B) IgG1 levels against rCpP2 at day 0 and 46 post initial immunization as measured by ELISA. Data were analyzed by using analysis of variance. Data are presented as the mean OD450 from each group of mice ± the standard deviation. (*, p<0.05).
Cellular immune response induced by CpP2-DNA vaccine
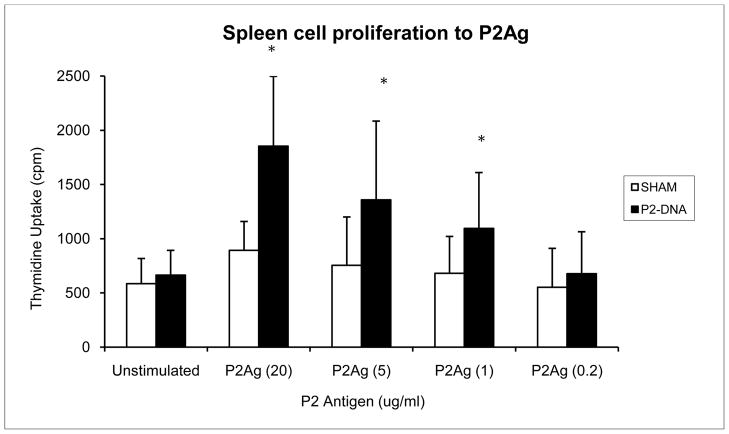
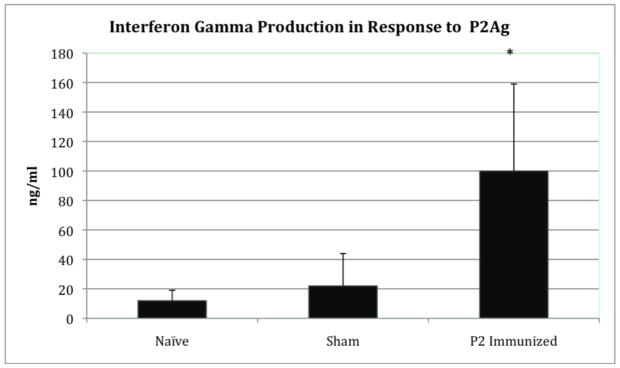
To examine the generation of CpP2-specific cellular immune responses in IL-12p40KO mice, proliferative responses of splenocytes to recombinant CpP2 protein were measured 2 wk after the last immunization. Splenocytes were stimulated in vitro with rCpP2, and the proliferative response was measured (Fig. 2). Our data show that stimulation of cells from CpP2-DNA immunized mice with different concentrations of rCpP2 protein resulted in a dose-dependent proliferative response. Stimulation index was also calculated and is as follows for each sample (ConA 14.3, LPS 15.3, P2 (20 μg/ml) 2.8; P2 (5 μg/ml) 2.1; P2 (1 μg/ml) 1.6; P2(0.2 μg/ml) 1.1. Increased levels of IFN-γ (3–5 fold higher) were observed in splenocyte cultures from mice immunized with CpP2-DNA compared with mice immunized with empty plasmid (sham) or from cultures from naïve mice (P<0.05) (Fig. 3).
FIG. 2.
In vitro proliferation of splenocytes from P2-DNA immunized IL-12p40KO mice. Female IL-12p40KO mice (5 mice per group) were immunized by injecting a total of 100 μg Cp23-DNA in a plasmid vector subcutaneously into each ear once every 14 days. Control mice received the vector only. Two weeks following the third immunization, mice were sacrificed and the spleens harvested. Single spleen cell suspensions were prepared and their proliferation against C. parvum recombinant P2 protein was measured by standard tritiated thymidine incorporation assay. Data were analyzed by Student’s t-test. Data is presented as the mean counts per minute ± the standard deviation. (*, p<0.05).
FIG. 3.
IFN-γ response in IL-12p40KO mice immunized with CpP2-DNA, vector control (sham) or nothing (naïve). Splenocytes from immunized mice were cultured with different stimuli as described for the lymphocyte proliferation assay. The response to P2Ag is shown above. Data were analyzed by Student’s t-test. Data is presented as ng/ml of IFN-γ ± the standard deviation (*, p<0.05) as measured by ELISA.
Effect of CpP2-DNA vaccine on oocyst shedding
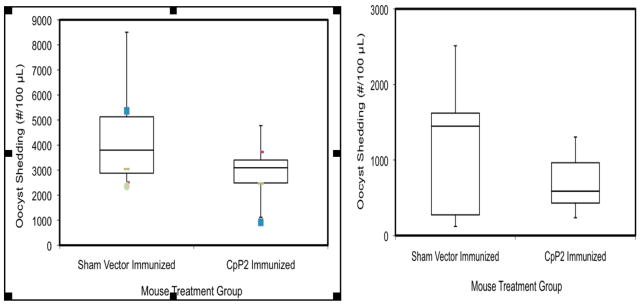
To address whether CpP2-DNA vaccine was able to induce protection against C. parvum infection, IL-12p40KO mice were challenged 2 weeks after the last immunization by oral exposure to 1 × 103 C. parvum oocysts. The parasite burden was assessed through day 14 post infection. Two replicate experiments are shown in Figure 4. Immunization of the mice with CpP2-DNA resulted in an approximate median reduction in the level of oocysts shedding of 40% and 60% respectively, compared to animals immunized with the control plasmid. For one study (left panel) a two sample (unequal variance) t-test indicated a statistically significant difference in oocyst shedding between the sham group and CpP2 group (p = 0.029), whereas in a second trial (right panel) no significant difference was found between the Sham and CpP2 groups (p = 0.179). Neither of the groups had statistically significant departures from normality, although p-value from the Shapiro-Wilk test for the Sham group was close to the nominal level (p = 0.051).
FIG. 4.
Box and whisker plot the median number of oocysts per group. The ends of the whisker are set at 1.5*IQR above the third quartile (Q3) and 1.5*IQR below the first quartile (Q1). Values outside this range are shown as outliers. Parasite oocyst burden in P2-DNA immunized IL-12p40 KO mice after challenge with C. parvum. Female IL-12p40 KO mice were immunized by injecting a total of 100 μg P2-DNA in a plasmid vector subcutaneously into both ears once every 14 days. Control mice injected with either PBS or the vector alone. Two weeks following the third immunization, mice were inoculated with 1000 C. parvum oocysts by oral gavage. Fecal samples were collected from individual mice at the days indicated, and assessed for parasite load by flow cytometry as described earlier (1). For one study (left panel) a two sample (unequal variance) t-test indicated a statistically significant difference in oocyst shedding between the Sham group and CpP2 group (p = 0.029), whereas in a second trial (right panel) no significant difference was found between the Sham and CpP2 groups (p = 0.179).
Effect of prime-boost on antibody responses and oocyst shedding
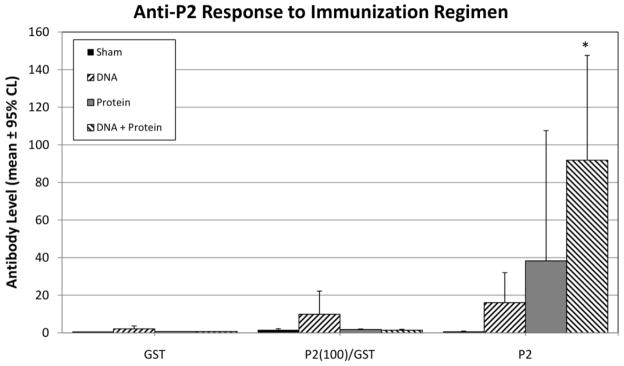
To determine if immunization with a DNA-vaccine vector followed by a protein boost could enhance the antibody response and induce greater protection, C57BL/6 IL-12KO mice were immunized with 100 μg of either pUMVC4b (control vector) or the recombinant DNA-vaccine vectors pUMVC4b-CpP2 at 2-week intervals, followed by immunization with 200 μg of recombinant P2 protein. In this experiment, we also compared humoral responses to the full-length recombinant protein to those targeting the carboxy-terminal P2 peptide (Fig. 5). Prime-boost immunization resulted in a significant increase in titers of IgG against the full length P2 recombinant protein but responses against the P2 carboxy-terminal peptide remained low (Fig. 5). While oocyst shedding levels were lower in prime-boost mice on day 7 post infection, no statistically significant differences in shedding were observed between sham and immunized groups (data not shown).
FIG. 5.
CpP2-specific serum immunoglobulin response in IL-12p40KO mice immunized with CpP2-DNA, protein, or CpP2-DNA followed with protein (prime-boost). Specific-IgG levels against GST (control), P2 peptide-GST, or rCpP2 as measured by multiplex bead assay, 2 weeks after final immunization. Data were analyzed by using analysis of variance. Data, OD450 are presented as square-root transformed means from each group of mice ± 95% confidence limits (*, p<0.05).
4. Discussion
Cryptosporidiosis represents a serious health problem. To date, there are no efficient vaccines and limited treatments for the disease, particularly among immunocompromised individuals. Consequently, the characterization of new parasite antigens capable of eliciting protective immune responses is an absolute priority. Immunization with DNA against other pathogens has been widely reported, and has been shown to induce humoral as well as antigen-specific T cell responses (24, 25, 26). Several studies have evaluated antigens of cryptosporidiosis using DNA immunization and demonstrated induction of specific immunogenic responses (27, 28, 29, 30, 31, 32, 33). However, the efficacies of these vaccines were not always evaluated or they were evaluated using a mouse model resistant to C. parvum infection (31, 32, 33, 34).
Our laboratory developed a C57BL/6 IL-12KO mouse model to evaluate C. parvum infection (35). This model offers a number of qualities essential for immunological studies, such as high susceptibility, development of a mixed Th1/Th2 mucosal response, resolution of the infection, and protection from re-infection (29, 36). In this study, we analyzed the immunogenic properties of the C. parvum ribosomal protein P2 antigen following subcutaneous DNA immunization in the IL-12KO mouse model. Our results demonstrate the efficacy of the CpP2-DNA vaccine to generate antigen-specific humoral and cellular responses. A significant IgG antibody response was observed in CpP2 immunized mice 6 wk after initial immunization. It has been demonstrated that DNA vaccines have the ability to preferentially generate Th1 responses in mouse models (37). However, immunization with CpP2-DNA apparently induces a predominant Th2 response, since the anti-P2 antibodies elicited were mainly of the IgG1 isotype. This observation could be attributed to the type of vector used and/or the innate properties of the antigen. Specific antigen expression has also been found to affect the profile of immune responses (38, 39, 40). Other parasitic ribosomal proteins such as the P0 have been reported to generate a strong Th1 antibody response, but this response was dependent on the type of adjuvant used and the mouse strain (41). Immune responses reported by Ehigiator et al (29), in which the Cp23 antigen from C. parvum was evaluated as a DNA vaccine using the pUMVC4b vector generated both IgG1 and IgG2a responses (though a larger IgG1 response was detected). Additionally, a recent report evaluating pVAX-15–23 as vaccine candidate (30) using the C57BL/6 IL-12KO model also generated a strong Th1 response and balanced Th1/Th2 antibody responses. The route of administration may also play a role in the specific immune response. It has been reported that the C. parvum Cp15 antigen elicited both IgG2a and IgA immune responses when administered mucosally (41). In our case, no detectable serum IgG2a or IgA were detected in subcutaneously immunized mice.
DNA immunization against other pathogens has elicited cellular immune responses in addition to antibody responses (42). Therefore, we evaluated the proliferative response of rCpP2 in immunized mice. Our data demonstrates a specific, dose-dependent stimulation response in splenocytes from CpP2-DNA immunized mice, albeit less pronounced than with Cp23 (29). The results also show that P2 immunized mice were able to enhance IFN-γ production compared to mice immunized with plasmid alone. Antigen-specific cellular responses elicited by the CpP2-DNA vaccine may be essential, as T-cell mediated responses play a major role in producing immunity against Cryptosporidium (43, 42).
The efficacy of the CpP2-DNA vaccine was evaluated in addition to specific CpP2 humoral and cellular responses. An overall trend in reduction in infection was observed in the mice immunized with CpP2-DNA compared to the control mice when challenged with a C. parvum inoculum. Independent experimental trials revealed variability in mouse responses. Lastly, a prime-boost regime was performed to enhance immunological responsiveness. Increased antibody titers were observed in the prime-boost method compared to cDNA alone. However, overall responses to the carboxy-terminal P2 peptide (the portion of the protein predominantly recognized by human infection sera) were low. It may be that strong antibody responses to this particular antigen requires repeated exposure over an extended length of time. Because anti-CpP2 IgG antibodies are found mainly among residents of countries in the developing world where Cryptosporidium infection occurs early in life and re-exposure is frequent, an immunization regime that mimics more closely the chronic exposure observed in developing countries may generate a better protective response.
Altogether, our data indicate that DNA immunization with the C. parvum P2 antigen induces an antigen-specific humoral and cellular response. As the results suggest, the CpP2 antigen could be a potential vaccine candidate when administered in a DNA formulation. Further evaluation using alternative vectors or mouse models may identify conditions that yield more potent and balanced responses. Development of an effective vaccine against Cryptosporidium infection may also be achieved by the inclusion of this antigen as part of a multiantigen vaccine, a strategy demonstrated to be effective against murine cutaneous leishmaniasis (45, 46, 47).
Highilights.
P2 (CpP2) is an important immunodominant marker in C. parvum infection
Mice immunized with a DNA vaccine encoding the C. parvum P2 antigen were able to elicit humoral and cellular responses
P2 antigen has potential to generate protective immunity against C. parvum infection
Acknowledgments
We thank Dr. Michael Arrowood (CDC, Atlanta) and Dr. Longti Xie (CDC, Atlanta) for production and purification of oocysts. We would like to thank Bramchetna Bedi (VA Medical Center, Decatur, GA) for technical assistance and Traci Leong (Emory, Atlanta) for help with statistical analysis. This work was supported, in part, by Grant RO1-AI-36680 from the National Institutes of Health, and Medical Research Service, U.S. Department of Veterans Affairs.
Footnotes
Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoxie NJ, Davis JP, Vergeront JM, Nashold RD, Blair KA. Cryptosporidiosis-associated mortality following a massive waterborne outbreak in Milwaukee, Wisconsin. Am J Public Health. 1997;87(12):2032–35. doi: 10.2105/ajph.87.12.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riggs MW. Recent advances in cryptosporidiosis: the immune response. Microbes Infect. 2002;4:1067–80. doi: 10.1016/s1286-4579(02)01631-3. [DOI] [PubMed] [Google Scholar]
- 3.Sadraei J, Rizvi MA, Baveja UK. Diarrhea, CD4+ cell counts and opportunistic protozoa in Indian HIV-infected patients. Parasitol Res. 2005;97(4):270–3. doi: 10.1007/s00436-005-1422-7. [DOI] [PubMed] [Google Scholar]
- 4.Levin MJ, Vazquez M, Kaplan D, Schijman AG. The Trypanosoma cruzi ribosomal P protein family: classification and antigenicity. Parasitol Today. 1993;9:381–4. doi: 10.1016/0169-4758(93)90088-w. [DOI] [PubMed] [Google Scholar]
- 5.Skeiky YAW, Benson DR, Parsons M, Elkon KB, Reed SG. Cloning and expression of Trypanosoma cruzi ribosomal protein P0 and epitope analysis of anti-P0 autoantibodies in Chagas’ disease patients. J Exp Med. 1992;176:201–11. doi: 10.1084/jem.176.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skeiky YAW, Benson DR, Guderian JA, Sleath PR, Parsons M, Reed SG. Trypanosoma cruzi acidic ribosomal P protein gene family. Novel P proteins encoding unusual cross-reactive epitopes. J Immunol. 1993;151:5504–15. [PubMed] [Google Scholar]
- 7.Brooks-Worrell BM, Splitter GA. Antigens of Brucella abortus S19 immunodominant for bovine lymphocytes as identified by one- and two-dimensional cellular immunoblotting. Infect Immun. 1992;60:2459–64. doi: 10.1128/iai.60.6.2459-2464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalrymple BP, Peters JM. Identification of L10e/L12e ribosomal protein genes in Babesia bovis. Nucleic Acid Res. 1992;20:2376. doi: 10.1093/nar/20.9.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkon K, Skelly S, Parnassa A, Moller W, Danho W, Weissbach H, et al. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1986;83:7419–23. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkon K, Bonfa E, Llovet R, Danho W, Weissbach H, Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci USA. 1988;85:5186–9. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, Uchiumi T, Ozawa T, Kikuchi M, Nakano M, Kominami R, et al. Autoantibodies against ribosomal proteins found with high frequency in patients with systemic lupus erythematosus with active disease. J Rheumatol. 1991;18:1681–4. [PubMed] [Google Scholar]
- 12.Chatterjee S, Singh S, Sohoni R, Singh NJ, Vaidya A, Long C, et al. Antibodies against ribosomal phosphoprotein P0 of Plasmodium falciparum protect mice against challenge with Plasmodium yoelii. Infect Immun. 2000;68(7):4312–8. doi: 10.1128/iai.68.7.4312-4318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skeiky YAW, Benson DR, Elwasila M, Badaro R, Burns JM, Reed SG. Antigens shared by Leishmania species and Trypanosoma cruzi: immunological comparison of the acidic ribosomal P0 proteins. Infect Immun. 1994;62(5):1643–51. doi: 10.1128/iai.62.5.1643-1651.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skeiky YAW, Kennedy M, Kaufman D, Borges MM, Guderian JA, Scholler JK, et al. LeIF: a recombinant Leishmania protein that induces an IL-12-mediated Th1 cytokine profile. J Immunol. 1998;161(11):6171–9. [PubMed] [Google Scholar]
- 15.Zhang H, Lee EG, Liao M, Compaore MKA, Zhang G, Kawase O, et al. Identification of ribosomal phosphoprotein P0 of Neospora caninum as a potential common vaccine candidate for the control of both neosporosis and toxoplasmosis. Mol Biochem Parasitol. 2007;153(2):141–8. doi: 10.1016/j.molbiopara.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Rajeshwari K, Patel K, Nambeesan S, Mehta M, Sehgal A, Chakraborty T, Sharma S. The P domain of the P0 protein of Plasmodium falciparum protects against challenge with malaria parasites. Infect Immun. 2004;72(9):5515–21. doi: 10.1128/IAI.72.9.5515-5521.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iborra S, Abánades DR, Parody N, Carrión J, Risueño RM, Pineda MA, et al. The immunodominant T helper 2 (Th2) response elicited in BALB/c mice by the Leishmania LiP2a and LiP2b acidic ribosomal proteins cannot be reverted by strong Th1 inducers. Clin Exp Immunol. 2007;150(2):375–85. doi: 10.1111/j.1365-2249.2007.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priest JW, Kwon JP, Montgomery JM, Bern C, Moss DM, Freeman AR, et al. Cloning and characterization of the acidic ribosomal protein P2 of Cryptosporidium parvum, a new 17-kilodalton antigen. Clin Vaccine Immunol. 2010;17(6):954–65. doi: 10.1128/CVI.00073-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrowood MJ, Donaldson K. Improved purification methods for calf-derived Cryptosporidium parvum oocysts using discontinuous sucrose and cesium chloride gradients. J Eukaryot Microbiol. 1996;43(5):89S. doi: 10.1111/j.1550-7408.1996.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 20.Priest JW, Kwon JP, Moss DM, Roberts JM, Arrowood MJ, Dworkin MS, et al. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigen. J Clin Microbiol. 1999;37(5):1385–92. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith LM, Bonafonte MT, Mead JR. Cytokine expression and specific lymphocyte proliferation in two strains of Cryptosporidium parvum-infected gamma-interferon knockout mice. J Parasitol. 2000;86(2):300–7. doi: 10.1645/0022-3395(2000)086[0300:CEASLP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Priest JW, Moss DM, Visvesvara GS, Jones CC, Li A, Isaac-Renton JL. Multiplex assay detection of immunoglobulin G antibodies that recognize Giardia intestinalis and Cryptosporidium parvum antigens. Clin Vaccine Immunol. 2010;17(11):1695–707. doi: 10.1128/CVI.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrowood MJ, Hurd MR, Mead JR. A new method for evaluating experimental cryptosporidial parasite loads using immunofluorescent flow cytometry. J Parasitol. 1995;81(3):404–9. [PubMed] [Google Scholar]
- 24.Méndez S, Gurunathan S, Kamhawi S, Belkaid Y, Moga MA, Skeiky YAW, et al. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J Immunol. 2001;166(8):5122–8. doi: 10.4049/jimmunol.166.8.5122. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Ren J, Da’dara A, Harn D, Xu M, Si J, et al. The protective effect of a Schistosoma japonicum Chinese strain 23 kDa plasmid DNA vaccine in pigs is enhanced with IL-12. Vaccine. 2004;23(1):78–83. doi: 10.1016/j.vaccine.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Revilla C, Sonabend AM, Rosas G, Toledo A, Meneses G, Lopez-Casillas F, et al. Intrahepatic DNA vaccination: unexpected increased resistance against murine cysticercosis induced by non-specific enhanced immunity. J Parasitol. 2006;92(3):655–7. doi: 10.1645/GE-665R1.1. [DOI] [PubMed] [Google Scholar]
- 27.Sagodira S, Buzoni-Gatel D, Iochmann S, Naciri M, Bout D. Protection of kids against Cryptosporidium parvum infection after immunization of dams with CP15-DNA. Vaccine. 1999b;17(19):2346–55. doi: 10.1016/s0264-410x(99)00041-9. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins M, Kerr D, Fayer R, Wall R. Serum and colostrum antibody responses induced by jet-injection of sheep with DNA encoding a Cryptosporidium parvum antigen. Vaccine. 1995;13(17):1658–64. doi: 10.1016/0264-410x(95)00121-g. [DOI] [PubMed] [Google Scholar]
- 29.Ehigiator HN, Romagnoli P, Priest JW, Secor WE, Mead JR. Induction of murine immune responses by DNA encoding a 23-kDa antigen of Cryptosporidium parvum. Parasitol Res. 2007;101:943–50. doi: 10.1007/s00436-007-0565-0. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Luo J, Amer S, Guo Y, Hu Y, Lu Y, et al. Multivalent DNA vaccine induces protective immune responses and enhanced resistance against Cryptosporidium parvum infection. Vaccine. 2011;29(2):323–8. doi: 10.1016/j.vaccine.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 31.He H, Zhao B, Liu L, Zhou K, Qin X, Zhang Q, et al. The humoral and cellular immune responses in mice induced by DNA vaccine expressing the sporozoite surface protein of Cryptosporidium parvum. DNA Cell Biol. 2004;23(5):335–9. doi: 10.1089/104454904323090967. [DOI] [PubMed] [Google Scholar]
- 32.Yu Q, Li J, Zhang X, Gong P, Zhang G, Li S, Wang H. Induction of immune responses in mice by a DNA vaccine encoding Cryptosporidium parvum Cp12 and Cp21 and its effect against homologous oocysts challenge. Vet Parasitol. 2010;172(1–2):1–7. doi: 10.1016/j.vetpar.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 33.Sagodira S, Iochmann S, Mevelec MN, Dimier-Poisson I, Bout D. Nasal immunization of mice with Cryptosporidium parvum DNA induces systemic and intestinal immune response. Parasit Immunol. 1999a;21(10):507–16. doi: 10.1046/j.1365-3024.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 34.Takashima Y, Xuan X, Kimata I, Iseki M, Kodama Y, Nagane N, et al. Recombinant bovine herpesvirus-1 expressing p23 protein of Cryptosporidium parvum induces neutralizing antibodies in rabbits. J Parasitol. 2003;89(2):276–82. doi: 10.1645/0022-3395(2003)089[0276:RBHEPP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Ehigiator HN, McNair N, Mead JR. IL-12 knockout C57BL/6 mice are protected from reinfection with Cryptosporidium parvum after challenge. J Eukaryot Microbiol. 2003;50(Suppl):539–41. doi: 10.1111/j.1550-7408.2003.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 36.Ehigiator HN, Romagnoli P, Borgelt K, Fernandez M, McNair N, Secor WE, et al. Mucosal cytokine and antigen-specific responses to Cryptosporidium parvum in IL-12p40 KO mice. Parasite Immunol. 2005;27(1–2):17–28. doi: 10.1111/j.1365-3024.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 37.Gurunathan S, Stobie L, Prussin C, Sacks DL, Glaichenhaus N, Fowell DJ, et al. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T-cells. J Immunol. 2000;165:915–24. doi: 10.4049/jimmunol.165.2.915. [DOI] [PubMed] [Google Scholar]
- 38.Iochmann S, Sagodira S, Mévélec MN, Répérant JM, Naciri M, Coursaget P, et al. Comparison of the humoral and cellular immune responses to two preparations of Cryptosporidium parvum CP15/60 recombinant protein. Microb Pathog. 1999;26(6):307–15. doi: 10.1006/mpat.1999.0276. [DOI] [PubMed] [Google Scholar]
- 39.Oran AE, Robinson HL. DNA vaccines, combining form of antigen and method of delivery to raise a spectrum of IFN-γ and IL-4-producing CD4+ and CD8+ T-cells. J Immunol. 2003;171:1999–2005. doi: 10.4049/jimmunol.171.4.1999. [DOI] [PubMed] [Google Scholar]
- 40.Espino AM, Osuna A, Gil R, Hillyer GV. Fasciola hepatica: humoral and cytokine responses to a member of the saposin-like protein family following delivery as a DNA vaccine in mice. Exp Parasitol. 2005;110(4):374–83. doi: 10.1016/j.exppara.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Iborra S, Carrión J, Anderson C, Alonso C, Sacks D, Soto M. Vaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c mice. Infect Immun. 2005;73:5842–52. doi: 10.1128/IAI.73.9.5842-5852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donnelly JJ, Ulmer JB, Liu MA. Review article: immunization with DNA. J Immunol Meth. 1994;176(2):145–52. doi: 10.1016/0022-1759(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Harp JA, Harmsen AG. Requirements for CD4+ cells and gamma interferon in resolution of established Cryptosporidium parvum infection in mice. Infect Immun. 1993;61(9):3928–32. doi: 10.1128/iai.61.9.3928-3932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald V, Robinson HA, Kelly JP, Bancroft GJ. Cryptosporidium muris in adult mice: adoptive transfer of immunity and protective role of CD4 versus CD8 cells. Infect Immun. 1994;62(6):2289–94. doi: 10.1128/iai.62.6.2289-2294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campos-Neto A, Webb JR, Greeson K, Coler RN, Skeiky YAW, Reed SG. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion protein confers protection against Leishmania major infection in susceptible BALB/c mice. Infect Immun. 2002;70(6):2828–36. doi: 10.1128/IAI.70.6.2828-2836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coler RN, Skeiky YAW, Bernards K, Greeson K, Carter D, Cornellison CD, Modabber F, Campos-Neto A, Reed SG. Immunization with a polyprotein vaccine consisting of the T-cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect Immun. 2002;70(8):4215–25. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rafati S, Salmanian AH, Taheri T, Vafa M, Fasel N. A protective cocktail vaccine against murine cutaneous leishmaniasis with DNA encoding cysteine proteinases of Leishmania major. Vaccine. 2001;19:3369–75. doi: 10.1016/s0264-410x(01)00081-0. [DOI] [PubMed] [Google Scholar]