Abstract
Increased red cell distribution width (RDW), a marker of anisocytosis, has been associated with adverse outcomes in multiple settings. Whether RDW is predictive of mortality in patients with peripheral artery disease (PAD) is unknown. We studied 13,039 consecutive outpatients (age 69.5 ±12.0 years, 60.9% men, 97.6% white) with PAD identified by non-invasive lower-extremity arterial testing at Mayo Clinic from 1/97 to 12/07, with follow-up through 9/09. We defined PAD as low (≤0.9) or high (≥1.4) ankle-brachial index (ABI). Cardiovascular risk factors and comorbidities were ascertained using electronic medical record (EMR)-based algorithms. RDW was obtained from the complete blood count drawn around the time of arterial evaluation. Mortality was ascertained using the Mayo EMR and Accurint® database. The association of RDW with all-cause mortality was analyzed by multivariable Cox proportional hazards regression. During a median follow-up of 5.5 years, 4039 (31.0 %) deaths occurred (28.7% in low and 38.9% in high ABI subsets). After adjustment for age, sex, cardiovascular risk factors and comorbidities, patients in the highest quartile of RDW (>14.5%) had 66% greater risk of mortality compared to the lowest quartile (<12.8%) (P<0.0001); a 1% increment in RDW was associated with 10% greater risk of all-cause mortality (hazard ratio, 1.10, 95% confidence interval [CI], 1.08 to 1.12, P<0.0001). The adjusted hazard ratio was similar in the low (1.10, 1.08-1.12) and high (1.09, 1.06-1.12) ABI subsets. In conclusion, RDW, a routinely available measure, is an independent prognostic marker in patients with PAD.
Introduction
Peripheral arterial disease (PAD), a surrogate for systemic atherosclerotic vascular disease, affects approximately 8 million individuals in USA, and is associated with increased mortality and morbidity.1,2 Predictors of mortality in patients with PAD are not well defined and would be valuable for risk stratification and clinical decision making, especially if routinely and inexpensively obtained. The ankle-brachial index (ABI) is an established non-invasive test for PAD, which is defined as a low (≤ 0.9) or high (≥ 1.4) ABI. Low ABI results from arterial lumen narrowing due to atherosclerosis3 and high ABI results from medial arterial calcification and poorly-compressible arteries.4 Whether RDW is associated with mortality in PAD is not known, and we therefore sought to investigate the prognostic value of RDW in patients with PAD.
Methods
From January 1st 1997 to December 31st 2007, 20,996 outpatients aged ≥ 18 years were referred to the non-invasive vascular laboratory of the Gonda Vascular Center for lower-extremity arterial evaluation. ABI was measured using an established protocol.5 We excluded patients who refused participation in research (n=760) and patients with non-atherosclerotic vascular diseases (n=1154), such as vasculitides, by using a set of appropriate International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) diagnosis and procedure codes. We identified 13,039 patients with PAD defined as 1) ABI ≤ 0.9 at rest or 1 min after exercise or 2) presence of poorly compressible arteries (any ABI in one leg ≥ 1.4 or lower extremity blood pressure ≥ 255 mmHg or non reproducible ankle systolic blood pressure). The index date was the date of lower-extremity arterial testing with abnormal ABI measurement. Follow-up was started at the index date and ended on 30th September 2009. The study was approved by the Institutional Review Board of the Mayo Foundation.
ICD-9 CM codes up to 6 months following the index date, and natural language processing were used to ascertain demographic information, smoking status, medications, conventional cardiovascular risk factors and comorbidities from the Mayo electronic medical record (EMR) as previously described.6 The outpatient complete blood count and other laboratory variables closest to the index date were obtained from the laboratory database. RDW was extracted as part of the result of complete blood count, obtained in the Central Clinical Laboratory, using Sysmex XE-5000 hematology analyzer (Mundelein, Illinois, Sysmex America, Inc.). Resting systolic and diastolic blood pressure levels were obtained as structured observations from the EMR. Patients were identified as smokers if they were actively smoking or had discontinued smoking within 1 year before the index date. Hypertension was defined as either systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥ 90 mmHg during two serial measurements within 3 months closest to the index date, or a prior diagnosis of hypertension with use of antihypertensive medication. Diabetes was defined as fasting blood glucose ≥126 mg/dL or random glucose >200 mg/dl or hemoglobin A1c >6.5%, or a prior diagnosis with any use of oral antidiabetic agent and/or insulin. Dyslipidemia was defined as total cholesterol >220 mg/dl, or high-density cholesterol <40 mg/dl in men or <45 mg/dl in women, or triglycerides >200 mg/dl or use of a lipid-lowering medication.
We used the following ICD-9 CM codes to identify the following comorbidities: coronary heart disease including ischemic heart disease 410.××-414.××, or history of percutaneous coronary intervention or coronary artery bypass surgery 36.10-36.14; heart failure 428; cerebrovascular disease 430.××-438.×× or history of carotid stenting or endarterectomy 00.61, 00.63 and 38.10; chronic obstructive pulmonary disease 490-496, 500-505, 506.4, chronic kidney disease 582-583.7, 585,586, 588, malignancy 140-208. Estimated glomerular filtration rate was calculated based on the Modification of Diet in Renal Disease Study formula.7 All-cause mortality was ascertained from the Mayo EMR and the Accurint® database.8
Continuous variables are reported as mean ± standard deviation or median. Categorical variables are reported as frequencies and percentage. RDW was examined as a continuous variable and also categorized into quartiles using the following cutoffs: < 12.8%, 12.8%-13.5%, 13.5%-14.5% and > 14.5%. We compared distribution of demographic characteristics, conventional cardiovascular risk factors, comorbidities and laboratory variables across RDW quartiles by analysis of variance for continuous variables and chi-square test for categorical variables. Statistically significant differences for the variables were assessed after adjustment for age, sex and body-mass index, using multivariable logistic regression. Cox proportional hazards regression was performed to evaluate the association of RDW with all-cause mortality, adjusting for age and sex (model 1), and additionally adjusting for body mass index, smoking status, hypertension, dyslipidemia, diabetes, cerebrovascular disease, coronary heart disease, heart failure, chronic obstructive pulmonary disease, chronic kidney disease, malignancy, aspirin and statin use, and hemoglobin level (model 2) in the overall sample and the two ABI subsets. Kaplan-Meier method was used to compare survival in different quartiles of RDW groups, using the log-rank test. Hypothesis testing was 2-tailed with P < 0.05 considered statistically significant. Statistical analyses were performed with JMP and SAS software version 8.2 (SAS Institute Inc, Cary, NC).
Results
Among 13,039 patients with PAD, the majority were white (97.6%), 60.9% were men and mean age was 69.5±12.0 years. The prevalence of low ABI was 77.4% and of high ABI was 22.6%. The mean RDW was 13.9±1.9% in patients with PAD, 13.8±1.8 in low ABI and 14.3±2.1 in high ABI group (P < 0.001, after adjustment for age and sex). A greater proportion of patients in high ABI group (18.9% in high ABI vs 10.3% in low, P < 0.001, after adjustment for age and sex) had RDW >15.6% (upper limit of Mayo Clinic normal values). Clinical characteristics of patients with PAD in quartiles of RDW are presented in Table 1. Patients in the highest RDW quartile were older and with higher prevalence of cardiovascular risk factors and comorbidities, except for cerebrovascular disease and malignancy. There was no statistical significant difference in aspirin use across the quartiles (P = 0.25).The higher quartile was associated with lower mean corpuscular volume, hemoglobin and estimated glomerular filtration rate. After adjustment for age and sex, the high ABI subset had more patients in the highest quartile (35.7% in high vs 23.1% in low ABI, P < 0.001) and lesser patients in the lowest quartile of RDW (17.8% in high vs 24.6% in low ABI).
Table 1.
Baseline Characteristics of Peripheral Artery Disease Patients by quartiles of Red Cell Distribution Width
| Quartiles | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Variable | (n=3008) | (n=3378) | (n=3273) | (n=3380) |
| Age (years) | 67.8 (12.9%) | 69.4 (11.9%) | 70.6 (11.3%) | 70.4 (11.6%) |
| Men | 1758 (58.4%) | 2134 (63.2%) | 2014 (61.5%) | 2039 (60.3%) |
| Body-mass index (kg/cm2) | 28.5 (5.1%) | 28.9 (5.4%) | 29.4 (5.8%) | 29.2 (6.6%) |
| Low ankle-brachial index | 2482 (82.5%) | 2729 (80.8%) | 2550 (77.9%) | 2326 (68.8%) |
| High ankle-brachial index | 526 (17.5%) | 649 (19.2%) | 723 (22.1%) | 1054 (31.2%) |
| Risk factors | ||||
| *Dyslipidemia | 2336 (77.7%) | 2646 (78.3%) | 2577 (78.7%) | 2573 (76.1%) |
| Hypertension | 2132 (70.9%) | 2506 (74.2%) | 2527 (77.2%) | 2543 (75.2%) |
| Diabetes | 960 (31.9%) | 1070 (31.7%) | 1178 (36.0%) | 1416 (41.9%) |
| Smoking | 2079 (79.3%) | 2494 (82.8%) | 2388 (80.5%) | 2392 (79.6%) |
| Comorbidities | ||||
| *Cerebrovascular disease | 910 (30.3%) | 1078 (31.9%) | 1053 (32.2%) | 1035 (30.6%) |
| Coronary heart disease | 1480 (49.2%) | 1826 (54.1%) | 1926 (58.9%) | 2051 (60.7%) |
| Heart failure | 267 (8.9%) | 386 (11.4%) | 539 (16.5%) | 969 (28.7%) |
| Chronic obstructive pulmonary disease |
408 (13.6%) | 530 (15.6%) | 580 (17.7%) | 647 (19.2%) |
| Chronic kidney disease | 141 (4.7%) | 202 (6.0%) | 317 (9.7%) | 644 (19.1%) |
| *Malignancy | 536 (17.8%) | 669 (19.8%) | 703 (21.5%) | 729 (21.6%) |
| Medications | ||||
| *Aspirin use | 1345 (44.7%) | 1549 (45.9%) | 1546 (47.2%) | 1556 (46.0%) |
| Statin use | 1021 (33.9%) | 1283 (38.0%) | 1306 (39.9%) | 1300 (38.5%) |
| Lab variables | ||||
| Red cell distribution width (%) | 12.3 (0.3) | 13.1 (0.2) | 13.9 (0.3) | 16.2 (2.1) |
| Erythrocyte count (1012/L) | 4.3 (0.5) | 4.3 (0.6) | 4.2 (0.6) | 4.1 (0.7) |
| Mean corpuscular volume (fL) | 92.0 (4.1) | 91.6 (4.5) | 90.9 (5.0) | 89.3 (8.1) |
| Hemoglobin (g/dL) | 13.5 (1.6) | 13.5 (1.7) | 13.1 (1.8) | 12.1 (1.9) |
| Creatinine (mg/dL) | 1.2 (0.5) | 1.3 (0.6) | 1.7 (0.7) | 1.7 (1.5) |
| eGFR (ml/min/1.73cm2) | 62.1 (18.0) | 60.9 (17.8) | 58.1 (19.4) | 53.6 (24.3) |
Continuous variables are expressed as mean (standard deviation); categorical variables are expressed as n (%); age, sex and body mass index adjusted the measures across the quartiles are significant at P < 0.01, except for the variables marked with *; coronary heart disease was defined as history of myocardial infarction, documented angina, abnormal stress test or coronary revascularization.
The association of RDW with mortality in the entire sample and in the two ABI subsets during the median follow-up of 5.5 years is presented in Table 2. Patients who died were older (73.5±10.3 years vs 67.8±12.3 years for patients alive), more often men (63.6%) and had higher prevalence of all the cardiovascular risk factors and comorbidities. After adjustment for age and sex, the decedents had higher RDW (14.3±2.1 vs 13.7±1.6 for patients alive, P < 0.001), lower statin use (25.1% vs 34.5% for patients alive, P < 0.001) and lower aspirin use (29.9% vs 31.9% for patients alive, P < 0.001). A 1% increment in RDW was independently associated with a 15% greater risk of all-cause mortality and similar risk in the two ABI subsets. After additional adjustment for body mass index, cardiovascular risk factors, comorbidities, malignancy, medications and hemoglobin level, RDW remained an independent predictor of all-cause mortality in these groups (Table 2). When RDW was analyzed according to quartiles, patients in the higher quartile (RDW > 13.5%) had higher mortality compared to those in the referent quartile (RDW < 12.8%, Table 3). We also found a graded increased risk of mortality in these patients, including patients in the fourth quartile with 45% higher and those in the third quartile with 14% higher risk of death, compared to the next lower quartile. As shown in the Kaplan Meier curves (Figure 1), patients in the highest quartile of RDW (>14.5%) had the poorest survival during a median follow up of 4.5 years (Log-rank P < 0.0001).
Table 2.
Multivariable Adjusted Hazard Ratios for Mortality with 1% Increase in Red Cell Distribution Width
| Deaths / n (%) | Model 1 | Model 2 | |
|---|---|---|---|
| Variable | HR (95% CI) | HR (95% CI) | |
| Peripheral Artery Disease | 4039 / 13039 (31.0%) | 1.15 (1.13-1.16) | 1.10 (1.08-1.12) |
| Low ankle-brachial index | 2891/10087 (28.7%) | 1.15 (1.13-1.17) | 1.10 (1.08-1.12) |
| High ankle-brachial index | 1148 / 2952 (38.9%) | 1.13 (1.11-1.15) | 1.09 (1.06-1.12) |
Model 1 adjusted for age and sex.
Model 2 additionally adjusted for body mass index, hypertension, dyslipidemia, diabetes, smoking, coronary heart disease, heart failure, cerebrovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, malignancy, statin and aspirin use, and hemoglobin level.
HR = hazard ratio
CI = confidence interval
Table 3.
Association of quartiles of baseline Red Cell Distribution Width with All-cause Mortality in Patients with Peripheral Artery Disease
| Quartiles | N | Deaths | HR (95% CI) | P value |
|---|---|---|---|---|
| 1 | 3008 | 779 (25.9%) | Referent | / |
| 2 | 3378 | 891 (26.4%) | 1.06 (0.95-1.18) | 0.94 |
| 3 | 3273 | 975 (29.8%) | 1.20 (1.09-1.34) | 0.0005 |
| 4 | 3380 | 1394 (41.2%) | 1.66 (1.49-1.85) | <0.0001 |
Adjustments as in model 2 in Table 2.
Q1 = red cell distribution width <12.8%; Q2 = red cell distribution width 12.8%–13.5%; Q3 = red cell distribution width 13.5% –14.5%; Q4 = red cell distribution width > 14.5%
Figure 1.
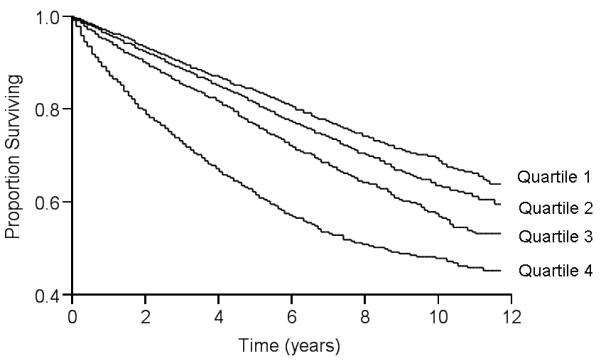
Kaplan-Meier survival curves according to quartiles of RDW in patients with PAD (P < 0.0001 by log-rank test for overall comparison among groups). Quartile 1: RDW < 12.8%; Quartile 2 : RDW 12.8% - 13.5%; Quartile 3 : RDW 13.5% - 14.5%; Quartile 4 : RDW >14.5%.
Discussion
The main finding of our study is that higher RDW is associated with greater all-cause mortality in patients with PAD identified in the non-invasive vascular laboratory. After adjustment for potential confounders such as age, sex, body mass index, hemoglobin level, cardiovascular risk factors and comorbidities, the association between RDW and mortality remained statistically significant. The hazard of all-cause mortality was similar in the low and high ABI subsets. Thus, in patients with PAD, RDW provides prognostic information incremental to cardiovascular risk factors and comorbid conditions.
Previous studies9-14 have reported RDW to be an independent predictor of mortality in community-dwelling adults and patients with cardiovascular disease. In 4,111 patients with history of myocardial infarction followed for a mean of 54 months, the adjusted hazard ratio (HR) for all-cause mortality was 1.14 per 1% increase in RDW (95% confidence interval [CI]: 1.05 – 1.24). 13 In 2,679 symptomatic chronic heart failure patients followed for a median of 34 months, the adjusted HR for all-cause mortality was 1.12 (1.03 – 1.20) per 1-SD increase in RDW.10 Two studies14,15 in patients with acute heart failure also found RDW to be a predictor of mortality. The adjusted HR of RDW per 1% increase was 1.03 (95% CI: 1.00 – 1.06) and 1.072 (1.023 – 1.123) respectively.
We found a 10% greater risk of mortality for a 1% increase in RDW, confirming the prognostic importance of RDW in the setting of PAD. Our study provides robust estimates of risk of mortality associated with RDW, after adjustment for multiple confounders including the hemoglobin level. Compared to previous studies enrolling patients from randomized clinical trials and volunteers from communities, patients in our study were older and had multiple chronic conditions at baseline. Therefore, our findings may be generalized to an older population with significant comorbidity.
Increased RDW results from heterogeneity of erythrocyte size and erythrocyte fragmentation in the circulation.16,17 Factors that contribute to increased erythrocyte size heterogeneity include iron or vitamin B12/folate deficiency, decreased erythrocyte life-span, impaired erythropoiesis and factors that contribute to erythrocyte fragmentation including increased fragility and destruction of red cells.18,19 Whether atherosclerosis, the main cause of stroke, myocardial infarction and PAD, directly leads to anisocytosis is not known. Possible mechanisms may include chronic inflammation and oxidative stress.20-22 RDW has been associated with inflammatory markers such as soluble tumor necrosis factor receptors23 and C-reactive protein in the setting of atherosclerosis and other chronic diseases. 24,25 Proinflammatory cytokines may contribute to the heterogeneity of erythrocyte population through several pathways.20,26 Inflammation and oxidative stress are closely related through the activation of nuclear factor kappa B by reactive oxygen species.27 Anti-oxidants selenium and carotenoids were associated with RDW at baseline and predicted RDW over one year follow up in community-dwelling elderly women28 and this may result from protection of glutathione peroxidase in the red cell.29,30
Although the association of RDW with mortality has been established in different patient settings, whether RDW has a causal role in leading to mortality, rather than being a marker of different pathophysiological processes, needs further investigation. A limitation of the present study is that cause of death was not available and we were unable to ascertain whether RDW was differentially associated with cardiovascular vs. noncardiovascular mortality. Medications such as diuretics use were not included in our analysis at present. However, we adjusted for hemoglobin level and other comorbidities that may be affected by the medication use. RDW remained as a significant predictor for mortality in spite of these adjustments. Additional potential limitations of our study also need to be considered. Our study is retrospective and the risk factors and comorbidities were ascertained from the EMR. Although ICD-9-CM codes are easily available at a relatively low cost, systematic misclassification and exclusion of conditions or procedures not pertinent to reimbursement may affect the prevalence of risk factors and comorbidities in the study. Also, although we adjusted for multiple factors and prevalent diseases, it is possible that there may be underlying confounding from occult diseases not included in the analysis.
Acknowledgments
Financial Support: This work was supported by grants HL-81331 and UL1-RR024150 (Center for Translational Science Activities) from the National Institutes of Health, Bethesda, MD.
Footnotes
Disclosure: The authors have nothing to disclose and have no relationships to industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. Jama. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, Bhatt DL, Wilson PW, D’Agostino R, Sr., Ohman EM, Rother J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S. One-year cardiovascular event rates in outpatients with atherothrombosis. Jama. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 3.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 4.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 5.Jouni H, Rodeheffer RJ, Kullo IJ. Increased Serum N-Terminal Pro-B-Type Natriuretic Peptide Levels in Patients With Medial Arterial Calcification and Poorly Compressible Leg Arteries. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kullo IJ, Fan J, Pathak J, Savova GK, Ali Z, Chute CG. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome-wide association study of peripheral arterial disease. J Am Med Inform Assoc. 2010;17:568–574. doi: 10.1136/jamia.2010.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 8.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 9.Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–108. doi: 10.1016/j.jns.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 11.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–523. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 14.van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL., Jr. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur J Heart Fail. 2010;12:129–136. doi: 10.1093/eurjhf/hfp179. [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Figal DA, Bonaque JC, Redondo B, Caro C, Manzano-Fernandez S, Sanchez-Mas J, Garrido IP, Valdes M. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009;11:840–846. doi: 10.1093/eurjhf/hfp109. [DOI] [PubMed] [Google Scholar]
- 16.Karnad A, Poskitt TR. The automated complete blood cell count. Use of the red blood cell volume distribution width and mean platelet volume in evaluating anemia and thrombocytopenia. Arch Intern Med. 1985;145:1270–1272. doi: 10.1001/archinte.145.7.1270. [DOI] [PubMed] [Google Scholar]
- 17.Hammarsten O, Jacobsson S, Fu M. Red cell distribution width in chronic heart failure: a new independent marker for prognosis? Eur J Heart Fail. 2010;12:213–214. doi: 10.1093/eurjhf/hfp208. [DOI] [PubMed] [Google Scholar]
- 18.Nagajothi N, Braverman A. Elevated red cell distribution width in the diagnosis of thrombotic thrombocytopenic purpura in patients presenting with anemia and thrombocytopenia. South Med J. 2007;100:257–259. doi: 10.1097/01.smj.0000257403.04625.36. [DOI] [PubMed] [Google Scholar]
- 19.Saigo K, Jiang M, Tanaka C, Fujimoto K, Kobayashi A, Nozu K, Iijima K, Ryo R, Sugimoto T, Imoto S, Kumagai S. Usefulness of automatic detection of fragmented red cells using a hematology analyzer for diagnosis of thrombotic microangiopathy. Clin Lab Haematol. 2002;24:347–351. doi: 10.1046/j.1365-2257.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- 20.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 21.Franchini M, Zaffanello M, Veneri D. Advances in the pathogenesis, diagnosis and treatment of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Thromb Res. 2006;118:177–184. doi: 10.1016/j.thromres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 23.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Lee WS, Kim TY. Relation between red blood cell distribution width and inflammatory biomarkers in rheumatoid arthritis. Arch Pathol Lab Med. 2010;134:505–506. doi: 10.5858/134.4.505.c. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Chaparro MA, Calvo-Bonacho E, Gonzalez-Quintela A, Cabrera M, Sainz JC, Fernandez-Labandera C, Aguado LQ, Meseguer AF, Valdivielso P, Roman-Garcia J. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care. 2010;33:e40. doi: 10.2337/dc09-1707. [DOI] [PubMed] [Google Scholar]
- 26.Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood. 2002;100:474–482. doi: 10.1182/blood-2002-01-0136. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Zhang X, Li JJ. The role of NF-kappaB in the regulation of cell stress responses. Int Immunopharmacol. 2002;2:1509–1520. doi: 10.1016/s1567-5769(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 28.Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, Fried LP. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin Nutr. 2010;29:600–604. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus A, Roth HP, Kirchgessner M. Supplementation with vitamin C, vitamin E or beta-carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc-deficient rats. J Nutr. 1997;127:1290–1296. doi: 10.1093/jn/127.7.1290. [DOI] [PubMed] [Google Scholar]
- 30.Alfthan G, Xu GL, Tan WH, Aro A, Wu J, Yang YX, Liang WS, Xue WL, Kong LH. Selenium supplementation of children in a selenium-deficient area in China: blood selenium levels and glutathione peroxidase activities. Biol Trace Elem Res. 2000;73:113–125. doi: 10.1385/BTER:73:2:113. [DOI] [PubMed] [Google Scholar]


