Abstract
Objective
To describe urban-rural differences in breast cancer incidence in Gharbiah, Egypt and to investigate if these differences could be explained by known risk factors of breast cancer.
Methods
We used data from the population-based cancer registry of Gharbiah, Egypt to assess breast cancer incidence from 1999 through 2006. The Egyptian census provided data on district-specific population, age, and urban-rural classification. Incidence patterns of breast cancer by district and age-specific urban-rural differences were analyzed.
Results
Overall, incidence rate of breast cancer was three to four times higher in urban areas than in rural areas (60.9/105/year for urban areas versus 17.8/105/year for rural areas; IRR = 3.73, 95% CI = 3.30, 4.22). Urban areas had consistently higher incidence of breast cancer across all age-groups for all years. Higher incidence of breast cancer was also seen in the more developed districts of Tanta and El-Mehalla.
Conclusions
Higher incidence of breast cancer in urban and more developed populations might be related to higher xenoestrogens, as well as other endocrine disruptors and genotoxic substances.
Keywords: Breast cancer, incidence, urban-rural, xenoestrogens, Egypt
INTRODUCTION
Breast cancer (BC) is the most common lethal malignancy accounting for nearly 23% of all female cancers worldwide, with more than a million new cases each year.1 The incidence rates (IRs) of BC vary worldwide, with high rates in North America, Northern and Western Europe, intermediate rates in South America and Southern Europe, and low rates in Africa and Asia.2 Developing countries show a higher incidence of BC in urban than in rural areas, a pattern that has not been fully explained.1–3 For example BC incidence in urban areas of Shanghai was 27.2/105 respectively compared to 9.1/105 in the nearby rural area of Jiashan.2 Similarly in India, BC incidence in rural registry of Barshi was 7.2/105 compared to the 31.3/105 in adjoining city of Mumbai (Bombay).3 While hereditary causes account for only 5–10% of BC risk, a significant portion of BC risk is environmental or non-hereditary in nature as shown by studies of women who migrate from low incidence to high incidence settings.4,5 Known risk factors of BC including environmental risk factors that modulate a woman’s exposure to endogenous estrogens, such as age of menarche, age of first full term pregnancy, number of children, duration of breastfeeding, and age of menopause, only explain up to little more than half of BC risk.6,7 It is reasonable to hypothesize that other unknown exogenous estrogenic factors may contribute to elevated incidence in industrialized countries since BC is associated with estrogenic exposures, and endogenous estrogens cannot explain the total non-hereditary risk. 6,7
Exogenous estrogens, or xenoestrogens, are environmental chemicals that mimic the action of hormones or directly affect pathways of endogenous hormones. Important among such chemicals because of their ubiquity and activity strength are those estrogen mimetics present in plastics such as bisphenol-A (BPA), phthalates and polyvinyl chloride (PVC), pesticides and insecticides like DDTs, polychlorinated biphenyls (PCBs) etc. and parabens, and placental extracts, aromatic amines, and industrial solvents like benzene and toluene.8 Mounting evidence from various parts of the world shows that use and exposure to xenoestrogens accompanies economic development. Many studies have demonstrated that various xenoestrogens are more abundant in urban areas,9–17 but widespread exposure in developed countries makes epidemiological investigation difficult.18
Comparisons between urban and rural populations that are fairly genetically homogeneous in developing countries such as Egypt, are ideally suited for studying environmental exposures, since differential rates of economic development translates into differential exposure to environmental risk factors of BC. Within Egypt, data on environmental exposures is very limited. However it is known that intensive agricultural practices have led to the use of thousands of metric tons of various pesticides which are present in large quantities in soil and water.19 Many of these pesticides such as toxaphene, endrin, DDT and lindane are persistent organic pollutants (POPs) as well as xenoestrogens.19 This has led to extensive human exposure to various chemicals, mainly in the Nile Delta Region (NDR) with most pesticides found in appreciable quantities in human milk as observed even in our own studies.20,21 We documented lower levels of these pesticides in Egyptian women who breastfed more.21
Thus it is quite possible that urban women, who have fewer children and breastfeed less than rural women, are exposed to higher levels of these xenoestrogens.21 Given the probable high exposure of urban populations to various xenoestrogens in addition to changing reproductive and lifestyle-related risk factors, we hypothesized that BC incidence is higher in urban than in rural areas of Egypt.
MATERIAL AND METHOD
Study Population
The study population consisted of all women diagnosed with primary BC during the eight years from 1999 through 2006, who were in the Gharbiah Population-Based Cancer Registry. For each case, the following information from routinely collected registry data was obtained: registry number, age at diagnosis, address, address code, smoking status, occupation, basis of diagnosis, tumor grade, stage, morphology, medical record number, and place of referral. Data were stripped of all personal identifiers and their analyses were approved by the University of Michigan Institutional Review Board and the Gharbiah Cancer Center Ethics Committee.
Gharbiah Population-Based Cancer Registry
The Gharbiah population-based cancer registry, founded in 1998 as a part of the Middle East Cancer Consortium (MECC) and funded by the U.S. National Cancer Institute (NCI), is located in Tanta, the capital of Gharbiah province.22, 23 Through an active registration process, data on cancer cases are collected from various sources in the province. For this study, most BC cases came from three locations; the Tanta Cancer Center (40–50%), Gharbiah Cancer Society (10–12%) and Tanta University Hospital (10–12%). Data obtained from these hospitals and centers using the International Agency for Research on Cancer (IARC) software CanReg4. Registrars were trained in data extraction and entry methods, and are periodically monitored by faculty of Emory School of Public Health, IARC, and the MECC.22, 23
Most of the BC cases in the registry (95.8%) were diagnosed by histopathological confirmation of the primary tumor.24 Cases were registered with the American Joint Committee on Cancer (AJCC) staging from 2003 onwards, while those from 1999–2002 employed the SEER staging, but were replaced by AJCC staging.
Gharbiah Province
The Gharbiah province is an administrative region located 90 kilometers north of Cairo in the Nile delta region and has eight districts, each with a capital city (Figure 1). Tanta city also serves as the capital of the province. Gharbiah has a population of more than 4 million people, 49% of whom are women. Approximately 30% of the population resides in urban areas and almost 47% of the female population is below the age of 20, according to the 2006 Central Agency for Public Mobilization and Statistics (CAPMAS) national census of Egypt.25
Figure 1.
Map of Nile Delta Region showing location of eight districts of Gharbiah with the respective overall incidence rates of breast cancer in each district.
Census Data
The 1996 and 2006 CAPMAS censuses were used to obtain data on women residing in Gharbiah,26 and linear regression was used to estimate the population during each study year. The linear growth rates of eight districts were applied to the urban and rural populations within those districts to determine urban and rural populations from 1999 through 2006. Twelve age categories were obtained from the census (one representing less than 24 years of age and eleven subsequent categories each comprising a 5-year interval). These population figures per age interval formed the denominators to calculate the various age-specific incidence rates of BC.
Urban-Rural Classification
Urban and rural designations were made according to the CAPMAS definitions.25 Urban areas consisted of all the capital cities of the eight districts of the province, while the remaining areas in the province were considered rural. Each case in the registry was assigned a residence code based on their residential address that follows the CAPMAS coding which was used to classify cases as urban or rural.
Statistical Analysis
Descriptive statistics and rate analyses were completed using SAS (Version 9; SAS Institute, Cary, NC). Yearly raw and age-adjusted incidence rates were calculated for Gharbiah province, each of the eight districts, and urban and rural areas for the province. Crude annual incidence rates were calculated by dividing the number of cases each year by the respective population estimate for that year. Age-specific incidence rates for the entire study area and for urban vs. rural areas were calculated for each of the twelve age categories. Direct age-adjusted incidence rates were calculated by using Gharbiah’s 2006 population as the standard. Trends in BC incidence were compared overall, and by urban-rural status, age categories and districts.
Incidence Rate Ratios (IRRs) and P-values for trend were calculated using negative binomial regression by the GENMOD procedure in SAS. Although age, histology and stage at diagnosis are potential confounders, histology was uniform in distribution across urban-rural strata and stage at diagnosis did not affect IRRs by more than 10%. Therefore, we computed age-standardized IRRs and 95% confidence intervals (CI). However, stage at diagnosis was a confounder for overall incidence trend and therefore we reported the IRR for overall incidence after adjusting for age, stage and year of diagnosis. In addition we also controlled for urban-rural status when computing P-value for trend for overall incidence.
To control for known reproductive factors that may have contributed to urban-rural differences, the following formula26 was used:
Where
Inc(Urban): Urban incidence rate of BC
Inc(Rural): Rural incidence rate of BC
n(Urbanj) and n (Ruralj): The number of urban and rural women respectively in the jth risk factor category
n(Urban) and n(Rural): The total number of urban and rural women respectively
RRj: The risk ratio or odds ratio (OR) associated with jth risk factor category
Age-adjusted urban and rural incidences of BC, ORs from an earlier Egyptian case-control study of BC27 and prevalence data from the Egyptian Health and Demographic Survey (EDHS)28 were employed in the formula. Due to potential differences in reproductive habits and diet, we investigated age at first birth or age at first full term pregnancy (FFTP), number of children, and duration of breastfeeding as potential variables to control for urban-rural incidence difference. However, the case-control study27 and EDHS28 indicated that the number of children and duration of breastfeeding did not confer much risk for BC, nor were these factors different for urban and rural women in Egypt. Therefore, we controlled only for age at FFTP.
RESULTS
A total of 4,794 female cases of BC with an average age of 50 (± 11.4) years were identified (Table 1). Tanta and El Mehalla, the two largest districts of Gharbiah, contributed the most cases, their contributions being 35.0% and 30.6% of cases, respectively. Most cases were either stage II (33.7%) or stage III (45.9%) while 4.4% and 16% of cases were in Stage I and IV respectively. Majority of cases had been diagnosed by pathological confirmation (94.4%) (Table 1).
Table 1.
Descriptive information of the registry study population in Gharbiah, Egypt, 1999–2006
| Variable | Descriptive Category | Urban No. (%) | Rural No. (%) | Overall No. (%) |
|---|---|---|---|---|
| Total Cases | 3043 (63.48) | 1688 (35.21) | 4794 (100) | |
| Year of Diagnosis | 1999 | 395 (70.54) | 165 (29.46) | 560 (11.68) |
| 2000 | 377 (69.69) | 164 (30.31) | 541 (11.29) | |
| 2001 | 378 (65.51) | 199 (34.49) | 577 (12.04) | |
| 2002 | 431 (69.40) | 190 (30.60) | 621 (12.95) | |
| 2003 | 349 (57.69) | 251 (41.49) | 605 (12.62) | |
| 2004 | 388 (59.06) | 254 (38.66) | 657 (13.71) | |
| 2005 | 347 (59.22) | 221 (37.71) | 586 (12.22) | |
| 2006 | 378 (58.42) | 244 (37.71) | 647 (13.50) | |
| Age | 0–24 | 14 (60.87) | 9 (39.13) | 23 (0.48) |
| 25–29 | 52 (55.91) | 41 (44.09) | 93 (1.94) | |
| 30–34 | 143 (55.43) | 115 (44.57) | 258 (5.38) | |
| 35–39 | 283 (57.52) | 209 (42.48) | 492 (10.26) | |
| 40–44 | 446 (60.85) | 287 (39.15) | 733 (15.29) | |
| 45–49 | 529 (64.99) | 285 (35.01) | 814 (16.98) | |
| 50–54 | 542 (67.41) | 262 (32.59) | 804 (16.77) | |
| 55–59 | 377 (65.34) | 200 (34.66) | 577 (12.04) | |
| 60–64 | 279 (65.04) | 150 (34.97) | 429 (8.95) | |
| 65–59 | 179 (68.32) | 83 (31.68) | 262 (5.47) | |
| 70+ | 110 (70.06) | 47 (29.94) | 157 (3.28) | |
| Districta | Tanta | 1213 (72.25) | 466 (27.75) | 1679 (35.02) |
| El-Mehalla | 1097 (74.88) | 368 (25.12) | 1465 (30.56) | |
| Kafr El-Zayat | 204 (57.96) | 148 (42.05) | 352 (7.34) | |
| Zefta | 136 (48.23) | 146 (51.77) | 282 (5.88) | |
| Samanoud | 124 (53.91) | 106 (46.09) | 230 (4.80) | |
| El Santa | 98 (34.51) | 186 (65.49) | 284 (5.92) | |
| Kotour | 77 (39.09) | 120 (60.91) | 197 (4.11) | |
| Basyoon | 103 (47.25) | 115 (57.75) | 218 (4.55) | |
| Stageb | I | 94 (62.67) | 56 (37.33) | 150 (4.39) |
| II | 695 (60.28) | 458 (39.72) | 1153 (33.73) | |
| III | 935 (59.55) | 635 (40.45) | 1570 (45.93) | |
| 1V | 290 (53.21) | 255 (46.79) | 545 (15.95) | |
| Basis of Diagnosisc | Histology | 2060 (62.18) | 1253 (37.82) | 3313 (77.14) |
| FNAC | 494 (66.67) | 247 (33.33) | 741 (17.25) | |
| Others | 184 (76.35) | 57 (23.65) | 241 (5.61) |
1.5% of cases had missing residence information.
28.7% of cases had missing or unknown AJCC stage information.
10.4% of cases had missing information on basis of diagnosis.
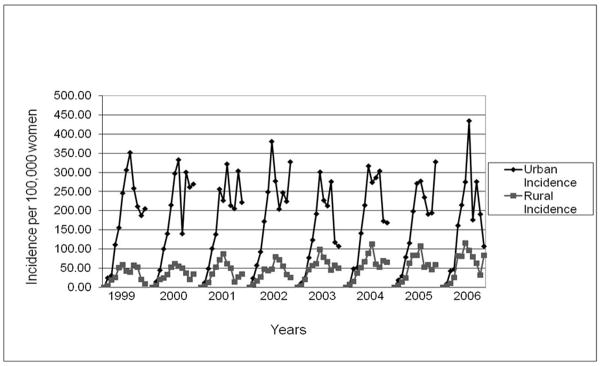
Overall incidence of BC in Gharbiah ranged from 30.2 per 100,000 women in 1999 to 34.5 per 100,000 women in 2006 and increased during the study period (P = 0.02) (Table 2) (Figure 2). We also observed a trend of decreasing breast cancer incidence in urban areas (P < 0.0001) while breast cancer incidence seems to be increasing in rural areas (P = 0.11) (Table 2). Age-specific BC incidence rates increased in the younger age categories, peaking around 45–55 years, and then declined in ages over 55 years. This pattern was consistent across the eight years of study (Figure 3). We also observed an increasing age of peak incidence across the eight years of this study (Figure 3). Incidence of BC across the eight districts of Gharbiah was highest in Tanta and lowest in Kotour and Zefta (Table 3) (Figure 1).
Table 2.
Breast cancer incidence rates by year for the entire region, and age-standardized rates for urban, rural and urban-rural incidence rate ratio in Gharbiah, Egypt, 1999–2006
| Year | No Cases | Overall Incidence per 100,000 | Age-Standardized Urban Incidence | Age-Standardized Rural Incidence | Urban-Rural IRR (95% CI)a |
|---|---|---|---|---|---|
| 1999 | 560 | 31.75 | 65.81 | 14.22 | 4.63 (3.20, 6.69) |
| 2000 | 541 | 30.22 | 62.57 | 13.90 | 4.50 (3.09, 6.57) |
| 2001 | 577 | 31.81 | 61.58 | 16.76 | 3.67 (2.55, 5.29) |
| 2002 | 621 | 33.67 | 68.97 | 15.62 | 4.42 (3.09, 6.31) |
| 2003 | 613 | 32.93 | 53.42 | 20.34 | 2.63 (1.84, 3.75) |
| 2004 | 665 | 35.11 | 59.87 | 20.13 | 2.97 (2.11, 4.20) |
| 2005 | 617 | 31.81 | 55.81 | 19.71 | 2.83 (1.97, 4.06) |
| 2006 | 681 | 34.49 | 59.21 | 21.87 | 2.71 (1.91, 3.83) |
| Overall | 4794 | 32.15 | 60.90 | 17.82 | 3.73 (3.30, 4.22)b |
| P for trend | 0.02b, c | <0.0001b | 0.11b |
IRR = Incidence Ratio. CI = Confidence Interval.
Adjusted for age, stage at diagnosis and year of diagnosis.
Also adjusted for urban-rural status.
Figure 2.
Breast cancer incidence (per 100,000 women) in Gharbiah, Egypt from 1999–2006 with linear trend-line for incidence (P for trend = 0.02).
Figure 3.
Overall age-specific incidence of breast cancer in Gharbiah, Egypt from 1999–2006. There are 12 categories of age: 0–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, >75. Each point on the lines of the graph corresponds to one age category. The sequence of age categories for each year is as given above.
Table 3.
Comparison of breast cancer incidence ratesa and incidence rate ratios between districts in Gharbiah, Egypt from 1999–2006.
| Years | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Districts | Inc† | IRR† (95% CI) |
Inc | IRR (95% CI) |
Inc | IRR (95% CI) |
Inc | IRR (95% CI) |
Inc | IRR (95% CI) |
Inc | IRR (95% CI) |
Inc | IRR (95% CI) |
Inc | IRR (95% CI) |
| Tanta | 49.66 | 1.95(1.32, 2.90) | 42.34 | 1.48(1.01, 2.15) | 50.31 | 2.48(1.61, 3.81) | 51.99 | 2.22(1.49, 3.30) | 48.09 | 1.88(1.26, 2.80) | 50.10 | 2.06(1.40, 3.04) | 46.16 | 2.31(1.50, 3.56) | 46.69 | 2.11(1.40, 3.17) |
| El Mehalla | 34.19 | 1.35 (0.90, 2.01) | 30.35 | 1.06 (0.72, 1.56) | 35.15 | 1.74 (1.13, 2.70) | 51.22 | 1.75 (1.17, 2.62) | 36.12 | 1.41 (0.94, 2.12) | 46.61 | 1.92 (1.30, 2.82) | 40.94 | 2.05 (1.33, 3.16) | 43.30 | 1.95 (1.30, 2.93) |
| Kafr El-Zayat | 39.91 | 1.10 (0.69, 1.76) | 32.88 | 0.98 (0.63, 1.53) | 23.54 | 1.16 (0.70, 1.93) | 35.13 | 1.50 (0.95, 2.36) | 23.68 | 0.93 (0.57, 1.50) | 19.93 | 0.82 (0.50, 1.34) | 17.21 | 0.86 (0.50, 1.48) | 25.93 | 1.17 (0.72, 1.89) |
| Zefta | 29.41 | 0.81 (0.50, 1.32) | 21.99 | 0.66 (0.41, 1.06) | 19.01 | 0.94 (0.56, 1.58) | 18.17 | 0.78 (0.47, 1.28) | 17.36 | 0.68 (0.41, 1.12) | 16.55 | 0.68 (0.42, 1.12) | 16.15 | 0.81 (0.47, 1.38) | 16.36 | 0.74 (0.44, 1.23) |
| Samanoud | 15.20 | 0.60 (0.34, 1.06) | 22.44 | 0.78 (0.51, 1.29) | 17.67 | 0.87 (0.49, 1.54) | 13.74 | 0.59 (0.33, 1.06) | 20.59 | 0.80 (0.48, 1.36) | 29.97 | 1.23 (0.77, 1.98) | 17.34 | 0.87 (0.49, 1.54) | 29.10 | 1.31 (0.80, 2.14) |
| El Santa | 21.04 | 0.83 (0.50, 1.36) | 23.16 | 0.81 (1.29, 0.51) | 28.23 | 1.39 (0.84, 2.29) | 20.68 | 0.89 (0.54, 1.46) | 15.74 | 0.62 (0.36, 1.05) | 19.46 | 0.80 (0.49, 1.31) | 19.59 | 0.98 (0.58, 1.67) | 20.99 | 0.95 (0.57, 1.57) |
| Kotour | 21.59 | 0.85 (0.50, 1.43) | 28.11 | 0.98 (0.61, 1.58) | 17.97 | 0.88 (0.50, 1.57) | 14.71 | 0.63 (0.35, 1.12) | 12.99 | 0.51 (0.28, 0.92) | 12.05 | 0.50 (0.27, 0.90) | 22.04 | 1.10 (0.64, 1.90) | 16.60 | 0.75 (0.43, 1.31) |
| Basyoonb | 25.42 | 1.00 | 28.64 | 1.00 | 20.30 | 1.00 | 23.42 | 1.00 | 25.60 | 1.00 | 24.30 | 1.00 | 19.97 | 1.00 | 22.17 | 1.00 |
All incidences are per 100,000 women.
Inc = Incidence, IRR = Incidence ratio
Basyoon is the reference district
Urban-rural BC incidence rates showed a consistent pattern with urban rates being higher than rural rates (1999 – IRR = 4.63, 95% CI = 4.04, 5.31 and 2006 – IRR = 2.71, 95% CI = 1.91, 3.83) (Table 2). Overall, and throughout the eight years, the urban incidence rates were higher than rural incidence rates (Overall IRR = 3.73, 95% CI = 3.30, 4.22) (Table 2). Urban populations showed higher age-specific incidence of BC than rural locations, for all age categories (Figure 4). On adjusting for age at FFTP, we observed a reduction in urban-rural IRR by 8.9%.
Figure 4.
Urban-rural age-specific incidence of breast cancer in Gharbiah, Egypt from 1999–2006. There are 12 categories of age: 0–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, >75. Each point on the lines of the graph corresponds to one age category. The sequence of age categories for each year is as given above.
DISCUSSION
This first study ever investigating BC trends in Egypt from a population-based cancer registry provided evidence of increasing BC incidence during the study period. Previously, a small-scale, hospital-based study from Alexandria, Egypt had suggested an increase in incidence rate of BC there.29 Rising BC incidence has been reported from most places in the world,1 with rapid increases observed in developing countries30, including those in the Middle-East.31 Apart from increasing exposure to known risk factors of BC it is likely that the Gharbiah registry is relatively new and the increased BC incidence observed in our study was due to an increase in the number of diagnostic and treatment centers in Gharbiah.32
We observed three-to-four times higher incidence of BC in urban than in rural areas. The higher urban incidence was consistent across eight years and for all age groups. Although known risk factors might be responsible for the observed higher urban incidence, EDHS findings indicated that urban and rural Egyptian women had similar reproductive risk factors.28 Furthermore, when we controlled for FFTP, one of the most important reproductive risk factors affecting BC risk, the urban-rural IRR changed slightly.
We considered that the elevated BC incidence in urban areas relative to rural areas could be due to limited access to diagnostic facilities in rural areas, possibly causing many rural women to die with BC undiagnosed. However, we did not find any significant difference in stage distribution of BC between urban and rural areas (Table 4) which ruled out late detection of cases from rural areas. Primary healthcare coverage in Egypt is reported to be 100%, with rural areas possibly having good access to physicians and primary care hospitals.22 Also, rural areas in Gharbiah are no further than 50 kilometers from Tanta, the capital city, and are mostly well-connected by readily available, inexpensive public transportation. Thus, difficulties in health care access and non-detection of cases cannot explain urban-rural or district-level differences reported in this study. EDHS results28 and our recent follow-up study (unpublished data) indicate that health seeking behavior of women in northern Egypt does not differ significantly between urban and rural areas. As such, health education programs related to breast health awareness must be incorporated equally in both urban and rural areas.
Table 4.
Distribution of the proportion of breast cancer cases by AJCC stage in Gharbiah, Egypt from 1999–2006
| AJCC Stage |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | |||||||||
| Year | Urban | Rural | Overall | Urban | Rural | Overall | Urban | Rural | Overall | Urban | Rural | Overall |
| 1999 | 3.45 | 1.60 | 2.80 | 25.43 | 20.80 | 23.81 | 50.00 | 52.80 | 50.98 | 21.12 | 24.80 | 22.41 |
| 2000 | 5.09 | 8.33 | 6.32 | 23.15 | 22.73 | 22.99 | 49.07 | 46.21 | 47.99 | 22.69 | 22.73 | 22.70 |
| 2001 | 3.70 | 3.64 | 3.68 | 31.69 | 31.52 | 31.62 | 48.56 | 40.61 | 45.34 | 16.05 | 24.24 | 19.36 |
| 2002 | 4.96 | 4.70 | 4.86 | 28.51 | 28.86 | 28.64 | 47.93 | 49.66 | 48.59 | 18.60 | 16.78 | 17.90 |
| 2003 | 5.53 | 3.88 | 4.79 | 36.76 | 34.95 | 35.95 | 41.11 | 45.15 | 42.92 | 16.60 | 16.02 | 16.34 |
| 2004 | 3.23 | 4.09 | 3.61 | 45.52 | 31.36 | 39.28 | 41.58 | 43.18 | 42.28 | 9.68 | 21.36 | 14.83 |
| 2005 | 5.56 | 3.08 | 4.52 | 35.56 | 41.54 | 38.06 | 50.37 | 41.03 | 46.45 | 8.52 | 14.36 | 10.97 |
| 2006 | 5.73 | 3.30 | 4.68 | 44.44 | 40.09 | 42.57 | 44.09 | 46.70 | 45.21 | 5.73 | 9.91 | 7.54 |
| Overall | 4.67 | 3.99 | 4.39 | 34.51 | 32.62 | 33.73 | 46.43 | 45.23 | 45.93 | 14.40 | 18.16 | 15.95 |
Urban- rural differences in BC incidence in Egypt and other developing countries are qualitatively analogous to the pattern of differences in incidence reported between developed and developing countries. This analogy is consistent with the patterns seen in age-specific BC incidence, where urban age-specific BC incidence is higher for all ages with patterns similar to developed countries. In contrast, the lower incidence in rural areas in this study showed a decrease in incidence in later years of life, similar to that seen in developing countries.30 We pose that the absence of a decline in incidence in older women in developed countries and urban areas could be due to sustained increased exposure to estrogenic factors throughout the lifetime. The increasing peak age of BC incidence that we observed is consistent with the above explanation since chronic estrogenic exposures throughout life increases BC incidence in later years of life.
We also observed an apparent decrease in urban BC incidence while rural BC incidence seemed to increase steadily across the study period though not significantly. The decrease in urban BC incidence occurred in the last four years (2003–2006) of our study and we believe that it is associated with 3–5% annual missing cases in these years since the data for these years wasn’t completely available at the time of our analysis. According to our assessment of the source of these cases, almost all of these cases were urban. The steady increase in rural BC incidence is consistent with our hypothesis since rural women are increasingly adopting urban lifestyles, reproductive habits and are also increasingly exposed to similar environmental factors as urban women due to economic development. Thus, it is quite probable that the urban-rural gap in BC incidence will become narrower in the coming decades.
BC incidence between the different Gharbiah districts also varied by as much as three-folds. Since the geographic distance between an incident case’s dwelling and the registry does not appear to affect the probability that the case will be detected and, by the procedures in place to track records, it does not affect the registration, we propose that perhaps exposures related to the relative economic development and industrialization between the districts are more relevant in causing these inter-district differences. Tanta and El-Mehalla, are the largest cities and are home to most of the industries and commercial centers of the province. Therefore, we speculate that women in these two districts may experience greater exposure to environmental risk factors such as xenoestrogens, a hypothesis that needs further investigation.
Although the link between xenoestrogens and BC has not been thoroughly explored, the evidence available suggests that exposure to xenoestrogens is high and increasing across the world. World pesticides sales have increased most in developing countries, and are two to three times higher than the current world average.33 Of further concern is exposures to plastics, which contain BPA and phthalates, is increasing in urban areas.34 These compounds are being detected in the urine of people in developed countries, 35–38 universally across the population. This can probably be ascribed to massive increases in plastic usage worldwide.39, 40
Short-acting xenoestrogens are also seen in other categories of products, such as food preservatives, cosmetics, and detergents.41–43 Recently, many studies have shown the greater presence and exposure to xenoestrogens in urban areas across many parts of the world.9–17 In our own work in Egypt, we previously discovered that urban women had higher levels of 7,8-dihydro-8-oxo-2’-deosyguanine (8-oxo-dG),44 suggesting greater exposure to carcinogenic influences. Within Gharbiah province, studies have shown dangerously high levels of heavy metals and inorganic pollutants in the Damietta branch of Nile River that flows along the east border of the province.45 There is also additional evidence of sewage and industrial wastewater polluting the Nile River mostly in the urban areas of Gharbiah province.46, 47 Thus, in addition to xenoestrogens, carcinogenic exposures in urban areas might also involve other endocrine-disruptors and genotoxic substances. Further research is clearly warranted about these environmental risk factors in Egypt and other developing countries.
One of the biggest strengths of this study derives from the fact that we saw a consistent pattern across eight years between urban-rural populations, in all age categories and districts, based on data from a population registry. However, this study also had a few limitations. As mentioned earlier, we had 3–5% of cases were missing for the years 2003–2006 and we determined that most of these missing cases were urban (results not shown). This could have resulted in a seeming reduction in the urban BC incidence and a consequent underestimation of the urban-rural IRR for the years 2003–2006. Also, the absence of information on individual risk factors as well as environmental exposures related to BC is a limitation. However, such information is not usually a part of the data collected by cancer registries, so this limitation is not particular to our study.
To our knowledge, no previous studies in developing countries have yet shown such a stark contrast in BC incidence between urban and rural populations. Future studies investigating the association of environmental risk factors such as xenoestrogens and BC at the individual level must consider that urban-rural populations in developing countries provide an ideal setting in terms of contrasting populations to analyze such exposures.
Acknowledgments
We are grateful to Dr. Hoda Gad, Mr. Khaled Daboos and other personnel of Tanta Cancer Center and Gharbiah Cancer Society for the valuable assistance they provided for this project. We would also like to thank Dr. Catherine Schairer for her valuable inputs regarding additional analyses.
Funding
This work was supported by the Middle East Cancer Consortium, National Cancer Institute, Bethesda [R25 CA112383, R03 CA117350,5 P30 CA46592], the Burroughs Wellcome Fund [SDM], and the Breast Cancer Research Foundation [SDM]. Block Grant of the Department of Epidemiology, University of Michigan School of Public Health; and the Travel Grant of the Rackham Graduate School of the University of Michigan to [SD].
Selected Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- BC
breast cancer
- BPA
bisphenol-A
- CAPMAS
Central Agency for Public Mobilization and Statistics
- CI
confidence interval
- DDT
Dichloro-Diphenyl-Trichloroethane
- EDHS
Egyptian Health and Demographic Survey
- FFTP
first full term pregnancy
- IARC
International Agency for Research on Cancer
- IR
incidence rate
- IRR
incidence rate ratio
- MECC
Middle East Cancer Consortium
- NCI
National Cancer Institute
- NDR
Nile Delta Region
- PCB
polychlorinated biphenyls
- PVC
polyvinyl chloride
- RR
risk ratio
- UN
United Nations
- US
United States
Footnotes
Conflict of Interest
The authors declare that they have no commercial or other associations that might pose a conflict of interest in connection with this article.
Ethical Approval:
This study used data stripped of all personal identifiers and was approved by the University of Michigan Institutional Review Board and the Gharbiah Cancer Center Ethics Committee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Fernández LM. Use of statistics to assess the global burden of breast cancer. Breast J. 2006;12 (Suppl 1):S70–80. doi: 10.1111/j.1075-122X.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents. VIII. International Agency for Research on Cancer; Lyon: 2002. [Google Scholar]
- 3.Cancer Atlas of India. National Cancer Registry Program. India: [Last accessed on: February 8, 2010.]. Url: https://canceratlasindia.org/chapter3_1.htm. [Google Scholar]
- 4.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 5.Maskarinec G. Breast cancer – interaction between ethnicity and environment. In Vivo. 2000;14(1):115–123. [PubMed] [Google Scholar]
- 6.Tavani A, Braga C, La Vecchia C, Negri E, Russo A, Franceschi S. Attributable risks for breast cancer in Italy: education, family history and reproductive and hormonal factors. Int J Cancer. 1997;70(2):159–163. doi: 10.1002/(sici)1097-0215(19970117)70:2<159::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Sprague BL, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Hampton JM, Newcomb PA. Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am J Epidemiol. 2008;168(4):404–411. doi: 10.1093/aje/kwn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray J, Evans N, Taylor B, Rizzo J, Walker M. State of the evidence: the connection between breast cancer and the environment. Int J Occup Environ Health. 2009;15(1):43–78. doi: 10.1179/107735209799449761. [DOI] [PubMed] [Google Scholar]
- 9.Gouin T, Jantunen L, Harner T, Blanchard P, Bidleman T. Spatial and temporal trends of chiral organochlorine signatures in Great Lakes air using passive air samplers. Environ Sci Technol. 2007;41(11):3877–3883. doi: 10.1021/es063015r. [DOI] [PubMed] [Google Scholar]
- 10.Harner T, Shoeib M, Diamond M, Stern G, Rosenberg B. Using passive air samplers to assess urban-rural trends for persistent organic pollutants Polychlorinated biphenyls and organochlorine pesticides. Environ Sci Technol. 2004;38(17):4474–4483. doi: 10.1021/es040302r. [DOI] [PubMed] [Google Scholar]
- 11.Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC. Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environ Sci Technol. 2004;38(1):34–41. doi: 10.1021/es034705n. [DOI] [PubMed] [Google Scholar]
- 12.Kitada Y, Kawahata H, Suzuki A, Oomori T. Distribution of pesticides and bisphenol A in sediments collected from rivers adjacent to coral reefs. Chemosphere. 2008;71(11):2082–2090. doi: 10.1016/j.chemosphere.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Chai Z, Sun H. Human hair as a potential biomonitor for assessing persistent organic pollutants. Environ Int. 2007;33(5):685–693. doi: 10.1016/j.envint.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Pentamwa P, Oanh NT. Levels of pesticides and polychlorinated biphenyls in selected homes in the Bangkok metropolitan region, Thailand. Ann N Y Acad Sci. 2008;1140:91–112. doi: 10.1196/annals.1454.005. [DOI] [PubMed] [Google Scholar]
- 15.Jafari A, Moeckel C, Jones KC. Spatial biomonitoring of persistent organic pollutants in Iran: a study using locally produced butter. J Environ Monit. 2008;10(7):861–866. doi: 10.1039/b802061b. [DOI] [PubMed] [Google Scholar]
- 16.Kumari B, Singh J, Singh S, Kathpal TS. Monitoring of butter and ghee (clarified butter fat) for pesticidal contamination from cotton belt of Haryana, India. Environ Monit Assess. 2005;105(1–3):111–120. doi: 10.1007/s10661-005-3159-2. [DOI] [PubMed] [Google Scholar]
- 17.Pant N, Shukla M, Kumar Patel D, et al. Correlation of phthalate exposures with semen quality. Toxicol Appl Pharmacol. 2008;231(1):112–116. doi: 10.1016/j.taap.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Brody JG, Moysich KB, Humblet O, Attfeild KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer. Cancer. 2007;109(12 Suppl):2667–2711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- 19.Mansour SA. Pesticide exposure - Egyptian scene. Toxicology. 2004;198:91–115. doi: 10.1016/j.tox.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Barakat AO. Assessment of persistent toxic substances in the environment of Egypt. Environ Int. 2004;30:309–322. doi: 10.1016/S0160-4120(03)00181-8. [DOI] [PubMed] [Google Scholar]
- 21.Soliman AS, Wang X, DiGiovanni J, et al. Serum organochlorine levels and history of lactation in Egypt. Environ Res. 2003;92:110–117. doi: 10.1016/s0013-9351(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 22.Freedman LS, Edwards BK, Ries LAG, Young JL. Cancer incidence in four member countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) compared with US SEER. National Cancer Institute. U.S. Department of Health and Human Services. National Institutes of Health; [Google Scholar]
- 23.Freedman LS, Al-Kayed S, Qasem MB, et al. Cancer registration in Middle East. Epidemiology. 2001;12(1):131–133. doi: 10.1097/00001648-200101000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim AS, Ismail K, Hablas A, Hussein H, Elhamzawy H, Ramadan M. Cancer in Egypt, Gharbiah. Triennial Report of 2000–2002. Gharbiah Population-Based Cancer Registry; Egypt: 2007. [Google Scholar]
- 25.CAPMAS Reports. Url: http://www.capmas.gov.eg/eng_ver/homee.htm.
- 26.Sturgeon SR, Schairer C, Gail M, McAdams M, Brinton LA, Hoover RN. Geographic variation in mortality from breast cancer among white women in the United States. J Natl Cancer Inst. 1995;87:1846–1853. doi: 10.1093/jnci/87.24.1846. [DOI] [PubMed] [Google Scholar]
- 27.Kishk N. Breast cancer in relation to some reproductive factors. J Egypt Public Health Assoc. 1999;74(5–6):547–566. [PubMed] [Google Scholar]
- 28.El-Zanaty F, Way A. Egypt Demographic and Health Survey 2005. Cairo, Egypt: Ministry of Health and Population, National Population Council, El-Zanaty and Associates, and ORC Macro; 2006. [Google Scholar]
- 29.Hosny G, Elkaffas SM. A prediction model for the incidence patterns of female breast cancers in Alexandria, Egypt. J Egypt Public Health Assoc. 2002;77(3–4):329–345. [PubMed] [Google Scholar]
- 30.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Saghir NS, Khalil MK, Eid T, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: a literature and registry analysis. Int J Surg. 2007;5(4):225–233. doi: 10.1016/j.ijsu.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Esteban D, Whelan S, Landico A, Parkin DM. Manual for cancer registry personnel. IARC Technical Report No. 10. International Agency for Research on Cancer; Lyon, France: 1995. [Google Scholar]
- 33.WRI. Pesticides and the Immune System: The Public Health Risks. World Resources Institute; Washington, DC: 1996. [PubMed] [Google Scholar]
- 34.Ndahi HB. The new world of plastics. The Technology Teacher. 2000;16 (1):18–22. [Google Scholar]
- 35.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113(4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittassek M, Wiesmuller GA, Koch HM, et al. Internal phthalate exposure over the last two decades – a retrospective human biomonitoring study. Int J of Hyg Environ Health. 2007;210(3–4):319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 38.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(2):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. [Last accessed February 18, 2010.]; Url: http://www.worldwatch.org/node/1499.
- 40. [Last accessed February 18, 2010.]; Url: http://reusablebags.com/facts.php.
- 41.Moses M. Designer poisons: How to protect your health and home from toxic pesticides. Pesticide Education Center; San Francisco: 1995. [Google Scholar]
- 42.Byford JR, Shaw LE, Drew MGB, Pope GS, Sauer MJ, Darbre PD. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2002;80:49–60. doi: 10.1016/s0960-0760(01)00174-1. [DOI] [PubMed] [Google Scholar]
- 43.Dabre PD, Byford JR, Shaw LE, et al. Estrogenic activity of benzylparaben. J Appl Toxicol. 2003;23:43–51. doi: 10.1002/jat.886. [DOI] [PubMed] [Google Scholar]
- 44.Soliman AS, Vulimiri SV, Kleiner HE, et al. High levels of oxidative DNA damage in lymphocyte DNA of premenopausal breast cancer patients from Egypt. Int J Environ Health Res. 2004;14(2):121–134. doi: 10.1080/0960312042000209534. [DOI] [PubMed] [Google Scholar]
- 45.Soltan ME, Awadallah RM. Chemical survery of the River Nile water from Aswan into the outlet. J Environ Sci Health. 1995;A30(8):1647–1658. [Google Scholar]
- 46.Abdel-Gaward S, Abdel-Shafy M. Pollution control of industrial wastewater from soap and oil industries: a case study. Water Sci Technol. 2002;46(4–5):77–82. [PubMed] [Google Scholar]
- 47.Awadallah RM, Soltan ME, Shabeb MSA, Moalla SMN. Bacterial removal of nitrate, nitrite and sulphate in wastewater. Wat Res. 1998;32(10):3080–3084. [Google Scholar]