Abstract
Fanconi anemia (FA) is an inherited disorder characterized by defective DNA repair and cellular sensitivity to DNA crosslinking agents. Clinically, FA is associated with high risk for marrow failure, leukemia and head and neck squamous cell carcinoma (HNSCC). Radiosensitivity in FA patients compromises the use of total-body irradiation for hematopoietic stem cell transplantation and radiation therapy for HNSCC. A radioprotector for the surrounding tissue would therefore be very valuable during radiotherapy for HNSCC. Clonogenic radiation survival curves were determined for pre- or postirradiation treatment with the parent nitroxide Tempol or JP4-039 in cells of four FA patient-derived cell lines and two transgene-corrected subclonal lines. FancG–/– (PD326) and FancD2–/– (PD20F) patient lines were more sensitive to the DNA crosslinking agent mitomycin C (MMC) than their transgene-restored subclonal cell lines (both P < 0.0001). FancD2–/– cells were more radiosensitive than the transgene restored subclonal cell line (ñ = 2.0 ± 0.7 and 4.7 ± 2.2, respectively, P = 0.03). In contrast, FancG–/– cells were radioresistant relative to the transgene-restored subclonal cell line (ñ = 9.4 ± 1.5 and 2.2 ± 05, respectively, P = 0.001). DNA strand breaks measured by the comet assay correlated with radiosensitivity. Cell lines from a Fanc-C and Fanc-A patients showed radiosensitivity similar to that of Fanc-D2–/– cells. A fluorophore-tagged JP4-039 (BODIPY-FL) analog targeted the mitochondria of the cell lines. Preirradiation or postirradiation treatment with JP4-039 at a lower concentration than Tempol significantly increased the radioresistance and stabilized the antioxidant stores of all cell lines. Tempol increased the toxicity of MMC in FancD2–/– cells. These data provide support for the potential clinical use of JP4-039 for normal tissue radioprotection during chemoradiotherapy in FA patients.
INTRODUCTION
Fanconi anemia (FA) is an autosomal recessive disorder characterized by dysfunctional DNA repair and increased incidence of aplastic anemia, myelodysplasia, acute myeloid leukemia, and epithelial malignancies (1–4), presenting at unusually early ages (2, 5–9). The overall median survival is 24 years (3, 4). FA patients who survive to their early adulthood years are 50 times more likely to develop solid tumors, particularly head and neck squamous cell carcinoma (HNSCC), compared to the general population (1, 4, 7, 10, 11). Thus FA patients may require radiotherapy for appropriate management (7, 8) despite their intrinsic cellular radiosensitivity (4–9, 12, 13).
Cells from FA patients display sensitivity to DNA crosslinking agents such as mitomycin C (MMC), cisplatin and diepoxybutane (9, 12, 14, 15). Chromosomal breakage in FA cells in response to alkylating agents is also frequent (9). The DNA repair dysfunction in FA has been attributed to mutations in one or more of 13 FA complementation groups (5). In the normal cellular response to DNA damage, FA proteins A, B, C, E, F, G, L and M form a nuclear complex that functions as a ubiquitin ligase, with FancL as its catalytic subunit for ubiquitination (5, 16). The L subunit then monoubiquinates the FancD2 and FancI proteins (17–19). Activated FancD2-FancI protein complex subsequently colocalizes with FA proteins (FancD1, FancN and FancJ) and other DNA repair proteins, including NBS1, to form nuclear foci that mediate DNA repair (5, 17–19).
Therapeutic approaches to the hematological defects in FA include allogeneic hematopoietic stem cell transplantation (20, 21). The problem of increased graft failure due to the need to use decreased doses of cyclophosphamide and total-body radiation in the preparative regimen of FA patients has been largely overcome by the addition of fludarabine (22). However, the adequacy of any transplantation regimen for FA patients with myelodysplasia or leukemia remains an issue (13–15, 20, 21, 23). Moreover, both young and older adult FA patients are developing HNSCC diagnosed either primarily or after hematopoietic stem cell transplantation. Most are treated with surgery alone, potentially contributing to the multiple primary cancers and relatively worse 2-year survival in comparison to non-FA HNSCC patients (1, 4, 7). In the cases where radiation therapy is used, the reduced dose may compromise tumor control and clinical outcome. A normal tissue radioprotective agent would be useful, but it must be safe in FA patients, not all of whom are equivalently radiosensitive (13–15, 23). In the present studies, we tested the mitochondrial targeted GS-nitroxide, JP4-039, for radiobiological effects on cell lines derived from four patients and in two FA patients compared to their transgene restored subclonal lines.
METHODS
Cell Lines
The Fanc-A patient cell line (OHSU-974) and Fanc-C patient cell line (VU1131)(52) were a gift from Dr. Hans Joenje, Vrige University Medical Center, Amsterdam, The Netherlands. The FA patient-derived cell lines, FancG–/– (PD326) and FA transgene-restored FancG subclone, were a gift from Dr. Christopher Bakkenist (University of Pittsburgh School of Medicine, Pittsburgh, PA). The FancD2–/– (PD20F) patient and transgene-restored FancD2 subclonal lines were a gift from Dr. Alan D'Andrea (Dana Farber Cancer Institute, Boston MA). The latter two cell lines have been described previously, including functional diagnosis by in vitro MMC sensitivity and its correction by transgene insertion (17, 24, 25). FancG–/– (PD326) and FancG transgene cells were maintained in a high-humidity incubator at 37°C and 95% air/5% CO2. Cells were cultured in Dulbecco's modified Eagle medium (DMEM) with 15% fetal bovine serum (FBS),1% l-glutamine and 1% penicillin-streptomycin (26) in T-75 flasks. FancD2–/– (PD20F) and FancD2 transgene-restored cells were maintained in the same incubator conditions but were cultured in RPMI 1640 medium with 15% FBS, 1% l-glutamine and 1% penicillin-streptomycin.
Assay for Cell Line Mitomycin C Sensitivity
FA and FA transgene-restored cells were grown in T-25 flasks. When the cells were 50% confluent, MMC was added in concentrations between 0 and 100 ng/ml daily for 3 days. Cells were then trypsinized and plated in 4-well plates at 1,000 cells per 15 ml. Eight days later, colonies of greater than 50 cells were stained with crystal violet solution and counted. Results are the means ± SEM of three separate experiments with at least six plates per point.
Radiosensitivity of FA and FA Transgene-Restored Cell Lines
Radiosensitivity of each FA and FA transgene-corrected fibroblast line was measured using a clonogenic colony-forming assay (27). Cells in logarithmic phase growth were irradiated with doses ranging from 0 to 8 Gy (J. L. Shepherd Mark V Irradiator, 70 cGy/min), then plated in Linbro 4-well tissue culture plates (MP Biomedicals, LLC, Solon, OH) at concentrations of 125, 250 or 1000 cells per 4.0 ml per well. Eight days later colonies of >50 cells were stained with crystal violet solution and counted using a GelCount cell colony counter (Oxford Optronix, Oxford, UK). The data were analyzed using linear-quadratic or single-hit, multitarget models (28, 29). Each experiment was repeated at least three times on three different days. The comparative protection and mitigation assay for JP4-039 and Tempol used comet assays as described (30, 31).
Synthesis of a Fluorophore-Tagged JP4-039 Analog
The detection of JP4-039 in cell tissue samples previously used ESR and MS methods (32, 33). For additional biological studies and to investigate the ability of these nitroxides to localize in the mitochondria, we desired a more direct fluorescence-based visualization and selected a BODIPY-FL® fluorophore for this purpose (34). An analog of JP4-039 with a BODIPY-FL® label in place of the N-Boc group was synthesized using a de novo synthesis approach (35). The direct synthesis of this labeled derivative from JP4-039 failed, since, after the Boc group of JP4-039 was successfully removed with HCI, treatment of the free amine with the N-hydroxysuccinimide activated ester of BODIPY-FL® (BODIPY-FL-NHS) provided an 83:17 mixture of the undesired bis-coupling product and the desired EMF338-008.
To avoid this unwanted side product, we intended to attach the BODIPY-FL® label to the alkene peptide isostere scaffold prior to addition of the TEMPO group. Accordingly, the carboxylic acid was protected as the methyl ester. Removal of the Boc-protecting group with trifluroacetic acid (TFA) followed by coupling to BODIPY-FL-NHS provided the conjugate in 82% yield. Saponification of the methyl ester under standard basic conditions resulted in extensive decomposition; therefore, pig liver esterase (PLE) in acetone/pH 7 phosphate buffer was used to effect a chemoselective transformation. Coupling of the resulting acid to 4-amino-tempo (4-AT) provided the desired BODIPY-FL®-labeled compound JP4-039-BODIPY (EMF338-008) in 56% yield over two steps (35).
Mitochondrial Localization of Fluorochrome-Labeled JP4-039 (BODIPY)
Fanc-D2 and FD20F cells were grown on glass cover slips for staining with MitoTracker Red (Molecular Probes-Invitrogen, Eugene, OR) and JP4-039-BODIPY. The cover slips were incubated with 10 μM JP4-039-BODIPY and 100 μM MitoTracker for 15 min at 37°C. The cells were washed with warm RPMI 1640 medium and fixed with 3.7% paraformaldehyde. The cover slips were mounted with either Prolong Gold/DAPI (Molecular Probes-Invitrogen) or gelvatol containing Hoechest and imaged using fluorescence and confocal microscopy.
Comet Assay
Measurement of DNA strand breaks after irradiation was performed as described previously (30, 31). Cells of the Fanc-D2, Fanc-G, PD20F and PD326 cell lines were irradiated to either 0 or 6 Gy and incubated at room temperature for 10 min, at which time the cells were rapidly chilled to 4°C to stop DNA repair. The cells were mixed in low melt agarose, and 500 cells were placed on the sample area of a CometSlide (Comet Assay 4250-050-K, Travigen, Inc., Gaithersburg, MD). The slides were rapidly chilled to 4°C and kept in the dark to prevent DNA repair. The slides were then placed in prechilled lysis solution and kept at 4°C for 60 min followed by washing in neutral electrophoresis buffer for 30 min at 4°C and electrophoresis at 21 V for 1 h at 4°C, then immersed in DNA precipitation solution for 30 min at room temperature, immersed in 70% ethanol for 30 min, and dried at 45°C for 15 min. The cells were then stained with SYBR Green 1 and examined under a fluorescence microscope, and the comet tails for each of 150 cells were quantified using the Comet Assay IV software.
Assays for Oxidative Stress Responses of Cell Lines to Radiation
The assay for intracellular glutathione stores using a Calbiochem Glutathione Assay Kit II (EMB Serono, Inc., Rockland, MA) has been published previously (36, 37). Cells of the Fanc-D2, Fanc-G, PD20F and PD326 cell lines were incubated for 1 h in JP4-039 (10 μM), irradiated to 0, 2 or 6 Gy, and collected by centrifugation 24 h later. The cells were homogenized in 50 mM phosphate buffer containing 1 mM EDTA and centrifuged at 10,000g for 15 min at 4°C. Protein levels were determined in each sample, 50 μl of each sample was added to individual wells in a 96-well plate, and 150 μl of cocktail mix was added to each well. Absorbance at 405 was determined 25 min later, and GSH concentration was determined using the End Point Method.
GS-Nitroxide and Tempol Treatment Effects on Radiosensitivity and Mitomycin C Sensitivity
Prior to or after irradiation or MMC addition, cells from each line were treated with 10 μM JP4-039 or Tempol. These concentrations have been shown to be optimal for radiation dose–response curves of human cells in vitro (27, 32). For protection experiments, drugs were added 1 h before irradiation or 1 h before adding MMC. For mitigation experiments, JP4-039 or Tempol was added immediately after irradiation. For MMC drug sensitivity, Tempol or JP4-039 was also added once daily for 3 days; these cells were then grown in nonsupplemented medium for an additional 5 days, and cell survival was scored on day 8. Irradiated or MMC-treated cells were transferred to tissue culture plates for the colony assay and at day 8 were stained using crystal violet solution. Colonies ≥ 50 cells were then counted. MMC-treated cell-derived colonies were scored on day 8.
Statistics
For radiosensitivity, the D0 and ñ were calculated for each group using means ± SD. Comparisons were made between any two groups with the two-sided two-sample t test. P ≤ 0.05 was regarded as significant, and P values were adjusted for multiple comparisons. For mitomycin C sensitivity, the data for number of colonies were summarized as means ± SD for each group at each MMC dose. The number of colonies were log-transformed, and a linear mixed model was fitted on the log-transformed data with group and MMC dose. Their interaction term was fixed as an explanatory variable within each subject variable, MMC dose, and used as a repeated measure. The F test was used to test for the significance of the interaction between each JP4-039- or Tempol treated group relative to each MMC dose. A significant interaction was defined as one showing a P < 0.05 in the MMC-induced decrease in colony numbers between groups.
RESULTS
FancG–/– and FancD2–/– Cell Lines are Sensitive to Mitomycin C
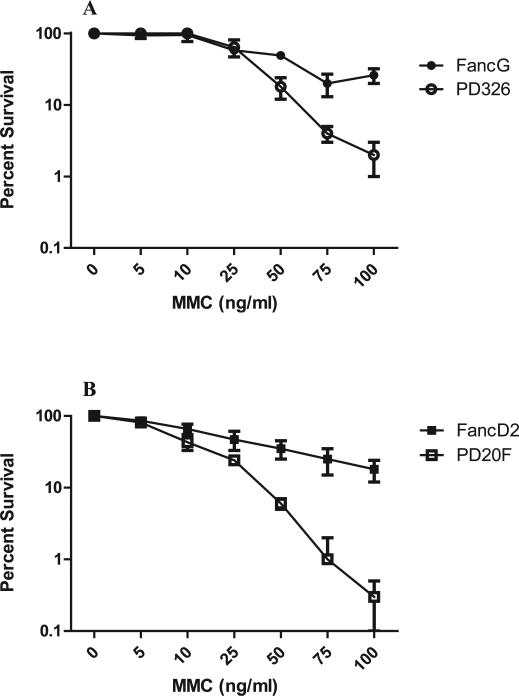
We first confirmed the diagnostic phenotype of the two FA patient cell lines. Comparison of the MMC sensitivity of each FA patient cell line with its respective transgene-restored cell line was carried out. FancG–/– (PD326) cells were more sensitive to MMC (P < 0.0001) than was the transgene-restored FancG cell line (Fig. 1A). FancD2–/– (PD20F) cells were also more sensitive to MMC (P < 0.0001) than the transgene-restored FancD2 cells (Fig. 1B). These results confirm the diagnostic FA phenotype in both lines and their normalization by restoration of the respective functional complementation genes (38).
FIG. 1.
Sensitivity of FA cell lines to MMC relative to the respective transgene-restored line. Cells were incubated in the presence of each concentration of MMC for 3 days. Cells were trypsinized and plated as described in the Methods. At day 8, colonies of ≥50 cells were stained with crystal violet solution and over 10,000 cells were counted. Panel A: FancG–/– (PD326) cells were significantly more sensitive to MMC than transgene-restored FancG cells (P < 0.0001). Panel B: FancD2–/– (PD20F) cells were significantly more sensitive to MMC than transgene restored FancD2 cells (P < 0.0001).
Radiosensitivity of FA Patient and FA Transgene-Restored Cell Lines
We evaluated the radiosensitivity of each FA cell line and its transgene-restored subclonal cell line. The FancG–/– (PD326) cell line was radioresistant relative to its respective transgene-corrected line (Table 1). In contrast, the FancD2–/– (PD20F) cell line was radiosensitive relative to its transgene restored line (Table 1). The Fanc-C and Fanc-A patient cells were more like the Fanc-D2–/– line in radiation sensitivity (Table 2). These results establish a clear heterogeneity in radiosensitivity between FA patient cell lines.
TABLE 1.
Effect of GS-Nitroxide JP4-039 Added before or after Irradiation on the Radiosensitivity of Fanconi Anemia D2–/– and G–/– Cell Lines
| Cell line | D0 (Gy) | ñ |
|---|---|---|
| FancG –/– (PD326) | 1.2 ± 0.3 | 9.4 ± 1.5 P = 0.001* (compared to FancG) |
| Tempol + PD326 before | 1.2 ± 0.1 | 14.7 ± 1.4 P = 0.01* (compared to PD326) |
| JP4-039 + PD326 before | 1.1 ± 0.2 | 20.5 ± 4.5 P = 0.02* (compared to PD326) P = 0.001* (compared to PD326 + Tempol) |
| PD326 + Tempol after | 1.1 ± 0.1 | 14.8 ± 1.5 P = 0.01* (compared to PD326) P = 0.001* (compared to FancG + Tempol) |
| PD326 + JP4-039 after | 1.1 ± 0.1 | 20.5 ± 4.5 P = 0.02* (compared to PD326) P = 0.03* (compared to FancG +J P4-039) |
| FancG restored | 1.2 ± 0.1 | 2.2 ± 0.5 |
| Tempol + FancG before | 1.3 ± 0.2 | 7.2 ± 0.7 P = 0.001* (compared to FancG) |
| JP4-039 + FancG before | 1.3 ± 0.1 | 20.5 ± 4.5 P = 0.002* (compared to FancG) |
| FancG + Tempol after | 1.0 ± 0.2 | 7.2 ± 0.7 P = 0.001* (compared to FancG) |
| FancG + JP4-039 after | 1.1 ± 0.1 | 6.8 ± 0.6 P = 0.001* (compared to FancG) |
| FancD2 –/– (PD20F) | 1.4 6 0.2 | 2.0 ± 0.7 P = 0.03* (compared to FancD2) |
| Tempol + PD20F before | 1.5 ± 0.3 | 4.5 ± 1.8 P = 0.02* (compared to PD320F) |
| JP4-039 + PD20F before | 1.7 ± 0.1 P = 0.04* (compared to PD320F) |
1.9 ± 0.3 |
| PD20F + Tempol after | 1.5 ± 0.3 | 4.0 ± 1.1 |
| PD20F + JP4-039 after | 1.6 ± 0.3 | 6.2 ± 0.7 P = 0.01* (compared to PD20F) |
| FancD2 restored | 1.0 ± 0.1 | 4.7 ± 2.2 |
| Tempol + FancD2 before | 1.1 ± 0.1 P = 0.06* (compared to Tempol + PD20F) |
5.1 ± 1.0 |
| JP4-039 + FancD2 before | 1.1 ± 0.1 P = 0.0009* (compared to JP4-039 + PD20F) |
7.6 ± 0.6 P < 0.0001* (compared to JP4-039 + PD20F) P = 0.04* (compared to FancD2) |
| FancD2 + Tempol Post | 1.2 ± 0.1 | 4.7 ± 1.2 |
| FancD2 + JP4-039 Post | 1.2 ± 0.1 | 7.8 ± 0.5 P = 0.02* (compared to FancD2) |
Notes. FancG, FancG –/– (PD326), FancD2 and FancD2–/– (PD20F) cells were irradiated with 0 to 8 Gy. The cells were plated in 4-well tissue culture plates and incubated in a high CO2 incubator for 8 days, and colonies of greater than 50 cells were counted. The data were analyzed by the linear-quadratic and single-hit, multitarget models; the D0 and ñ are shown. FancG transgene-restored cells were more radiosensitive than FancG–/– cells [decreased shoulder on the survival curve (ñ)], while FancD2–/– cells were more radiosensitive than transgene-restored FancD2 cells. The data are the average of three separate experiments on 3 separate days. Tempol or JP4-039 was added before or after irradiation and the D0 and ñ for each cell line were calculated as described in the Methods. Means ± SD of D0 and ñ in each group are shown. P values were calculated for the comparison between two groups with the two-sided two-sample t test. Significant P values are indicated by an asterisk. Each experiment was carried out a minimum of three times.
TABLE 2.
Effect of GS-Nitroxide, JP4-039 Added before or after Irradiation on the Radiosensitivity of Fanconi Anemia A and C Patient Cells
| Cell line | D0 (Gy) | ñ |
|---|---|---|
| Fanc-A–/– (OH-SU-974) | 1.6 ± 0.1 | 4.2 ± 1.1 |
| Tempol (10 μM) + Fanc-A–/– before | 2.5 ± 0.2 (P = 0.007) |
3.2 ± 1.3 |
| JP4-039 (10 μM) + Fanc-A–/– before | 1.9 ± 0.1 (P = 0.02) |
4.3 ± 0.6 |
| Fanc-A–/– + Tempol after | 2.1 ± 0.1 (P = 0.02) |
3.5 ± 1.5 |
| Fanc-A–/– + JP4-039 after | 2.1 ± 0.1 (P = 0.002) |
2.2 ± 0.7 |
| Fanc-C–/– (VU-113) | 1.6 ± 0.1 | 3.3 ± 0.3 |
| Tempol (10 μM) + Fanc-C–/– before | 1.9 ± 0.1 (P = 0.003) |
2.4 ± 0.2 |
| JP4-039 (10 μM) + Fanc-C–/– before | 2.3 ± 0.2 (P = 0.02) |
2.4 ± 1.0 |
| Fanc-C–/– + Tempol after | 1.6 ± 0.2 | 10.3 ± 1.0 (P = 0.003) |
| Fanc-C–/– + JP4-039 after | 1.5 ± 0.2 | 8.5 ± 1.1 (P = 0.01) |
GS-nitroxide, JP4-039, Increases Radioresistance of Both FA Cell Lines and Transgene-Restored Subline
The mitochondrial targeted GS-nitroxide drug JP4-039 or its parent non-mitochondrial targeted nitroxide, Tempol, were added either before irradiation (protection) or after irradiation (mitigation). Adding JP4-039 before irradiation increased the radioresistance, shown as an increase in the ñ of FancG–/– (PD326) (P = 0.02) as well as transgene-restored FancG cells (P = 0.002) (Table 1). Pretreatment with JP4-039 also increased the radioresistance of FancD2–/– cells (PD20F), seen as an increase in D0 (P = 0.04), and the transgene-restored subline FancD2, seen as an increase in ñ (P = 0.04) (Table 1).
In radiation mitigation experiments, JP4-039 added after irradiation increased the radioresistance of both FancG–/– (PD326) and FancG transgene-restored cells, as shown by an increase in ñ (P = 0.001 and 0.02, respectively) (Table 1). Tempol added after irradiation also increased radioresistance (P = 0.001 and 0.01, respectively) (Table 1). When added after irradiation, JP4-039 increased the radiation resistance of FancD2–/– (PD20F) and FancD2 transgene-restored cells, as shown by an increase in ñ (P = 0.001 and 0.02, respectively) (Table 1). Tempol was a less effective mitigator for FancD2–/– (PD20F) and FancD2 cells (Table 1). Both Fanc-A–/– and Fanc-C–/– cells were protected and mitigated by JP4-039 (Table 2).
JP4-039 Localization in Mitochondria Increases Effectiveness Compared to Tempol
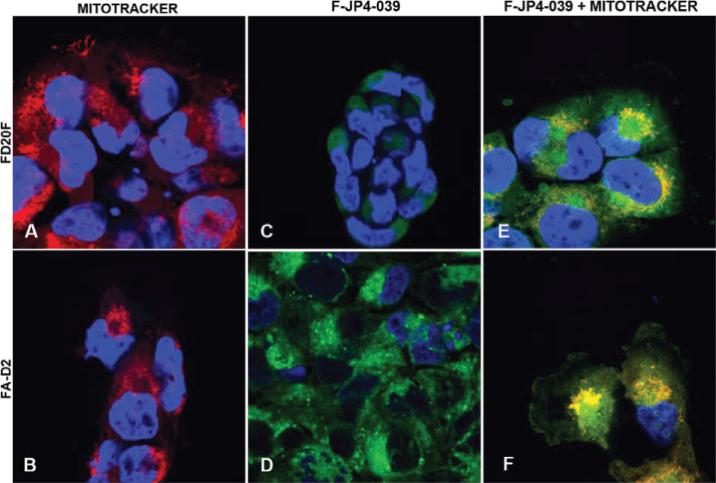
We evaluated the importance of mitochondrial localization of JP4-039, comparing it to Tempol. Table 3 shows greater radiation protection at lower concentrations of JP4-039 compared to Tempol. To confirm that mitochondrial localization was occurring, we targeted a fluorochrome-labeled analogue of JP4-039. Figure 2 shows that fluorophore-labeled-JP4-039 BODIPY but not the fluorophore alone (not shown) localized to mitochondria of both Fanc-D2–/– (PD20F) and transgene-restored Fanc-D2 cells. JP4-039-BODIPY was as effective as JP4-039 as a radioprotector and mitigator of Fanc-D2 cells (Table 4). Thus the fluorochrome labeling did not abrogate the radioprotective capacity of JP4-039.
TABLE 3.
JP4-039 is More Effective than Tempol in Radioprotective Capacity
| Cell line | Concentration (μM) | ñ |
|---|---|---|
| FancG | 0 | 2.5 ± 0.3 |
| Tempol + FancG | 0.1 | 4.5 ± 1.3 |
| 1 | 4.9 ± 0.9 | |
| 10 | 7.2 ± 0.7 (P = 0.001) |
|
| JP4-039 + FancG | 0.1 | 4.0 ± 0.1 (P = 0.04) |
| 1 | 5.3 ± 0.1 (P = 0.01) |
|
| 10 | 20.5 ± 4.5 (P = 0.002) |
Notes. FancG cells were preincubated in 0, 0.1, 1.0 or 10 μM tempol or JP4-039 for 1 h and then irradiated with 0–8 Gy as described in the Methods. Colonies were stained with crystal violet 7 days later, and colonies of greater than 50 cells were counted. The data were analyzed by linear-quadratic and single-hit, multitarget models. At 0.1, 1 and 10 μM, the cells incubated with JP4-039 had a significantly increased ñ compared to cells treated with the same molar concentration of Tempol. At 0.1 or 1.0 μM, Tempol was not radioprotective. The mitochondrial localization of JP4-039 was >10-fold more effective than Tempol by molar concentration.
FIG. 2.
JP4-039-BODIPY colocalizes to mitochondria in Fanc-D2 and Fanc-D2–/– (PD20F) cells. Cells were stained with MitoTracker Red and JP4-039-BODIPY. Panels A and B: MitoTracker only (red); panels C and D: JP4-039-BODIPY only (green); panels E and F: JP4-039-BODIPY/MitoTracker colocalization (yellow). Original magnification 1000×.
TABLE 4.
JP4-039-BODIPY is as Effective a Radioprotector of Fanc-D2 Cells as JP4-039
| Condition | D0 (Gy) |
|---|---|
| FancD2 control | 1.0 ± 0.1 |
| DMSO + FancD2 | 1.0 ± 0.1 |
| Tempol + FancD2 | 1.1 ± 0.1 |
| JP4-039 + FancD2 | 1.1 ± 0.1 (P = 0.003) |
| JP4-039-Bodipy + FancD2 | 1.1 ± 0.1 (P < 0.0001) |
Notes. FancD2 cells were incubated for 1 h in DMSO (control), tempol (10 μM), JP4-039 (10 μM) or JP4-039-Bodipy (10 μM), then irradiated with 0 to 8 Gy, plated in 4-well tissue culture plates, incubated for 7 days at 37°C in a CO2 incubator, and stained with crystal violet. Colonies of greater than 50 cells were counted as described in the Methods. Data were analyzed using linear-quadratic or single-hit, multitarget models. FancD2 cells treated with JP4-039 or JP4-039-BODIPY had significantly increased survival compared to control irradiated FancD2 cells. There was no significant difference between JP4-039 and JP4-039-BODIPY.
JP4-039 Ameliorates Reduction of Glutathione by Radiation in Fanconi Anemia Cell Lines
To determine whether the radioprotective and mitigative capacity of JP4-039 was associated with its function as a free radical scavenger, we used established assays for radiation-mediated depletion of antioxidant stores (36). Table 5 shows that JP4-039 treatment maintained high intracellular antioxidant stores after 2 Gy or 6 Gy irradiation.
TABLE 5.
Glutathione Levels in Irradiated Fanconi Anemia Patient Cell Lines are Preserved by JP4-039 Treatment
| Cell line | 0 Gy | 0 Gy + JP4-039 | 2 Gy | 2 Gy + JP4-039 | 6 Gy | 6 Gy + JP4-039 |
|---|---|---|---|---|---|---|
| FancD2 | 10.8 ± 0.3 | 16.3 ± 0.1 (P = 0.002)*** |
14.8 ± 1.1 | 22.1 ± 3.0 (P = 0.15)* |
6.5 ± 0.1 | 10.0 ± 0.1 (P = 0.0007)** |
| PD20F | 18.2 ± 0.1 | 22.5 ± 1.0 (P = 0.01)*** |
19.4 ± 0.3 | 21.2 ± 0.5 (P = 0.08)* |
22.8 ± 0.8 | 37.1 ± 2.2 (P = 0.03)** |
| FancG | 8.9 ± 0.3 | 18.2 ± 1.0 (P = 0.01)*** |
7.6 ± 0.2 | 8.5 ± 0.1 (P = 0.01)* |
8.0 ± 0.2 | 11.8 ± 9.1** (P = 0.002) |
| PD326 | 5.5 ± 0.1 | 6.5 ± 0.1*** (P = 0.003) |
16.8 ± 2.0 | 17.0 ± 5.1 (P = 0.98)* |
0.8 ± 0.7 | 6.3 ± 0.1 (P = 0.017)** |
Notes.
Compared to 0 Gy
compared to 2 Gy
compared to 6 Gy.
Fanc-D2, PD20F, Fanc-G, and PD326 cells were incubated for 1 h in 10 μM JP4-039, irradiated to 0, 2 or 6 Gy, and then cells harvested 24 h later. Glutathione concentrations were determined as described in the Methods. Results demonstrated that all cell lines treated with JP4-039 had increased antioxidant store levels compared to irradiated control cells at each radiation dose.
JP4-039 Does Not Alter MMC Sensitivity of FA Patient Cell Lines
We tested the effects of JP4-039 and Tempol on the sensitivity of FA cell lines and respective transgene-restored cell lines to MMC. As shown in Fig. 3, there was no significant effect of JP4-039 or Tempol on the relative sensitivity of FancG–/– (PD326) or FancG cell lines to MMC. However, Tempol increased the sensitivity of both FancD–/– (PD20F) and FancD2 transgene-restored cells to MMC (P = 0.005 and 0.04) (Fig. 3).
FIG. 3.
JP4-039 does not alter the sensitivity of FA patient cell lines to MMC. Cells of FA patient cell lines and FA transgene-restored cell lines were incubated in each concentration of MMC for 3 days. JP4-039 or Tempol (10 μM) was added daily starting 1 h before initial incubation, then daily for 7 days. Cells were trypsinized and replated at day 3 and then after 8 days were stained with crystal violet solution and colonies of ≥50 cells counted. Panel A: Neither JP4-039 nor Tempol altered the sensitivity of FancG transgene-restored cells to MMC (P = 0.6 and 0.1, respectively). Panel B: Neither JP4-039 or Tempol altered the sensitivity of FancG–/– (PD326) cells to MMC (P = 0.71 and 0.58,=respectively). Panel C: JP4-039 did not alter the sensitivity of FancD2 restored cells to MMC (P = 0.4); however, Tempol increased the sensitivity to MMC (P = 0.005). Panel D: JP4-039 did not alter the sensitivity of FancD2 transgene-restored cells (P =1.0) while Tempol increased the sensitivity to MMC (P = 0.04).
Relative Radioresistance of Fanc-G–/– (PD326) Cells Correlates with Decreased Radiation-Induced DNA Double-Strand Breaks
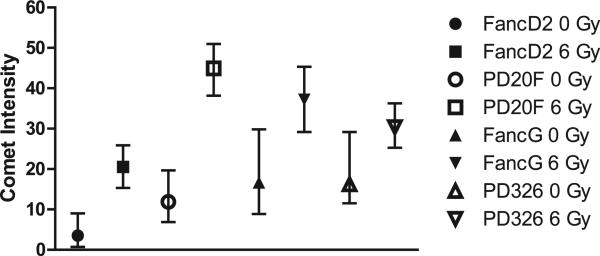
We measured DNA double-strand breaks after 6 Gy irradiation by a comet assay in Fanc-G–/– (PD326) and Fanc-G cells compared to Fanc-D2–/– (PD20F) and Fanc-D2 transgene-restored cells. The two cell lines with the most DNA strand breaks were the Fanc-D2–/– (PD20F) and Fanc-G cells (Fig. 4), which were also more radiosensitive as determined by clonogenic survival curve analysis.
FIG. 4.
Decreased radiation induced DNA strand breaks by comet assay correlates with relative radioresistance of Fanc-G–/– (PD326) cells compared to Fanc-D2–/– (PD20F) cells. Cells were irradiated to 0 or 6 Gy and collected 10 min after irradiation. The cells were prepared for the neutral comet assay, which measured double-stranded DNA breaks by plating in low-melt agarose, then treated with protease and RNase to leave the DNA only. The DNA was electrophoresed and stained with a fluorescent dye as described in the Methods. The tail intensity was determined, with a higher tail intensity indicating increased DNA strand breaks. The Fanconi anemia cell line comet intensity was compared for eight groups (Fanc-D2 0 Gy, Fanc-D2 6 Gy, Fanc-G 0 Gy, Fanc-G 6 Gy, PD326 0 Gy, PD326 6 Gy, PD20F 0 Gy, PD20F 6 Gy). Because there were multiple 0 values in some of the groups, and because the test for normality was rejected for most of the groups, the two-sided Wilcoxon rank sum test was used to test the difference between any two groups. Data were summarized by median and interquartile range (IQR) correspondingly. In each group, data are restated as median, interquartile range (IQR), and sample size (n). P values were calculated for the comparison between any two groups with the two-sided Wilcoxon rank sum test. For all tests, P values of less than 0.05 were regarded as significant. All the tests had significant P values except the one comparing PD326 and Fanc-G cells at 0 Gy. Fanc-D2–/– (PD20F) cells had more DNA strand breaks (comet intensity) than Fanc-G–/– (PD326) cells (P < 0.0001). Gene-corrected Fanc-D2 cells and Fanc-G cells were more radioresistant or radiosensitive than parent lines (P < 0.0001), consistent with clonogenic survival curve data.
Radiation survival curves demonstrated that the Fanc-D2 transgene-restored cells were more radioresistant than PD20F cells. The nonirradiated PD20F cells also had more DNA double-strand breaks than did the nonirradiated transgene-restored Fanc-D2 cells (P = 0.002). Comet assay data demonstrated that PD20F cells had more DNA double-strand breaks after 6 Gy than did the Fanc-D2 gene-restored cells (P < 0.0001). Fanc-D2 cells were relatively radioresistant compared to PD20F cells by clonogenic survival curve assay.
The comet assay data also agreed with the clonogenic radiation survival curve data for Fanc-G and PD326 cells. Fanc-G cells had increased comet intensity after 6 Gy irradiation compared to PD326 cells (P < 0.0001); however, in contrast to the Fanc-D2–/– (PD20F) and transgene-restored cells, there was no significant difference between the unirradiated Fanc-G and PD326 cells (P = 0.06).
DISCUSSION
Given the limitations that intrinsic radiosensitivity places on the tolerance for fractionated external-beam radiotherapy (1, 4, 10, 11), there is increasing concern for the high incidence of oral cavity and oropharyngeal cancers occurring both de novo (39) and in previously transplanted FA patients (39). Recently available organ-specific radio-protective agents can increase esophageal radiation tolerance in non-small-cell lung cancer patients (32, 33, 40) and oral cavity/oropharynx tissue tolerance in HNSCC patients (41). Whether tumor-adjacent tissue radioprotectors can be effective in patients with an intrinsic defect in DNA repair is unknown.
In the present studies, we tested a novel small molecule mitochondrial-targeted GS-nitroxide (JP4-039) (27, 32, 33) for its radiobiological effect on cells of four Fanconi anemia patient cell lines. A cell line from a FancD2–/– patient was radiosensitive relative to its transgene-restored subline. In contrast, a cell line from a patient with the FancG–/– genotype was relatively radioresistant compared to its transgene-restored subline. Whether this result with FancG–/– cells represents selection for a secondary mutation during cell line establishment that confers radioresistance without MMC resistance is unknown. Studies with other FancG–/– patient cell lines or freshly removed cells may provide additional information. In both FA cell lines and their transgene-restored subclonal lines, JP4-039 added before or after irradiation increased radioresistance in clonogenic survival curve assays. Cell lines from two other patients with the Fanc-A or Fanc-C genotype were similar in radiosensitivity to Fanc-D2–/– cells and were also protected and mitigated by JP4-039. Fluorophore-labeled JP4-039 (BODIPY) localized to the mitochondria of these cell lines. JP4-039 was more effective at lower concentrations than Tempol and more effectively preserved cellular antioxidant stores, further confirming its free radical scavenging. Thus JP4-039 is potentially a valuable radioprotector for FA patient cells independent of their intrinsic radiosensitivity.
An explanation for the radiosensitivity of the FancD2–/– (PD20F) cell line might be related to other FA pathway components. In the PD20F (FancD2–/–) cell line, the core complex is formed but does not monoubiquinate the missing FancD2 protein and thus cannot repair through its normal pathway, or the associated pathways, to the extent seen in the FancG–/– (PD326) cell line. This mechanism may explain why the PD20F cell line was more radiosensitive (ñ was smaller) than the FancG–/– (PD326) cell line. FancD2–/– cells have greater genetic instability than other FA cells, which may correlate with the higher incidence of tumors in the mouse model and the reduced numbers of hematopoietic stem and progenitor cells compared to FancC–/– cells (42). Genome instability in the FancD2–/– genotype may also contribute to instrinsic radiosensitivity of PD20F cells.
The radioresistance of the FancG–/– (PD326) cell line was unexpected. The FancC protein presides over the cellular apoptotic threshold by repressing proapoptotic signals. Hsp70 mediates protection from cellular stresses, including radiation, TNF-α and caspase activation (43–45), binds to hsp70, and prevents apoptosis by suppressing PKR activation (46, 47). Once activated, PKR phosphorylates the α-subunit of eIF2, a protein synthesis initiation factor, and thus inhibits translation, upregulates the proapoptotic proteins Bax and Fas, and induces apoptosis (48). In the FancG–/– (PD326) cells, the FA core complex may not have formed, and an alternative pathway of FancC binding to hsp70 may have been upregulated, which may have upregulated hsp70, suppressing PKR (48, 49). Further studies will be required to explain the relative radioresistance of PD326 cells compared to the transgene-restored FancG cells.
Ionizing radiation induces reactive oxygen species, which cause lipid peroxidation at the mitochondrial membrane and initiate a cascade of biochemical events, leading to an increase in mitochondrial membrane permeability, the release of cytochrome c into the cytoplasm, and caspase-3 activation, leading to apoptotic death (32). The targeting of JP4-039 to mitochondria (33) decreases apoptosis and increases radioresistance (32). While JP4-039 and Tempol induced radioresistance, the DNA crosslinking effect of MMC was not altered in FA cells or transgene-restored sublines (50). These results may reflect a short duration of action of JP4-039 and Tempol relative to MMC or may indicate that the mechanism of cell killing by MMC was not altered by antioxidants. MMC-treated cells (50) may have overwhelmed JP4-039 by ongoing generation of ROS induced by MMC. While Tempol has been shown to be effective as a tumor inhibitor in one mouse model of Fanconi anemia (51), Tempol increased the sensitivity of FancD2–/– (PD20F) cells to MMC while JP4-039 did not. This result emphasizes the potential importance of mitochondrial targeting for a safe radiation-protective effect in the setting of other chemotherapeutic drugs used in HNSSC patients.
The present data support the use of an organ-specific radioprotector for the oral cavity in Fanconi anemia patients and in other patients with genetically determined defects in DNA repair, including Bloom syndrome and ataxia telangiectasia, who may require external-beam radiotherapy. Studies with radiosensitive FancD2–/– mice (10) bearing orthotopic tumors are in progress using a novel formulation of JP4-039/F15, which has proven successful in radioprotection of the esophagus (33).
ACKNOWLEDGMENTS
This project was supported by Grants T32AG21885 and U191A168021-01 from the National Institutes of Health.
REFERENCES
- 1.Rosenberg PS, Socié G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 2.Auerbach AD, Allen RG. Leukemia and preleukemia in Fanconi anemia patients. A review of the literature and report of the International Fanconi Anemia Registry. Cancer Genet Cytogenet. 1991;51:1–12. doi: 10.1016/0165-4608(91)90002-c. [DOI] [PubMed] [Google Scholar]
- 3.Alter BP, Greene MH, Velazquez I, Rosenberg PS. Cancer in Fanconi anemia. Blood. 2003;101:2072. doi: 10.1182/blood-2002-11-3597. [DOI] [PubMed] [Google Scholar]
- 4.Alter BP. Fanconi's anemia and malignancies. Am J Hematol. 1996;53:99–110. doi: 10.1002/(SICI)1096-8652(199610)53:2<99::AID-AJH7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.D'Andrea AD. Susceptibility pathways in Fanconi's anemia and breast cancer. N Engl J Med. 362:1909–19. doi: 10.1056/NEJMra0809889. 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101:1249–56. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 7.Masserot C, Peffault de Latour R, Rocha V, Leblanc T, Rigolet A, Pascal F, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer. 2008;113:3315–22. doi: 10.1002/cncr.23954. [DOI] [PubMed] [Google Scholar]
- 8.Lowy DR, Gillison ML. A new link between Fanconi anemia and human papillomavirus-associated malignancies. J Natl Cancer Inst. 2003;95:1648–50. doi: 10.1093/jnci/djg125. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki MS, Tonomura A. A high susceptibility of Fanconi's anemia to chromosome breakage by DNA cross-linking agents. Cancer Res. 1973;33:1829–36. [PubMed] [Google Scholar]
- 10.Parmar K, Kim J, Sykes SM, Shimamura A, Stuckert P, Zhu K, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. 2010;28:1186–95. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinciguerra P, Godinho SA, Parmar K, Pellman D, D'Andrea AD. Cytokinesis failure occurs in Fanconi anemia pathway-deficient murine and human bone marrow hematopoietic cells. J Clin Invest. 2010;120:3834–42. doi: 10.1172/JCI43391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auerbach AD, Rogatko A, Schroeder-Kurth TM. International Fanconi Anemia Registry: relation of clinical symptoms to diepoxybutane sensitivity. Blood. 1989;73:391–6. [PubMed] [Google Scholar]
- 13.Gluckman E, Devergie A, Dutreix J. Radiosensitivity in Fanconi anemia: application to the conditioning regimen for bone marrow transplantation. Br J Haematol. 1983;54:431–40. doi: 10.1111/j.1365-2141.1983.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 14.Mohseni Meybodi A, Mozdarani H. DNA damage in leukocytes from Fanconi anemia (FA) patients and heterozygotes induced by mitomycin C and ionizing radiation as assessed by the comet and comet-FISH assay. Iran Biomed J. 2009;13:1–8. [PubMed] [Google Scholar]
- 15.Mohseni-Meybodi A, Mozdarani H, Vosough P. Cytogenetic sensitivity of G0 lymphocytes of Fanconi anemia patients and obligate carriers to mitomycin C and ionizing radiation. Cytogenet Genome Res. 2007;119:191–5. doi: 10.1159/000112060. [DOI] [PubMed] [Google Scholar]
- 16.Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–70. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 17.Näf D, Kupfer GM, Suliman A, Lambert K, D'Andrea AD. Functional activity of the Fanconi anemia protein FAA requires FAC binding and nuclear localization. Mol Cell Biol. 1998;18:5952–60. doi: 10.1128/mcb.18.10.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi K, Taniguchi T, Ranganathan V, New HV, Moreau LA, Stotsky M, et al. Interaction of FANCD2 and NBS1 in the DNA damage response. Nat Cell Biol. 2002;4:913–20. doi: 10.1038/ncb879. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Andreassen PR, D'Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol Cell Biol. 2004;24:5850–62. doi: 10.1128/MCB.24.13.5850-5862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mudra KK, Morris C, DeLaat C, Sambrano J, Masterson M, Mueller R, et al. Bone marrow transplantation in Fanconi anemia using matched sibling donors. Blood. 1994;84:2050–4. [PubMed] [Google Scholar]
- 21.Gluckman E, Auerbach AD, Horowitz MM, Sobocinski KA, Ash RC, Bortin MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856–62. [PubMed] [Google Scholar]
- 22.MacMillan ML, Wagner JE. Haematopoietic cell transplantation for Fanconi anaemia – when and how? Br J Haematol. 2010;149:14–21. doi: 10.1111/j.1365-2141.2010.08078.x. [DOI] [PubMed] [Google Scholar]
- 23.Djuzenova CS, Rothfuss A, Oppitz U, Spelt G, Schindler D, Hoehn H, et al. Response to X-irradiation of Fanconi anemia homozygous and heterozygous cells assessed by the single-cell gel electrophoresis (comet) assay. Lab Invest. 2001;81:185–92. doi: 10.1038/labinvest.3780226. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Higuera I, Kuang Y, Näf D, Wasik J, D'Andrea AD. Fanconi anemia proteins FANCA, FANCC, and FANCG/XRCC9 interact in a functional nuclear complex. Mol Cell Biol. 1999;19:4866–73. doi: 10.1128/mcb.19.7.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang Y, Garcia-Higuera I, Moran A, Mondoux M, Digweed M, D'Andrea AD. Carboxy terminal region of the Fanconi anemia protein, FANCG/XRCC9, is required for functional activity. Blood. 2000;96:1625–32. [PubMed] [Google Scholar]
- 26.Mirchandani KD, McCaffrey RM, D'Andrea AD. The Fanconi anemia core complex is required for efficient point mutagenesis and Rev1 foci assembly. DNA Repair (Amst) 2008;7:902–11. doi: 10.1016/j.dnarep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rwigema JCM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, et al. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80:860–8. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JA, Zhan Q, Kufe DW, et al. Manganese superoxide dismutase (SOD2) inhibits radiation-induced apoptosis by stabilization of the mitochondrial membrane. Radiat Res. 2002;157:568–77. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Epperly MW, Bray JA, Carlos TM, Prochownik E, Greenberger JS. Biology of marrow stromal cell lines derived from long-term bone marrow cultures of Trp53-deficient mice. Radiat Res. 1999;152:29–40. [PubMed] [Google Scholar]
- 30.Epperly MW, Bernarding M, Gretton J, Jefferson M, Nie S, Greenberger JS. Overexpression of the transgene for manganese superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-alpha, IL-3 withdrawal, and ionizing radiation. Exp Hematol. 2003;31:465–74. doi: 10.1016/s0301-472x(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 31.Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggit D, et al. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, et al. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo. 2009;23:717–26. [PMC free article] [PubMed] [Google Scholar]
- 33.Epperly MW, Goff J, Li S, Gao X, Wipf P, Wang H, et al. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo. 2010;24:811–21. [PMC free article] [PubMed] [Google Scholar]
- 34.Ulrich G, Ziessel R, Harriman A. The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed. 2008;47:1184–201. doi: 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]
- 35.Frantz MC, Forbeck EM, Davoren JE, Wang Z, Epperly MW, Greenberger JS, et al. Synthesis and biological analysis of mitochondria-targeted nitroxide conjugates based on Gramacidin S. J Am Chem Soc. Forthcoming [Google Scholar]
- 36.Epperly MW, Osipov AN, Martin I, Kawai K, Borisenkko GG, Jefferson M, et al. Ascorbate as a “redox-sensor” and protector against irradiation-induced oxidative stress in 32D cl 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys. 2004;58:851–61. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Epperly MW, Wegner R, Kanai AJ, Kagan V, Greenberger EE, Nie S, et al. Irradiated murine oral cavity orthotopic tumor antioxidant pool destabilization by MnSOD-plasmid liposome gene therapy mediates tumor radiosensitization. Radiat Res. 2007;267:289–97. doi: 10.1667/RR0761.1. [DOI] [PubMed] [Google Scholar]
- 38.Fagerlie S, Lensch MW, Pang Q, Bagby GC., Jr The Fanconi anemia group C gene product: signaling functions in hematopoietic cells. Exp Hematol. 2001;29:1371–81. doi: 10.1016/s0301-472x(01)00755-x. [DOI] [PubMed] [Google Scholar]
- 39.Park JW, Pitot HC, Strati K, Spardy N, Duensing S, Grompe M, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–68. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarhini AA, Belani C, Luketich JD, Ramalingam SS, Argiris A, Gooding W, et al. A phase I study of concurrent chemotherapy (Paclitaxel and Carboplatin) and thoracic radiotherapy with swallowed manganese superoxide dismutase (MnSOD) plasmid liposome (PL) protection in patients with locally advanced stage III non-small cell lung cancer. Hum Gene Ther. 2011;22:336–43. doi: 10.1089/hum.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo HL, Seixas-Silva JA, Epperly MW, Gretton JE, Shin DM, Greenberger JS. Prevention of irradiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat Res. 2003;159:361–70. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang QS, Marquez-Loza L, Eaton L, Duncan AW, Goldman DC, Anur P, et al. Fancd2–/– mice have hematopoietic defects that can be partially corrected by Resveratrol. Blood. 2010;116:5140–51. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–71. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 44.Rathbun RK, Christianson TA, Faulkner GR, Jones G, Keeble W, O'Dwyer M, et al. Interferon-gamma-induced apoptotic responses of Fanconi anemia group C hematopoietic progenitor cells involve caspase 8-dependent activation of caspase 3 family members. Blood. 2000;96:4204–11. [PubMed] [Google Scholar]
- 45.Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, Jäättelä M, et al. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995;95:926–33. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang Q, Keeble W, Christianson TA, Faulkner GR, Bagby GC. FANCC interacts with Hsp70 to protect hematopoietic cells from IFN-gamma/TNF-alpha-mediated cytotoxicity. EMBO J. 2001;20:4478–89. doi: 10.1093/emboj/20.16.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang Q, Christianson TA, Keeble W, Koretsky T, Bagby GC. The anti-apoptotic function of Hsp70 in the interferon-inducible double-stranded RNA-dependent protein kinase-mediated death signaling pathway requires the Fanconi anemia protein, FANCC. J Biol Chem. 2002;277:49638–43. doi: 10.1074/jbc.M209386200. [DOI] [PubMed] [Google Scholar]
- 48.Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123–38. doi: 10.1016/s1357-2725(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 49.Cumming RC, Lightfoot J, Beard K, Youssoufian H, O'Brien PJ, Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med. 2001;7:814–20. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- 50.Pritsos CA, Sartorelli AC. Generation of reactive oxygen radicals through bioactivation of mitomycin antibiotics. Cancer Res. 1986;46:3528–32. [PubMed] [Google Scholar]
- 51.Zhang QS, Eaton L, Snyder ER, Houghtaling S, Mitchell JB, Finegold M, et al. Tempol protects against oxidative damage and delays epithelial tumor onset in Fanconi anemia mice. Cancer Res. 2008;68:1601–8. doi: 10.1158/0008-5472.CAN-07-5186. [DOI] [PubMed] [Google Scholar]
- 52.van Zeeburg HJT, Snijders PJF, Pals G, Hermsen MAJA, Rooimans MA, Bagby G, et al. Generation and molecular characterization of head and neck squamous cell lines of Fanconi anemia patients. Cancer Res. 2005;65:1271–6. doi: 10.1158/0008-5472.CAN-04-3665. [DOI] [PubMed] [Google Scholar]