Abstract
Necrotizing herpetic stromal keratitis (HSK) in mice rapidly improved after amniotic membrane transplantation (AMT). In this study we determined the fate of polymorphonuclear neutrophils (PMN) after AMT. AMT or tarsorrhaphy (T) was performed in BALB/c mice with ulcerative HSK. After 2 days, corneas were studied histologically and by transmission electron microscopy (TEM). CD11b, Gr-1, and TUNEL-positive cells were identified. Macrophages were depleted by subconjunctival injection of dichloromethylene-diphosphonate-liposomes (Cl2MDP-LIP) before AMT. Corneas were studied for interleukin (IL)-1α, IL-2, interferon (IFN)-γ, CXCL1, CXCL2, and tumor necrosis factor (TNF)-α production by ELISA. PMN-enriched cell preparations co-cultured with amniotic membrane (AM) or with AM and such recombinant (r) cytokines as rIL-1α, rIL-2, and rTNF-α or supernatants from activated lymphocytes were investigated by flow cytometry (Annexin-V/7-AAD and TUNEL), and a dimethylthiazolyl-diphenyltetrazolium-bromide (MTT)-viability assay. Corneas in the AMT mice had less inflammation, fewer PMN-like cells and fewer CD11b+, and Gr-1+ cells (P < 0.01), but a higher ratio of apoptotic to viable PMN-resembling cells (P < 0.01) than the T mice. Phagocytic removal of apoptotic PMN-like cells by macrophages was evident in the AMT group. After Cl2MDP-LIP treatment, the corneas had more cell debris and apoptotic cells with PMN-like morphology. The concentrations of IL-1 α, IL-2, CXCL1, and TNF-α were reduced in corneas of the AMT group as compared to that of the T group, while the concentration of CXCL2 was increased. Apoptosis of PMN-resembling cells was detected following cocultivation with AM, even when proinflammatory cytokines were present. Resolution of corneal inflammation in mice with necrotizing HSK after AMT is associated with increased apoptosis of PMN-like cells, reduction of pro-inflammatory cytokines, an increase of CXCL2, and increased removal of apoptotic PMN-like cells by macrophages.
Keywords: amniotic membrane transplantation, herpetic stromal keratitis, apoptosis, neutrophils, macrophage, macrophage depletion, infection, cytokines
1. Introduction
Herpetic stromal keratitis (HSK) is an immune mediated disease of the HSV-1-infected cornea that may result in visual loss (Streilein et al., 1997; Thomas and Rouse, 1997). T-lymphocytes are mediating this form of corneal inflammation, and CD4 + T-cell derived cytokines such as interferon (IFN)-γ and interleukin (IL)-2 are critical for the development of the disease (Hendricks et al., 1992; Niemialtowski and Rouse, 1992; Tang et al., 1997; Tang and Hendricks, 1996). Polymor-phonuclear neutrophils (PMN) are the most prominent infiltrating cell type seen in HSK (Meyers-Elliott and Chitjian, 1981) and are associated with elimination of HSV-1 from the infected eye (Thomas et al., 1997; Tumpey et al., 1996). Nonetheless, PMN infiltration leads to corneal destruction and opacity (Daheshia et al., 1998).
Amniotic membrane transplantation (AMT) used as a “graft” has been advocated as a basement membrane substitute (Shimazaki et al., 1998; Tseng et al., 1997). It has been reported that even deep corneal ulcers can be successfully treated with AMT (Hanada et al., 2001; Kruse et al., 1999). Anti-inflammatory action has been seen when AMT was used as a “patch” to cover the ocular surface (Hanada et al., 2001). A broad collection of potent anti-inflammatory substances has been found in AM to down-regulate factors for chemokine expression in human keratocytes (Bültmann et al., 1999). Diverse anti-inflammatory proteins like interleukin-10 and interleukin-1 receptor antagonist has also been found (Fidel et al., 1994; Hao et al., 2000). In addition AM also contains inhibitors of metalloproteinases and inhibitors of nitric oxide synthase in keratocytes (Hao et al., 2000; Kim et al., 2000).
We have recently shown that human AMT as a “patch” promotes rapid reepithelialization and reduces stromal inflammation and ulceration in a mouse model of HSV-1 necrotizing keratitis (Heiligenhaus et al., 2001). We noted that AMT covered mouse corneas show rapid resolution of corneal inflammation consistent with rapid reduction of PMN (Heiligenhaus et al., 2001). Previously, it has been suggested that apoptosis of PMN is a new strategy to clear a large number of PMN and their potentially histotoxic contents to achieve rapid resolution of acute inflammation (Meagher et al., 1992; Stern et al., 1996). Therefore, we re-examined this mouse model of HSK-induced necrotizing keratitis and investigated the mechanism explaining the fate of PMN after AMT.
2. Materials and methods
These studies were carried out in accordance with the Institutional Animal Care and Use Committee and Ethic Committee, with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and with the Declaration of Helsinki. All experiments were performed on 6–8 week-old female BALB/c mice (Charles River, Germany) (Heiligenhaus et al., 1999).
2.1. Virus and corneal infection
The KOS strain of HSV-1 was prepared as described previously (Bauer et al., 2000; Heiligenhaus et al., 1999).
For infection, the mice were anesthetized intraperitoneally with ketamine-hydrochloride (2 mg) and mepivacaine-hydrochloride (400 ng). The epithelium of the right eye was scratched in a criss-cross pattern for 8 times. Five µl of KOS strain of HSV-1 (105 PFU) were applied on the cornea (Bauer et al., 2000; Heiligenhaus et al., 1999).
2.2. Preparation of preserved human amniotic membrane
Briefly, human placenta was collected at elective cesarean delivery (Heiligenhaus et al., 2001; Lee and Tseng, 1997; Prabhasawat et al., 1997; Tseng et al., 1998). The amniotic membrane was flattened onto nitrocellulose paper (Hybond N+, Amersham, UK), with the epithelium surface up. The AM samples were stored at −80 °C in DMEM/glycerol 1:1 (v/v) until use.
2.3. Study design
Mice (n = 32) with ulcerating HSK at day 14 after HSV infection were used. The cornea was completely covered with human AM as a temporary patch (n = 16) and was secured by tarsorrhaphy (T) as described previously (Heiligenhaus et al., 2001). Mice that received T without AMT served as a control (n = 16). Two days after surgery, the AM was removed. The eyes were then enucleated and were immediately frozen in liquid nitrogen or fixed in formalin.
2.4. In vivo macrophage depletion
Cl2MDP, kindly provided by Roche Diagnostics (Mannheim, Germany), was prepared as a suspension using liposomes termed Cl2MDP-LIP as previously described (Van Rooijen and Sanders, 1994). Directly before AMT, 25 µl of Cl2MDP-LIP were injected subconjunctivally. Control mice received AMT and PBS-liposome (PBS-LIP) (n = 5 for both groups) (Bauer et al., 2000).
2.5. Histology and immunohistochemistry
Eyes for light microscopy were fixed in Mc-Dowells solution, rinsed in cacodylate buffer, dehydrated with ethanol, and embedded in paraffin (n = 5). A series of five-micron sections was prepared for studying the number of infiltrating cells and for the detection of apoptosis by TUNEL assay (depicted in Section 3). The paraffin sections were stained with hematoxylin-eosin. The number of PMN-like cells was counted by means of bright-field microscopy in three representative areas. Another set of eyes (n = 5) was collected, snap-frozen in liquid nitrogen and embedded in OCT compound for immuno-histochemical detection of cells using the avidine-biotinimmunoperoxidase technique as previously published (Heiligenhaus et al., 2001) based on cell markers: CD11b, Gr-1, and CD3 (Pharmingen, Hamburg, Germany).
Sections with toluidine blue-borax staining (see transmission electron microscopy) were used for morphological observation and counting of infiltrating as viable cells with ring-shaped nuclei or apoptotic cells with shrinkage and condensed nuclei (Kroemer et al., 1998; Savill et al., 1989; Tang et al., 1997).
2.6. TUNEL assay
Fragmented DNA was determined in paraffin sections (n = 5, each group), whole mounted murine cornea sections (n = 5, each group) or peritoneal lavages by the terminal deoxyribonucleotidyl transferase-mediated (TUNEL) labelling method according to the manufacturer’s instructions using TdT in situ, TACS™ Fluorescein in situ or FlowTACS™ in situ Apoptosis Detection Assay (R&D Systems, Germany).
2.7. Transmission electron microscopy
Eyes (n = 5, each group) were immediately fixed in 2.5% glutaraldehyde for 6 h at room temperature, and then fixed in OsO4 (2%) in cacodylate buffer for 1 h. Tissues were dehydrated with acetone and embedded in Durcupan (Fluka, Germany). Sections (0.5 µm or 1 um) were obtained with Ultracut E (Reichert Jung). Semisections (1 µm) were stained with toluidine blue-borax solution and were examined by light microscopy. Sections for TEM (0.5 µm) were contrasted with Pb-citrate 1% for 1 min, and stained with uranyl-acetate 5% in 50% ethanol for 10 min. Specimens were examined in a Zeiss EM 900 transmission electron microscope in a masked fashion.
2.8. Isolation of peritoneal cells
Cell suspensions enriched with PMN were obtained from peritoneal lavages 24 h after inoculation of 3% starch solution (1 ml) in BALB/c mice. For MTT-test peritoneal cells were further purified by Percoll gradient (Sigma), and by a 4 h incubation step for 4 h in 24-well plates at 37 °C to remove the adherent cell population.
2.9. Cytokine quantification by ELISA
Individual corneas (n = 10, each group) were prepared after complete removal of the limbal tissue and iris. Samples harvested 2 days after AMT or T were stored at −80 °C until assayed. Corneas were thawed, homogenized and the supernatants were studied for the content of IL-1α, IL-2, IFN-γ, CXCL2 (R&D Systems, Germany), CXCL1, and TNF-α with the use of commercially available ELISA kits (Pharmingen, Hamburg, Germany).
2.10. Flow cytometric evaluation
Peritoneal lavages containing PMN were obtained and 1.5 × 107 cells/3 ml were co-cultured with AM (3.5 cm diameter) or medium for 24 h in 6 well dishes (Falcon3046, Becton Dickinson, Franklin Lakes, NJ, USA). The adherent cells of the peritoneal cell suspension (e.g. macrophages) were removed by a cultivation step on plastic dishes for 4 h at 37 °C. Cells were stained with an antibody directed against Gr-1. Signs of early apoptosis on these cells were detected by the use of the Annexin V-PE Apoptosis Detection Kit (BD Pharmingen), which localizes membrane phospholipid phosphatidylserine (PS). With the counter nuclear staining by 7-AAD, the double staining allowed us to detect intact cells (Annexin V−, 7-AAD−), early apoptotic (Annexin V+, 7-AAD−) and late apoptotic or necrotic cells (Annexin V+, 7-AAD+) (Fadok et al., 1992). Fragmented DNA in GR1+ cells was furthermore determined by TUNEL assay.
2.11. Preparation of corneal tissues
The eyes were collected and the corneas including a limbal ring were excised. Neighbour tissue (e.g. iris or lens) were removed. The corneas were cut at the rim with four to five incisions in order to avoid tension of the tissue, and to allow flat-mounting of the specimen. The corneas were then embedded in OCT-compound, frozen on smooth OCT block and sectioned on a cryostat.
2.12. Immunostaining of corneal tissues
For staining, the cornea sections were fixed for 10 min in acetone, followed by three washing steps with PBS. To avoid un-specific staining, the corneal tissue was blocked for 20 min with 0.05 mg/ml Fc-Block (BD). After the blocking procedure was performed the corneal tissue was incubated with the CD11b antibody conjugated to Alexa-Fluor 647 (0.005 µg/ml, Serotec) at 4 °C for 2 h. Subsequently, the sections were then washed with PBS, the nuclei were stained with 1 µg/ml Hoechst 33342, and tissues were washed three times with PBS. Sections were mounted with glycerol-gelatine containing 0.375 µg/ml p-phenyldiamine (Sigma) and coverslipped. Controls were performed using 0.05 mg/ml Alexa-Fluor 647 conjugated isotype control instead of CD11b specific antibody. If immunostaining was combined with TUNEL-staining, the immunostaining was performed first, and the TUNEL protocol was done thereafter. Negative controls for the TUNEL assay were processed with the same protocol just with omitted TdT enzyme in the reaction mix.
2.13. Confocal microscopy
Whole mounted murine corneas were analyzed using a confocal laser scanning microscope (LSM 510, Zeiss, Goettingen, Germany). The images were taken at medium-power magnification with a 20 × objective. The specimens were scanned with an Argon laser at 488 nm, a Helium-Neon 2 laser at 633 nm and an Enterprise Argon UV-Laser at 364 nm wavelength respectively. The three different scans were combined to a merge.
2.14. MTT viability assay
To determine the effects of AM on viability of PMN enriched cell suspension (see above), cells were treated with medium (control) or AM (3.5 cm diameter) for 24 h. Some wells were treated with recombinant IL-1α, IL-2, or TNF-α, or with supernatants collected from concanavalin A activated lymphocytes. For the determination of cell viability, MTT solution, which was prepared by dissolving MTT (5 mg/ml) in RPMI-1640 and filtering via a 0.2 µm filter, was added and incubated for 3 h. During incubation at 37 °C, the yellow dimethylthiazolyl-diphenyltetrazolium-bromide (MTT) was reduced to purple formazan in the mitochondria of living cells. At the end of the incubation period, cells and dye crystals were dissolved by adding N,N-dimethylformamide. Absorbance was measured at 570 nm (reference wavelength at 690 nm) in a MRX – ELISA-Reader (Dynatech Laboratories) and the results were expressed as optical density (OD).
2.15. Statistical analysis
The same experiment was repeated twice. Student’s t-test was used to test the differences between the experimental groups with respect to the cell numbers in the histological and immunohistochemical staining, the TUNEL assay, the MTT-viability assay, and the ELISA. Fisher’s protected least significant difference test was used to analyze the statistical differences between mean values of clinical keratitis scores. P < 0.05 was considered as statistically significant.
3. Results
3.1. Reduction of cells resembling PMN by morphology in corneas after AMT
Before intervention, all corneas showed stromal necrosis and ulceration at day 14 after infection. Consistent with our previous report (Heiligenhaus et al., 2001), clinical ulceration and stromal inflammation was dramatically improved in two days after AMT when compared to the control treated with T alone (Heiligenhaus et al., 2001) (Fig. 1A,B). The central cornea was infiltrated by a high number of cells resembling PMN (Table 1). Two days after AMT, infiltration of PMN-like cells in the central cornea significantly regressed as compared to the control group (P < 0.01).
Fig. 1.
Influence of the amniotic membrane (AM) on the histology of severe HSK in BALB/c mice. Fourteen days after corneal infection with HSV-1, corneas with 4+ severity were patched with an AM for 2 days (A) or tarsorrhaphy (T) (B). While severe PMN-like cell infiltration was present in the central cornea in the T eyes, it was markedly reduced after AMT. Toluidine blue-borax staining. Magnification 150×; the scale bar indicates 100 µm. (C) Cornea with AMT. Non-viable PMN-like cells with shrinked and condensed nuclei are marked with arrows. Magnification 1000×. The scale bar indicates 20 µm. (D) Cornea with T. Cornea contains viable PMN-resembling cells with ring-shaped nuclei. (E) Murine cornea with AMT. Many TUNEL+ cells are present within the area of PMN-like cell infiltration in the superficial stroma. Typical TUNEL+ cells are indicated with arrows. (F) Murine cornea with T. TUNEL+ cells were abundant at the edges and the base of ulceration; only few TUNEL+ cells were found in inflammatory cell infiltration. Typical TUNEL− cells are indicated with arrows. The scale bar indicates 100 µm.
Table 1.
Inflammatory cell infiltration in the cornea in BALB/c mice with HSK after AMT
| Treatment | None | T | AMT |
|---|---|---|---|
| Time point | Day 0 | Day 2 | Day 2 |
| Severity of HSKa | 4.0 | 3.1 ± 1.1 | 1.2 ± 0.8* |
| PMNb-like cells | 455 ± 19 | 342 ± 27 | 84 ± 18* |
| CD11b | 357 ± 38.4 | 257 ± 67 | 52.5 ± 6.3* |
| Gr-1 | 417 ± 43.1 | 370 ± 82.5 | 85.2 ± 42.7* |
| CD3 | 95.2 ± 17.1 | 72.3 ± 19.5 | 17.3 ± 2.8* |
P < 0.01.
BALB/c mice were infected with 105 PFU HSV-1 (KOS). Mice with ulcerating HSK on day 14 p.i. received an AMT or a tarsorrhaphy (T). Two days after treatment, the presence of ulceration and the severity of stromal keratitis were evaluated and then the eyes were removed. Paraffin sections were stained with standard HE staining. AMT improved HSK and reduced the number of infiltrating inflammatory cells. Severity of herpetic stromal keratitis (HSK) ± SD; 0; clear cornea; +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity, iris visible; and +4, opaque cornea, iris not visible, necrotizing stromal keratitis with ulceration.
PMN-like cells were studied on HE stained sections. Immunohistochemical stainings with antibodies targeting CD3, CD11b, or Gr-1-antigen. Cell numbers (mean ± SEM) were determined in a 10 × 10 grid in the central cornea.
The finding that the number of cells resembling PMN was decreased in the cornea after AMT was confirmed by the immunohistochemical staining with antibodies directed against CD11b and Gr-1. While high numbers of CD11b+ and Gr-1+ cells were found in the central cornea at day 14 after infection, the numbers were markedly diminished two days after AMT (P < 0.01). In contrast, the numbers of CD11b+ and Gr-1+ cells in the control group only decreased slightly but not significantly 2 days after the tarsorrhaphy (Table 1).
3.2. Phenotypic characterisation of PMN-like cells in corneas with HSK after AMT
The majority of cells resembling PMN morphology in the central cornea of the control group had a normal morphology with ring shaped nuclei (Fig. 1A,C). Following AMT, however, many of these cells in the central cornea were characterized by cell shrinkage and condensation of the nuclei (Fig. 1B,D). Such an abnormal morphology of PMN-resembling cells was especially abundant in the superficial layers of the stroma covered by the amniotic membrane. The ratio of PMN-like cells showing cell shrinkage and condensed nuclei in the total population of PMN-like cells in the central cornea increased after 2 days with AMT when compared to the control mice (Table 2, P < 0.05).
Table 2.
Influence of AMT on the viability of PMN-resembling cells
| Treatment | None | T | AMT |
|---|---|---|---|
| Time point | Day 0 | Day 2 | Day 2 |
| Total no. of inflammatory cells in central corneaa |
498.5 ± 64.2 | 434.0 ± 76.6 | 146.2 ± 22.8* |
| PMN-like cells in the central cornea |
342.9 ± 32.7 | 309.8 ± 42.9 | 122.2 ± 14.3* |
| Ratio (non-viable/viable)b PMN-like cells |
0.15 ± 0.05 | 0.19 ± 0.04 | 2.52 ± 0.41* |
P < 0.05; difference in the cell counts between T and AMT group by Student’s t-test.
BALB/c mice were infected with 105 PFU HSV-1 (KOS). Mice with ulcerating HSK on day 14 p.i. received an AMT or a tarsorrhaphy (T). Two days after treatment eyes were removed and sections were stained by toluidine blue borax staining. Magnification 500×. Number of infiltrating inflammatory cells in high-power field in the central cornea; mean ± SEM.
Non-viable PMN-like cells: cells with cell shrinkage and condensed nucleus. Viable PMN-like cells: cells with ring-shaped nuclei. Cell counting data are expressed as mean ± SEM (n = 5 corneas per group).
TUNEL assay was performed in paraffin sections (Fig. 1E,F). In the control, TUNEL positive cells were detected across the entire epithelium, at the edges and on the base of the ulcers. Only few TUNEL positive cells were seen in areas of inflammatory cell infiltration that consisted predominantly of PMN-resembling cells present deep in the stroma or in the anterior chamber. This finding was in a sharp contrast to that seen two days after AMT, where substantial numbers of TUNEL positive cells were found in the epithelium and in the superficial corneal stroma. TUNEL positive cells were primarily detected within the stromal areas of infiltrating PMN-resembling cells. In these areas, many PMN-like cells with shrunken morphology and condensed nuclei were present.
A similar observation was found in corneal flat mounts processed with FITC-TUNEL and anti-CD11b antibody. In the control (T) many CD11b positive cells were detected within the area of PMN-like cell infiltration in the superficial stroma. Only few of these cells were positive stained for FITC-TUNEL. In contrast we found in a comparable region in the superficial stroma of an AMT treated cornea that many CD11b+ cells were positively stained for TUNEL, suggesting that apoptotic cell death of these cells is present (Fig. 2).
Fig. 2.
Immunofluorescent staining of flat-mounts of central corneal stroma from BALB/c mice (age: 8 weeks) with ulcerative HSK after 12 h of treatment with AMT (A) or T (B). Five different specimens were stained for appearance of CD11b antigen (red), TUNEL (green), and the nuclei were stained with Hoechst 33342 (dark blue); combination of colours as green-blue indicate co-localisation. Original magnification, 200-fold. The scale-bar indicates 100 µm. (A) Many CD11b+, TUNEL+ cells were present within the area of PMN-resembling cell infiltration in the stroma. Typical green-red TUNEL+ CD11b+ cells are indicated with arrows. (B) CD11b+ TUNEL negative cells that were typically found in the central cornea are indicated with arrowheads.
Transmission electron microscopy (TEM) was performed in eyes with HSK at 12 h after AMTor in the control group. In the control group, PMN-like cells in the heavy cellular infiltration had normal ultra-structural characteristics similar to specimens obtained from mice with ulcerating keratitis 14 days after corneal HSV infection. At the edges of the stromal ulceration, occasional necrotic PMN-like cells were found, with visible cellular debris, disintegrated plasma membranes, invisible cytoplasma, and dispersed chromatin within the swollen nuclei (Fig. 3B). In contrast, the ultra-structural appearance was profoundly different after AMT. Macrophage-like-cells were abundant (Fig. 3A), and many PMN-resembling cells appeared smaller, with abnormal round morphology and prominent blebbing. Additionally, there were groups of phagocytes with pseudopodia directed towards PMN-like cells, suggesting phagocytosis of these cells (Fig. 3A,C). Apoptotic bodies were seen within macrophage-like-cells (Fig. 3C).
Fig. 3.
Influence of the amniotic membrane (AM) on PMN-like cells in BALB/c mice with severe HSK. Fourteen days after corneal infection with HSV-1, superficial stroma of the corneas with 4+ severity received AMT (A) or patched with tarsorrhaphy (T) for 12 h (B). (A) After AMT, two macrophage-like-cells (M) with phagocytosis of a PMN-resembling cell. (B) Mature PMN-like cells in the T mice. (C) A macrophage with apoptotic bodies (AB) within phagosomes after AMT. TEM; scale bar indicates 10 µm.
3.3. Macrophages in corneas after AMT
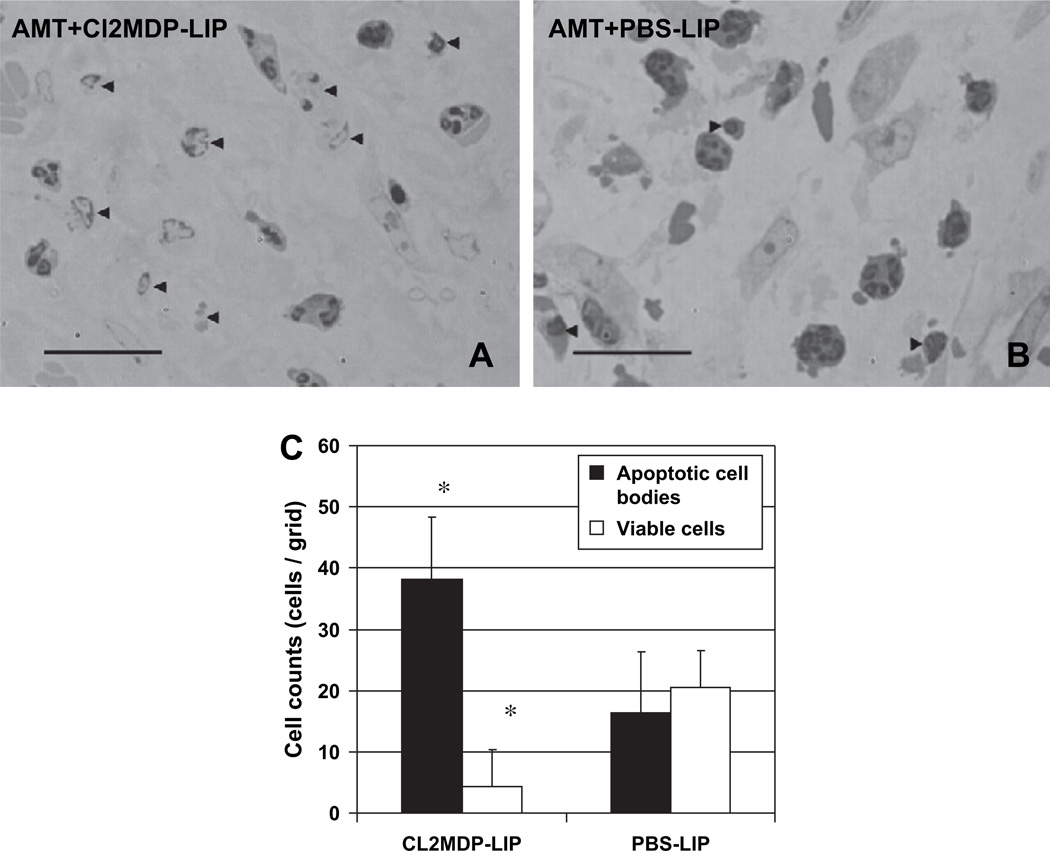
To test the hypothesis that macrophages are involved in the rapid removal of the PMN-like cells from HSV-1-infected mouse corneas after AMT, we depleted macrophages by subconjunctival injection of Cl2MDP-LIP immediately before AMT. The histological analysis of these mouse corneas revealed a large number of shrunken cells, cellular debris, and pyknotic nuclei characteristic of apoptotic bodies (Fig. 4A,C). In contrast, only few such cells were found in mice of the control group that were pre-treated with PBS-LIP (Fig. 4B,C).
Fig. 4.
Macrophage depletion by subconjunctival Cl2MDP-LIP immediately before AMT in mice with HSK. (A) Subconjunctial Cl2MDP-LIP prior to AMT. (B) Subconjunctival PBS-LIP prior to AMT. Histopathology at 12 h after treatment. (C) Cells counts in corneas with HSK and AMT after Cl2MDP-LIP or PBS-LIP-treatment. The data are expressed as mean ± SEM (*P < 0.05). Toluidine blue-borax staining. Magnification 500×. Scale bar indicates 100 µm. Arrowheads indicate apoptotic cell bodies.
3.4. Expression of pro-inflammatory cytokines and chemokines in the cornea
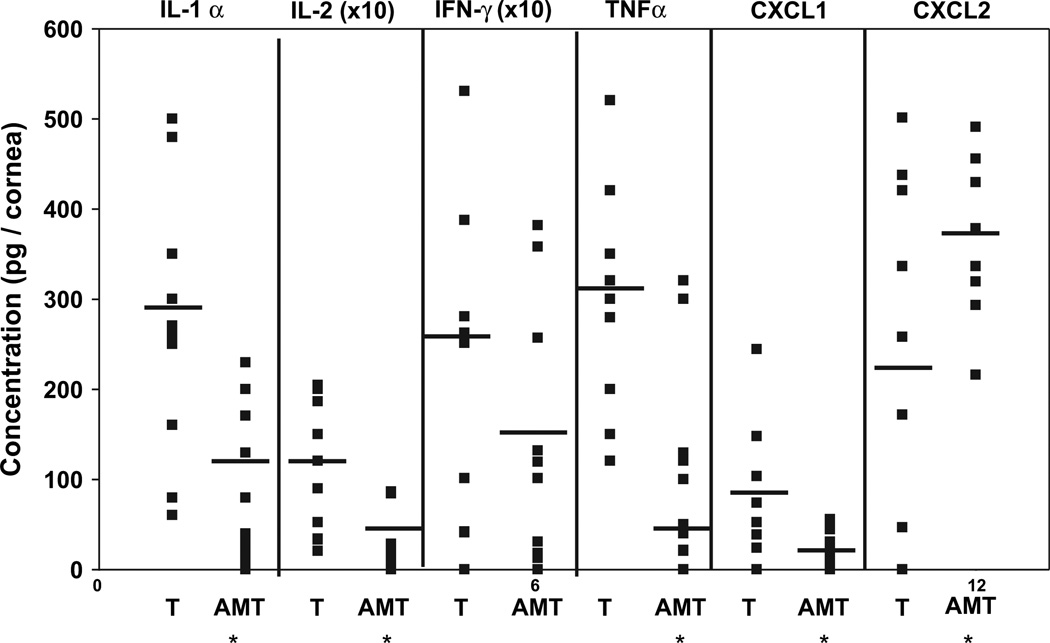
Previously, it has been shown that PMN survival can be prolonged and PMN invasion can be induced by certain proinflammatory cytokines (Keel et al., 1997; Murray et al., 1997). The chemokines CXCL1 and CXCL2 are important modulators of PMN invasion. Our data revealed that two days after AMT, the concentration of IL-1α, IL-2 and TNF-α, but not IFN-γ, in the corneas was significantly lower than those in the control mice (Fig. 5). The level of the chemokine CXCL1 was also reduced. However, the amount of CXCL2, a chemokine that is secreted by macrophages (Wolpe et al., 1989) and resident corneal cells to promote PMN invasion into the HSV-1-infected cornea (Yan et al., 1998), was significantly increased when compared to the control mice (Fig. 5, P < 0.05).
Fig. 5.
Effect of human AMT on the expression of IL-1α, IL-2, IFN-γ, TNF-α, CXCL1, and CXCL2 in the cornea. Mice with HSK were treated with AMT as a patch, or received tarsorrhaphy (T). Lysates from individual corneas (n = 10, each group) were prepared and measured by ELISA. Compared to the control group, the secretion of IL-1α, IL-2, TNF-α, CXCL1 was reduced after AMT. The expression of CXCL2 was increased *P < 0.05.
3.5. In vitro influence of amniotic membrane on PMN-like cells
To determine whether AM affected PMN-resembling cells directly, we used mouse peritoneal lavages 24 h after inoculation of a starch solution, a 4 h adherent step and co-cultivated them with AM. After 24 h of co-cultivation, cells were subjected to immunostaining against Gr1, and to Annexin-V/7-AAD or FITC-TUNEL staining for flow cytometry. For analysis, a live gate excluding most of the lymphocytes, was defined (18.4% or 26.2% of the cells were in the live gate). The results showed that the number of Gr1+ cells decreased following a coculture with AM (Gr1+ cells: 24.2% with medium vs 15.5% with AM coculture) (Fig. 6A). The further analysis of Gr1+ cells for apoptotic features showed that more Gr1+ cells were also positively stained for Annexin V and 7-AAD (32.1% with medium vs 40.9% with AM coculture) following AM coculture. Also, after coculture with AM more Gr1+ cells were stained positively for TUNEL (17.8% with medium vs 29.1% with AM) (Fig. 6A).
Fig. 6.
Flow cytometry analysis with Annexin V/7-AAD or TUNEL staining of Gr1 + PMN-like cells cocultured with AM. After 24 h (A) typical signs of apoptosis were detected in cells cocultured with AM. The MTT assay measures the conversion of the yellow dye to a purple formazan by mitochondria of living cells. Reduction of cell viability was measured by reduced appearance of formazan (B) after 24 h of cocultivation with AM. OD at 570 nm was clearly reduced (W/O = without). The data are expressed as mean ± SEM; *P < 0.05, Difference in the optical density (OD) between medium and AM-group by Student’s t-test.
MTT assay showed that poor cell viability was detected in enriched neutrophil suspensions 24 h after co-cultivation with AM (Fig. 6B). Addition of 10 and 100 U/ml of rIL-1α, rIL-2, or rTNF-α or supernatants collected from concanavalin A activated splenocytes were not able to rescue PMN-resembling cells in peritoneal lavages from cell death induced by AM. The same was found following coculturing of PMN-like cells and AM together with supernatants obtained from homogenized corneas with HSK. In contrast, MTT assay revealed high cell viability in PMN-resembling cells of the control.
4. Discussion
Recently, we have shown that application of AMTas a patch significantly improves necrotizing HSK in both experimental and clinical settings (Heiligenhaus et al., 2001, 2003). The present study investigates the influence of AMT on PMN-like cells. It is well known that PMN express Gr-1 (Fleming et al., 1993; Hestdal et al., 1991) and CD11b (Hickstein et al., 1987; Ho and Springer, 1982; Leenen et al., 1994; Rosen and Gordon, 1987; Springer et al., 1979), but both markers are not unique to PMN and the relation of both markers to PMN-resembling cells is therefore ambiguous. Previous findings strongly suggest that the Gr-1+ neutrophils are the predominant infiltrating cells in the corneas with HSK (Stumpf et al., 2002; Thomas et al., 1997). Our results demonstrate that the PMN numbers, i.e., of CD11b+, and Gr-1+ cells, were profoundly reduced in murine corneas with HSK and AMT and that this correlated well with apoptosis of PMN in the cornea after AMT. Induction of PMN apoptosis could also be reproduced in vitro by co-culturing isolated murine PMN enriched cell suspensions with human AM using flow cytometry to detect the markers Annexin-V, 7-AAD, and by detecting fragmentation of DNA by TUNEL-staining.
An earlier study found that AM obtained from clinical samples contains varying degrees of inflammatory cells in the AM stroma, and that these include CD14 positive monocytes/macrophages, and CD4- and CD8-positive lymphocytes (Shimmura et al., 2001). In addition, many of these cells were TUNEL positive, suggesting an enhanced apoptotic cell death. In our mouse model of HSK, however, we noted that the attraction of PMN to the AM was not the primary reason for the elimination of PMN from the inflamed cornea because AM did not show significant numbers of PMN or TUNEL positive inflammatory cells (data not shown).
Our findings show that the increased apoptosis of infiltrating PMN in the stroma of HSV-induced keratitis after AMT is unique and may be explained by other action mechanisms. One possibility is that AMT reduces production of pro-inflammatory cytokines and chemokines from the corneal stroma. Previously, others have found that the expression of IL-8, Gro-alpha, and epithelial cell derived neutrophil attractant (ENA) was suppressed in keratocytes cultured on AM stromal matrix (Bültmann et al., 1999; Solomon et al., 2001). Herein, we noted that expression of IL-2, TNF-α, and IL-1α was decreased in the cornea after AMT. A recent study showed that the number of T lymphocytes was also reduced in murine eyes with HSK following AMT (Heiligenhaus et al., 2003). Previously it has been shown that IL-2 mediates corneal inflammation by regulating local IFN-γ production, establishing a PMN chemotactic gradient and maintaining PMN viability in the cornea (Tang et al., 1997). TNF-α is essential for the recruitment of PMN into tissue by inducing expression of adhesion molecules ICAM-1 and VCAM-1 in vascular endothelial cells (McHale et al., 1999). Interleukin-1 can inhibit PMN apoptosis, and a decrease of IL-1 in the microenvironment was followed by a rapid apoptosis of neutrophils (Watson et al., 1998). At the site of inflammation, apoptosis is often delayed while the survival is increased by such factors as IFN-γ, IL-1α, IL-2 and TNF-α (Keel et al., 1997; Murray et al., 1997). However, in vitro we found that recombinant IL-2, TNF-α, IL-1 or supernatants harvested from concanavalin A-activated murine lymphocytes or supernatants from HSK-corneas could not rescue PMN from apoptotic cell death caused by AM. Collectively, these results indicate that PMN apoptosis induced by AM is independent of cytokines commonly released at the site of inflammation.
The other possibility is the reduction of chemokines by AMT. The chemokine CXCL1 is involved in neutrophil chemotaxis and activation, and its synthesis is induced by TNF-α. Herein, we noted that the level of CXCL1 in the cornea was markedly reduced after AMT. Nevertheless, the level of CXCL2, a well-known inducer of PMN migration and of tissue injury in the cornea following HSV-1 infection (Yan et al., 1998), was significantly increased in the cornea after AMT. We speculated that the increased level of CXCL2 might result from an increasing number of macrophages that was detected early after AMT. Indeed, it has been recently shown that CXCL2 is secreted from peritoneal macrophages after ingestion of apoptotic T cells likewise to support leukocyte infiltration to the site of apoptosis in some situations (Uchimura et al., 1997). Our observation was further supported by transmission electron microscopy which showed abundant macrophage-like-cells in the cornea with HSK as early as 12 h after AMT, and their phagocytosis of apoptotic PMN. Furthermore, depletion of macrophages by subconjunctival injection of Cl2MDP-LIP induced a rapid accumulation of apoptotic PMN and cell debris. Taken together, these data support the notion that macrophages infiltrating the cornea with necrotizing HSV-1 keratitis after AMT play an important role in the elimination of cell debris and apoptotic PMN from the cornea.
The aforementioned phagocytosis of apoptotic PMN by macrophages might have profound effects on controlling inflammation generated by HSK. Several reports have shown that such macrophages ingesting apoptotic cells not only fail to induce inflammation but also actively suppress an inflammatory immune response (Fadok et al., 1998, 2000). Macrophages with ingested apoptotic cells markedly decrease expression of CD40, a co-stimulatory factor for T-lymphocytes (Barker et al., 1999) of proinflammatory cytokines and increased production of prostaglandin E2 and transforming growth factor-b1 (Fadok et al., 1998), both factors involved as regulators of physiological T cell homeostasis, activation, differentiation, and effector function. Recently, it has been shown that cells of a macrophage cell-line cocultured with AM only underwent apoptosis when activated with IFN-γ, while apoptosis was not found when IFN-γ was absent (Li et al., 2006).
Future studies are warranted to determine whether this mechanism may play an important role in resolving HSV-infected corneal inflammation by AMT, and measures augmenting such a mechanism will help us manage HSK more effectively.
Acknowledgements
The authors thank Sandra Schindler-Drongowski, Department of Ophthalmology, University of Duisburg-Essen for her excellent technical assistance.
Footnotes
Grant: DFG: Ba 2248/1-1, He 1877/12-1, Ernst und Berta Grimmke Foundation. Disclosure codes: DB, SW, PH, MH, KM, DM, NvR, KPS, AH: N. SCG T: TissueTech, Inc.; Bio-Tissue, Inc. (F, I, E, C, P).
References
- Barker RN, Erwig L, Pearce WP, Devine A, Rees AJ. Differential effects of necrotic or apoptotic cell uptake on antigen presentation by macrophages. Pathobiology. 1999;67:302–305. doi: 10.1159/000028085. [DOI] [PubMed] [Google Scholar]
- Bauer D, Mrzyk S, van Rooijen N, Steuhl KP, Heiligenhaus A. Macrophage-depletion influences the course of murine HSV-1 keratitis. Current Eye Research. 2000;20:45–53. [PubMed] [Google Scholar]
- Bültmann S, You L, Spandau U, Rohrschneider K, Völcker HE, Kruse FE. Amniotic membrane down-regulates chemokine expression in human keratocytes. ARVO Abstract. 1999:3044. [Google Scholar]
- Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Experimental Eye Research. 1998;67:619–624. doi: 10.1006/exer.1998.0565. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/ paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of Clinical Investigation. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macro-phages. Journal of Immunology. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Fidel PL, Jr, Romero R, Ramirez M, Cutright J, Edwin SS, LaMarche S, Cotton DB, Mitchell MD. Interleukin-1 receptor antagonist (IL-1ra) production by human amnion, chorion, and decidua. American Journal of Reproductive Immunology. 1994;32:1–7. doi: 10.1111/j.1600-0897.1994.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. Journal of Immunology. 1993;151:2399–2408. [PubMed] [Google Scholar]
- Hanada K, Shimazaki J, Shimmura S, Tsubota K. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. American Journal of Ophthalmology. 2001;131:324–331. doi: 10.1016/s0002-9394(00)00825-4. [DOI] [PubMed] [Google Scholar]
- Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Bauer D, Meller D, Steuhl KP, Tseng SC. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Investigative Ophthalmology & Visual Science. 2001;42:1969–1974. [PubMed] [Google Scholar]
- Heiligenhaus A, Bauer D, Wasmuth S, Steuhl KP. Amniotic membrane transplantation improves herpetic keratitis by local and not by systemic effects. Ophthalmologe. 2003;100:209–215. doi: 10.1007/s00347-002-0720-z. [DOI] [PubMed] [Google Scholar]
- Heiligenhaus A, Bauer D, Zheng M, Mrzyk S, Steuhl KP. CD4 + T-cell type 1 and type 2 cytokines in the HSV-1 infected cornea. Graefes Archive for Clinical and Experimental Ophthalmology. 1999;237:399–406. doi: 10.1007/s004170050251. [DOI] [PubMed] [Google Scholar]
- Hendricks RL, Tumpey TM, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. Journal of Immunology. 1992;149:3023–3028. [PubMed] [Google Scholar]
- Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. Journal of Immunology. 1991;147:22–28. [PubMed] [Google Scholar]
- Hickstein DD, Ozols J, Williams SA, Baenziger JU, Locksley RM, Roth GJ. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. The Journal of Biological Chemistry. 1987;262:5576–5580. [PubMed] [Google Scholar]
- Ho MK, Springer TA. Mac-1 antigen: quantitative expression in macrophage populations and tissues, and immunofluorescent localization in spleen. Journal of Immunology. 1982;128:2281–2286. [PubMed] [Google Scholar]
- Keel M, Ungethum U, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin-10 counterregulates proinflammatory cyto-kine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–3363. [PubMed] [Google Scholar]
- Kim JC, Kim YJ, Song HJ, Cho JH. Down regulation of metal-loproteinases and inducible nitric oxide synthase in keratocyte cultured with amniotic membrane extract. ARVO Abstract. 2000:1393–B768. [Google Scholar]
- Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annual Review of Physiology. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- Kruse FE, Rohrschneider K, Volcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106:1504–1510. doi: 10.1016/S0161-6420(99)90444-X. discussion 1511. [DOI] [PubMed] [Google Scholar]
- Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. American Journal of Ophthalmology. 1997;123:303–312. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. Journal of Immunological Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Li W, He H, Kawakita T, Espana EM, Tseng SC. Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Experimental eye research. 2006;82:282–292. doi: 10.1016/j.exer.2005.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale JF, Harari OA, Marshall D, Haskard DO. TNF-alpha and IL-1 sequentially induce endothelial ICAM-1 and VCAM-1 expression in MRL/lpr lupus-prone mice. Journal of Immunology. 1999;163:3993–4000. [PubMed] [Google Scholar]
- Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. Journal of Leukocyte Biology. 1992;52:269–273. [PubMed] [Google Scholar]
- Meyers-Elliott RH, Chitjian PA. Immunopathogenesis of corneal inflammation in herpes simplex virus stromal keratitis: role of the polymor-phonuclear leukocyte. Investigative Ophthalmology and Visual Science. 1981;20:784–798. [PubMed] [Google Scholar]
- Murray J, Barbara JA, Dunkley SA, Lopez AF, Van Ostade X, Condliffe AM, Dransfield I, Haslett C, Chilvers ER. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. Journal of Immunology. 1992;149:3035–3039. [PubMed] [Google Scholar]
- Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104:974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- Rosen H, Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. Journal of Experimental Medicine. 1987;166:1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. Journal of Clinical Investigation. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki J, Shinozaki N, Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. British Journal of Ophthalmology. 1998;82:235–240. doi: 10.1136/bjo.82.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408–413. doi: 10.1097/00003226-200105000-00015. [DOI] [PubMed] [Google Scholar]
- Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. British Journal of Ophthalmology. 2001;85:444–449. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T, Galfre G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. European Journal of Immunology. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. American Journal of Pathology. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunology Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- Stumpf TH, Case R, Shimeld C, Easty DL, Hill TJ. Primary herpes simplex virus type 1 infection of the eye triggers similar immune responses in the cornea and the skin of the eyelids. Journal of General Virology. 2002;83:1579–1590. doi: 10.1099/0022-1317-83-7-1579. [DOI] [PubMed] [Google Scholar]
- Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. Journal of Immunology. 1997;158:1275–1283. [PubMed] [Google Scholar]
- Tang Q, Hendricks RL. Interferon gamma regulates platelet endothe-lial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. Journal of Experimental Medicine. 1996;184:1435–1447. doi: 10.1084/jem.184.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stro-mal keratitis. Journal of Immunology. 1997;158:1383–1391. [PubMed] [Google Scholar]
- Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunologic Research. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Archives of Ophthalmology. 1998;116:431–441. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. American Journal of Ophthalmology. 1997;124:765–774. doi: 10.1016/s0002-9394(14)71693-9. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. Journal of Virology. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura E, Kodaira T, Kurosaka K, Yang D, Watanabe N, Kobayashi Y. Interaction of phagocytes with apoptotic cells leads to production of pro-inflammatory cytokines. Biochemical and Biophysical Research Communications. 1997;239:799–803. doi: 10.1006/bbrc.1997.7556. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macro-phages: mechanism of action, preparation of liposomes and applications. Journal of Immunological Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Watson RW, Rotstein OD, Parodo J, Bitar R, Marshall JC. The IL-1 beta-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1 beta. Journal of Immunology. 1998;161:957–962. [PubMed] [Google Scholar]
- Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XT, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Investigative Ophthalmology and Visual Science. 1998;39:1854–1862. [PubMed] [Google Scholar]