Abstract
Aim: To evaluate the effects of glutamine on the hepatic ischemia reperfusion injury in rats.
Methods: Thirty rats were divided into three groups as sham (Group 1), control (Group 2), and glutamine(gln) treatment group (Group 3). All rats underwent hepatic ischemia for 45 min followed by 60 min period of reperfusion. Rats were intraperitoneally infused with only 0.9% saline solution in group 2. Rats in group 3 received gln (0.75 g/kg) by intraperitoneal administration, before ischemia and before reperfusion. Blood samples and liver tissues were harvested from the rats, and then the rats were sacrificed. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) levels were determined. Total antioxidant capacity (TAC), catalase (CAT), total oxidative status (TOS), oxidative stress index (OSI) and myeloperoxidase (MPO) in hepatic tissue were measured. Also liver tissue histopathology was evaluated by light microscopy.
Results: The levels of liver enzymes in group 3 were significantly lower than those in the group 2. TAC in liver tissue was significantly higher in group 3 than in group 2. TOS, OSI and MPO in hepatic tissue were significantly lower in group 3 than the group 2. Histological tissue damage was milder in the gln treatment group than that in the control group.
Conclusion: Our results suggest that gln treatment protects the rat liver against to hepatic ischemia-reperfusion injury.
Keywords: glutamine, liver, ischemia-reperfusion, effects
Liver injuries, liver tumor resection, hemorrhagic shock with fluid resuscitation, and liver transplantation are responsible for liver injury caused by ischemia/reperfusion (I/R). Various mechanisms have been proposed to explain the mechanisms of ischemia-reperfusion (IR) injury. Temporary clamping of the portal triad, ie, inflow occlusion by the Pringle's maneuver, is a common strategy to minimize bleeding during hepatic resection. Unfortunately, this method causes hepatic ischemiareperfusion (I/R) injury and may result in postoperative functional disorder of the liver. Oxygen free radicals, produced on reperfusion, play a critical role in the injury caused by ischemia-reperfusion1. Reactive oxygen radicals lead to an inflammatory response and tissue damage by activating some mediators. It can also directly damage cell components2.
Glutamine (Gln) is a conditionally essential nutrient during serious injury or illness, and plays a vital role in tissue metabolism3,4. Recently, Gln has been demonstrated to protect against I/R injury of gut, heart and skeletal muscle and its possible mechanism of action is partially related to the preservation of the content of glutathione (GSH)5,6.
However, there are few studies dealing with the effect of Gln on hepatic I/R injury. Consequently, we designed this experiment in order to study the effect of Gln on hepatic I/R injury in rats and its possible mechanisms. Gln protects against ischemia-reperfusion injury of gut, heart and skeletal muscle by preserving the GSH content in the tissues5, 6. GSH is an important endogenous antioxidant that protects against oxygen free radical injuries and intravenous GSH administration during reperfusion of ischemic liver can prevent reperfusion injury in rats7. Therefore, it seems possible that the administration of Glutamine (Gln) might protect the liver against the ischemia reperfusion injury and we aimed to confirm this hypothesis. We investigated alterations in the oxidantantioxidant balance by measuring oxidant parameters such as total oxidative status (TOS), oxidative stress index (OSI) and myeloperoxidase (MPO), and antioxidant parameters, such as total antioxidant capacity (TAC) and catalase (CAT) in the liver tissue. Also we examined histopathological changes in the liver parenchyma.
Material and methods
Thirty male Sprague–Dawley rats weighing 250–300 g were used in the study. All of the experimental protocols were performed according to the guidelines for the ethical treatment of experimental animals.
Animals and experimental protocol
The rats were housed individually in cages, and allowed free access to standard rat chow and water before and after the experiments. The animal rooms were windowless and under controlled temperature (22 ± 2℃) and lighting conditions. The animals were fasted overnight before the experiments, but were given free access to water. They were anesthetized using 100 mg kg–1ketamine and 20 mg kg–1 xylazine body weight, i.p. The right femoral vein was cannulated to administer drugs and saline.
Animals were divided into three groups, sham group (Group 1), control group (Group 2), and Glutamine (Gln) treatment group (Group 3) (n=10,each). The abdomen was shaved and disinfected with 75% ethanol. A midline incision was performed; the first porta hepatis was exposed. An atraumatic clamp was used to prevent blood supply to the left lateral and median lobes of the liver. Forty-five minutes later, the ischemic liver was reperfused by opening the clamp, and reperfusion was achieved for 60 min. Sham control rats underwent the same protocol without vascular occlusion. Glutamine (Gln) was given to the rats in the treatment group, before ischemia and before reperfusion at a dose of 0.75 g/kg by intraperitoneal route. Rats in the control group were infused only with saline. After the above methods procedures were completed, the rats were sacrificed and blood and liver tissue samples were obtained.
Measuring serum liver enzymes
The abdominal aorta was punctured and 5 ml of blood was taken and put into heparinized tubes. Plasma was separated by centrifugation (3000 rpm for 10 min at room temperature) for biochemical studies. The activities of alanine aminotransferase (ALT, a specific marker for hepatic parenchymal injury), and aspartate Aminotransferase (AST, a nonspecific marker for hepatic injury) in plasma were determined in units per liter using standard auto-analyzer methods on an Abbott Aeroset (Abbott Laboratories, Abbott Park, IL, USA). Just before the rats were sacrificed, their livers were removed for histopathological evaluation.
Measurement of plasma endotoxin level
Plasma endotoxin level was measured by the colorimetric Limulus amebocyte lysate (LAL) test. The test was performed by using the Pyrochrome test kit (Pyroquant Diagnostik, M?rfelden, Germany). All glassware, solutions and surgical instruments used in the experiment were autoclaved at 121℃ for 15 min. The non-pyrogenicity of solutions was tested using the LAL test (Charles River Endosafe, Charleston, SC, USA).
Blood samples (3-4 mL) were collected using heparin- coated pyrogen-free disposable syringes. Plateletrich plasma (PRP) was prepared from the blood by centrifugation at 150 g for 10 min. Fifty microliters of PRP were transferred into a polystyrene plastic tube and kept frozen at -80℃ until the assay. Frozen PRP samples were kept at room temperature for about 30 min before the assay. Fifty microliters of 0.18 mol/L NaOH were added to 50 $L PRP, and incubated at 37℃ for 5 min. Next, 50 μL 0.32 mol/L perchloric acid was added, and incubated at 37℃ for a further 10 min. To dissolve the formed precipitate, 100 μL 0.18 mol/L NaOH was added, and vortexed. Twenty-five microliters of the solution was transferred into sterile non-pyrogenic microplates (Pyroquant Diagnostik), and 25 μL 0.2 mol/L Tris/HCl buffer (pH 8.0) was added to the wells(11). Finally, 50 μL pyrochrome test solution was added to all wells, and mixed for 30 s. Plates were incubated at 37℃. Optical density was read at 405 nm. Standard curves from 0.04 to 1.28 EU/mL were used to evaluate the concentration of endotoxin.
Measurement of total oxidant status
TOS of supernatant fractions was determined using a novel automated measurement method developed by Erel11. Oxidants present in the sample oxidize the ferrous ion–odianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of nmol H2O2 Equiv. per mg protein.
Measurement of the total antioxidant capacity
TAC of supernatant fractions was determined using a novel automated measurement method developed by Ere11. In this method, hydroxyl radical, which is the most potent biological radical, is produced. In the assay, ferrous ion solution, which is present in Reagent 1, is mixed with hydrogen peroxide, which is present in Reagent 2. The sequential produced radicals such as Brown colored dianisidinyl radical cation, produced by the hydroxyl radical, are also potent radicals. Using this method, antioxidative effect of the sample against the potent-free radical reactions, which is initiated by the produced hydroxyl radical, is measured. The assay has excellent precision values, lower than 3%. The results are expressed as nmol Trolox Equiv./mg protein12.
Oxidative stress index
Percent ratio of TOS level to TAC level was accepted as OSI. OSI value was calculated according to the following formula= OSI (arbitrary unit)¼TOS (nmol H2O2 Equiv. per mg protein)/TAC (nmol Trolox Equiv. per mg protein)8.
Determination of myeloperoxidase activity
The MPO (EC 1.11.1.7) activity was determined, using a 4-aminoantipyrine/phenol solution as the substrate for MPO mediated oxidation by H2O2 and changes in absorbance at 510 nm were recorded9. One unit of MPO activity was defined as that which degraded 1 mol H2O2 per min at 258℃. The results were expressed as mU per g protein.
Determination of catalase activity
Liver CAT activities were determined by Goth's colorimetric method, in which supernatant was incubated in H2O2 substrate and the enzymatic reaction stopped by the addition of ammonium molybdate. The intensity of the yellow complex formed by molybdate and H2O2 was measured at 405mm10.
Histopathological study
Each liver was divided into two pieces. One was placed in 10% formalin solution immediately, left overnight, and then embedded in paraffin blocks. The blocks were cut in 4 μm sections and stained with hematoxylin–eosin, using standard protocols. The severity of hepatic injury in the sections was evaluated using a pointcounting method on an ordinal scale as follows: grade 0, minimal or no evidence of injury; grade 1, mild injury consisting of cytoplasmic vacuolation and focal nuclear pyknosis; grade 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and grade 3, severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration13.
Statistical analysis
For statistical analyses, nonparametric independent group comparisons were made. For multiple comparisons, the Kruskal-Wallis was used for comparisons between groups and the Mann-Whitney test used if any statistical significance was found. A level of 5% (P < 0.05) was considered statistically significant. Data were expressed as mean and SD.
Results
TAC and CAT activities in liver tissue were significantly higher in Group 3 than those in Group 2 (P < 0.05 and P < 0.001, respectively). However, TAC and CAT activities in liver tissue were significantly lower in Group 2 than those in Group 1 (P < 0.05 and P < 0.01, respectively). TOS and OSI in hepatic tissue were significantly lower in Group 3 than those in Group 2 (P < 0.001 for both). Also MPO levels in hepatic tissue were significantly lower in Group 3 than those in Group 2 (P < 0.01). However, TOS, OSI and MPO levels in hepatic tissue were significantly higher in Group 2 than those in Group 1 (P < 0.01 for all). There were not statistically significant differences between the Gln treatment group and the sham group regarding to the oxidant and antioxidant parameters (P>0.05). The results are summarized in Table 1.
Table 1: Liver enzyme levels, oxidative and antioxidative parameters in all groups (n:10, mean± SD).
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; LDH: Lactate dehydrogenase; TAC: Total antioxidant capacity; TOS: Total oxidative status; OSI: Oxidative stress index; MPO: Myeloperoxidase; CAT: Catalase. a=P < 0.05, bP < 0.01, Sham groups vs I/R groups; d=P < 0.01, Sham groups vs I/R + NS groups; e=P < 0.05, f=P < 0.01, I/R groups vs I/R + Gln groups. 1P > 0.05, Sham groups vs I/R + Gln groups; 2P < 0.001, I/Rgroups vs I/R + Gln groups.
The ALT and AST levels were increased significantly in groups 2 and 3 in comparison with groups 1 (p=0.05 in all cases). However, the ALT and AST levels were decreased significantly in group 3 compared to group 2 (p<0.05).
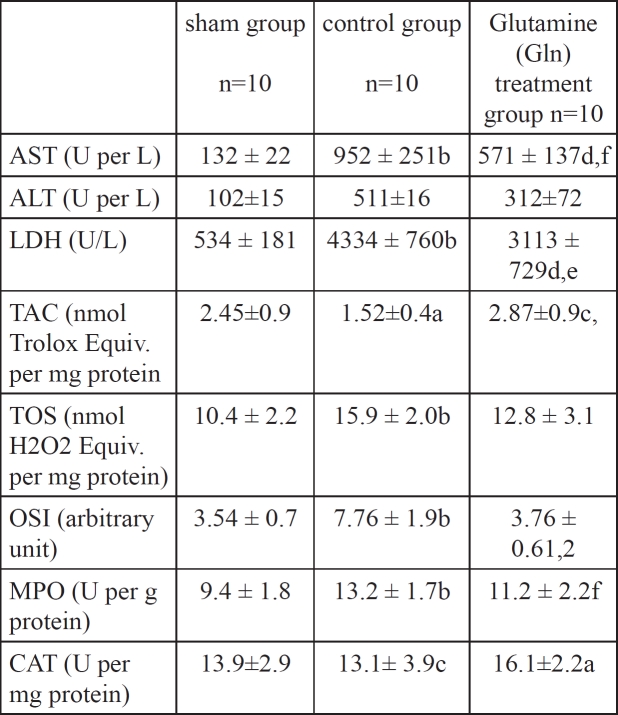
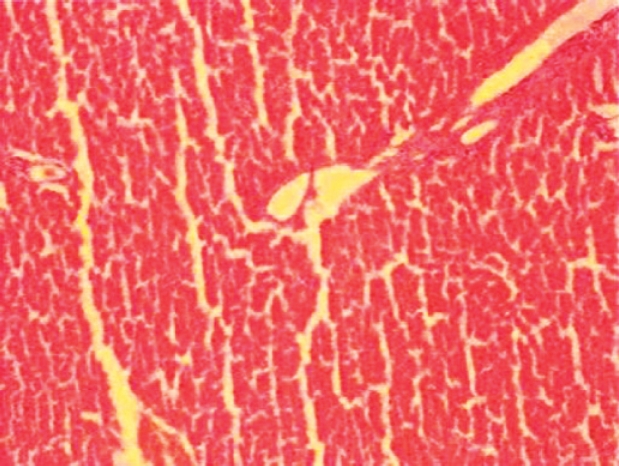
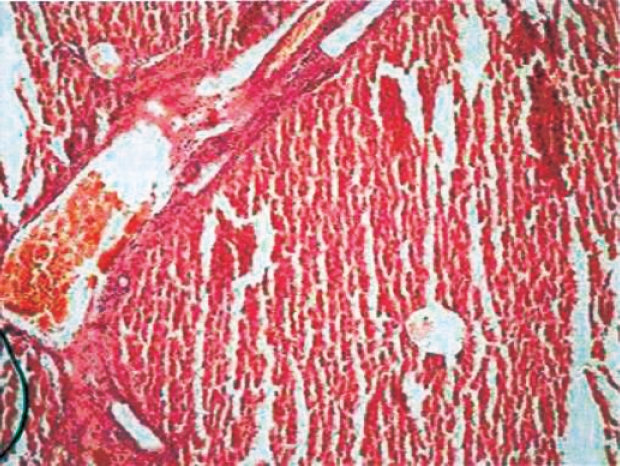
In histopathological evaluation, there were no pathological changes in liver tissue of the sham group (Figure 1). Liver specimens from rats after ischemia reperfusion exhibited focal necrosis and infiltration of leukocytes (Figure 2). Gln treatment significantly decreased these pathological changes (Figure 3).
Figure 1: Normal liver tissue.
Figure 2: Histopathological findings 60 min after reperfusion in the control group. Focal necrosis with sinusoidal congestion and neutrophil accumulation is seen.
Figure 3: Histopathological findings 60 min after reperfusion in gln treatment group. Focal necrosis got better, sinusoidal congestion and neutrophil accumulation diminished.
Histological tissue damage was milder in the Gln treatment group than that in the control group.
The liver histopathological scores were 0.1 ± 0.2, 2.8±0.1, and 1.1 ± 0.2 in groups 1 to 3, respectively. The histopathological scores were higher in groups 2 and 3 than in groups 1 (P < 0.05 in all cases). Moreover, the histopathological score was lower in group 3 than in group 2 (P < 0.05). Normal findings were obtained on histological examination of rats in groups 1 (Figure 1A). In group 2, focal necrosis with sinusoidal congestion and neutrophil accumulation were observed at the midzonal region (Figure 2). In contrast, these changes were markedly attenuated in Gln-treated livers (Figure 3).
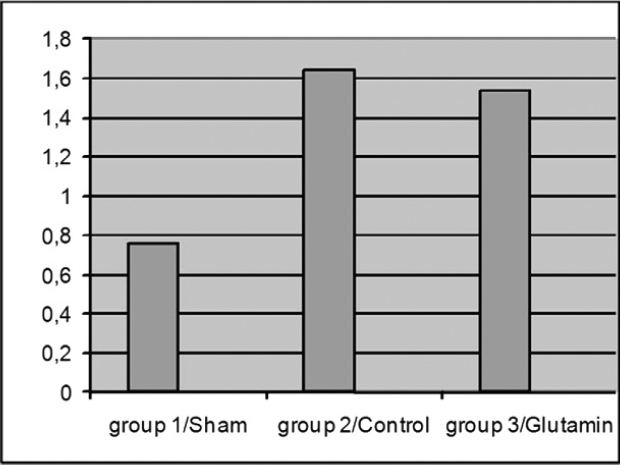
Figure 4: Plasma endotoxin levels.
Effect of Gln pretreatment on plasma endotoxin level during I/R injury
In the second series of experiments, plasma endotoxin level was evaluated after liver I/R injury. There was a statistically significant difference in plasma endotoxin level between the Sham (group I), and liver I/R injury (group 2) groups (0.76 EU/mL vs 1.64 EU/mL, P < 0.001). Gln pretreatment lowered the plasma endotoxin level (group 3), which was increased due to liver I/R injury (group 2) (1.54 EU/mL vs 1.64 EU/mL, P = 0.48). However, this decrease failed to reach statistical significance (Figure 4).
Discussion
Hepatic ischemia and reperfusion injury is observed following major liver surgery, transplantation, trauma and sepsis and may cause metabolic and structural hepatic damage14,15. This remains a significant problem in surgical procedures, and is a limiting factor in liver transplantation16. Hepatic injury caused by ischemia can be described as necrosis. It was reported that apoptosis of hepatocytes and sinusoidal endothelial cells is a critical mechanism contributing to hepatic I/R injury17. The mechanism of apoptosis in ischemia and reperfusion injury usually includes production of oxygen radical species and intracellular calcium overloading18. Translocation of bacteria and endotoxin from gut, well documented in hepatic I/R injury, also contributed to the induction of hepatocyte apoptosis. TNF-α induced by endotoxin also can induce hepatocyte apoptosis19.
Gln is one of the most abundant amino acid in plasma and plays an important role in the metabolism. The exact mechanisms of the protective effect of Gln against ischemia and reperfusion injury of organs and tissues are not yet completely understood. Gln protects against ischemia- reperfusion injury of gut, heart and skeletal muscle by preserving the GSH content in the tissues20,21. GSH is an important endogenous antioxidant that protects against oxygen free radical injuries and intravenous GSH administration during reperfusion of ischemic liver can prevent reperfusion injury in rats22. The content of GSH is useful for determination of the degree of tissue damage23.
Gln is a precursor for synthesis of nucleic acids and glutathione. It is the main fuel for rapid proliferating and dividing cells such as enterocytes and lymphocytes. It can maintain the metabolism of intestinal mucosal cells directly or indirectly, promote hyperplasia of epithelial cells of ileum and colon, maintain the structure and function of small intestinal mucosal and reduce the increment of intestina permeability24,25. Translocation of bacteria and endotoxin from gut, well documented in hepatic I/R injury, also contribute to the induction of hepatocyte apoptosis. TNF-α induced by endotoxin also can induce hepatocyte apoptosis26. In the present study Gln treatment markedly attenuated ALT, AST and LDH activities which are associated with hepatic parenchymal injury. The increase of AST, ALT and LDH activities observed in control groups can be elucidated by lipid peroxidation leading to cytolysis, which is caused by the oxygen free radical formed during the reperfusion phase27. The decrease of AST, ALT, and LDH activities observed in the rats treated with Gln, when compared to the rats in the control group, suggests a possible protective effect of Gln treatment in the hepatic ischemia/reperfusion condition. In the present study, we preferred to measure oxidants and antioxidant capacity simultaneously to assess oxidative stress more exactly. In the present study, we measured oxidative stress with OSI which was detected using both oxidative and antioxidative parameters. We evaluated TAC which reflects the antioxidative status and TOS to investigate oxidative status using a more recently developed measurement methods by Erel11,12.
Gln treatment significantly reduced OSI and TOS levels, which show oxidative stress, and increased TAC levels, which show antioxidant capacity, in liver tissue. Oxidative stress activates mechanisms that lead to the synthesis of proinflammatory cytokines and cell adhesion molecules. Therefore, oxidative stress may contribute to an inflammatory response induced by endotoxemia after hepatic ischemia reperfusion. Our data confirm that liver ischemia reperfusion increases oxidative stress, an effect that not only produces direct tissue damage, but also modulates production of toxic cytokines leading to inflammation and leukocyte infiltration, consistent with previous studies. In addition to this, Gln treatment alleviated pathological structural changes.
Catalase is an oxidoreductase enzyme, which transforms H2O2 into H2O and O2. It can protect cells from damage induced by ischemia reperfusion through scavenging reactive oxygen species28. The results of the present study showed that treatment with Gln can increase catalase activity, and this is consistent with its protective effect.
Infiltration of neutrophils into tissues is commonly assessed by changes in activity of MPO, which is an enzyme found primarily in neutrophils. Increased MPO activity in the liver of rats after I/R suggests activation of an inflammatory response. In our study, we observed increased MPO activity in the liver tissue, and this may indicate that neutrophil accumulation and lipid peroxidation contributes to ischemia reperfusion-induced liver injury. Previously, it has been reported that the activated neutrophils located in the inflammatory foci and secreting MPO into the extracellular space can convert hydroperoxides into free radicals, triggering lipid peroxidation29. This is consistent with the results of our study. The administration of iloprost prevented hepatic malfunction, inhibited the generation of free radicals, and improved hepatic microcirculatory impairment after hepatic I/R injury 30.
The results of the present study may have clinical applications to liver surgery associated with the Pringle maneuver and hepatic transplantation. Further studies are necessary to clarify the effects of different doses and administration periods of Gln on plasma endotoxin levels and histopathological changes in liver I/R.
References
- 1.Hassan-Khabbar S, Cottart CH, Wendum D, Vibert F, Clot JP, Savouret JF. Postischemic treatment by trans-resveratrol in rat liver ischemiareperfusion: a possible strategy in liver surgery. Liver Transpl. 2008;14:451–459. doi: 10.1002/lt.21405. [DOI] [PubMed] [Google Scholar]
- 2.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Pina E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–159. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melis GC, ter Wenge lN, Boelens PG, van Leeuwen PA. Glutamine: recent developments in research on the clinical significance of glutamine. Curr Opin Clin Nutr Metab Care. 2004;7:59–70. doi: 10.1097/00075197-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:772–776. doi: 10.1016/S0140-6736(98)02007-8. [DOI] [PubMed] [Google Scholar]
- 5.Harward TR, Coe D, Souba WW, Klingman N, Seeger JM. Glutamine preserves gut glutathione levels during intestinal ischemia/ reperfusion. J Surg Res. 1994;56:351–355. doi: 10.1006/jsre.1994.1054. [DOI] [PubMed] [Google Scholar]
- 6.Prem JT, Eppinger M, Lemmon G, Miller S, Nolan D, Peoples J. The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. Am J Surg. 1999;178:147–150. doi: 10.1016/s0002-9610(99)00148-8. [DOI] [PubMed] [Google Scholar]
- 7.Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864–870. doi: 10.3748/wjg.v10.i6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei H, Frenkel K. Relationship of oxidative events and DNA oxidation in SENCAR mice to in vivo promoting activity of phorbol ester-type tumor promoters. Carcinogenesis. 1993;14:1195–1201. doi: 10.1093/carcin/14.6.1195. [DOI] [PubMed] [Google Scholar]
- 10.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 11.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Ozturk H, Gezici A, Ozturk H. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induce oxidative stres in rats. Hepatol Res. 2006;34:76–83. doi: 10.1016/j.hepres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Shin T, Kuboki S, Huber N, Eismann T, Galloway E, Schuster R, et al. Activation of peroxisome proliferator-activated receptor-gamma during hepatic ischemia is age-dependent. J Surg Res. 2008;147:200–205. doi: 10.1016/j.jss.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gulik TM, de Graaf W, Dinant S, Busch OR, Gouma DJ. Vascular occlusion techniques during liver resection. Dig Surg. 2007;24:274–281. doi: 10.1159/000103658. [DOI] [PubMed] [Google Scholar]
- 16.He XS, Ma Y, Wu LW, Wu JL, Hu RD, Chen GH. Dynamical changing patterns of glycogen and enzyme histochemical activities in rat liver graft undergoing warm ischemia injury. World J Gastroenterol. 2005;11:2662–2665. doi: 10.3748/wjg.v11.i17.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun K, Liu ZS, Sun Q. Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect nof ischemic postconditioning. World J Gastroenterol. 2004;10:1934–1938. doi: 10.3748/wjg.v10.i13.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshimi Y, Miyazaki S. Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated cytosolic Ca2+ level. J Immunol. 1995;54:599–609. [PubMed] [Google Scholar]
- 19.Gantner F, Leist M, Jilg S, Germann PG, Freudenberg MA, Tiegs G. Tumor necrosis factor-induced hepatic DNA fragmentation as an early marker of T cell-dependent liver injury in mice. Gastroenterology. 1995;109:166–176. doi: 10.1016/0016-5085(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 20.Harward TR, Coe D, Souba WW, Klingman N, Seeger JM. Glutamine preserves gut glutathione levels during intestinal ischemia/ reperfusion. J Surg Res. 1994;56:351–355. doi: 10.1006/jsre.1994.1054. [DOI] [PubMed] [Google Scholar]
- 21.Prem JT, Eppinger M, Lemmon G, Miller S, Nolan D, Peoples J. The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. Am J Surg. 1999;178:147–150. doi: 10.1016/s0002-9610(99)00148-8. [DOI] [PubMed] [Google Scholar]
- 22.Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864–870. doi: 10.3748/wjg.v10.i6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armeni T, Ghiselli R, Balercia G, Goffi L, Jassem W, Saba V. Glutathione and ultrastructural changes in inflow occlusion of rat liver. J Surg Res. 2000;88:207–214. doi: 10.1006/jsre.1999.5781. [DOI] [PubMed] [Google Scholar]
- 24.Scheppach W, Loges C, Bartram P, Christl SU, Richter F, Dusel G, et al. Effect of free glutamine and alanyl-glutamine dipeptide n on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994;107:429–434. doi: 10.1016/0016-5085(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 25.Blikslager AT, Rhoads JM, Bristol DG, Roberts MC, Argenzio RA. Glutamine and transforming growth factor-alpha stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery. 1999;125:186–194. [PubMed] [Google Scholar]
- 26.Gantner F, Leist M, Jilg S, Germann PG, Freudenberg MA, Tiegs G. Tumor necrosis factor-induced hepatic DNA fragmentation as an early marker of T cell-dependent liver injury in mice. Gastroenterology. 1995;109:166–176. doi: 10.1016/0016-5085(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 27.Chouker A, Martignoni A, Schauer RJ, Dugas M, Schachtner T, Kaufmann I. Alpha-gluthathione S-transferase as an early marker of hepatic ischemia/reperfusion injury after liver resection. World J Surg. 2005;29:528–534. doi: 10.1007/s00268-004-7431-3. [DOI] [PubMed] [Google Scholar]
- 28.Yabe Y, Kobayashi N, Nishihashi T, Takahashi R, Nishikawa M, Takakura Y, et al. Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther. 2001;298:894–899. [PubMed] [Google Scholar]
- 29.Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. Nacetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:BR274–BR278. [PubMed] [Google Scholar]
- 30.Harada N, Okajima K, Kushimoto S, Isobe H, Tanaka K. Antithrombin reduces ischemia/reperfusion injury of rat liver by increasing the hepatic level of prostacyclin. Blood. 1999:157–164. [PubMed] [Google Scholar]