Abstract
We present a 5-year-old boy with pneumonia who complained of right lumbar pain on the 7th day of treatment with Ceftriaxone. Ultrasound examination revealed mild to moderate right hydronephrosis. Under spasmoanalgetic therapy and hydration there was spontaneous passage of three small calculi. Infrared spectroscopy showed that the calculi were composed of calcium-ceftriaxonate. Full metabolic investigation was performed and moderate hypercalciuria was detected, suggesting the role of hypercalciuria in ceftriaxone-associated nephrolithiasis.
Keywords: biliary pseudolithiasis, ceftriaxone, child, infrared spectroscopy, urolithiasis, hypercalciuria
Metabolic factors, urinary tract infection and obstruction are the most common etiologies of nephrolithiasis in children. Drug induced nephrolithiasis is rarely described in children1. The incriminated drugs are ceftriaxone (CTX), trimethoprim-sulfamethoxasole, topiramate, antiretroviral drugs (atazanavir, indinavir, sulfadizine). CTX is a third generation cephalosporin antibiotic widely prescribed for respiratory and urinary tract infections. Biliary pseudolithiasis and urolithiasis are rare complication of CTX therapy and both conditions are usually transient2-7. Cessation of the drug, spasmnoanalgetic therapy, good hydration and follow up ultrasound studies are sufficient treatment measures in patients with suspected CTX induced urolithiasis. Finally, infrared (IR) spectrum8 of the calculi is critical for establishing the correct diagnosis.
Case report
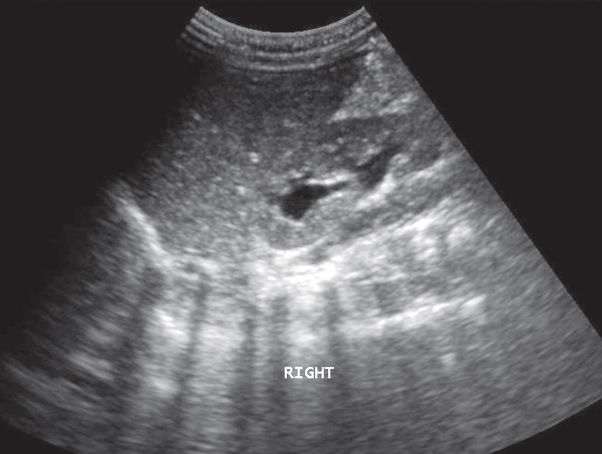
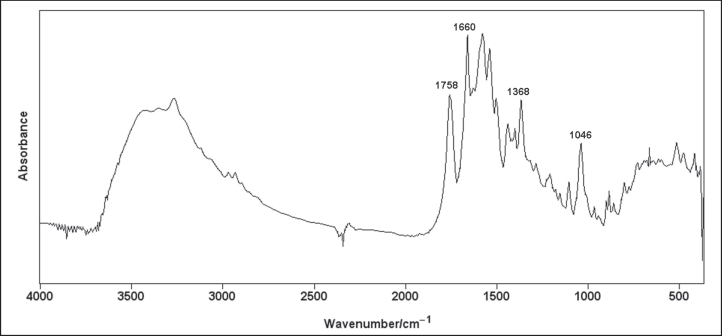
A 5-year-old boy was admitted to the Hospital for treatment of community acquired pneumonia. CTX was prescribed in intravenous infusion 1.0 g/day (45 mg/ kg). His general condition promptly normalized and on the 7th hospital day he was placed on oral cephalosporin. He suddenly complained of intensive right sided lumbar pain. Renal sucussion was positive on that side. Microscopic examination of the urinary sediment revealed numerous red blood cells (eumorphic). Ultrasound scan showed grade II hydronephrosis of the right kidney (Figure 1). No calculi were detected in the kidneys. The gallbladder examination did not reveal pseudolithiasis. The child was given intravenous spasmoanalgetics for pain. Full metabolic investigation was performed and normal serum levels of calcium, phosphate, magnesium and uric acid were observed. Urinary excretion of cystine, uric acid, oxalate and citrate was also within normal limits. There was moderate hypercalciuria (5.4 mg/kg/day). Within the next two hospital days under forced hydration there was spontaneous passage of three calculi. IR spectroscopy was performed and the recorded spectrum identified the calculi as calcium ceftriaxonate (Figure 2). Follow up ultrasound scans revealed prompt resolution of the right hydronephrosis. Hypercalciuria was confirmed several times during the follow up and increased hydration was advised.
Figure 1: Hydronephrosis of the right kidney.
Figure 2: Infrared spectrum of the calculi revealing characteristic peaks for the calcium-cefrtiaxonate.
Discussion
CTX is a widely used third generation cephalosporin antibiotic and clinicians should be aware of the possible appearance of CTX-induced precipitates within the gallbladder and urinary tract2-7. This complication is termed "biliary pseudolithiasis" or "reversible cholelithiasis". The majority of patients are asymptomatic but in few of them right upper abdominal quadrant pain, nausea, vomiting, and cholecystitis may develop. Cessation of CTX, symptomatic treatment and follow up ultrasound scan are usually sufficient interventions. Risk factors for CTX induced gallbladder precipitations are well established and include high doses of the drug, hypercalcemia, renal failure, total parenteral nutrition, bile stasis etc. CTX is filtered by the kidneys unchanged and forms insoluble salts with calcium in a 1:1 molar ratio. Risk factors for urolithiasis are not well established but certainly poor urine output, high doses of CTX and hypercalciuria may favor CTX induced nephrolithiasis. Stojanovic and Djuric Vijatovl recently reported a pediatric patient who developed nephrolithiasis in a kidney with congenital ureteropelvic junction obstruction9.
CTX induced urinary precipitates may be asymptomatic and detected on routine ultrasound scanning but in some patients may manifest hematuria or renal colic2, 10-17. Bilateral obstruction with calculi may lead to acute renal failure as in the patient reported by Prince and Senac10. Often there is coexistence with biliary pseudolithiasis and confusion may arise in a child with pain in the right upper abdominal quadrant during CTX treatment11. Thus, besides examination of the gallbladder, careful ultrasound scanning of the kidney and urinalysis should be performed.
The true prevalence of CTX induced nephrolithiasis is not known. The first case of CTX induced nephrolithiasis was reported by Shaad et al in 19882. In their series of 37 children treated with CTX, 16 developed biliary pseudolithiasis and one child had concurrent biliary and urinary calculi, manifesting renal colic and obstruction of the kidney. In the recent prospective study by Biner et al 156 children with various infections treated with CTX at the doses of 50mg/kg, 75mg/kg and 100mg/kg have been followed by ultrasound scan17. Twenty seven children (17%) developed biliary pseudolithiasis or sludge, while only one child (0.6%) had urolithiasis. Mohham et al prospectively followed 284 children with pyelonephritis (185 girls and 99 boys)15. Nephrolithiasis was identified in 4 children (1.4%). None of the children had a metabolic risk factor.
Although our patient received CTX at moderate therapeutic dosage for 7 days, hypercalciuria was the most likely factor favoring development of urinary CTX precipitates. Therefore, in all patients who receive CTX and manifest hematuria, renal colic or lithiasis one should examine the possibility of urinary CTX precipitates. Finally we would like to emphasize the role of IR spectroscopy for the confirmation of ceftriaxone-associated nephrolithiasis8.
Conflict of interests: None declared
References
- 1.Daudon M, Jungers P. Drug-induced renal calculi: epidemiology, prevention and management. Drugs. 2004;64:245–275. doi: 10.2165/00003495-200464030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone associated biliary pseudolithiasis in children. Lancet. 1988;2:1411–1413. doi: 10.1016/s0140-6736(88)90596-x. [DOI] [PubMed] [Google Scholar]
- 3.Bor O, Dinleyici EC, Kebapci M, Aydogdu SD. Ceftriaxoneassociated biliary sludge and pseudocholelithiasis during childhood: a prospective study. Pediatr Int. 2004;46:322–324. doi: 10.1111/j.1328-0867.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet JP, Abid L, Dabhar A, Levy A, Soulier Y, Blangy S. Early biliary pseudolithiasis during ceftriaxone therapy for acute pyelonephritis in children: a prospective study in 34 children. Eur J Pediatr Surg. 2000;10:368–371. doi: 10.1055/s-2008-1072393. [DOI] [PubMed] [Google Scholar]
- 5.Palanduz A, Yalcin I, Tonguc E, Guler N, Ones U, Salman N. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound. 2000;28:166–168. doi: 10.1002/(sici)1097-0096(200005)28:4<166::aid-jcu2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulou F, Efremidis S, Karyda S, Badouraki M, Karatza E, Panteliadis C, et al. Incidence of ceftriaxone-associated gallbladder pseudolithiasis. Acta Paediatr. 1999;88:1352–1355. doi: 10.1080/080352599750030077. [DOI] [PubMed] [Google Scholar]
- 7.Blais C, Duperval R. Biliary pseudolithiasis in a child associated with 2 days of ceftriaxone therapy. Pediatr Radiol. 1994;24:218–219. doi: 10.1007/BF02012198. [DOI] [PubMed] [Google Scholar]
- 8.Dao NQ, Daudon M. Infrared and Raman Spectra of Calculi. Paris: Elsevier; 1997. [Google Scholar]
- 9.Stojanovic V, Djuric Vijatov G. Nephrolithiasis caused by ceftriaxone in a 3-year-old child with ureteropelvic junction obstruction. Case Report Med. 2009 doi: 10.1155/2009/365962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prince JS, Senac MO., Jr Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis in a child. Pediatr Radiol. 2003;33:648–651. doi: 10.1007/s00247-003-0963-0. [DOI] [PubMed] [Google Scholar]
- 11.de Moor RA, Egberts AC, Schroder CH. Ceftriaxone associated nephrolithiasis and biliary pseudolithiasis. Eur J Pediatr. 1999;158:975–977. doi: 10.1007/s004310051261. [DOI] [PubMed] [Google Scholar]
- 12.Acun C, Erdem LO, Sogut A, Erdem CZ, Tomac N, Gundogdu S, et al. Gallbladder and urinary tract precipitations associated with ceftriaxone therapy in children: a prospective study. Ann Trop Paediatr. 2004;24:25–31. doi: 10.1179/027249304225013349. [DOI] [PubMed] [Google Scholar]
- 13.Avci Z, Koktener A, Uras N, Catal F, Karadag A, Tekin O. Nephrolithiasis associated with ceftriaxone therapy: a prospective study in 51 children. Arch Dis Child. 2004;89:1069–1072. doi: 10.1136/adc.2003.044156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochat P, Cochat N, Jouvenet M, Floret D, Wright C, Martin X, et al. Ceftriaxone-associated nephrolithiasis. Nephrol Dial Transplant. 1990;5:974–976. doi: 10.1093/ndt/5.11.974. [DOI] [PubMed] [Google Scholar]
- 15.Mohkam M, Karimi A, Gharib A, Daneshmand H, Khatami A, Ghojevand N. Ceftriaxone associated nephrolithiasis: a prospective study in 284 children. Pediatr Nephrol. 2007;22:690–694. doi: 10.1007/s00467-006-0401-2. [DOI] [PubMed] [Google Scholar]
- 16.Tasic V, Sofijanova A, Avramoski V. Nephrolithiasis in a child with acute pyelonephritis. Ceftriaxone-induced nephrolithiasis and biliary pseudolithiasis. Pediatr Nephrol. 2005;20:1510–1511. doi: 10.1007/s00467-005-1909-6. [DOI] [PubMed] [Google Scholar]
- 17.Biner B, Oner N, Celtik C, Bostancioğlu M, Tunçbilek N, Güzel A, et al. Ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound. 2006;34:217–222. doi: 10.1002/jcu.20228. [DOI] [PubMed] [Google Scholar]