Abstract
Essential hypertension is a pro-inflammatory, pro-constrictor disease coinciding with endothelial dysfunction and inward vessel remodeling. Using the skin circulation our aim was to determine if iNOS upregulation attenuates NO-dependent cutaneous vasodilation in hypertensive humans. We hypothesized that with hypertension (1) localized iNOS inhibition would restore vasodilation in response to NO-dependent stimuli and (2) iNOS expression would be increased and phosphorylated vasodilator-stimulated phosphoprotein (pVASP) would be decreased. In vivo protocols: four intradermal microdialysis (MD) fibers were placed in 9 hypertensive and 10 normotensive (SBP: 146 ± 4 vs.113 ± 2 mmHg, p<0.001) men and women. MD fibers served as control, iNOS-inhibited (1400W), nNOS-inhibited (NPLA), and non-selective NOS-inhibited (L-NAME). Cutaneous vascular conductance was calculated (%CVCmax; sodium nitroprusside) during standardized local heating (42°C) and acetylcholine (ach) dose-response protocols (0.01, 0.1, 1, 5, 10, 50, 100 mmol/L). The NO-dependent local heating response was attenuated at control (95 ± 2 vs. 76 ± 2 %CVCmax, p<0.05) and nNOS-inhibited sites (94 ± 4 vs. 77 ± 3 %CVCmax, p<0.01) in hypertensives. iNOS inhibition augmented the NO-dependent local heating response (93 ± 2 vs. 89 ± 10 %CVCmax). Ach-induced vasodilation was attenuated in control sites at doses ≥ 0.1mM Ach in hypertensives, and was restored with iNOS inhibition (0.1 mM p<0.05; 1, 5, 10 mM p<0.001; 50, 100 mM p<0.01). In vitro iNOS expression was increased (p=0.006) and pVASP/VASP was decreased in skin from hypertensive humans (p=0.04). These data suggest that iNOS is upregulated in essential hypertensive humans and contributes to reduced NO-dependent cutaneous vasodilation.
Keywords: nitric oxide, inducible nitric oxide synthase, skin blood flow, hypertension, microvascular dysfunction
Introduction
Hypertension-related vascular dysfunction involves a complex interaction of inflammation, endothelial dysfunction, including a loss of endothelial nitric oxide (NO), and an upregulation of proconstrictor pathways1–3. The pathogenesis of hypertension-associated microvascular dysfunction occurs simultaneously in multiple vascular beds 4, 5. The cutaneous circulation is an accessible, representative vascular bed for the assessment of mechanisms underlying microvascular dysfunction with essential hypertension5–9,10. Deficits in microvascular reactivity, including a loss of NO signaling, and inward vessel remodeling are clearly evident in essential hypertensive human skin and parallel vascular changes that occur in the renal circulation with essential hypertension 5. However, the precise mechanisms leading to this dysfunction are unclear.
One putative mechanism underlying hypertension-related microvascular dysfunction is through inducible nitric oxide synthase (iNOS)11, 12. iNOS generates high concentrations of NO which is easily converted to peroxynitrite and superoxide in the pro-oxidant environment characteristic in essential hypertension. In addition, iNOS also upregulates arginase activity which limits NO production through eNOS13 by preferentially utilizing the common substrate L-arginine and is capable of inducing NOS uncoupling 14. We have demonstrated that acute localized arginase inhibition augments NO-dependent vasodilation in essential hypertensive human skin 15, 16, however, alterations in NO production and the role of iNOS is unknown. Therefore, the purpose of the present study was to examine the contributions of constitutively expressed and inducible NOS isoforms in the cutaneous vasculature of essential hypertensive humans using both physiological and pharmacological stimuli to induce endothelium-dependent vasodilation17. We hypothesize that in vivo attenuated vascular reactivity in essential hypertensive humans results from compromised eNOS derived NO-dependent dilation, and that acute iNOS inhibition would augment NO-dependent vasodilation in hypertensive humans induced by local heating of the skin or perfusion of the endothelium-dependent agonist acetycholine. We further hypothesized that in vitro iNOS expression would be increased and the downstream indicator of NO vasodilatory function in vascular smooth muscle, vasodilatory-stimulated phosphoprotein (VASP), would be decreased in skin samples obtained from humans with essential hypertension.
Methods
Subjects
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent were voluntarily obtained from all subjects prior to participation. Subject characteristics are presented in Table 1. Blood pressure status was determined in accordance with the guidelines set forth by the American Heart Association 18 and further explored using 24 hour ambulatory blood pressure monitoring. Subjects underwent a complete medical screening and were otherwise healthy with the exclusion of stage I hypertension and were not taking any medications including antihypertensives. Seven out of the eight essential hypertensive subjects were nieve to antihypertensive pharmacotherapy and one subject had previously been taking antihypertensive drugs but had not taken the drug for over a year. All premenopausal women (N=2) were studied on days 2–7 of their menstrual cycle, and post-menopausal women (N=8) reported that it had been at least one year since the cessation of their last menses. No perimenopausal women were studied.
Table 1.
Participant characteristics.
| Characteristic | Normotensive | Hypertensive |
|---|---|---|
| Subjects (male, female) | (3, 7) | (7, 2) |
| Age (years) | 52 ± 1 | 53 ± 2 |
| BMI | 25 ± 1 | 27 ± 1 |
| Systolic BP (mmHg) | 113 ± 3 | 146 ± 4* |
| Diastolic BP (mmHg) | 73 ± 2 | 93 ± 2* |
| MAP (mmHg) | 86 ± 2 | 111 ± 3* |
| Glucose (mg·dL−1) | 90 ± 3 | 94 ± 3 |
| HDL (mg·dL−1) | 62 ± 5 | 57 ± 4 |
| LDL (mg·dL−1) | 110 ± 4 | 106 ±7 |
P<0.01 difference from the normotensive age match control group.
In Vivo Vasoreactive Studies
Protocols were performed in a thermoneutral laboratory with the subject semi-supine and the experimental arm at heart level. Four intradermal microdialysis fibers (MD 2000, Bioanalytical Systems) were inserted into the forearm skin as previously described 19. Microdialysis sites were perfused with Ringers to serve as control, 0.1 mmol/L 1400W (N-[3-(aminomethyl)benzyl] acetamidine, AG Scientific) to inhibit iNOS, 5 mmol/L NPLA (Nω-Propyl-L-arginine, Tocris) to inhibit nNOS, 20 mmol/L L-NAME (NG-nitro-L-arginine methyl ester, Tocris) to non-selective inhibit all NOS isoforms (FDA IND 105,572) at a rate of 2 μL/min (Bioanylitical Systems Bee hive and Baby Bee microinfusion pumps, West Lafayette, IN). All drugs were mixed just prior to usage, dissolved in lactated Ringer solution, and sterilized (Acrodisc, Pall, Ann Arbor, MI, USA). The efficacy of the isoform specific antagonism and concentrations of the pharmacological agents used in this study have been demonstrated in other microdialysis studies (1400W Ki = 7 nM, NPLA Ki = 57 nM) 20,21–26.
Local heating protocol
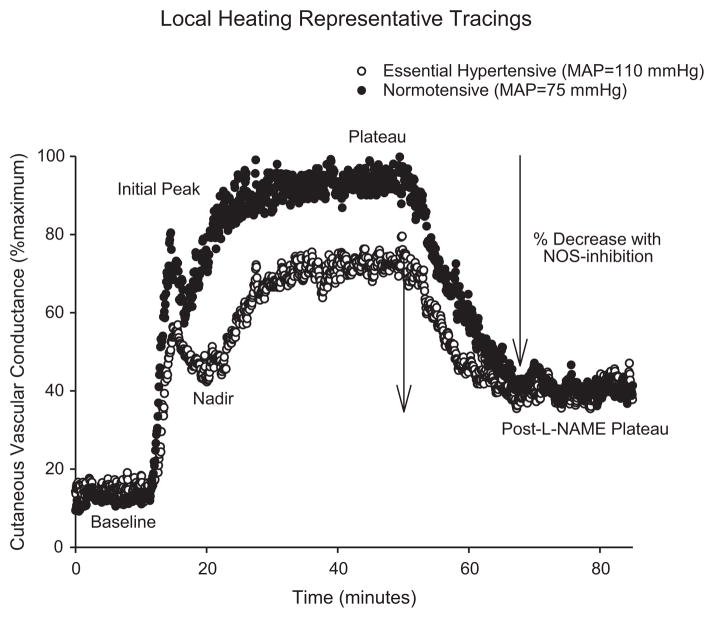
After subsidence of initial insertion trauma (60–120 minutes), local skin temperature was clamped at 33°C and laser Doppler probes attached over each site. Following baseline measurements, a standardized local skin warming protocol was performed to induce NO-dependent vasodilation 27. This protocol induced cutaneous vasodilation that is predominantly (~70%) mediated by the production of NO from eNOS17, 28, 29. No subjects reported pain or a burning sensation during skin heating. After skin blood flow reached an established plateau (30–40 minutes) 20mmol/L L-NAME was perfused to quantify NO-dependent vasodilation in all sites. A representative tracing illustrating the phases of the local heating response from a normotensive and a hypertensive subjects’ control sites are illustrated in Figure 1. This figure shows the phases of the local heating response including the initial peak and nadir which are primarily mediated by sensory nerve mechanisms with a small NO contribution, followed by the predominantly NO-dependent plateau as illustrated by the infusion of L-NAME to vasodilation due to the functional production of NO27. Following a new post-L-NAME stabilization in skin blood flow, local temperature was increased to 43°C and 28mmol/L sodium nitroprusside (SNP) was perfused to induce maximal cutaneous vasodilation (CVCmax) 22, 30. In our previous work and in pilot work this combination of heat and high concentration of SNP has been shown to induce maximal vasodilation 22.
Figure 1.
Representative skin blood flow tracings during local heating for a normotensive and essential hypertensive subject. The characteristic phases of the local heating response are labeled: Initial peak, nadir, local heating plateau, and post L-NAME plateau. The difference between the plateau and the post L-NAME plateau indicates the vasodilation due to the production of NO.
Acetylcholine dose response protocol
Subsidence of insertion trauma and baseline measurement followed the same procedure as for the local heating. Protocols were performed on the same arm and separated by at least one week to allow the skin to fully heal between trials. Following baseline measurement, local skin temperature remained clamped at 33°C during perfusion of seven ascending concentrations of acetylcholine (Ach) for 5 minutes each: 0.01, 0.1, 1, 5, 10, 50, and 100 mmol/L Ach. This amount of time allowed for a plateau in skin blood flow at each concentration of Ach. Each Ach concentration was mixed with the appropriate isoform specific NOS inhibitor and perfused at 2 μL/min. After completion of the Ach dose response, local skin temperature was increased to 43°C and 28mmol/L sodium nitroprusside (SNP) was perfused through all sites at a rate of 4 μL/min to induce maximal cutaneous vasodilatation (CVC) 22, 30.
In Vitro Skin Sample Analysis
Ventral forearm skin samples were obtained on a separate day from the in vivo functional assessment of vasoreactivity and on the opposite arm. Using sterile technique two 3mm diameter skin samples were obtained after anesthetization using 2% lidocaine without epinephrine. Samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Western blot analysis
After centrifugation of skin homogenates twice at 15000g at 4°C for 20 minutes, protein concentration was determined using a Bio-Rad DC protein assay. For Western blot analysis,25 μg proteins were fractionated by SDS/PAGE and electrotransferred to a nitrocellulose membrane (Hybond-ECL, Amersham Life Sciences). The membranes were blocked for 1 hour at room temperature(5% nonfat dry milk, in Tris Buffered Saline containing 0.1% Tween-20; TBST) and incubated with a primary antibody to eNOS (SantaCruz Biotech; 1:1000); nNOS (BD Bioscience, 1:1000); iNOS (BD Bioscience, 1:1000), pVASP (1:1000; Cell Signaling). Bound antibody was detected with HRP-conjugated IgG secondary antibody (1:1000) (Santa Cruz Biotechnology) and visualized using enhanced chemiluminescence. Next, the pVASP blot was stripped using Restore Plus Western Blot Stripping Buffer (Thermo Scientific) and reprobed with VASP antibody (Cell Signaling). GAPDH was used as loading control. Densitometry analyses were performed using Image J software (NIH).
Data and Statistical Analysis
Skin blood flow data were digitalized at 40 Hz, recorded and stored for offline analysis using Windaq software and Dataq data acquisition system (Windaq; Dataq Instruments, Akron, OH). Data were normalized to a percent of maximal CVC (%CVCmax). CVC data were averaged over a stable 5 minutes of baseline, local heating plateau, post L-NAME plateau, and maximal vasodilation. The initial peak and nadir CVC were visually identified as the highest and lowest values and averaged over 10 seconds. The vasodilation due to NO was calculated from the difference between the plateau and the post-L-NAME plateau (Figure 1). Absolute CVCmax in each site was calculated as the average of a stable plateau in laser- Doppler flux during 28mmol/L SNP infusion and local heating to 43°C divided by MAP. Because the late plateau phase of the local heating response is primarily dependent on NOS function, whereas the early phase has contributions from both sensory nerves and NOS, analysis and further discussion focus on the later phase of the cutaneous vasodilatory response.
Student’s unpaired t-tests were used to compare physical characteristics between subject groups and to examine potential differences in the densitometry analysis of the western blots. Absolute CVCmax data were analyzed using a two-way repeated measures ANOVA (group * pharmacological site, SPSS 19). %CVCmax were analyzed using three-way, mixed model, repeated measures ANOVA (group * pharmacological site * local heating VD phase or Ach dose; proc mix SAS 9.2). Specific planned comparisons were performed when appropriate to determine where differences between groups and pharmacological sites occurred with appropriate Bonfferoni correction. Significance was set at α = 0.05. Values are presented as means ± SEM.
Results
The physical characteristics of the subjects are presented in Table 1. Subjects were matched for age, body mass index, total cholesterol, and high and low density lipoproteins. Hypertensive subjects showed a significantly higher resting systolic, diastolic and mean arterial blood pressure compared to normotensive controls (p<0.001).
Figure 1 illustrates representative skin blood flow tracings during local heating for a normotensive and essential hypertensive subject. The characteristic phases of the local heating response are labeled.
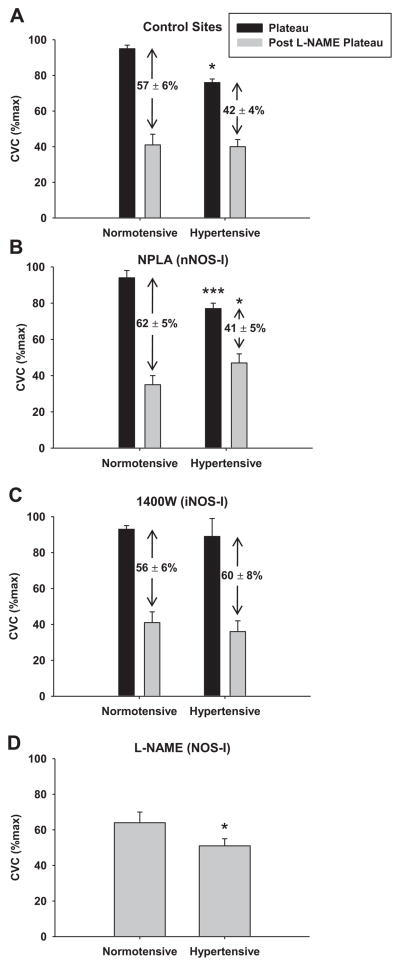
Figure 2 illustrates the %CVCmax at the NO-dependent plateau and after NOS inhibition with the non-specific NOS inhibitor L-NAME during local skin warming at different pharmacological sites in normotensive controls and essential hypertensive subjects. The difference between the plateau and the post-L-NAME plateau is a quantification of within site NO-dependent vasodilation during local heating and is labeled numerically on the graph. The NO-dependent plateau was attenuated in the hypertensive group at control (HT: 79±2 vs. NT: 95±2 %CVCmax; p<0.024) and nNOS inhibited (HT: 77±3 vs. NT: 94±4 %CVCmax; p=0.0004) sites compared to the normotensive group, but was restored in the hypertensive group at the iNOS inhibited sites (HT: 89±9 vs. NT: 93±2; p=0.39). A greater decrease in %CVCmax with NOS inhibition was present in the normotensive group at the control (HT: 42±4 vs. NT: 57±6 %CVCmax; p=0.039) and nNOS inhibited (HT: 41±5 vs. NT: 62±5 %CVCmax; p<0.001) sites, but no difference was present at the iNOS inhibited site (HT: 60±8 vs. NT: 56±6 %CVCmax; p=0.39). The plateau in %CVCmax during local heating at the continuous L-NAME site (non-specific NOS inhibited) was greater in the normotensive compared to hypertensive group (Figure 2D; p<0.001). A significant difference in %CVCmax between groups was observed during local heating at the iNOS inhibited site (1400W) during the initial peak (HT: 60±10 vs. NT: 49±4 %CVCmax; p=0.04) and nadir (HT: 48±8 vs. NT: 30±3 %CVCmax;p=0.0002). No differences in %CVCmax were present between groups at any of the other pharmacological sites for baseline, initial peak, or nadir during local heating. There were also no differences in absolute maximal CVC with localized microdialysis treatment or between groups (p>0.05).
Figure 2.
Cutaneous vascular conductance (%CVCmax) at the plateau in skin blood flow during local warming and after NOS inhibition with L-NAME in normotensive and essential hypertensive subjects in the (A.) control site, (B.) the nNOS-inhibited site, (C.) the iNOS-inhibited site, and (D.) non-specific NOS inhibited site. The black bars indicate %CVCmax at the plateau and the grey bar indicates %CVCmax at the post L-NAME plateau. NO-dependent vasodilation during local heating is the difference between the bars and is shown (mean±SEM). Values significantly different from normotensive group: * p<0.05, ** p<0.001.
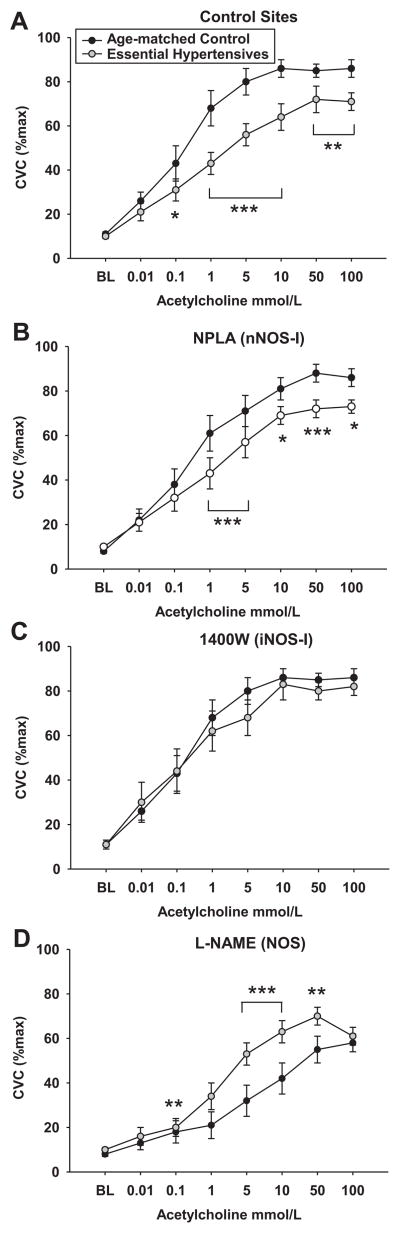
Figure 3 shows the mean Ach dose response curves for both groups in each drug treatment site. Ach-induced vasodilation was attenuated at all concentrations of Ach greater than 0.1 mmol/L in the control sites and greater than 1 mmol/L Ach in the nNOS inhibited sites in the hypertensive compared to normotensive group (all p<0.05 see Figure 3). Ach-induced vasodilation was restored with iNOS inhibition in the hypertensive group compared to their control sites. In addition, there were no longer differences between the hypertensive and the normotensive groups in %CVCmax in the sites where iNOS was inhibited. Vasodilation was attenuated at all Ach concentrations with non-specific NOS inhibition in both groups compared to their respective control sites, but vasodilation was greater in the hypertensive compared to normotensive group at all doses from 1 to 50 mmol/L Ach (1.0 mmol/L p<0.01, 5 and 10 mmol/L p<0.001, 50 mmol/L p<0.01), but not 100 mmol/L Ach.
Figure 3.
Cutaneous vascular conductance (%CVCmax) in normotensive and essential hypertensive subjects during acetylcholine-induced vasodilation across a series of concentrations in the (A.) control site, (B.) the nNOS-inhibited site, (C.) the iNOS-inhibited site, and (D.) non-specific NOS inhibited site (L-NAME). Values significantly different from normotensive group: * p<0.05, ** p<0.01, ** p<0.001.
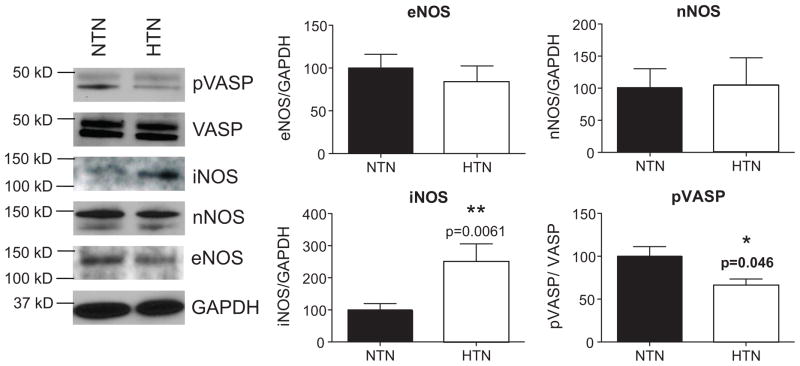
Figure 4 shows densitometric analysis and representative western blots of nNOS, iNOS, eNOS, and pVASP from the skin biopsy samples for both groups. There were no differences in eNOS or nNOS expression between the groups. iNOS expression was increased (p=0.006) and pVASP (p=0.046) was decreased in skin samples from the essential hypertensive group.
Figure 4. NOS1, NOS2, NOS3, VASP, and pVASP.
Expression of the three NOS enzymes, and VASP, and pVASP activity were determined by western blotting. GAPDH was used as loading control. Sample blot is shown in first panel. Densitometry analysis was performed using ImageJ software (NIH). * P<0.05 difference from the normotensive group.
Discussion
The major new findings of the present study were that cutaneous NO-dependent vasodilation to both physiological and pharmacological stimuli are attenuated in essential hypertensive human skin and acute iNOS inhibition with 1400W increased NO-dependent vasodilation likely through eNOS-mediated mechanisms. In response to endothelium dependent agonists, vasodilation was increased in the essential hypertensives when all NOS isoforms were inhibited compared to the normotensive group. In vitro analysis of skin biopsy tissue showed that iNOS protein expression was increased but there were no differences in the constitutively expressed NOS isoforms. Furthermore, pVASP was reduced in the hypertensive skin samples suggesting that functional NOS activity at the level of the vascular smooth muscle is decreased. Together these findings suggest that reduced functional NO production from eNOS contributes to cutaneous microvascular dysfunction in essential hypertensive humans and may be related to an upregulation in iNOS.
The present data identify iNOS upregulation as a possible target contributing to attenuated NO-dependent vasodilation in essential hypertensive humans. One putative mechanism that may be contributing to hypertension-associated microvascular dysfunction is a iNOS-mediated upregulation of arginase activity. Arginase is the final enzyme of the urea cycle and competes with eNOS for the common substrate L-arginine, resulting in reduced NO bioavailability, and partially mediating eNOS uncoupling 14, where eNOS produces superoxide instead of NO31. In animal models of essential hypertension increased arginase activity contributes to endothelial dysfunction and inward vessel remodeling through the downstream production of polyamines 32, 33. Because the affinity of arginase for L-arginine is considerably lower than eNOS, direct substrate competition is unlikely in healthy individuals 34, 35, as such acute arginase inhibition is ineffective at increasing NO-dependent vasodilation in young and middle-aged healthy humans36. However, recent work has established an iNOS-dependent upregulation of arginase activity through S-nitrosylation as a mechanism for limiting NO production through eNOS. This post-translational mechanism acts to stabilize arginase and alter its substrate affinity, allowing it to compete with eNOS for L-arginine 13, 37. Although we did not measure arginase activity in the present data because of the limited protein obtained from the skin biopsy samples, we have shown in other studies that acute arginase inhibition augments NO-dependent vasodilation in essential hypertensive human skin 19, 38. Further research into the link between augmented iNOS, arginase, and microvascular dysfunction is necessary.
In vitro analysis of the skin biopsies showed that the downstream vascular smooth muscle NO target pVASP was decreased in samples obtained from the hypertensive group. This is consistent with the functional in vivo findings of attenuated NO-dependent vasodilation during local heating and pharmacological perfusion of the endothelium-dependent agonist acetylcholine. Because pVASP is a general downstream vasodilatory molecule there are limits to the mechanistic interpretation of these data. However, these data together suggest that NO synthesized by iNOS is not having a vasodilatory effect on the vascular smooth muscle. Instead, in the pro-oxidant environment characteristic of hypertension, it is more likely that iNOS synthesized NO is rapidly oxidized and converted to peroxynitrite. This potential mechanism is supported by the increase in NO-dependent vasodilation observed when high concentrations of the non-specific antioxidant ascorbate are acutely administered in hypertensive humans 39.
In the present study we have initially focused on characterizing the involvement of the NOS isoforms in attenuated vasodilation with essential hypertension. Our current findings suggest that increased iNOS expression has potential downstream interaction through the constitutive NOSs (mainly eNOS). However, the initial stimulus (or stimuli) that increase(s) iNOS in hypertensive cutaneous microvasculature remain unclear. Possible candidates include a host of immune modulators including tumor necrosis factor α (TNFα), IL-6, COX-2, and elevated angiotensin II40. As such, potential pharmacological targets for the treatment of hypertension-induced microvascular dysfunction include the angiotensin pathway and novel anti-inflammatory compounds such as acromalin which decreases iNOS and COX-2 expression by altering TNFα and IL-641.
An additional interesting finding from the present data set was that vasodilation was increased in the hypertensive group when all NOS isoforms were inhibited in response to the endothelium-dependent agonist acetylcholine (compared to the normotensive group). There are many possible explanations for this finding in addition to decreased functional NO bioavailability. First, there may be other non-NO dependent mechanisms that are upregulated with hypertension acting as a compensatory mechanism for decreased eNOS activity, including endothelium derived hyperpolarization factors and both vasoconstrictor and vasodilatory products of the arachadonic acid pathway. Secondly, increased O2.− production from uncoupled eNOS and other hypertension-associated elevations in the activity of enzymes NAD(P)H oxidase and xanthine oxidase 1, 2 may lead to a relative vasoconstriction. Superoxide is capable of inducing vasoconstriction through the Rho/ROCK pathway in the vascular smooth muscle and has been identified mechanistically as the signal inducing vasoconstriction in human skin42–44. Furthermore this mechanism is implicated in microvascular dysfunction with primary aging45–47. Thus, inhibiting the uncoupled eNOS with L-NAME would decrease superoxide production and result in increased blood flow with increasing concentrations of acetylcholine.
In the present study we did not find a significant difference in absolute maximal cutaneous vascular conductance as we and others have demonstrated using similar techniques in unmedicated essential hypertensive humans38, 39. This difference likely reflects the relatively young age and stage I hypertensive status of our population. Moreover, it suggests the disease progression begins with a relative endothelial dysfunction and then moves on toward vascular smooth muscle hypertrophy and inward vessel remodeling.
We have utilized the cutaneous circulation to examine a potential mechanism underlying hypertension-induced microvascular dysfunction in humans. Using both physiologically relevant and pharmacological stimuli our results are consistent with findings from other regional circulations 48 and demonstrate a globalized endothelial dysfunction. Although the cutaneous circulation is being recognized as a useful model for examining hypertension-induced microvascular dysfunction it should be acknowledged that the sympathetic neural innervation is specialized for thermoregulatory functions and does not significantly contribute to overall blood pressure control. However, recent findings specific to essential hypertensive vascular pathology suggest that the changes occuring in the cutaneous circulation parallel those that occur in the renal vascular bed5 which is important in blood pressure regulation.
Limitations
Men and women demonstrate differences in cardiovascular risk and several studies have demonstrated important differences in endothelial function between men and women. In this study we included both men and women but the groups were not evenly matched for sex and two premenopausal women were included (one in each group). It would have been ideal to match the groups evenly for sex and menopausal status. However, while sex differences have been observed in the reflex regulation of thermoregulatory skin blood flow due to differences in sympathetic control, little evidence exists for potential differences in the regulation of skin blood flow during local heating or the perfusion of endothelium-dependent agonists.
We did not measure a total index of direct NOS activity in the skin samples. Due to the limited number of skin samples per subjects and protein recovery per sample we were not able to successfully measure total NOS activity. One reason for this was that there are several endogenous enzyme-independent sources of NO in the skin which increase with ultraviolent light exposure 49, leading to an unfavorable signal to noise ratio. Instead, we measured a downstream vascular smooth muscle indicator of NOS activity demonstrating that it is decreased in samples from essential hypertensive humans. Finally, we attempted to probe for phosphorylated eNOS, however due to the time lapse between the initial analysis and labile nature of these proteins they were not detectable.
Perspectives
Hypertension-associated vascular dysfunction is a complex, multifaceted condition which occurs simultaneously in multiple vascular beds. Attenuated NO-dependent dilation in the cutaneous circulation may precede alterations in conduit vessels9, 38, 39, 50–53, making the skin an easily accessible, generalizable vascular bed for assessing in vivo vascular function and dysfunction in pre-clinical and cardiovascular disease groups4, 54–56. The present data indicate a mechanistic link between inflammation and attenuated NO-dependent vasodilation in hypertensive humans. Because iNOS inhibition restored locally-mediated vasodilation in the hypertensive group, iNOS may be a potential molecular target in the treatment of essential hypertension-induced vascular dysfunction.
In summary, cutaneous NO-dependent vasodilation to both physiological and pharmacological stimuli are attenuated in essential hypertensive human skin. iNOS expression was increased in skin samples from essential hypertensive humans and acute iNOS inhibition restored NO-dependent vasodilation, likely through eNOS mechanisms. In addition, the downstream functional NO target pVASP was reduced in hypertensive skin samples. Together these findings suggest that reduced functional NO production from eNOS contributes to cutaneous microvascular dysfunction in essential hypertensive humans and may be related to an upregulation in iNOS.
Table 2.
Absolute maximum cutaneous vascular conductance (CVC) at each drug site for normotensive and hypertensive subjects during the local heating protocol. There were no differences between groups or with localized microdialysis drug interventions (two-way repeated measures ANOVA). Values are presented as mean ± SEM.
| Group | Maximum CVC
|
|||
|---|---|---|---|---|
| Control | nNOS Inhibited | iNOS Inhibited | NOS Inhibited | |
| Normotensive | 1.79±0.28 | 1.70±0.19 | 1.86±0.23 | 1.81±0.12 |
| Hypertensive | 1.56±0.15 | 1.90±0.28 | 1.53±0.25 | 1.56±0.34 |
Acknowledgments
The authors would like to thank Jane Pierzga for her technical assistance and Jessica Dahmus for her assistance with data collection. We would also like to thank W. Larry Kenney for his input with the manuscript.
Sources of Funding
Supported by National Institutes of Health grant RO1-HL093238-02 (LAH).
Footnotes
Disclosures
All authors have read and approved the submission of the manuscript; the manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an abstract.
Dan Berkowitz is the scientific founder and consultant for Arginetix Inc., a biotechnology company dedicated to the development of therapeutics targeting arginase in diseases in which endothelial dysfunction is an important contributing factor. The remaining authors have nothing to disclosure or conflicts of interest to report.
References
- 1.Feletou M, Verbeuren TJ, Vanhoutte PM. Endothelium-dependent contractions in shr: A tale of prostanoid tp and ip receptors. Br J pharmacol. 2009;156:563–574. doi: 10.1111/j.1476-5381.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42:574–581. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser doppler flowmetry correlates with renal resistive index. J Hum Hypertens. 2011 doi: 10.1038/jhh.2010.117. In Press. [DOI] [PubMed] [Google Scholar]
- 6.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34:655–658. doi: 10.1161/01.hyp.34.4.655. [DOI] [PubMed] [Google Scholar]
- 7.Debbabi H, Bonnin P, Ducluzeau PH, Leftheriotis G, Levy BI. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens. 2010;23:541–546. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- 8.Debbabi H, Bonnin P, Levy BI. Effects of blood pressure control with perindopril/indapamide on the microcirculation in hypertensive patients. Am J Hypertens. 23:1136–1143. doi: 10.1038/ajh.2010.115. [DOI] [PubMed] [Google Scholar]
- 9.Cohuet G, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: Therapeutic potential. Pharmacol Ther. 2006;111:81–98. doi: 10.1016/j.pharmthera.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2007;105:370–2. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 11.Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.umar U, Chen J, Sapoznikhov V, Canteros G, White BH, Sidhu A. Overexpression of inducible nitric oxide synthase in the kidney of the spontaneously hypertensive rat. Clin Exp Hypertens. 2005;27:17–31. doi: 10.1081/ceh-200044249. [DOI] [PubMed] [Google Scholar]
- 13.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible no synthase dependent s-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC, Berkowitz DE. Arginase inhibition restores nos coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holowatz LA, Thompson CS, Kenney WL. L-arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006;574:573–581. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol. 2006;291:H2965–2970. doi: 10.1152/ajpheart.00648.2006. [DOI] [PubMed] [Google Scholar]
- 17.Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol. 2008;295:H123–129. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AHA. Heart disease and stroke statistics-2006 update. American Heart Assocication; 2006. [DOI] [PubMed] [Google Scholar]
- 19.Holowatz LA, Kenney WL. Local ascorbate administration augments no- and non-no-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol. 2007;293:H1090–1096. doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 20.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible no synthase dependent s-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol. 2007;293:H2161–167. doi: 10.1152/ajpheart.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJR, Knowles RG. 1400w is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 24.Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation. 2008;15:569–579. doi: 10.1080/10739680802091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by n omega-propyl-l-arginine. J Med Chem. 1997;40:3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]
- 26.Cooper GR, Mialkowski K, Wolff DJ. Cellular and enzymatic studies of n[omega]-propyl--arginine and s-ethyl-n-[4-(trifluoromethyl)phenyl]isothiourea as reversible, slowly dissociating inhibitors selective for the neuronal nitric oxide synthase isoform. Arch Biolchem Biophys. 2000;375:183–194. doi: 10.1006/abbi.1999.1658. [DOI] [PubMed] [Google Scholar]
- 27.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 28.Kellogg DL, Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol. 2008;586:847–857. doi: 10.1113/jphysiol.2007.144642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol. 2009;107:1438–1444. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol. 1986;6:337–346. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 31.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]
- 33.Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens. 2005;23:971–978. doi: 10.1097/01.hjh.0000166837.78559.93. [DOI] [PubMed] [Google Scholar]
- 34.Di Costanzo L, Sabio G, Mora A, Rodriguez PC, Ochoa AC, Centeno F, Christianson DW. Crystal structure of human arginase i at 1.29-angstrom resolution and exploration of inhibition in the immune response. Proc Natl Acad Sci U S A. 2005;102:13058–13063. doi: 10.1073/pnas.0504027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith OW, Stuehr DJ. Nitric oxide synthases: Properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 36.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J Physiol. 2011;589:2093–2103. doi: 10.1113/jphysiol.2010.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunn J, Gutbrod S, Webb A, Pak A, Jandu SK, Bhunia A, Berkowitz DE, Santhanam L. S-nitrosation of arginase 1 requires direct interaction with inducible nitric oxide synthase. Mol Cell Biochem. 2011;355:83–89. doi: 10.1007/s11010-011-0841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol. 2007;581:863–872. doi: 10.1113/jphysiol.2007.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holowatz LA, Kenney WL. Local ascorbate administration augments no- and non-no-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol. 2007;293:H1090–1096. doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 40.Kajita M, Murata T, Horiguchi K, Iizuka M, Hori M, Ozaki H. Inos expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H1021–1031. doi: 10.1152/ajpheart.00563.2009. [DOI] [PubMed] [Google Scholar]
- 41.Chanput W, Mes J, Vreeburg RA, Savelkoul HF, Wichers HJ. Transcription profiles of lps-stimulated thp-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010;1:254–261. doi: 10.1039/c0fo00113a. [DOI] [PubMed] [Google Scholar]
- 42.Holowatz LA. Human cutaneous microvascular ageing: Potential insights into underlying physiological mechanisms of endothelial function and dysfunction. J Physiol. 2008;586:3301. doi: 10.1113/jphysiol.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by rho kinase in vivo in human skin. Am J Physiol. 2006;292:H1700–5. doi: 10.1152/ajpheart.01078.2006. [DOI] [PubMed] [Google Scholar]
- 44.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Rho kinase-mediated local cold-induced cutaneous vasoconstriction is augmented in aged human skin. Am J Physiol. 2007;293:H30–36. doi: 10.1152/ajpheart.00152.2007. [DOI] [PubMed] [Google Scholar]
- 45.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: Role of alpha2c-adrenoceptor translocation. Circ Res. 2004;94:1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 46.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol. 2005;289:H243–250. doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- 47.Lang JA, Jennings JD, Holowatz LA, Kenney WL. Reflex vasoconstriction in aged human skin increasingly relies on rho-kinase dependent mechanisms during whole-body cooling. Am J Physiol. 2009;297:H1792–1797. doi: 10.1152/ajpheart.00509.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 49.Mowbray M, McLintock S, Weerakoon R, Lomatschinsky N, Jones S, Rossi AG, Weller RB. Enzyme-independent no stores in human skin: Quantification and influence of uv radiation. J Invest Dermatol. 2009;129:834–842. doi: 10.1038/jid.2008.296. [DOI] [PubMed] [Google Scholar]
- 50.Lauer T, Heiss C, Preik M, Balzer J, Hafner D, Strauer BE, Kelm M. Reduction of peripheral flow reserve impairs endothelial function in conduit arteries of patients with essential hypertension. J Hypertens. 2005;23:563–569. doi: 10.1097/01.hjh.0000160213.40855.b7. [DOI] [PubMed] [Google Scholar]
- 51.Rizzoni D, Agabiti-Rosei E. Endothelial factors and microvascular hypertensive disease. J Cardiovasc Pharmacol. 2001;38 (Suppl 2):S15–18. doi: 10.1097/00005344-200111002-00005. [DOI] [PubMed] [Google Scholar]
- 52.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: A new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 53.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 54.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 55.Rossi M, Carpi A, Galetta F, Franzoni F, Santoro G. The investigation of skin blood flowmotion: A new approach to study the microcirculatory impairment in vascular diseases? Biomed Pharmacother. 2006;60:437–442. doi: 10.1016/j.biopha.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol. 2004;287:H2687–2696. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]