Abstract
Robust associations have been identified between impulsive personality characteristics and cigarette smoking during adolescents, indicating that impulsive behavior may play an important role in the initiation of cigarette smoking. The present study extended this research by using laboratory behavioral assessments to explore relationships between three specific dimensions of impulsive behavior (impulsive decision-making, inattention and disinhibition) and adolescent cigarette smoking. Participants were male and female adolescent smokers (n = 50) and nonsmokers (n = 50). Adolescent smokers were more impulsive on a measure of decision-making; however, there were significant smoking status by gender interaction effects for impulsive inattention and disinhibition. Male smokers were most impulsive on the measure of inattention, but male smokers were least impulsive on the measure of disinhibition. Correlations between biomarkers of smoking and impulsive inattention and disinhibition were found for females but not males. The current findings, coupled with previous findings (Reynolds et al., 2007), indicate there may be robust gender difference in associations between certain types of impulsive behavior and cigarette smoking during adolescence.
Impulsivity is a multidimensional construct that is often linked to various forms of addiction (including cigarette smoking, e.g., Fillmore & Rush, 2002; Mitchell, 1999; Reynolds, 2006b) as well as a variety of other clinical diagnoses (e.g., Gauggel, Rieger & Feghoff, 2004; Heerey et al., 2007). Impulsivity has been broadly defined as “human behavior without adequate thought, the tendency to act with less forethought than do most individuals of equal ability and knowledge, or the predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions” (International Society for Research on Impulsivity; http://impulsivity.org/). Research specifically targeting associations between impulsivity and adolescent cigarette smoking has identified robust associations between self-report assessments of impulsivity and smoking. Adolescent smokers generally score more impulsively on these assessments than adolescent nonsmokers (e.g., Forgays, 1986; Tercyak et al., 2003; Zuckerman et al., 1990). Such robust associations may indicate that being highly impulsive is a risk factor for the initiation of smoking.
Little adolescent cigarette smoking research related to impulsivity has included laboratory behavioral assessments of impulsive behavior. These behavioral assessments utilize tasks that assess the actual behaviors of interest rather than a participant’s self reports of behavior. Recent research using behavioral assessments provide evidence that impulsivity can be conceptualized as at least three separate dimensions of behavior that include decision-making, inattention and disinhibition (Reynolds, Penfold & Patak, 2008). Typically, correlations between behavioral and self-report measures of impulsivity are low (e.g., Heyman & Gibb, 2006; Lane et al., 2003) or not significant (e.g., Krishnan-Sarin et al., 2007; Reynolds et al., 2004; Reynolds, Penfold & Patak, 2008). This lack of association may be due to differences in the breadth/specificity of behaviors assessed by behavioral and self-report methods, with behavioral assessments modeling more specific behavioral processes (see Reynolds et al., 2006). However, as with self-report assessments, behavioral measures confirm that adult addicted populations are often more impulsive than non-addicted control participants (e.g., Bickel et al., 1999; Crean et al., 2000; Lejuez et al., 2003; Madden et al., 1997; Mitchell, 1999; Moeller & Dougherty, 2002; Petry & Casarella, 1999; Reynolds, 2006; Reynolds et al., 2004; Vuchinich & Simpson, 1998), thus demonstrating the relevance of these procedures for drug use and abuse research.
Of these three behavioral dimensions, impulsive decision-making includes assessments that require the individual to decide between delayed versus immediate or probabilistic versus certain outcomes (i.e., delay discounting or risk taking). A number of studies have found a relationship between these types of measures and cigarette smoking status, with smokers generally being more impulsive than nonsmokers. For measures of delay discounting, these findings have been especially robust—as demonstrated for a variety of discounted commodities such as monetary or health outcomes (see Bickel & Marsch, 2001, and Reynolds, 2006a, for reviews). For example, adult smokers discount the value of delayed monetary rewards more than never smokers (e.g., Baker, Johnson & Bickel, 2003; Bickel, Odum, & Madden, 1999; Mitchell, 1999; Ohmura, Takahashi, & Kitamura, 2005; Reynolds, 2006b; Reynolds et al., 2004). This finding also has been replicated among adolescent smokers and nonsmokers (Audrain-McGovern et al., 2004; Reynolds et al., 2007). Adult smokers also discount the value of health gains and losses more steeply by delay than never smokers (Odum, Madden & Bickel, 2002); and smokers are more impulsive on measures of risk taking, which involves decisions about uncertain outcomes but does not involve delay (e.g., Lejuez et al., 2003).
While impulsive decision-making has been well researched in the context of substance abuse, and cigarette smoking more specifically, impulsive inattention and disinhibition have received much less emphasis. Of these two dimensions, inattention involves the inability to maintain alertness and receptivity for a particular set of stimuli or stimuli changes over time (e.g., Davies, Jones, & Taylor, 1984). Measures of inattention differentiate individuals diagnosed with attention deficit hyperactivity disorder (ADHD) from non-clinical control participants (e.g., Losier, McGrath, & Klein, 1996; Epstein et al., 2003); however, there has been little research on the association between impulsive inattention and substance abuse. One study has shown that overnight abstinence from smoking (among adult smokers) increases omission and commission errors, representing higher levels of inattention (Sacco et al., 2005). Also, another study found that adult smokers diagnosed with ADHD had poorer performance on a measure of inattention during a period of smoking abstinence than non-ADHD control smokers who also were abstaining (McClernon et al., 2008). Additionally, there have been acute nicotine effects on inattention. For example, adult smokers being treated with a nicotine patch had significantly better attention compared to smokers in a placebo condition (Poltavski & Petros, 2006). Collectively, these studies demonstrate acute withdrawal or nicotine effects on inattention, but no research has more generally compared smokers and nonsmokers using laboratory assessments of inattention.
By contrast, impulsive disinhibition emphasizes ability to inhibit inappropriate or unwanted behaviors, which has been related to conditions such as ADHD (e.g., Castellanos et al., 2000), externalizing disorders (Krueger et al., 2002; Krueger et al., 2007) and drug addiction (e.g., Fillmore & Rush, 2002). As with inattention, very little research has compared smokers and nonsmokers with measures of disinhibition, but acute nicotine effects on this facet of behavior have been found in participants diagnosed with ADHD—with nicotine acutely reducing disinhibition in this population (Potter & Newhouse, 2004). For the one study that has compared smokers and nonsmokers there was an un-hypothesized interaction between gender and smoking status: male smokers had significantly shorter stop reaction times (i.e., less impulsive responding) than male nonsmokers, a pattern that was not observed for female smokers and nonsmokers (Reynolds et al., 2007). The authors suggested the interaction may have reflected nicotine-based improvements in task performance for male smokers only.
Using laboratory behavioral tasks, the current study compared these three dimensions of impulsive behavior (i.e., impulsive decision-making, inattention and disinhibition) in male and female adolescent smokers and nonsmokers. Secondarily, we explored possible gender interactions as this has not been examined extensively in the previous literature. Two self-report assessments of impulsivity also were included for comparison purposes. Because of the lack of similar previous research, it was generally hypothesized that adolescent smokers would be more impulsive than nonsmokers across these behavioral and self-report assessments.
METHOD
Participants
Participants in this study were male and female adolescent smokers and nonsmokers recruited from the central Ohio area through posted advertisements, newspaper advertisements and word of mouth referrals. An initial phone screening was conducted to determine eligibility. Participants in this study ranged in age between 13 and 17 years and self-reported smoking four or more cigarettes per day for at least the preceding three months (smokers) or never smoking (nonsmokers). Once at the laboratory, each participant provided samples of breath and urine to verify smoking status. The breath sample was tested for CO content using a Micro 4 Smokerlyzer (Bedford Scientific, Kent, United Kingdom), and the urine sample was tested for cotinine content using a homogenous enzyme immunoassay (Graham-Massey Analytical Labs, New haven, CT). Participants that were classified as smokers had cotinine levels of ≥ 200 ng/ml. Participants classified as nonsmokers had CO levels of ≤ 5 ppm and cotinine levels of ≤ 50 ng/ml.
Measures
Laboratory Behavioral Assessments
Experiential Discounting Task (EDT; Reynolds & Schiffbauer, 2004)
The EDT is a real-time adjusting amount procedure designed to assess delay discounting (i.e., impulsive decision-making). EDT assessments have been compared between adult smokers and nonsmokers (Reynolds, 2006b); however, performance on this measure has not yet been compared between adolescent smokers and nonsmokers. Participants completed four blocks of choices between a delayed and probabilistic (35% chance of receiving) standard amount ($0.30 delayed by 0, 7, 14, or 28s) and an adjusting smaller amount that was immediate and certain. Each block of choices assessed discounting for one of the four delays, and the order of the choice blocks was counterbalanced across participants. As a real-time assessment all choice consequences (delay, probabilities and rewards) were experienced during the testing session, that is, while the participant was still making choices. Following each reward delivery, participants “banked” their money to proceed to the next choice. Immediately following each banking response the money was delivered from a coin dispenser located on the table at which the participant was seated, and the participant kept all of the earned money as part of his or her participation payment. Indifference points were determined for each delay assessed, which were used to determine a discounting curve for each participant. See Reynolds and Schiffbauer (2004) for more description of the EDT and participant instructions for this measure. Participants earned approximately $12 from completing the EDT.
Conners’ Continuous Performance Test – II (CPT -II; Conners, 2004)
The CPT-II is a computerized task designed to measure sustained attention. Participants were asked to left click a computer mouse as quickly as possible when letters other than the letter X were presented (target stimulus) on the screen and to refrain from responding when the letter X (non-target stimulus) was presented. The time between each stimulus (target and non-target) was varied between 1, 2 or 4 seconds, and the task took approximately 15 minutes to complete. The outcome measures used in this study were designed to indicate inability to sustain attention and included number of omissions, number of commissions, and hit reaction time (raw scores). High numbers of omission errors (not responding to target stimuli) and/or commission errors (responding to non-target stimuli) as well as high hit reaction times (slow rate of response) reflect inattention.
Go/Stop Task (Dougherty et al., 2003)
The Go/Stop Task was designed to assess impulsive disinhibition (see Dougherty, Mathias, Marsh, & Jagar, 2005). Participants were presented a series of three digit numbers on a computer screen (e.g. …436 …256 …256 …822) with a 1s blank screen separating each three digit number. Participants completed 240 trials (2 blocks of 120) in which they were instructed to respond as quickly as possible by left clicking a computer mouse when a matching three digit number appeared (go signal), which occurred for 50% of the numbers. Participants earned $0.05 for each go-signal response that occurred while the matching number was visible (400ms) but lost $0.05 for “late” responses that occurred after the number disappeared (after 400ms). The participant lost $0.10 for responses to non-matching numbers.
For a randomly selected 25% of the go-signal trials, the second matching number changed colors from black to red, thus indicating a stop trial. Participants were instructed to inhibit left-click responses when the go-signal numbers changed colors (stop-signal). Participants earned $0.05 for each successfully inhibited response following a color change but lost $0.05 for failures to inhibit. Stop-signal color changes happened after different intervals within 400 ms of a go signal. These stop-signal intervals adjusted according to task performance. Interval lengths decreased (i.e., occurred more quickly during the go-signal) following failures to inhibit, thus making it easier to stop for the next trial; however, interval lengths increased following successful inhibition, thus making it more difficult to stop for the next trial. Stop-signal intervals continued to adjust in this way until the participant was able to successfully inhibit on approximately 50% of the stop-signal trials. At this 50% criterion, the stop signal reaction time (SSRT) was calculated by subtracting the stop-signal delay (at which inhibition was at approximately 50%) from the go reaction time (Go RT; the average interval for a participant to respond to go signals). From this calculation, longer SSRT values (measured in milliseconds) reflected behavioral disinhibition and impulsivity. See Dougherty et al. (2003) for participant instructions. Participants earned between $0 and $4 for completing the Go/Stop Task.
Self-Report Assessments
Barratt Impulsiveness Scale – Adolescent (BIS-11-A; Fossati et al., 2002)
The BIS-11-A is a 30 item self report questionnaire designed to measure impulsiveness. Items are on a 4-point scale (1 = rarely/never to 4 = almost always/always). The BIS-11-A is an adaptation of the adult BIS-11 (Patton, Stanford, & Barratt, 1995). The original adult version of the BIS-11 consisted of three sub-factors (motor impulsiveness, nonplanning impulsiveness, and attentional impulsiveness); however, due to the high intercorrelations among the sub-factors for adolescents, it has been recommended that total scores are the most appropriate index of impulsivity for this age group. Higher total scores reflect greater impulsivity. Past research has found that the BIS-11-A has good internal consistency in adolescent samples (alpha = 0.78; Fossati et al., 2002). Alpha for the current data set was lower (alpha = 0.58).
Conners-Wells’ Adolescent Self Report Scale – Long Form (CASS: L; Conners, 2001)
The CASS:L is an 87 item self-report questionnaire designed for use with adolescents to measure factors related to ADHD symptoms. Respondents rated items on a 4-point rating scale (0 = “not true at all” to 3 = “very often, very frequently”). The CASS:L has been shown to have high internal reliability (alpha = 0.75 – 0.95), good test-retest reliability (r = 0.73 – 0.89) and good criterion validity (Conners et al., 1997). Internal consistency for the current data set was high (alpha = 0.96).
For the purposes of this study, the ADHD index score and the DSM-IV symptom subscales (inattention and hyperactivity) from the CASS:L were used. The ADHD index is used to identify adolescents at risk for having ADHD. The DSM-IV symptom subscales correspond to the official diagnostic criteria in the Diagnostic and Statistical Manual – Fourth Edition (DSM-IV) for ADHD separated into the two categories of inattention and hyperactivity (Conners, 2001). Results of a confirmatory factor analysis (Conners et al., 1997) provide evidence for the two distinct factors. However, these two categories also can be combined to derive an overall ADHD index score. Raw scores on these subscales are converted to T-scores, with higher scores reflecting more attention or hyperactivity problems.
Procedure
All data collection took place in a human-behavior laboratory at the Research Institute at Nationwide Children’s Hospital, Department of Pediatrics, The Ohio State University. Institutional Review Board approved consent and assent forms were reviewed and signed by all participants. Following parental consent/adolescent assent, participants were tested for breath CO levels and then completed a brief demographic questionnaire, the self-report measures as well as a widely used measure to estimate IQ, the Kaufman Brief Intelligence – Second Edition (KBIT2; Kaufman & Kaufman, 2004). Following completion of self-report measures, participants completed the laboratory behavioral tasks, with task order counterbalanced across participants. Participants were then escorted to a private restroom where they were asked to provide a urine sample (unobserved) in a provided collection cup. Urine samples were frozen until they could later be assayed for cotinine content. Participants were then debriefed and paid for their participation. All laboratory sessions were conducted between the hours of 12:00 p.m. and 7:00 p.m.
Statistical Analyses
An area-under-the-curve (AUC) method, as specified by Myerson, Green, & Warusawitharana (2001), was used to characterize data from the EDT. From the AUC method, smaller AUC values reflect greater discounting and impulsivity. The AUC data were inspected for normality using Shapiro-Wilk tests and were transformed using a log-10 function to improve normality.
All analyses were performed using SPSS 15.0. Demographic characteristics were compared using one-way ANOVAs for continuous variables and Chi-square for categorical variables. Outcomes from the laboratory behavioral measures (AUC value for the EDT, CPT-II subscale scores and SSRT values from the Go/Stop Task), and also the self-report measures of impulsivity, were compared using separate between subject two-way ANOVAs. Smoking status and gender were the grouping variables. Significant effects were further explored using LSD post hoc analyses. Pearson correlations were performed to examine associations between study variables.
As secondary analyses, the between subject two-way ANOVASs were re-run with any identified demographic group differences controlled as covariates. These analyses were intoned to explore the significance of findings related to the laboratory behavioral measures above and beyond what might be accounted for by group differences in demographic characteristics.
RESULTS
Participants
Participant demographic data are presented in Table 1. No differences were found between groups on age or ethnicity. In terms of participant median income, male nonsmokers had significantly higher median annual income than male smokers and females (smokers and nonsmokers). There were no significant differences between any of the other groups. With respect to IQ, male nonsmokers had significantly higher IQ scores than male smokers and females (smokers and nonsmokers). Female smokers had significantly lower IQ scores than female nonsmokers. No other group differences were found for IQ.
Table 1.
Demographics, Smoking Indicators, and Self-Report Measures (N = 100)
| Smokers | Nonsmokers | |||
|---|---|---|---|---|
| Male (n = 17) | Female (n = 33) | Male (n = 17) | Female (n = 33) | |
| Demographics | ||||
| Age [years; M(SD)] | 15.59 (1.18) | 15.69 (1.01) | 15.06 (1.03) | 15.15 (1.15) |
| Ethnicity (n; white:black:other) | 7:9:1 | 15:17:1 | 13:3:1 | 10:19:4 |
| Median Annual Household Income [$; M (SD)]a | 55629 (25033) a, b | 48257 (24278) b | 75795 (28327)a | 50230 (27310) b |
| KBIT [IQ; M (SD)] | 91.06 (16.91) a | 87.15 (10.81) a | 106.82 (15.29) b | 97.15 (12.96) b |
| Smoking Indicators | ||||
| Carbon Monoxide [ppm; M (SD)] | 10.53 (6.19) a | 11.30 (8.16) a | 2.24 (1.52) b | 1.73 (1.21) b |
| Cotinine [ng/ml; M (SD)] | 1131.69 (682.78) a | 1348.21 (897.82) a | 3.53 (14.55) b | 0.58 (3.13) b |
| Self-report Measures | ||||
| BIS-11-A | 73.68 (14.83) a | 68.03 (10.19) b | 63.18 (9.76)b | 63.73 (9.92) b |
| CAAS: L Inattention | 53.75 (11.01) | 51.24 (10.91) | 50.76 (9.69) | 46.97 (10.20) |
| CAAS: L Hyperactivity | 51.00 (11.94) | 49.48 (9.09) | 46.71 (7.47) | 47.00 (9.58) |
| CAAS: L ADHD | 52.12 (11.44) | 51.21 (10.33) | 47.88 (8.92) | 47.27 (8.49) |
Note. Means in the same row that do not share the same subscript differ at p < .05.
The median annual household income was calculated based on census track data for postal zip code region of the participant’s residence.
In comparing smokers and nonsmokers, smokers had significantly higher CO and cotinine levels compared to the nonsmokers, thus providing verification of smoking status classifications. Also, some of the self-report measures differentiated smokers and nonsmokers. The smokers were more impulsive on the BIS-11-A, and they also had higher overall scores on the ADHD subscale of the CAAS:L assessment. There were no significant interactions between smoking status and gender for these measures.
Dependent Measures
EDT
There was no significant interaction between gender and smoking status for the EDT; nor was there any main effect for gender for this measure. However, the smokers discounted significantly more (i.e., performed more impulsively) than did the nonsmokers (Figure 1; F = 3.81, p < .05). However, after controlling for group differences in IQ and median income level, the smoking status effect found for the EDT was no longer statistically significant.
Figure 1.
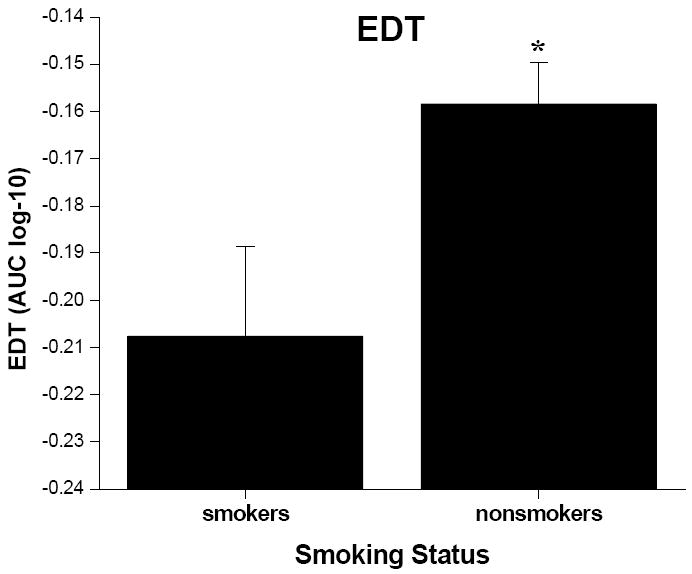
Mean (+ SEM, denoted by the error bars) AUC values from the EDT for smokers and nonsmokers.* indicates significant difference from smokers at p < .05 level.
CPT-II
A significant smoking status x gender interaction effect was found for number of omissions (Figure 2; F = 6.96, p < .05). Male smokers had significantly more omissions than male nonsmokers (p < .001) and females [both smokers and nonsmokers (p’s < .001)]. There were no significant differences among any of the other groups for number of omissions. Also, a significant main effect of smoking status was found for omissions (F = 10.10, p <.01). Specifically, smokers had a greater number of omissions than nonsmokers, which would be accounted for by the high number of omissions committed by male smokers. There also was a significant effect of gender on number of omissions (F =12.52, p <.001). Males made more errors of omission than females, again accounted for by male smokers.
Figure 2.
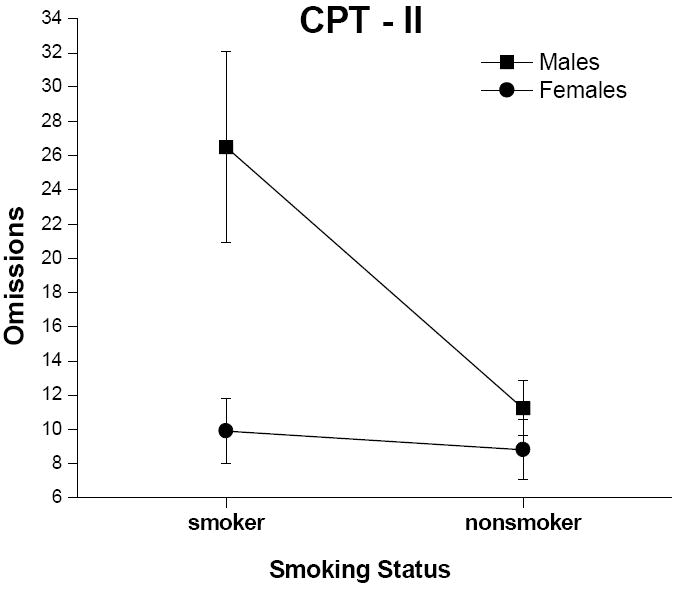
Mean (+ SEM, denoted by the error bars) number of omissions from the CPT-II for male and female smokers and nonsmokers.
There was no interaction or main effect of smoking status found for number of commissions. However, there was a significant group effect for gender (Figure 3; F = 15.81, p < .001). Males made more errors of commission than females.
Figure 3.
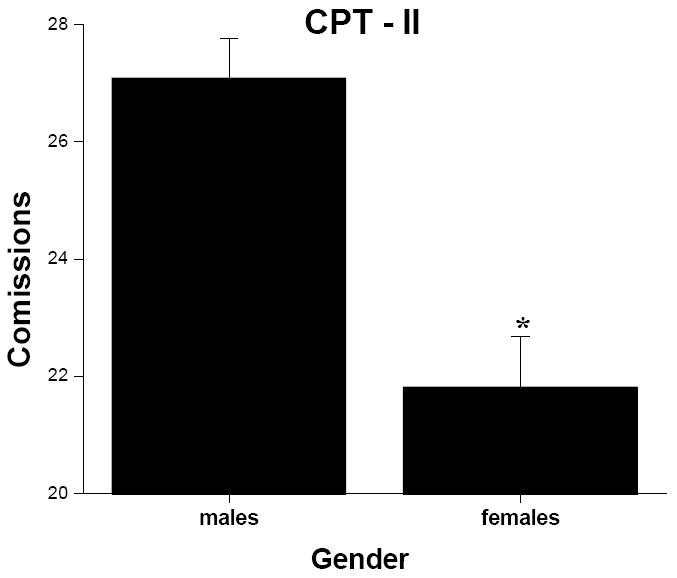
Mean (+ SEM, denoted by the error bars) number of comissions from the CPT-II for males and females. * indicates significant difference from males at p < .05 level.
As with omissions, there was a significant smoking status x gender interaction for hit reaction time (Figure 4; F = 7.18, p < .01). Male nonsmokers had significantly faster hit reaction times than male smokers (p < .05) and females [both smokers (p < .05) and non-smokers (p < .001)]. There were no significant differences among any of the other groups on hit reaction time. Also, there was no main effect of smoking status for hit reaction time; however, there was a significant group effect for gender (F = 4.55, p < 0.05), with males having faster hit reaction times than females. All findings for the CPT-II remained significant after controlling for group differences in IQ and median income level.
Figure 4.
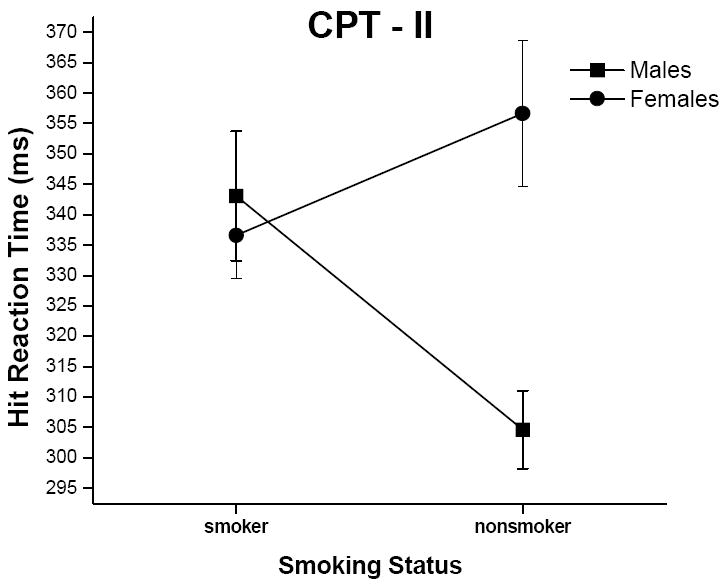
Mean (+ SEM, denoted by the error bars) hit reaction time values (in ms) from the CPT-II for male and female smokers and nonsmokers.
Go/Stop Task
A significant smoking status x gender interaction effect also was found for SSRT values (Figure 5; F = 11.34, p < .001). Male nonsmokers had significantly slower SSRT values (i.e., more impulsive) than male smokers (p < .05), female smokers (p < .05), and female nonsmokers (p < .05). Also, female smokers had significantly slower stop signal reaction times than female nonsmokers (p < .05) and male smokers (p < .05). There were no main effects for smoking status or gender for the Go/Stop Task. Again, all findings for the Go/Stop Task remained statistically significant after controlling for group differences in IQ and median income level.
Figure 5.
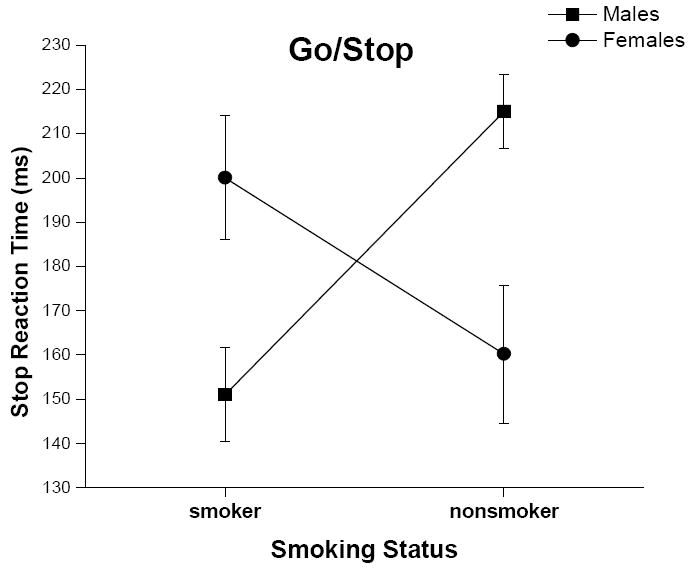
Mean (+ SEM, denoted by the error bars) stop reaction time from the Go/Stop Task for male and female smokers and nonsmokers.
As a secondary analysis to determine if these gender effects might be attributed to gender differences in the number of cigarettes smoked or average time since last cigarette, we conducted one-way ANOVAs for smoking rate and time since last cigarette with gender as the grouping variable. Males and females did not differ significantly in average number of cigarettes smoked (F = 0.001, p = 0.98) or average time since last cigarette (F = 1.38, p = 0.25).
Correlation Analyses
Breath CO was not correlated with any of the measures of impulsivity; however, cotinine level was positively correlated with number of omissions on the CPT-II (r = 0.24, p < .05) and SSRT values on the Go/Stop Task (r = 0.21, p < .05). As a set of secondary analyses, these correlations were calculated separately for males and females. Again, CO was not correlated with any of the measures of impulsivity. However, level of cotinine was positively correlated with number of omissions (r = 0.49, p < .01) and SSRT values (r = 0.50, p < .01) in females. These associations were not observed in males.
Table 2 shows the Pearson correlation matrix for the measures of impulsivity. The EDT was not correlated with any other measures. All subscales of the CPT-II were positively correlated. Also, number of omissions was positively correlated with the inattention and hyperactivity subscales of the CAAS: L; however, neither number of commissions nor hit reaction time was correlated with any of the self-report measures. The Go/Stop Task was not correlated with any other measures. All self-report measures were correlated with each other.
Table 2.
Correlation Matrix for All Measures of Impulsivity
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. log EDT | 1.0 | |||||||
| 2. CPT Omissions | -0.058 | 1.0 | ||||||
| 3. CPT Commissions | 0.163 | 0.314** | 1.0 | |||||
| 4. CPT Hit Reaction Time | -0.176 | 0.239* | -0.386** | 1.0 | ||||
| 5. Go/Stop SSRT | -0.128 | 0.017 | 0.102 | -0.131 | 1.0 | |||
| 6. BIS-11-A | 0.071 | 0.075 | 0.132 | -0.053 | -0.039 | 1.0 | ||
| 7. CAAS:L – ADHD | 0.072 | 0.138 | 0.019 | 0.145 | -0.049 | 0.544** | 1.0 | |
| 8. CAAS:L – inattention | 0.054 | 0.275** | 0.145 | 0.120 | -0.072 | 0.637** | 0.703** | 1.0 |
| 9. CAAS:L - hyperactivity | -0.116 | 0.352** | 0.095 | 0.113 | 0.015 | 0.515** | 0.673** | 0.620** |
Note:
p < .05,
p < .01
DISCUSSION
The current study was conducted to compare behavioral assessments of impulsive decision-making, inattention and disinhibition, as well as self-report measures of impulsivity, between male and female adolescent smokers and nonsmokers. It was hypothesized that adolescent smokers would perform more impulsively on the behavioral and self-report measures. The results indicated that on some of the different laboratory behavioral assessments the relationships between smoking status and impulsivity were dependent on the gender of the participant. However, with the self-report measures there were smoking status main effects as hypothesized with no interaction effects involving gender.
Across the different behavioral dimensions of impulsivity assessed for this study, varying effects were identified. For delay discounting, smokers discounted more by delay (i.e. performed more impulsively) than nonsmokers, and there were no significant gender effects or gender by smoking status interaction effects. This finding is consistent with what has been previously reported for the EDT in adult smokers and nonsmokers (Reynolds, 2006b). For impulsive inattention, male smokers appeared to be most inattentive. That is, male smokers had more errors of omission (more impulsive inattention) than all other groups (male nonsmokers and female smokers and nonsmokers). A similar effect was found for hit reaction time on the CPT-II: Male smokers had slower hit reaction time values than all other groups except female nonsmokers. By contrast, for the behavioral measure of disinhibition (Go/Stop Task), male smokers appeared to be the least impulsive group. That is, male smokers had shorter SSRT values than all other groups (male nonsmokers and female smokers and nonsmokers).
For the self-report measures of impulsivity, results were more directly aligned with smoking status. Smokers had higher scores than nonsmokers on both the BIS-11-A and the ADHD subscale of the CASS:L. While these results are different from those of the behavioral measures for inattention and disinhibition, this difference should perhaps not be surprising. For the current data set, the self-report measures of impulsivity correlated highly with each other but did not correlate as highly with any of the behavioral measures. Similar previous findings have been reported from studies comparing self-report and behavioral measures of impulsivity. For example, studies specifically exploring associations between the BIS-11-A and the CPT-II and Go/Stop Task have found little association between these measures (e.g., Reynolds et al., 2007; Reynolds, Penfold, & Patak, 2008). This lack of association between self-report and behavioral assessments is consistent with the view that these methods measure different aspects of impulsive behavior, or different levels of breadth/specificity. The divergent findings based on smoking status between self-report and behavioral methods also are consistent with this conclusion.
The interaction effects between gender and smoking status observed with some of the behavioral measures are noteworthy. The relationship between smoking status and impulsive inattention has been relatively unexplored. Therefore the finding that male smokers were more impulsive on this dimension of behavior is a new finding and may indicate that inattention is more a risk factor for smoking in males than females. However, the findings with behavioral disinhibition are consistent with previously reported results (Reynolds et al., 2007). Specifically, male smokers had significantly shorter SSRT values (less impulsive) on the Go/Stop task than male nonsmokers. It was suggested previously that the effects of nicotine may have improved inhibition in male smokers. This suggestion may still be tenable; however, in the current study a significant correlation between cotinine content (a metabolite of nicotine) and SSRT values was found for females but not for males. Higher cotinine levels (i.e., associated with heavier smoking) were associated with poorer performance in females. This finding would suggest that heavier smoking among females may be linked with reduced inhibition, an effect not observed in male smokers. Alternatively, greater disinhibition in females may lead to heavier smoking, again an association that would not be observed in males.
It is notable that there was no interaction between smoking status and gender on the EDT, suggesting that among smokers or nonsmokers males and females had similar patterns of discounting. One difference between the EDT as measure of decision-making and the other behavioral measures included in this study (CPT-II and Go/Stop Task) is that making choices on the EDT was not time-contingent. That is, participants could take as long as they liked to make a choice between the two choice options. By contrast, response times, or ability to withhold responses, were timed for the CPT-II and Go/Stop Task. This timed performance aspect of these measures may have increased the likelihood of gender differences or nicotine effects for these measures over the EDT. Future research might explore the relationship between gender and performance on time-contingent procedures.
Research is limited on the interaction between smoking and gender using these behavioral measures of impulsivity; still, some research has examined gender and nicotine interactions. For example, in humans, females administered acute nicotine have higher brain metabolism during a Continuous Performance Task (similar to the CPT-II of the current study) than males in a variety of brain regions (e.g. cortical and subcortical prefrontal system, dorsal prefrontal cortex, posterior medial thalamus, nucleus accumbens, etc.; Fallon et al., 2005). Using animal models, female rats have different rates of nicotine metabolism and receptor changes than male rats after nicotine exposure (Koylu et al., 1997; Pogun, 2001). Also, independent of nicotine, there are differences between males and females in brain activation during Stop paradigm procedures (Similar to the Go/Stop Task of the current study). Specifically, males and females differed in the region of brain activation while completing a Stop Task, despite having similar patterns of behavioral performance. Furthermore, males used a greater number of brain regions than females during this task (Li et al., 2006). Taken together, these results lend support to the idea that there are likely different mechanisms of action for nicotine, and different regions of brain activation associated with impulsive inattention and disinhibition, between males and females that may contribute to the gender specific findings of the current and previous studies (Reynolds et al., 2007). Future prospective research (initiated prior to any substantial use of nicotine) is needed to determine if, in fact, the initiation and progression of cigarette smoking during adolescence is associated with (a) inattention in males and (b) deterioration of behavioral inhibition in females. Conversely, such research may identify improved inhibition in males as well. However, this study did not find associations between biomarkers of smoking and impulsive disinhibition in males.
For the current study, the smoking status effect was no longer significant for the EDT when participant IQ and median income were controlled as covariates. This finding indicates that these variables may contribute to this smoking status effect. While this is an important consideration when interpreting these results, this finding does not eliminate delay discounting as a factor related to adolescent cigarette smoking. Of these variables (i.e., discounting, IQ, and income), it is notable that delay discounting is the only variable that can be considered a behavior. As such, findings related to delay discounting may provide comparatively more information for the tailoring of cigarette smoking prevention or treatment strategies, even if rate of delay discounting is partially driven by a person’s IQ and/or income.
This study is not without limitations. While all measures indicating smoking status (i.e., CO and cotinine) showed that males and females smoked similarly, there were no measures of level of withdrawal or level of addiction. It is possible, for example, that males and females differed systematically in degree of withdrawal, although there were also no gender differences in time since last cigarette. Future studies should examine the effect of withdrawal, or level of addiction, on the interaction between gender and smoking status on these behavioral measures. Another important limitation of this study was the use of a cross-sectional design, which limits ability to posit cause or affect relationships. Future prospective research may help to determine causal directions between amount of smoking and task performance.
In conclusion, we found differing and unexpected results between laboratory behavioral measures of impulsivity and self-report measures. These findings highlight the importance of including both types of assessments in research exploring relationships between impulsive behavior and cigarette smoking, or dug use more generally. In addition to distinctions between behavioral and self-report assessments, previous research indicates that among the behavioral assessments the construct of impulsivity is made up of several distinct dimensions (Lane et al., 2003; Reynolds, Penfold, & Patak, 2008; Sonuga-Barke, 2002). From the current study, the different patterns of findings across the measures of impulsive decision-making, inattention and disinhibition underscore the importance of considering associations between these different dimensions of impulsive behavior and cigarette smoking. This more inclusive approach may stand to more fully characterize the complex relationships between impulsive behaviors and cigarette smoking during adolescence.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant R21 DA020423.
We would like to thank Michele Patak, Palak Shroff, Sara Imhoff, and Shane Melanko for their assistance on this project.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha.
References
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP. Applying a Behavioral Economic Framework to Understanding Adolescent Smoking. Psychology of Addictive Behaviors. 2004;18:64–73. doi: 10.1037/0893-164X.18.1.64. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson M, Bickel W. Delay discounting in current and never-before cigarette smokers; Similarities and differences across commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch L. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel W, Odum A, Madden G. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Marvasti FF, Ducharme JL, Walter JM, Israel ME, Krain A, Pavlovsky C, Hommer DW. Executive function oculomotor tasks in girls with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:644–650. doi: 10.1097/00004583-200005000-00019. [DOI] [PubMed] [Google Scholar]
- Conners CK, Wells KC, Parker JDA, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for the assessment of adolescent psychopathology: Factor structure, reliability, validity and diagnostic sensitivity. Journal of Abnormal Child Psychology. 1997;25:487–497. doi: 10.1023/a:1022637815797. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales-Revised (CRS-R): Technical manual. Toronto: MHS; 2001. [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test II. Toronto: MHS; 2004. [Google Scholar]
- Crean JP, de Wit H, Richards JB. Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Experimental and Clinical Psychopharmacology. 2000;8:155–162. doi: 10.1037//1064-1297.8.2.155. [DOI] [PubMed] [Google Scholar]
- Davies DR, Jones DM, Taylor A. Selective and sustained attention tasks: Individual and group differences. In: Parasuraman R, Davies DR, editors. Varieties of Attention. Academic Press; New York: 1984. pp. 395–447. [Google Scholar]
- Dougherty DM, Bjork JM, Moeller FG, Harper RA, Marsh DM, Mathias CW, Swann AC. Familial transmission of Continuous Performance Test behavior: attentional and impulsive response characteristics. The Journal of General Psychology. 2003;130:5–21. doi: 10.1080/00221300309601271. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behavior Research Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. Journal of Abnormal Child Psychology. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Fallon J, Keator D, Mbogori J, Taylor D, Potkin S. Gender: a major determinant of brain response to nicotine. International Journal of Neuropsychopharmacology. 2005;8:17–26. doi: 10.1017/S1461145704004730. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Forgays DG. Personality characteristics and self-abusive behavior. National Institute on Drug Abuse: Research monograph series. Mono. 1986;74:45–58. [PubMed] [Google Scholar]
- Fossati A, Barratt ES, Acquarini E, Di Ceglie A. Psychometric properties of an adolescent version of the Barratt Impulsiveness Scale – 11 (BIS-11-A) in a sample of Italian high school students. Perceptual and Motor Skills. 2002;95:621–635. doi: 10.2466/pms.2002.95.2.621. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, Feghoff TA. Inhibition of ongoing responses in patients with Parkinson’s disease. Journal of Neurology, Neurosurgury, and Psychiatry. 2004;75:539–44. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RR, Gold JM. Delay discounting in schizophrenia. Cognitive Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman G, Gibb S. Delay discounting in college cigarette chippers. Behavioural Pharmacology. 2006;17:669–679. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition: Manual. Circle Pines: AGS; 2004. [Google Scholar]
- Koylu E, Demirgoren S, London ED, Pöğün S. Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sciences. 1997;61:185–190. doi: 10.1016/s0024-3205(97)00665-6. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig A, Smith A, Liss T, McFetridge A, Cavallo D, Carroll K, Potenza M. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality; Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S, Cherek DR, Rhodes HM, Pietras CJ, Techeremissine OV. Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependence. Addictive Disorders and Their Treatment. 2003;2:33–40. [Google Scholar]
- Lejeuz CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Li C, Huang C, Constable R, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: A meta-analytic review. Journal of Child Psychology and Psychiatry. 1996;37:971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Madden G, Petry N, Badger G, Bickel W. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:56–62. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- McClernon F, Kollins S, Lutz A, Fitzgerald D, Murray D, Redman C, Rose J. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology. 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- Mitchell S. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM. Impulsivity and substance abuse: what is the connection? Addictive Disorders & Their Treatment. 2002;1:3–10. [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Bickel WK. Discounting of delayed health gains and losses by current, never- and ex-smokers of cigarettes. Nicotine and Tobacco Research. 2002;4:295–303. doi: 10.1080/14622200210141257. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry N, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug and Alcohol Dependence. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Pogun S. Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning. International Journal of Psychophysiology. 2001;42:195–208. doi: 10.1016/s0167-8760(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiology and Behavior. 2006;87:614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention deficit/hyperactivity disorder. Psychopharmacology. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behavioural Pharmacology. 2006a;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B. Do high rates of cigarette consumption increase delay discounting? A cross-sectional comparison of adolescent smokers and young-adult smokers and nonsmokers. Behavioral Processes. 2004;67:545–549. doi: 10.1016/j.beproc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Reynolds B. The Experiential Discounting Task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behavioral Pharmacology. 2006b;17:133–142. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Karraker K, Horn K, Richards JB. Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behavioural Processes. 2003;64:333–344. doi: 10.1016/s0376-6357(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Patak M, Shroff P, Penfold R, Melanko S, Duhig A. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2007;15:264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: laboratory behavioral assessments. Experimental and Clinical Psychopharmacology. 2008;16:124–131. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behavioural Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotine receptor mechanisms. Archives of General Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke EJ, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD—A dual pathway model of behavior and cognition. Behavior and Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Audrain-McGovern J. Personality differences associated with smoking experimentation among adolescents with and without comorbid symptoms of ADHD. Substance Use and Misuse. 2003;38:1953–1970. doi: 10.1081/ja-120025121. [DOI] [PubMed] [Google Scholar]
- Vuchinich R, Simpson C. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Ball S, Black J. Influences of sensations seeking, gender, risk appraisal, and situational motivation on smoking. Addictive Behaviors. 1990;15:209–220. doi: 10.1016/0306-4603(90)90064-5. [DOI] [PubMed] [Google Scholar]


