Abstract
Cryptorchidism and scrotal heating result in abnormal spermatogenesis, but the mechanism(s) prescribing this temperature sensitivity are unknown. It was previously reported that the AKR/N or MRL/MpJ-+/+ mouse testis is more heat-resistant than the testis from the C57BL/6 strain. We have attempted to probe into the mechanism(s) involved in heat sensitivity by examining global gene expression profiles of normal and heat-treated testes from C57BL/6, AKR/N, and MRL/MpJ-+/+ mice by microarray analysis. In the normal C57BL/6 testis, 415 and 416 transcripts were differentially expressed (at least 2-fold higher or lower) when compared with the normal AKR/N and MRL/MpJ-+/+ testis, respectively. The AKR/N and MRL/MpJ-+/+ strains revealed 268 differentially expressed transcripts between them. There were 231 transcripts differentially expressed between C57BL/6 and 2 purported heat-resistant strains, AKR/N and MRL/MpJ-+/+. Next, the testes of C57BL/6 and AKR/N mice were exposed to 43°C for 15 minutes and harvested at different time points for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) studies and microarrays. An increase of TUNEL-positive germ cell numbers was significant 8 hours after heat exposure in the C57BL/6 mouse. However, this increase was not observed in the AKR/N mouse until 10 hours after heat exposure. All tubules showed germ cell loss and disruption in C57BL/6 testis 24 hours after heat shock. In contrast, although a number of seminiferous tubules showed an abnormal morphology 24 hours post–heat shock in the AKR/N mouse, many tubules still retained a normal structure. Numerous transcripts exhibited differential regulation between the 2 strains within 24 hours after heat exposure. The differentially expressed transcripts in the testes 8 hours after heat exposure were targeted to identify the genes involved in the initial response rather than those attributable to germ cell loss. Twenty transcripts were significantly down-regulated and 19 genes were up-regulated by hyperthermia in C57BL/6 and did not show a parallel change in the AKR/N testis. Conversely, heat shock resulted in 30 up-regulated transcripts and 31 down-regulated transcripts in AKR/N that were not similarly regulated in C57BL/6. A number of genes shared similar differential expression patterns and differential regulation by hyperthermia in both strains of mice. Taken together, the results of the present study indicate that the diverse genetic backgrounds in the 3 strains lead to major differences in normal testis gene expression profiles, whereas the differences in heat shock responses involve a significantly smaller number of genes. The data generated may provide insights regarding gene networks and pathways involved in heat stress and their relationship to spermatogenesis.
Keywords: Heat stress, testis, mouse strain, AKR/N, C57BL/6, microarray analysis
Most mammalian testes must descend from the abdominal cavity for normal development to occur (Moore and Chase, 1923). Scrotal temperature in mammals is 2°C to 8°C lower than the temperature of the rest of the body (Banks et al, 2005). Exposure to higher temperatures such as those found in the rest of the body results in documented degenerative changes in several species, such as mouse (Hand et al, 1979; Reid et al, 1981; Gasinska and Hill, 1990), rat (Fridd et al, 1975; Khan and Brown, 2002), pig (Stone, 1981), bull (Ross and Entwistle, 1979; Kastelic et al, 1997), ram (Waites and Setchell, 1964; Setchell et al, 1971), and human (Robinson et al, 1968; Nistal et al, 1980; Zorgniotti, 1980, 1988). The elevated testicular temperature impairs spermatogenesis and causes not only a temporary reduction in relative testis weight accompanied by a period of partial or complete infertility, but also a reduction in progressive sperm motility and viability and a lower in vitro fertilization rate of oocytes by sperm from heat-shocked males (Setchell et al, 1988, 1998; Jannes et al, 1998). Precoital testicular heating leads to a transient retardation in embryo growth and an increase in the rate of embryonic degeneration (Setchell et al, 1988, 1998; Mieusset et al, 1992; Jannes et al, 1998). Scrotal heat stress also impairs sperm DNA integrity and causes a sex ratio distortion in the offspring (Perez-Crespo et al, 2007).
One of the adverse effects of heat on the mammalian testis is germ cell loss. The most vulnerable cell types are primary spermatocytes (meiotic spermatogenic cells) and round spermatids (early haploid cells) (Blackshaw et al, 1973; Parvinen, 1973; Blackshaw and Massey, 1978; Steinberger, 1989; Yin et al, 1997). Spermatogonia have a slightly lower susceptibility to elevated temperature (Moore and Chase, 1923; Chowdhury and Steinberger, 1964; Davis and Firlit, 1966; Perez-Crespo et al, 2007). Cataldo et al (1997) found that the initiation of translation in pachytene spermatocytes and Sertoli cells is inhibited by exposure to abdominal temperature and that elongated spermatids are much more resistant to thermal stress.
Although the physiological and cellular responses of testis to heat stress have been well documented, the molecular mechanisms through which these responses are directed remain largely unknown. Studies indicate that the testicular germ cell loss observed in cryptorchidism occurs via apoptosis (Yin et al, 2002). Rockett et al (2001) found that several groups of genes, such as heat shock genes (HSPs), non-HSP stress response genes, and cell-signaling genes, were induced by heat stress. It has been reported that heat stress impairs DNA, RNA, and protein synthesis and may cause protein denaturation, abnormal chromatin packing, and reduction of DNA integrity (Steinberger, 1991; Sailer et al, 1997; Banks et al, 2005; Perez-Crespo et al, 2007).
Recently, Kon and Endoh (2001) and Kazusa et al (2004) reported heat-shock resistance in the experimental cryptorchid testes of some strains of mice. An increase of apoptotic cells after heat stress was detected in the mouse strains of A/J, BALB/c, CBA/N, C3H/He, C57BL/6, ddY and ICR, whereas mouse strains of AKR/N, MRL/MpJ-+/+, and MRL/MpJ-lpr/lpr showed relative resistance to experimental cryptorchidism (Kon and Endoh, 2001; Kazusa et al, 2004). A mutation of the exonuclease 1 gene was found in MRL/MpJ-+/+, suggesting a possible relation to the heat resistance of spermatocytes in the MRL/MpJ-+/+ mouse (Namiki et al, 2003, 2005).
The heat resistance observed in the testes from a specific strain of mice provides an excellent in vivo model system for studying the underlying mechanisms of heat response. In this study, the gene expression in the testes of 3 different strains of mice was monitored by microarray analysis, and then 2 of these strains, 1 normal (C57BL/6) and 1 reported to be heat-resistant (AKR/N), were subjected to scrotal heat exposure. The effect of hyperthermia was assessed by comparing global gene expression and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses in hope of identifying the key molecular components involving in the heat response.
Materials and Methods
Animals
The C57BL/6, AKR/N, and MRL/MpJ-+/+ strains of mice aged 8 weeks were used in this study. AKR/N and MRL/MpJ-+/+ mice were purchased from NCI Frederick (Frederick, Maryland). All strains of mice were housed in a standard animal facility under controlled temperature (21°C to 22°C) and photoperiod (12L:12D) with free access to food and water. All animal procedures were conducted according to the guidelines stated in the United States Public Health Service’s Guide for the Care and Use of Laboratory Animals.
Heat Treatment
To examine the effects of heat stress on the testis of AKR/N and C57BL/6 strains of mice, the animals were subjected to a short exposure of elevated temperature. Scrotal heat stress was performed as described previously (Lue et al, 1999) with minor modifications. Briefly, mice were anesthetized with an IP injection of ketamine-xylazine mixture at a dose of 0.1 mg/kg (ketamine) and 0.05 mg/kg (xylazine). The testes were secured into the scrotum and lowered into a 43°C water bath so that the scrotum and one-fourth of the body were completely immersed for 15 minutes. Control mice were treated the same way except that the testes were not immersed. Fourteen mice of each strain were assigned to 7 groups of 2 mice each. One group of mice was assigned to each of the following time points: 4, 6, 8, 10, 12, and 24 hours posttreatment and control (8 hours after manipulation). One testis from each animal was placed in Trizol reagent (Invitrogen, Carlsbad, California) for RNA extraction, and the other testis was fixed by immersion in Bouin solution (Bailey et al, 2002) for histological analysis. To eliminate the variation caused by minor differences of temperature, the mice for each time point from both strains were put in the same water bath at the same time and taken out simultaneously. The experiment was performed twice.
Immunohistochemistry and TUNEL Analysis
One testis from each mouse was immersed in Bouin solution overnight, then dehydrated in graded ethanol and embedded in paraffin. The paraffin-embedded testis was cut into 5-μm sections and mounted on Probe-on-Plus microscope slides (Fisher Scientific Co, Pittsburgh, Pennsylvania). To assess apoptosis in germ cells, in situ end labeling of DNA strand breaks was performed on testis sections by TUNEL techniques using a BD ApoAlert DNA Fragmentation Assay kit (BD Biosciences, Palo Alto, California) according to the manufacturer’s instructions. Briefly, sections were deparaffinized in xylenes, rehydrated in a graded series of ethanol solutions, and rinsed in PBS; this was followed by proteinase K treatment. Terminal transferase was used for the tailing reaction to label DNA fragments with FITC. Quantitation of apoptosis was carried out by counting TUNEL-positive germ cells visualized with a Nikon Microphot-FX (Meridian Instrument Company Inc, Kent, Washington) microscope. The rate of germ cell apoptosis was expressed as the number of apoptotic germ cells per tubule and the number of tubules containing increased apoptotic germ cells. Data are shown as the mean ± SEM from 3 different mice for each time point of each strain. Photomicrographs were taken with an Olympus OLY-200 digital camera (Olympus America Inc, Melville, New York) using Olympus MagnaFire Camera Imaging and Control version 1.0.
Preparation of Total Testis RNA and Microarray Processing
Total RNA was extracted from whole testes homogenized in Trizol in accordance with the manufacturer’s protocol (Invitrogen). The quality of the RNA was assessed by gel electrophoresis and the A260:A280 ratio. A minimum A260:A280 ratio of 1.8 was required for microarray hybridization. All microarray hybridization, scanning, and analysis was performed in the LBB1 service unit at Washington State University as described previously (Shima et al, 2004). Briefly, 5 μg total RNA of posttreatment or control from C57BL/6 or AKR/N or MRL/MpJ-+/+ testis was used to generate biotinylated cRNA target from an oligo-deoxythymidine–primed reverse transcription reaction using the MEGAScript kit (Ambion, Austin, Texas). The cRNA target was fragmented and hybridized to the Affymetrix Mouse genome 430 2.0 arrays (Affymetrix, Santa Clara, California) in duplicate, and stained in accordance with the manufacturer’s protocol. The arrays were washed utilizing the Affymetrix GeneChip Fluidics Station 400 and scanned using a GeneArray Scanner 2500A (Agilent, Palo Alto, California).
Absolute and Comparison Analysis
After scanning, microarray output was viewed to examine the presence of excessive background hybridization and physical anomalies. GeneChip Operating Software (GCOS; Affymetrix) was used to do absolute analysis. The default GCOS statistical values were used for all analyses, and all probe sets were scaled to a mean target signal intensity of 125. Relative expression levels of each transcript (signal) were determined based on signal and detection (present, absent, and marginal). After the initial analysis, data were exported and loaded into GeneSpring 6.1 (Silicon Genetics, Redwood City, California) for further analysis. The data comprising C57BL/6, AKR/N and MRL/MpJ-+/+ with or without heat treatment were normalized in GeneSpring using the default/recommended normalization methods (Shima et al, 2004; Small et al, 2005; Zhou et al, 2005). To describe the differences in transcript levels observed between the normal, untreated testis of the 3 strains of mice used in this study, the term differentially expressed gene is used. A differentially expressed gene was required to have a raw signal value of no less than 50 in at least 1 strain, a 2-fold or higher difference in signal between 2 strains, and statistical significance based on the 1-way analysis of variance (ANOVA) test. For example, a transcript described as being differentially expressed between C57BL/6 and AKR/N required a raw signal value of no less than 50 in either C57BL/6 or AKR/N, a 2-fold or higher difference in signal between these 2 strains, and a statistically significant difference based on the 1-way ANOVA test. The term heat-regulated gene describes those transcripts that are different between the normal testis and the heat-treated testis of the same strain. Once again, these transcripts must have a raw signal value of no less than 50 in either the control or the heat-treated samples, a 2-fold or higher difference in signal between the control and treated samples, and a statistically significant difference based on the 1-way ANOVA test. Regulated in both strains includes the transcripts up-regulated or down-regulated similarly in both strains. Differentially regulated denotes the transcripts differentially regulated between 2 unique strains. For example, differentially up-regulated in C57BL/6 by heat exposure indicates those transcripts that have a raw signal value of no less than 50 in the heat-treated samples and are up-regulated 2-fold or greater by heat in C57BL/6 but down-regulated or not regulated in AKR/N mice. In the tables, listed genes were those with the highest fold change in each comparison.
Functional Characterization and Cell Type Analysis
Ontological analysis was performed using Onto-Express (http://vortex.cs.wayne.edu) from the Intelligent Systems and Bioinformatics Laboratory at Wayne State University. A list of selected transcripts was classified based on biological function using the Onto-Express ontology classification. Briefly, the Affymetrix probe ID of the selected transcripts was saved as a .txt file and input into the onto-tool for the analysis. The expression levels of particular transcripts in different cell types were analyzed utilizing the database available on the Griswold laboratory web page (http://www. wsu.edu/~griswold/microarray/ under “Mouse germ cells reference samples M430 2.0”).
Results
The public can access the microarray data generated in the present study on the Griswold laboratory web page (http://www.wsu.edu/~griswold/microarray/).
Comparison Analysis of Gene Expression in Normal Testes of C57BL/6, AKR/N, and MRL/MpJ-+/+ Mice
To examine testis gene expression in the 3 murine strains, the raw data generated from the microarrays were imported into GeneSpring after initial analysis. The testes from different strains of mice showed divergent gene expression. About 416 transcripts were differentially expressed between normal C57BL/6 testis and normal MRL/MpJ-+/+ testis (228 higher in C57BL/6 testis and 188 higher in MRL/MpJ-+/+ testis). The biological functions of 185 out of these 416 transcripts are unknown, and 36 are involved in transport, 24 in DNA-dependent regulation of transcription, 15 in metabolic processes, 13 in protein amino acid phosphorylation, and 5 in apoptosis. The AKR/N and MRL/MpJ-+/+ testes revealed 268 differentially expressed transcripts between them (128 higher in AKR/N testis and 140 higher in MRL/MpJ-+/+ testis). The biological processes of 134 out of the 268 transcripts are unknown, and 21 are involved in transport, 6 in cell cycle, 6 in cell differentiation, and 3 in apoptosis. About 415 transcripts were differentially expressed between normal C57BL/6 and AKR/N testis, including 229 transcripts higher in C57BL/6 testis and 186 higher in AKR/N testis. The biological functions of 190 out of these 415 transcripts are unknown, and 25 are involved in DNA-dependent regulation of transcription, 4 in immune response, and 12 in lipid metabolism/biosynthetic process. Table 1 contains selected genes differentially expressed between different strains of mice.
Table 1.
Selected genes differentially expressed between different strains of mice: C57BL/6 (C57), AKR/N (AKR) and/or MRL/MpJ–+/+(MRL)a
| Gene | Gene Symbol | GenBank Accession No. | Fold Change |
|---|---|---|---|
| Differentially expressed between C57 and MRL | C57/MRL | ||
| RIKEN cDNA 3322402L07 gene | 3322402L07Rik | NM_023727 | 68.7 |
| Defensin beta 11 | Defb11 | NM_139221 | 46.2 |
| Complement factor H-related 3 | Cfhr3 | AK015277 | 20.3 |
| PFTAIRE protein kinase 1 | Pftk1 | NM_011074 | −26.8 |
| Cytochrome P450, family 2, subfamily f, polypeptide 2 | Cyp2f2 | NM_007817 | −23.5 |
| Serine peptidase inhibitor, clade A, member 3N | Serpina3n | NM_009252 | −9.3 |
| Differentially expressed between C57 and AKR | C57/AKR | ||
| RIKEN cDNA 1700021K10 gene | 1700021K10Rik | BB611374 | 229.5 |
| RIKEN cDNA 1700084F23 gene | 1700084F23Rik | AK006991 | 93.7 |
| Coiled-coil domain containing 123 | Ccdc123 | NM_028120 | 22.3 |
| Melanoma antigen | Mela | NM_008581 | −125.2 |
| RIKEN cDNA 9430014N10 gene | 9430014N10Rik | AK020418 | −22.6 |
| Sfi1 homolog, spindle assembly associated (yeast) | Sfi1 | NM_030207 | −11.8 |
| Differentially expressed between MRL and AKR | MRL/AKR | ||
| Spondin 1, (f-spondin) extracellular matrix protein | Spon1 | BQ175871 | 26.6 |
| Dual adaptor for phosphotyrosine and 3-phosphoinositides 1 | Dapp1 | NM_011932 | 14.3 |
| PFTAIRE protein kinase 1 | Pftk1 | NM_011074 | 8.1 |
| RIKEN cDNA 1700003F17 gene | 1700003F17Rik | AV281513 | −140.8 |
| Defensin beta 11 | Defb11 | NM_139221 | −34.2 |
| Cardiomyopathy associated 5 | Cmya5 | XM_912335 | −24.3 |
All genes must have a raw signal no less than 50 in at least 1 strain and 2-fold or more differences in expression between 2 strains compared.
Genes differentially expressed between C57BL/6 and 2 purported heat-resistant strains, AKR/N and MRL/MpJ-+/+, were also examined. Some 104 transcripts showed significantly higher levels in both AKR/N and MRL/MpJ-+/+ mice than in C57BL/6 mice, and 127 showed significantly lower levels in heat-resistant strains (Table 2 lists selected transcripts showing the greatest differences in expression levels). Within these 231 transcripts, 101 have unknown biological function; 21 are involved in transport, 15 in DNA-dependent regulation of transcription, 14 in oxidation and reduction, and 8 in metabolic processes; and 6 are related to apoptosis or antiapoptosis.
Table 2.
Selected genes differentially expressed between C57BL/6 (C57) and 2 purported heat-resistant strains, AKR/N (AKR) and/or MRL/MpJ–+/+ (MRL)a
| Affymetrix Fold Change |
||||
|---|---|---|---|---|
| Gene | Gene Symbol | GenBank Accession No. | AKR/C57 | MRL/C57 |
| Apolipoprotein C-II | Apoc2 | NM_009695 | 55.48 | 97.22 |
| Lactoperoxidase | Lpo | NM_080420 | 35.58 | 44.00 |
| Caspase 9 | Casp9 | NM_015733 | 21.77 | 21.81 |
| Cytochrome P450, family 2, subfamily c, polypeptide 55 | Cyp2c55 | NM_028089 | 17.68 | 23.04 |
| Vanin 1 | Vnn1 | NM_011704 | 15.77 | 15.05 |
| Histidine ammonia lyase | Hal | NM_010401 | 12.91 | 11.70 |
| Mitogen-activated protein kinase 14 | Mapk14 | NM_011951 | 12.24 | 11.74 |
| Transcribed locus | — | BM211445 | −312.41 | −77.71 |
| RIKEN cDNA 4930589P08 gene | 4930589P08Rik | AK017048 | −236.58 | −101.88 |
| RIKEN cDNA 1110020P15 gene | 1110020P15Rik | NM_197979 | −37.00 | −56.96 |
| Potassium channel tetramerization domain containing 14 | Kctd14 | NM_001010826 | −20.52 | −24.36 |
| Carboxypeptidase N, polypeptide 1 | Cpn1 | NM_030703 | −17.39 | −21.18 |
| EF hand domain containing 2 | Efhd2 | NM_025994 | −16.97 | −6.93 |
All genes must have a raw signal no less than 50 in at least 1 strain and 2-fold or more differences in expression between C57BL/6 and the other 2 strains.
Different Responses to Single Heat Treatment in Testes of AKR/N and C57BL/6 Mice
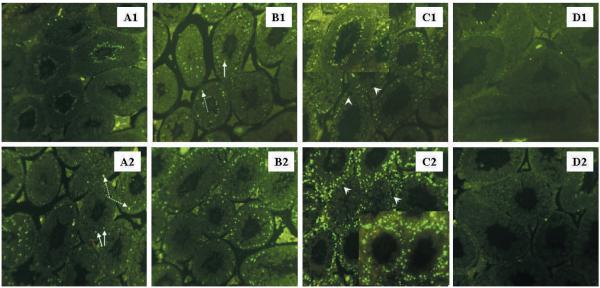
To investigate the response of C57BL/6 and AKR/N testes to heat exposure, germ cell apoptosis was monitored at different time points after heat stress by TUNEL analysis (Figure 1). No increase in apoptotic cells was observed in either AKR/N or C57BL/6 mice at 6 hours post–heat stress compared with the untreated control testis (data not shown). At 8 hours after heat exposure, an increase of TUNEL-positive cells was detected in C57BL/6 testis, and this increase continued through 10 and 24 hours. By 24 hours, all tubules in the C57BL/6 showed a dramatic increase in the number of apoptotic cells and were visibly disrupted. However, in the AKR/N strain, no increase in germ cell apoptosis was detectable until 10 hours after heat exposure. Even after 24 hours, there were tubules that did not contain apoptotic cells or spaces in seminiferous epithelium because of germ cell loss. Although the increase of apoptotic cells appeared at different time points between the 2 strains, the same cell types (primary spermatocytes and round spermatids) were the first cell types to enter into programmed cell death in both strains (Figure 1B1 and A2).
Figure 1.
Analysis of apoptosis using TUNEL assay of testis cross sections from AKR/N (upper panel) and C57BL/6 (lower panel). (A1) and (A2), 8 hours after heat exposure; (B1) and (B2), 10 hours after heat exposure; (C1) and (C2), 24 hours after heat exposure. (D1) and (D2) depict sections from non–heat-treated testes used as negative controls. TUNEL-positive cells appear bright green. The C57BL/6 testis exhibits a significant increase in TUNEL-positive cells at 8 hours (A2) compared with negative control (D2). Apoptotic cells continue to increase at 10 hours (B2). At 24 hours (C2), at least 5 positive cells per tubule are observed in nearly every tubule (see embedded picture). In contrast, an increase of apoptotic cells is not detected until 10 hours (B1) after heat exposure in the AKR/N testis. At 24 hours, although large portions of tubules contain increased apoptotic cells, there are still tubules with less than 2 apoptotic cells present without a sign of germ cell loss (C1, see embedded picture). Spermatocytes (indicated by dashed white arrow) and round spermatids (indicated by solid white arrow) were the first cell types that were TUNEL-positive in both strains. At 24 hours after heat shock, some tubules in both strains contain spaces in seminiferous epithelium because of germ cell loss (indicated by white arrow head) Autofluorescence was detected in heads of elongated spermatids. Original magnification ×100 for all pictures except embedded pictures in (C1) and (C2) (×200).
To quantify the differences between the strains using germ cell apoptosis after heat stress, tubules with TUNEL-positive cells were counted at different time points after heat exposure. As shown in Table 3, tubules were divided into 3 groups: 1) tubules with 0–2 TUNEL-positive cell(s) per tubule, considered intact tubules; 2) tubules with 3–5 TUNEL-positive cells, considered slightly damaged tubules; and 3) tubules with more than 5 TUNEL-positive cells per tubule, considered damaged tubules. Nearly all tubules contained 0–2 apoptotic cell(s) in control animals of both strains. Therefore, no differences between the 2 strains were noticed before heat treatment. However, at 8 hours post–heat shock, 35% of the tubules in C57BL/6 had an increase in TUNEL-positive cells, whereas in the AKR/N testis, only 1% of the tubules showed a similar effect. The data clearly show that the AKR/N strain is more heat-resistant than the C57BL/6 strain.
Table 3.
Damage to seminiferous tubules of C57BL/6 and AKR/N mice after heat treatmenta
| Strain | Time Post–Heat Treatment, h |
% Tubules With 0–2 Positive Cells/Tubule |
% Tubules With 3–5 Positive Cells/Tubule |
% Tubules With >5 Positive Cells/Tubule |
|---|---|---|---|---|
| C57BL/6 | 8 | 63.5 ± 5.1 | 2.2 ± 0.7 | 35.2 ± 2.8 |
| 10 | 5.0 ± 1.0 | 13.2 ± 2.5 | 82.3 ± 2.9 | |
| 12 | 0 ± 0 | 8.2 ± 1.0 | 91.4 ± 1.2 | |
| 24 | 0 ± 0 | 0 ± 0.02 | 99.9 ± 0.1 | |
| No heat treatment | 97.8 ± 1.6 | 2.2 ± 1.0 | 0 ± 0.5 | |
| AKR/N | 8 | 98.4 ± 1.8 | 1.4 ± 0.9 | 0.9 ± 0.1 |
| 10 | 72.2 ± 2.1 | 5.9 ± 0.3 | 23.6 ± 3.8 | |
| 12 | 35.1 ± 5.3 | 6.2 ± 2.0 | 61.1 ± 3.2 | |
| 24 | 31.2 ± 6.1 | 4.3 ± 1.1 | 62.5 ± 4.6 | |
| No heat treatment | 99.2 ± 0.08 | 0.2 ± 0.05 | 0.6 ± 0.1 |
All genes must have a raw signal no less than 50 in at least 1 strain and 2-fold or more differences in expression between C57BL/6 and the other 2 strains. Tubules with only 0–2 positive cell(s) were considered normal, whereas tubules with more than 5 positive cells were considered damaged. In C57BL/6, damaged tubules increased dramatically at 8 and 10 h after exposure to heat. After 24 h, all tubules showed signs of damage. In contrast, the damage was delayed in AKR/N and even after 24 h, about 30% of the tubules showed no sign of germ cell loss.
Overall Gene Regulation in Testes of AKR/N and C57BL/6 Mice After a Single Heat Exposure
Testicular samples were collected at different times from treated animals and controls from both strains, and RNA was isolated for microarray analysis on the 430 2.0 chips. Correlation coefficients between the replicates of controls or heat-treated samples calculated using Microsoft Excel (Microsoft, Redmond, Washington) were greater than 0.98 (range, 0.985–0.995). Therefore, no significant differences were observed between any replicates.
Approximately 45 100 transcripts are present on the 430 2.0 array, representing a large percentage of the total murine genome. Based on analysis in GCOS, 47% and 44% of the transcripts were designated as present or marginally present in the C57BL/6 control and AKR/N control samples, respectively.
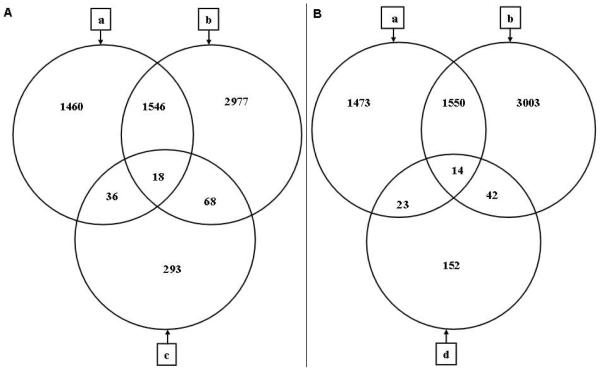
The testis samples from 8, 10, 12, and 24 hours after heat exposure and controls were examined by microarrays and analyzed by GeneSpring. As shown in Figure 2, a total of 4609 transcripts exhibited significant changes in expression levels as defined by our parameters between any time points and control in C57BL/6 testes, whereas 3060 transcripts showed differences in AKR/N testes. There were 1564 transcripts that displayed significant changes in expression in both strains, whereas 1496 transcripts showed change only in AKR/N testes and 3045 transcripts in C57BL/6 testes. As many as 68 transcripts that were differentially expressed between C57BL/6 and AKR/N controls also changed in expression levels only in C57BL/6 after heat exposure, whereas 36 showed significant change in expression in AKR/N after heat shock (Figure 2A). Some 23 transcripts that were differentially expressed between C57BL/6 control testis and 2 purported heat-resistant strains also changed in expression levels only in AKR/N after heat treatment, and 42 only in C57BL/6 after heat treatment (Figure 2B).
Figure 2.
Venn diagram showing distribution of transcripts before and after heat exposure. (A) shows control C57BL/6 and AKR/N testes; (B) shows control C57BL/6 testis and 2 heat-resistant stains: AKR/N and MRL/MpJ-+/+. Circle a: number of transcripts regulated within 24 hours by heat, including transcripts that exhibited significant changes in expression levels as defined by our parameters between any time points (8, 10, 12 and 24 hours) and control, in AKR/N mouse testis. Circle b: number of transcripts regulated within 24 hours by heat, including transcripts that exhibited significant changes between any time points (8, 10, 12, and 24 hours) and control in C57BL/6 mouse testis. Circle c: number of transcripts differentially expressed between control C57BL/6 and AKR/N testes. Circle d: number of transcripts differentially expressed between control C57BL/6 testis and 2 heat-resistant stains, AKR/N and MRL/MpJ-+/+.
Gene Regulation in Testes of AKR/N and C57BL/6 Mice 8 Hours After Heat Shock
As shown in Table 3, levels of germ cell apoptosis in the C57BL/6 and AKR/N strains started to show differences 8 hours after heat exposure. Many tubules contain apoptotic cells and spaces in seminiferous epithelium because of germ cell loss within 24 hours after heat shock in both C57BL/6 and AKR/N strains (Figure 1). To identify those genes demonstrating regulation as a direct result of heat exposure and not germ cell loss, the global gene expression data from the 8-hour time point were utilized. The transcripts similarly heat regulated (both go up or both go down) in both strains or diametrically heat regulated (1 up, 1 down or not regulated) between 2 strains were further analyzed using Onto-Express for biological function. Table 4 shows the number of transcripts in the 2 strains of mice altered by scrotal hyperthermia. About 15 transcripts were similarly up-regulated in both strains 8 hours after heat shock. These include 9 (60%) heat shock proteins (Hsp) or HSP-related proteins: Hspa1a (heat shock protein 1A), Hspb1 (Hsp27, heat shock protein 1), Hspe1 (mtcpn10, heat shock protein 1, chaperonin 10), Hsph1 (heat shock protein 105/110 kDa protein 1), Hspa8 (Hsc70, heat shock protein 8), Hspa9a (mortalin, heat shock protein 9A), Cryab (crystallin, alpha B), Dnaja1 (DnaJ (Hsp40) homolog, subfamily A, member 1), Dnaja4 (DnaJ (Hsp40) homolog, subfamily A, member 4); and 3 transcription factors, Egr1 (early growth response 1), Jun (Jun oncogene), and Fos (FBJ osteosarcoma oncogene). A number of transcripts exhibited different regulation levels in the 2 strains. For example, Egr1 was up-regulated almost 27-fold by heat in C57BL/6 testes but less than 4-fold in AKR/N mice, although this gene was up-regulated by heat in both strains. Of the 4 transcripts similarly down-regulated in both strains, no known biological function could be assigned. Table 5 lists selected genes commonly regulated by heat shock in C57BL/6 and AKR/N.
Table 4.
Number of transcripts regulated in 2 mouse strains after heat treatmenta
| Transcripts Regulated in C57BL/6 Testis 8 h After Heat Exposure |
Transcripts Regulated in AKR/N Testis 8 h After Heat Exposure |
|||
|---|---|---|---|---|
| Total No. | Differentially Regulated | Total No. | Differentially Regulated | |
| No. of transcripts up-regulated in either strainb | 37 | 19 | 48 | 30 |
| No. of transcripts down-regulated in either strainc | 28 | 20 | 39 | 31 |
The criteria for a “heat-regulated” gene requires a raw signal value of no less than 50 in either control or the heat-treated samples, a 2-fold or higher change in signal between the control and treated samples, and statistically significant difference based on 1-way analysis of variance test.
No. of transcripts up-regulated in both strains = 15.
No. of transcripts down-regulated in both strains = 4.
Table 5.
Selected genes commonly regulated by heat stress in both C57BL/6 and AKR/Na
| Affymetrix Fold Change |
||||
|---|---|---|---|---|
| Genes | Gene Symbol | GenBank Accession No. | C57BL/6 | AKR/N |
| Up-regulated in both strains | ||||
| Heat shock protein 1A | Hspa1a | NM_010479 | 46.4 | 12.7 |
| Early growth response 1 | Egr1 | NM_007913 | 26.6 | 3.8 |
| FBJ osteosarcoma oncogene | Fos | NM_010234 | 17.5 | 4.7 |
| Suppressor of cytokine signaling 3 | Socs3 | NM_007707 | 12.4 | 5.2 |
| Heat shock protein 8 | Hspa8 | NM_031165 | 9.7 | 4.7 |
| Jun oncogene | Jun | NM_010591 | 4.9 | 4.5 |
| DnaJ (Hsp40) homolog, subfamily A, member 4 |
Dnaja4 | NM_021422 | 3.5 | 3.0 |
| Down-regulated in both strains | ||||
| Similar to DNA segment, Chr 7, ERATO Doi 680, expressed (LOC384685), mRNA |
BB748743 | −6.8 | 22.4 | |
| RIKEN cDNA 2210401K01 gene (SCY1-like 1) |
2210401K01Rik | AK020483 | −2.2 | −2.1 |
| Transcribed sequences | BB451601 | −2.5 | −2.7 | |
| RIKEN full-length enriched library, clone:2210017G18 |
2210017G18Rik | AK008743 | −4.4 | −3.5 |
All genes must have a raw signal no less than 50 in both strains and 2-fold or more regulation in expression by heat compared with the control samples in both strains.
There were a total of 67 transcripts identified as being heat-regulated in C57BL/6. Of those, 37 were up-regulated and 28 down-regulated. Nineteen (51.3%) of the up-regulated transcripts were up-regulated in C57BL/6 but not in AKR/N. Of these 19 differentially up-regulated transcripts, 4 are involved in steroid or sterol biosynthesis: Sc4mol (Sterol-C4-methyl oxidase-like), Sc5d (sterol-C5-desaturase [fungal ERG3, delta-5-desaturase] homolog [S cerevisae]), Star (steroidogenic acute regulatory protein), and Cyp11a1 (cytochrome P450, family 11, subfamily a, polypeptide 1). Two are transcription factors: Btg2 (B-cell translocation gene 2, antiproliferative) and Stat3 (the signal transducer and activator of transcription 3). One gene was involved in FASL biosynthesis: Phlda1 (pleckstrin homology-like domain, family A, member 1). About 20% of the transcripts were unknown or their biological function was unknown. Of the 28 transcripts that were down-regulated in C57BL/6 after heat shock, 20 (71.5%) were differentially down-regulated in C57BL/6 and not in AKR/N. Twelve (60%) of the transcripts were unknown or without a known function.
There were 87 heat-regulated transcripts identified in the AKR/N testis. Of those, 48 were up-regulated and 39 down-regulated. About 30 (62.5%) of the up-regulated transcripts were up-regulated only in AKR/N and not in C57BL/6. Four were transcription factors, and 4 of the transcripts respond to unfolding proteins and apoptosis: Hspb8 (heat shock protein 8), Hsp90ab1 (Hspcb, heat shock protein 1, beta), Cebpb (CCAAT/enhancer binding protein, beta), and Gadd45b (the growth arrest and DNA-damage–inducible 45 beta). Approximately 40% of the differentially up-regulated transcripts were unknown or their biological functional was unknown. Thirty-one transcripts were differentially down-regulated in AKR/N, with over 50% being unknown transcripts. About 13% were transcription factors, and 10% were related to protein folding, including Fkbp3 (FK506 binding protein3) and Fkbp5 (FK506 binding protein 5). Table 6 lists some selected genes differentially regulated by heat shock in C57BL/6 and AKR/N.
Table 6.
Selected genes differentially regulated by heat stress in C57BL/6 and AKR/Na
| Genes | Gene Symbol | GenBank Accession No. | Affymetrix Fold Change |
|---|---|---|---|
| Differentially up-regulated in C57BL/6 | |||
| Pleckstrin homology-like domain, family A, member 1 | Phlda1 | NM_009344 | 3.8 |
| TYRO protein tyrosine kinase binding protein | Tyrobp | NM_011662 | 3.0 |
| Steroidogenic acute regulatory protein | Star | NM_011485 | 3.0 |
| B-cell translocation gene 2, antiproliferative | Btg2 | NM_007570 | 2.5 |
| Differentially down-regulated in C57BL/6 | |||
| Aminopeptidase-like 1 | Npepl1 | NM_213733 | −3.8 |
| Histone 1, H2ae | Hist1h2ae | NM_175660 | −3.0 |
| Mus musculus resistance to inhibitors of cholinesterase 3 homolog (C. elegans) (Ric3) |
Ric3 | NM_001038624 | −2.9 |
| Hypoxia up-regulated 1 | Hyou1 | NM_021395 | −2.5 |
| Differentially up-regulated in AKR/N | |||
| Heat shock protein 1, beta | Hsp90ab1 | NM_008302 | 4.0 |
| Cysteine and glycine-rich protein 1 | Csrp1 | NM_007791 | 3.4 |
| Phospholipase A2, group VI | Pla2g6 | NM_016915 | 2.7 |
| CCAAT/enhancer binding protein (C/EBP), beta | Cebpb | NM_009883 | 2.5 |
| Differentially down-regulated in AKR/N | |||
| Kalirin, RhoGEF kinase | Kalrn | XM_001001454 | −7.7 |
| Smoothened homolog (Drosophila) | Smo | NM_176996 | −3.1 |
| Sperm acrosome associated 3 | Spaca3 | NM_029367 | −3.0 |
| Helicase with zinc finger domain | Helz | NM_198298 | −3.0 |
All genes must have a raw signal no less than 50 in at least 1 of the strains and 2-fold or more regulation in expression between 2 strains.
Comparison Analysis of Gene Expression and Gene Regulation
Six transcripts differentially expressed between untreated C57BL/6 and AKR/N testes were found to be differentially regulated in C57BL/6 and AKR/N 8 hours after heat exposure. These genes included Strbp (spermatid perinuclear RNA binding protein), Sc4mol, D3Ertd300e (p38 interacting protein), Tra2a (transformer 2 alpha homolog [Drosophila]), Rerg (RAS-like estrogen-regulated growth inhibitor), and 1 gene with unknown biological function. Five out of these 6 genes (except Rerg) were found also differentially expressed between C57BL/6 control testis and 2 heat-resistant strains.
Discussion
The effect of cryptorchidism on testis function can differ, depending on the strain of mouse. Some strains, including C57BL/6, were found to be sensitive to heat stress, whereas other strains, such as AKR/N, MRL/MpJ-+/+, and MRL/MpJ-lpr/lpr, were relatively resistant to heat stress, as determined by the testis weight ratio and apoptotic cell number after cryptorchidism for at least 7 days (Kon and Endoh, 2001; Kazusa et al, 2004). The weight of the cryptorchid testis in the AKR/N strain was approximately 70% of that of the intact testis 14 days after experimentally induced cryptorchidism, whereas the testis weight was reduced to less than 50% in other heat-stress–sensitive strains (Kon and Endoh, 2001; Kazusa et al, 2004). The current study attempted to reduce the complexity of experimental cryptorchidism by simply subjecting the testes to a single scrotal heat stress under tightly controlled conditions. To our knowledge, this is the first attempt to use global gene expression profiling to dissect the effect of a heat exposure in the murine testis by comparing a normal strain to heat-resistant strains. First of all, a perusal of the global gene expression in the normal testis of 3 strains clearly demonstrates how much genetic background alone can affect the transcriptome. This becomes critical when gene expression studies between different strains are compared. About 415 transcripts showed significant differences in expression levels between C57BL/6 and AKR/N control mice, including several immune response–associated genes, which is consistent with the fact that AKR/N has various immunological defects (http://web.ncifcrf.gov/research/animal_production_ program/strain_information). It is not very surprising that the number of transcripts differentially expressed between AKR/N and MRL/MpJ-+/+ mice (268) is much less than that of transcripts between C57BL/6 and AKR/N (415) or MRL/MpJ-+/+ (416), because the AKR/N mouse shares some genetic background with the MRL/MpJ-+/+ mouse, as stated by Mouse Genome Informatics (http://www.informatics.jax.org).
Our results confirm that primary spermatocytes and round spermatids are the most vulnerable to heat stress in C57BL/6 and AKR/N, because DNA damage examined by TUNEL assay was detected first in these cells. However, DNA damage in AKR/N testis was delayed compared with C57BL/6, and a large portion of spermatocytes and spermatids in AKR/N mice didn’t show obvious cell damage even 24 hours after heat shock (Figure 1). Although a large number of apoptotic cells were seen among the first metaphase spermatocytes in stage XII of seminiferous tubules in normal MRL/MpJ-+/+ mice (not heat treated) but not in AKR/N mice, similar heat-resistant pachytene spermatocytes and round spermatids were detected in MRL/MpJ–+/+ mice after cryptorchidism (Kazusa et al, 2004). This result suggests that the same mechanisms may be involved in heat resistance in AKR/N and MRL/MpJ-+/+ mice. Therefore, some of the transcripts differentially expressed between C57BL/6 and 2 heat-resistant strains, AKR/N and MRL/MpJ-+/+, may play very important roles in this process. For example, 6 genes were involved in the apoptosis or antiapoptosis processes, with some of them highly expressed in the heat-resistant strains, including caspase 9 (Casp9), vanin 1 (Vnn1), tumor necrosis factor, and alpha-induced protein 8 (Tnfaip8), whereas some of the genes were highly expressed in C57BL/6, including serine/threonine kinase 17b, apoptosis-inducing (Stk17b), peroxiredoxin 2 (Prdx2), and RNA binding motif and ELMO domain 1 (Rbed1). Vnn1 was one of the antiapoptosis genes that showed dramatic differences between C57BL/6 and heat-resistant strains and is highly expressed in pachytene spermatocytes and round spermatids in the 129 mouse strain according to our array data. Further studies are needed to determine which genes and related proteins in this list are involved in heat sensitivity in the testes of different strains.
To identify the factors associated with different heat sensitivity between 2 strains of mice, we examined gene expression profiles of controls and heat-treated testes. Numerous transcripts exhibited differential regulation between 2 strains 24 hours after heat treatment. Because many tubules were visibly disrupted at 24 hours in both strains, the changes in gene expression most likely include many secondary responses to heat stress and mask the responses of the genes directly affected by the insult. Therefore, the data from the 8-hour time point was emphasized because the difference in response between the 2 strains as indicated by the TUNEL assay was measurable but no obvious morphology change was detectable. A relatively small number of transcripts were observed to be differentially regulated by heat stress between testes of C57BL/6 and AKR/N strains. Twenty transcripts were found to be differentially down-regulated and 19 genes differentially up-regulated in C57BL/6 but not in AKR/N following hyperthermia. Out of 19 differentially up-regulated genes, only 6 have a raw signal above 100 in pachytene spermatocytes, 5 in round spermatids, and 12 in Sertoli cells, according to the cell-specific array data available from the Griswold lab. This suggests that somatic cells may play an important role in early response to hyperthermia in heat-sensitive strain C57BL/6 testis. Among 30 differentially up-regulated transcripts in AKR/N, 19 have a raw value above 100 in pachytene spermatocytes and 14 in round spermatids, whereas 18 are present in Sertoli cells. One of the most differentially up-regulated genes in AKR/N was Hsp90ab1, which has high expression levels in all 3 cell types. The data generated indicate that both somatic cells and germ cells contribute to the heat resistance in AKR/N testis. Further effort is needed to determine the importance of a specific cell type in heat resistance and how a unique transcript affects heat sensitivity in these strains.
The biggest gene family commonly up-regulated in both strains was the heat shock proteins (Hsp). Expression of as many as 9 different Hsps or Hsp-related proteins was increased at least 2-fold at 8 hours after a single heat exposure. Aguilar-Mahecha et al (2001) reported that HSPs-chaperones were expressed in spermatogenic cells and that a number of Hsps are expressed in a developmentally regulated fashion from pachytene spermatocyte to elongated spermatids. Most HSPs have strong cytoprotective effects and behave as molecular chaperones for other cellular proteins (for reviews, see Bukau and Horwich, 1998; Garrido et al, 2001). However, the increase in expression of these Hsps didn’t prevent testicular cells from apoptosis, at least not in the C57BL/6 testis. The possible reasons are as follows: 1) The expression of Hsps is cell-specific in testis (Meinhardt et al, 1995; Ogi et al, 1999; Aguilar-Mahecha et al, 2001) so that the regulated transcription of Hsps may also be cell-specific and cannot be discerned when whole testis was used for microarray analysis. 2) It has been recognized that HSPs regulate apoptosis differently; for example, HSP27 and HSP70 are antiapoptotic whereas HSP60 and HSP10 are proapoptotic. It has also been shown that HSPs function at multiple points in the apoptotic signaling pathway (for reviews, see Creagh et al, 2000; Lebret et al, 2003; Didelot et al, 2006). Even additional heat-inducible HSP70 in pachytene spermatocytes and round spermatids does not appear to protect male germ cells from the harmful effects of elevated temperature on spermatogenic cells (Chowdhury and Steinberger, 1970). Therefore, different HSPs regulated by heat shock may function through different pathways and either protect cells from apoptosis or induce certain cells into programmed cell death. It seems that regulation of HSPs is not associated with different responses to hyperthermia, because these Hsps were up-regulated in both strains. However, several Hsps exhibited different expression levels and different regulation levels in the 2 strains. For example, Hspa1a (Hsp70) and Egr1 showed differences of over 7-fold between 2 strains, suggesting that some HSPs may be involved in heat resistance seen in AKR/N.
Four genes involved in steroid biosynthesis were differentially up-regulated in C56BL/6 after heat exposure: Star, Cyp11a1, Sc4mol, and Sc5d. Star also showed differential expression levels between controls from C56BL/6 and AKR/N testes, and Sc4mol was also differentially expressed between C57BL/6 and 2 heat-resistant strains. It has been reported that steroid biosynthesis was disturbed in the cryptorchid testis (Damber et al, 1980; Bergh and Damber, 1984; Bergh et al, 1984) and the concentration of testosterone within the cryptorchid testis is reduced compared with the normal scrotal testis (Keel and Abney, 1981; Farrer et al, 1985). This suggests a deleterious effect on the function of Leydig cells by heat stress in heat-sensitive strains. However, Star showed higher expression levels in AKR/N control mouse, and low resting steroid levels in plasma were noticed in this strain (http://web.ncifcrf.gov/research/animal_production_program/strain_information). The reason and significance for the disturbed steroid biosynthesis in heat sensitivity is unknown. Further study is warranted to determine whether the difference in regulation of transcripts associated with steroid biosynthesis in different strains of mice is related to the differences in the sensitivity of germ cells to heat exposure in these strains of mice.
Two major pathways, intrinsic and extrinsic, are involved in the process of caspase activation and apoptosis in mammalian cells (for reviews, see Adams and Cory, 1998; Ashkenazi and Dixit, 1998; Green, 2000; Hengartner, 2000, 2001; Reed, 2000). Members of the BCL-2 family play a major role in governing the intrinsic pathway, which is the mitochondria-dependent apoptotic pathway, with proteins such as caspase 9 (Apaf-3) and BAX functioning as inducers and proteins such as BCL-2 as suppressors of cell death. The extrinsic pathway for apoptosis involves ligation of the death receptor (such as Fas) to its ligand (FasL), and activation of caspase 8 or 10 and caspases 3 and 7, which results in cellular disassembly. The endoplasmic reticulum (ER) has also shown to be involved in apoptotic execution (Nakagawa et al, 2000). As shown in Table 2, several genes involving in the process of apoptosis were more highly expressed in both AKR/N and MRL/MpJ–+/+ than in C57BL/6, such as caspase 9, Mapk14, and Map3k12 (for reviews see Cho and Choi, 2002). High expression levels of these genes are unlikely to contribute to the metaphase-specific apoptosis noticed in the MRL/MpJ–+/+ mouse (Kon, 2005), because no abnormal programmed cell death was observed in the AKR/N control testis when compared with the C57BL/6 testis. Further study is needed to determine whether these genes are involved in the heat resistance in both the AKR/N and MRL/MpJ–+/+ strains. Testicular germ cell loss observed with exposure to abdominal heat stress occurs by apoptosis (Yin et al, 1997). Studies indicated that the mitochondria-dependent and possibly also the ER-dependent pathways are the key apoptotic pathways for heat-induced germ cell death (Hikim et al, 2003) and Fas/FasL signaling may be dispensable for heat-induced germ cell apoptosis (Hikim et al, 2003; Sinha Hikim et al, 2003; Vera et al, 2004). Therefore, the occurrence of apoptosis, at least at the earlier time points after heat exposure, is more likely driven by activation of intrinsic and/or extrinsic signaling pathways involving pre-existing proteins in germ cells than related to global changes in gene expression. In this study, apoptosis was used as an indicator to indicate: 1) germ cell apoptosis in testes of control C57BL/6 and AKR/N mice; 2) different responses to heat stress in different mouse strains; and 3) a relatively early time point of response to heat shock. However, a few genes were noticed to be differentially regulated by heat stress in the testes of either strain. For example, a small increase in expression of the Bcl2a1a gene (B-cell leukemia/lymphoma 2 related protein A1a) was observed in the AKR/N mouse. Phlda1 (pleckstrin homology-like domain, family A, member 1, Tdag51), a gene that plays a key role in Fas up-regulation and apoptosis in T cells (Park et al, 1996; Gomes et al, 1999), was also differentially up-regulated in C57BL/6 testis after heat shock. Bid (BH3 interacting domain death agonist) was slightly up-regulated by heat at 24 hours in C57BL/6 but not in AKR/N. Further studies are needed to address the relationship between the heat resistance and the gene regulation involved in apoptotic pathways.
The microarray data also identified many other groups of genes, as well as numerous unknown transcripts not mentioned above, whose expression was differentially regulated by heat exposure in either strain of mouse, suggesting multiple possible mechanisms contributing to various heat responses from dissimilar strains of mice.
Taken together, the combination of histological and molecular biological analyses of testes from 3 strains and heat response from 2 strains of mice has demon-strated that: 1) diverse genetic backgrounds lead to inherent differences in testis gene expression profiles; 2) germ cells from the AKR/N testis are relatively heat-resistant compared with those of the C57BL/6 testis; and 3) multiple mechanisms are likely involved in the different responses of testes to heat shock between 2 strains of mice. This study represents the first transcriptome analysis and comparison of testes from different strains of mice showing varied heat sensitivity. Genes and gene networks identified as significant by microarrays provide important leads for pursuing a more complete understanding of temperature regulation in spermatogenesis. However, the analysis of the current data is by no means conclusive and final. Because of the stringent nature of the analysis, some genes involved in the regulation of heat sensitivity may be omitted in the present report, either because they have a lesser fold change (less than 2-fold) or because they are unable to pass the statistical tests performed in the present analysis. Another possibility is that certain genes are cell type–specific and the expression levels may be diluted in the assays by examining expression within the whole testis. Thus, further analyses of the array data by employing different approaches and cutoff criteria are necessary to use fully the wealth of information contained in this study. It is also noteworthy that the data obtained from the present experiment represent an integrated information or response by at least 3 major testicular somatic cells and numerous developing germ cells after heat stress. Each testicular cell type could play a unique role in heat sensitivity corresponding to its specialized function. Further work to classify changes in expression levels of a unique transcript into specific cell type is in progress and could provide information about functions of specific cell type in heat response. In addition, further studies on proteins correlating with message levels, in combination with their functions, could deepen our understanding of the mechanism regarding sensitivity of testis to hyperthermia.
Acknowledgment
The authors would like to thank Derek Pouchnik for microarray hybridization and processing.
Supported by HD 10808 from NICHD.
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Aguilar-Mahecha A, Hales BF, Robaire B. Expression of stress response genes in germ cells during spermatogenesis. Biol Reprod. 2001;65:119–127. doi: 10.1095/biolreprod65.1.119. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock–initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- Banks S, King SA, Irvine DS, Saunders PT. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. 2005;129:505–514. doi: 10.1530/rep.1.00531. [DOI] [PubMed] [Google Scholar]
- Bergh A, Ason BA, Damber JE, Hammar M, Selstam G. Steroid biosynthesis and Leydig cell morphology in adult unilaterally cryptorchid rats. Acta Endocrinol (Copenh) 1984;107:556–562. doi: 10.1530/acta.0.1070556. [DOI] [PubMed] [Google Scholar]
- Bergh A, Damber JE. Local regulation of Leydig cells by the seminiferous tubules. Effect of short-term cryptorchidism. Int J Androl. 1984;7:409–418. doi: 10.1111/j.1365-2605.1984.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw AW, Hamilton D, Massey PF. Effect of scrotal heating on testicular enzymes and spermatogenesis in the rat. Aust J Biol Sci. 1973;26:1395–1407. doi: 10.1071/bi9731395. [DOI] [PubMed] [Google Scholar]
- Blackshaw AW, Massey PF. The effect of cryptorchidism on the quantitative histology, histochemistry and hydrolytic enzyme activity of the rat testis. Aust J Biol Sci. 1978;31:53–64. doi: 10.1071/bi9780053. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Cataldo L, Mastrangelo MA, Kleene KC. Differential effects of heat shock on translation of normal mRNAs in primary spermatocytes, elongated spermatids, and Sertoli cells in seminiferous tubule culture. Exp Cell Res. 1997;231:206–213. doi: 10.1006/excr.1996.3447. [DOI] [PubMed] [Google Scholar]
- Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35:24–27. doi: 10.5483/bmbrep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- Chowdhury AK, Steinberger E. A quantitative study of the effect of heat on germinal epithelium of rat testes. Am J Anat. 1964;115:509–524. doi: 10.1002/aja.1001150307. [DOI] [PubMed] [Google Scholar]
- Chowdhury AK, Steinberger E. Early changes in the germinal epithelium of rat testes following exposure to heat. J Reprod Fertil. 1970;22:205–212. doi: 10.1530/jrf.0.0220205. [DOI] [PubMed] [Google Scholar]
- Creagh EM, Sheehan D, Cotter TG. Heat shock proteins—modulators of apoptosis in tumour cells. Leukemia. 2000;14:1161–1173. doi: 10.1038/sj.leu.2401841. [DOI] [PubMed] [Google Scholar]
- Damber JE, Bergh A, Janson PO. Leydig cell function and morphology in the rat testis after exposure to heat. Andrologia. 1980;12:12–19. doi: 10.1111/j.1439-0272.1980.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Davis JR, Firlit CF. The germinal epithelium of cryptorchid testes experimentally induced in prepubertal and adult rats. Fertil Steril. 1966;17:187–200. doi: 10.1016/s0015-0282(16)35884-8. [DOI] [PubMed] [Google Scholar]
- Didelot C, Schmitt E, Brunet M, Maingret L, Parcellier A, Garrido C. Heat shock proteins: endogenous modulators of apoptotic cell death. Handb Exp Pharmacol. 2006;172:171–198. doi: 10.1007/3-540-29717-0_8. [DOI] [PubMed] [Google Scholar]
- Farrer JH, Sikka SC, Xie HW, Constantinide D, Rajfer J. Impaired testosterone biosynthesis in cryptorchidism. Fertil Steril. 1985;44:125–132. doi: 10.1016/s0015-0282(16)48689-9. [DOI] [PubMed] [Google Scholar]
- Fridd CW, Marphy J, Linke CA, Bonfiglio TA. Response of rat testis to localized induced hyperthermia. Urology. 1975;5:76–82. doi: 10.1016/0090-4295(75)90308-8. [DOI] [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Gasinska A, Hill S. The effect of hyperthermia on the mouse testis. Neoplasma. 1990;37:357–366. [PubMed] [Google Scholar]
- Gomes I, Xiong W, Miki T, Rosner MR. A proline- and glutaminerich protein promotes apoptosis in neuronal cells. J Neurochem. 1999;73:612–622. doi: 10.1046/j.1471-4159.1999.0730612.x. [DOI] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Hand JW, Walker H, Hornsey S, Field SB. Effects of hyperthermia on the mouse testis and its response to X-rays, as assayed by weight loss. Int J Radiat Biol Relat Stud Phys Chem Med. 1979;35:521–528. doi: 10.1080/09553007914550631. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. Apoptosis: corralling the corpses. Cell. 2001;104:325–328. doi: 10.1016/s0092-8674(01)00219-7. [DOI] [PubMed] [Google Scholar]
- Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH, Soeng K, Wang C, Swerdloff RS. Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology. 2003;144:3167–3175. doi: 10.1210/en.2003-0175. [DOI] [PubMed] [Google Scholar]
- Jannes P, Spiessens C, Van der Auwera I, D’Hooghe T, Verhoeven G, Vanderschueren D. Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum Reprod. 1998;13:372–375. doi: 10.1093/humrep/13.2.372. [DOI] [PubMed] [Google Scholar]
- Kastelic JP, Cook RB, Coulter GH. Scrotal/testicular thermoregulation and the effects of increased testicular temperature in the bull. Vet Clin N Am Food Anim Pract. 1997;13:271–282. doi: 10.1016/s0749-0720(15)30340-6. [DOI] [PubMed] [Google Scholar]
- Kazusa K, Namiki Y, Asano A, Kon Y, Endoh D, Agui T. Differences in spermatogenesis in cryptorchid testes among various strains of mice. Comp Med. 2004;54:179–184. [PubMed] [Google Scholar]
- Keel BA, Abney TO. Alterations of testicular function in the unilaterally cryptorchid rat. Proc Soc Exp Biol Med. 1981;166:489–495. doi: 10.3181/00379727-166-41096. [DOI] [PubMed] [Google Scholar]
- Khan VR, Brown IR. The effect of hyperthermia on the induction of cell death in brain, testis, and thymus of the adult and developing rat. Cell Stress Chaperones. 2002;7:73–90. doi: 10.1379/1466-1268(2002)007<0073:teohot>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon Y. Morphogenetic investigation of metaphase-specific cell death in meiotic spermatocytes in mice. Anat Sci Int. 2005;80:141–152. doi: 10.1111/j.1447-073x.2005.00117.x. [DOI] [PubMed] [Google Scholar]
- Kon Y, Endoh D. Heat-shock resistance in experimental cryptorchid testis of mice. Mol Reprod Dev. 2001;58:216–222. doi: 10.1002/1098-2795(200102)58:2<216::AID-MRD11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Lebret T, Watson RW, Fitzpatrick JM. Heat shock proteins: their role in urological tumors. J Urol. 2003;169:338–346. doi: 10.1016/S0022-5347(05)64123-7. [DOI] [PubMed] [Google Scholar]
- Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Parvinen M, Bacher M, Aumuller G, Hakovirta H, Yagi A, Seitz J. Expression of mitochondrial heat shock protein 60 in distinct cell types and defined stages of rat seminiferous epithelium. Biol Reprod. 1995;52:798–807. doi: 10.1095/biolreprod52.4.798. [DOI] [PubMed] [Google Scholar]
- Mieusset R, Quintana CP, Partida LG Sanchez, Sowerbutts SF, Zupp JL, Setchell BP. Effects of heating the testes and epididymides of rams by scrotal insulation on fertility and embryonic mortality in ewes inseminated with frozen semen. J Reprod Fertil. 1992;94:337–343. doi: 10.1530/jrf.0.0940337. [DOI] [PubMed] [Google Scholar]
- Moore CR, Chase HD. Heat application and testicular degeneration. Anat Rec. 1923;26:344–403. [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Namiki Y, Endoh D, Kon Y. Genetic mutation associated with meiotic metaphase-specific apoptosis in MRL/MpJ mice. Mol Reprod Dev. 2003;64:179–188. doi: 10.1002/mrd.10208. [DOI] [PubMed] [Google Scholar]
- Namiki Y, Kon Y, Kazusa K, Asano A, Sasaki N, Agui T. Quantitative trait loci analysis of heat stress resistance of spermatocytes in the MRL/MpJ mouse. Mamm Genome. 2005;16:96–102. doi: 10.1007/s00335-004-2424-y. [DOI] [PubMed] [Google Scholar]
- Nistal M, Paniagua R, ez-Pardo JA. Histologic classification of undescended testes. Hum Pathol. 1980;11:666–674. doi: 10.1016/s0046-8177(80)80078-5. [DOI] [PubMed] [Google Scholar]
- Ogi S, Tanji N, Iseda T, Yokoyama M. Expression of heat shock proteins in developing and degenerating rat testes. Arch Androl. 1999;43:163–171. doi: 10.1080/014850199262454. [DOI] [PubMed] [Google Scholar]
- Park CG, Lee SY, Kandala G, Lee SY, Choi Y. A novel gene product that couples TCR signaling to Fas(CD95) expression in activation-induced cell death. Immunity. 1996;4:583–591. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Observations on freshly isolated and accurately identified spermatogenic cells of the rat. Early effects of heat and short-time experimental cryptorchidism. Virchows Arch B Cell Pathol. 1973;13:38–47. doi: 10.1007/BF02889295. [DOI] [PubMed] [Google Scholar]
- Perez-Crespo M, Pintado B, Gutierrez-Adan A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev. 2007;75:40–47. doi: 10.1002/mrd.20759. [DOI] [PubMed] [Google Scholar]
- Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid BO, Mason KA, Withers HR, West J. Effects of hyperthermia and radiation on mouse testis stem cells. Cancer Res. 1981;41:4453–4457. [PubMed] [Google Scholar]
- Robinson D, Rock J, Menkin MF. Control of human spermatogenesis by induced changes of intrascrotal temperature. JAMA. 1968;204:290–297. [PubMed] [Google Scholar]
- Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65:229–239. doi: 10.1095/biolreprod65.1.229. [DOI] [PubMed] [Google Scholar]
- Ross AD, Entwistle KW. The effect of scrotal insulation on spermatozoal morphology and the rates of spermatogenesis and epididymal passage of spermatozoa in the bull. Theriogenology. 1979;11:111–129. doi: 10.1016/0093-691x(79)90064-5. [DOI] [PubMed] [Google Scholar]
- Sailer BL, Sarkar LJ, Bjordahl JA, Jost LK, Evenson DP. Effects of heat stress on mouse testicular cells and sperm chromatin structure. J Androl. 1997;18:294–301. [PubMed] [Google Scholar]
- Setchell BP, D’Occhio MJ, Hall MJ, Laurie MS, Tucker MJ, Zupp JL. Is embryonic mortality increased in normal female rats mated to subfertile males? J Reprod Fertil. 1988;82:567–574. doi: 10.1530/jrf.0.0820567. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Ekpe G, Zupp JL, Surani MA. Transient retardation in embryo growth in normal female mice made pregnant by males whose testes had been heated. Hum Reprod. 1998;13:342–347. doi: 10.1093/humrep/13.2.342. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Voglmayr JK, Hinks NT. The effect of local heating on the flow and composition of rete testis fluid in the conscious ram. J Reprod Fertil. 1971;24:81–89. doi: 10.1530/jrf.0.0240081. [DOI] [PubMed] [Google Scholar]
- Shima JE, McLean DJ, McCarrey JR, Griswold MD. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod. 2004;71:319–330. doi: 10.1095/biolreprod.103.026880. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Lue Y, az-Romero M, Yen PH, Wang C, Swerdloff RS. Deciphering the pathways of germ cell apoptosis in the testis. J Steroid Biochem Mol Biol. 2003;85:175–182. doi: 10.1016/s0960-0760(03)00193-6. [DOI] [PubMed] [Google Scholar]
- Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD. Profiling gene expression during the differentiation and development of the murine embryonic gonad. Biol Reprod. 2005;72:492–501. doi: 10.1095/biolreprod.104.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger A. Temperature and enviromental effects on the testis. Plenum; New York, NY: 1989. [Google Scholar]
- Steinberger A. Effects of temperature on the biochemistry of the testis. Adv Exp Med Biol. 1991;286:33–47. doi: 10.1007/978-1-4684-5913-5_4. [DOI] [PubMed] [Google Scholar]
- Stone BA. Thermal characteristics of the testis and epididymis of the boar. J Reprod Fertil. 1981;63:551–557. doi: 10.1530/jrf.0.0630551. [DOI] [PubMed] [Google Scholar]
- Vera Y, az-Romero M, Rodriguez S, Lue Y, Wang C, Swerdloff RS, Sinha Hikim AP. Mitochondria-dependent pathway is involved in heat-induced male germ cell death: lessons from mutant mice. Biol Reprod. 2004;70:1534–1540. doi: 10.1095/biolreprod.103.024661. [DOI] [PubMed] [Google Scholar]
- Waites GM, Setchell BP. Effect of local heating on blood flow and metabolism in the testis of the conscious ram. J Reprod Fertil. 1964;8:339–349. doi: 10.1530/jrf.0.0080339. [DOI] [PubMed] [Google Scholar]
- Yin Y, Hawkins KL, DeWolf WC, Morgentaler A. Heat stress causes testicular germ cell apoptosis in adult mice. J Androl. 1997;18:159–165. [PubMed] [Google Scholar]
- Yin Y, Stahl BC, DeWolf WC, Morgentaler A. P53 and Fas are sequential mechanisms of testicular germ cell apoptosis. J Androl. 2002;23:64–70. doi: 10.1002/jand.2002.23.1.64. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD. Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod. 2005;72:1010–1019. doi: 10.1095/biolreprod.104.035915. [DOI] [PubMed] [Google Scholar]
- Zorgniotti AW. Testis temperature, infertility, and the varicocele paradox. Urology. 1980;16:7–10. doi: 10.1016/0090-4295(80)90321-0. [DOI] [PubMed] [Google Scholar]
- Zorgniotti AW. Role of temperature in regulation of spermatogenesis. Fertil Steril. 1988;50:677–678. doi: 10.1016/s0015-0282(16)60212-1. [DOI] [PubMed] [Google Scholar]