Abstract
OBJECTIVES
Opioid prescription use is increasing. Narcotic bowel syndrome (NBS) refers to chronic abdominal pain aggravated by narcotic use. Despite increasing narcotic use, NBS may be under-recognized. The aim of this study was to assess whether gastrointestinal (GI) symptoms in the community are associated with chronic narcotic use and estimate the likely prevalence of NBS.
METHODS
Validated self-report GI symptom questionnaires were mailed to 4,898 randomly selected people in the community. The medical charts of all respondents were reviewed to identify participants who had used narcotics and to determine whether they were taking an opioid for > 5 weeks for the treatment of chronic pain (malignant or nonmalignant). NBS was defined as abdominal pain developing in those taking chronic narcotics. The associations between GI symptoms and chronic narcotics use were assessed using logistic regression analysis.
RESULTS
A total of 2,913 respondents returned a completed questionnaire (overall response rate 59%, mean age 62, 52% female); 117 participants (4.1%, 95% confidence interval (CI): 3.3, 4.5) were taking narcotics. Five participants (0.17%; 95% CI: 0.06, 0.40%) met the criteria for NBS. Participants using narcotics had an increased use of laxatives (17 vs. 8% in those not using narcotics, P < 0.05). GI symptom reporting was more common in participants on narcotics, although the adjusted (for age, gender, somatic symptom complaints, and use of laxatives) odds ratios (ORs) were significantly increased only for frequent abdominal pain and stool frequency.
CONCLUSIONS
NBS may be relatively uncommon. Those on narcotics report additional GI symptoms (abdominal pain and stool frequency) and use more laxatives.
INTRODUCTION
Opioid analgesics are the mainstay of therapy in patients with chronic cancer pain and may represent the only source of relief for many patients with moderate-to-severe nonmalignant pain (1,2). However, adverse effects can compromise the usefulness of these agents for analgesia. As opiates affect gastrointestinal (GI) motility through the mu-receptor (1,3-5), bowel dysfunction is the most important and distressing adverse effect. Opioid bowel (or GI) dysfunction is manifested by symptoms, such as constipation, nausea, bloating, ileus, and sometimes worsening abdominal pain (4,6). The effects of opioids on bowel function have been best studied in patients with cancer pain. For example, in a prospective study of 1,635 cancer patients referred to a pain clinic, cancer-related constipation was reported by 33% of patients (7). However, the prevalence of adverse GI effects has not been extensively evaluated in outpatients with chronic nonmalignant pain.
Narcotic bowel syndrome (NBS) is a recognized subset of opioid bowel dysfunction that is characterized by chronic or frequently recurring abdominal pain that worsens with continued or escalating dosages of narcotics (8,9). This syndrome is thought to be under-recognized, but is probably becoming more prevalent because of an increase in the use of narcotics for chronic nonmalignant painful disorders in United States (4,6, 10), and the paradoxical development of increased pain in those chronically taking opiates associated with maladaptive behaviors (11, 12). However, to our knowledge, there are no epidemiological data on the prevalence of NBS in the US community or elsewhere.
Thus, we aimed to evaluate whether bowel dysfunction is associated with chronic narcotic use and estimate the prevalence of NBS in the general US population.
METHODS
Participants
The Olmsted County (MN) population comprises 124,277 persons (US Census 2000 data), of whom 89% are white; socio-demographically, the community is very similar to the US white population (13). Over 95% of County residents receive their medical care from one of the two group practices (Mayo Medical Center and Olmsted Medical Center). The Mayo Clinic has maintained a common medical record system with its two affiliated hospitals (Saint Marys and Rochester Methodist) for over 90 years. Recorded diagnoses and surgical procedures are indexed, including the diagnoses made for outpatients seen in office or clinic consultations, emergency room visits or nursing home care, as well as the diagnoses recorded for hospital inpatients. This system was further developed by the Rochester Epidemiology Project (14). Annually, over 80% of the entire population is attended by one or both of these two practices, and 96% of the entire population is seen at least once during any given 4-year period (13). The REP medical records linkage system provides what is essentially an enumeration of the population, from which random samples can be drawn. Following approval by the institutional review boards of the Mayo Clinic and Olmsted Medical Center, we used this system to draw a series of random samples of Olmsted County residents stratified by age (5-year intervals between 20 and 94 years) and gender (equal numbers of men and women).
A randomly identified subset of Olmsted County residents were mailed a revision of the study questionnaire (14- 18). Specifically participants who had died, moved from the County, and those who had not responded (or explicitly refused to respond to an earlier follow-up survey (19)) were not eligible for this study survey. A total of 4,954 residents of Olmsted County were mailed the study questionnaire in 2002–2004, and reminder letters were mailed at weeks 2, 4, and 7 to non-responders. Participants who indicated at any point that they did not wish to complete the survey were not contacted further. Otherwise, non-responders were contacted by telephone at week 10 to request their participation and verify their residence within the County. A completed questionnaire was returned by 2,913 participants, giving a response rate of 59%.
Case definition of NBS and data collection
We reviewed all medication lists in the clinical documentation of the 2,913 participants who completed the questionnaire, in 2002–2004. We found 117 participants who were taking any prescription narcotics. The clinical records of all these participants were reviewed by one physician (RSC) from the first date of narcotic prescription until December 31, 2007, to identify all potential cases of NBS. To identify all potential cases of NBS, demographic data as well as clinical data of participants taking narcotics were reviewed from the complete medical records, including every inpatient and outpatient visit.
Chronic narcotic use and NBS were defined by specific criteria, as follows:
Chronic narcotic use. This refers to taking any opioid drug for ≥5 weeks for the treatment of chronic or acute pain of malignant or nonmalignant origin (4).
- NBS. This refers to episodes of abdominal pain that resulted in inpatient or outpatient visits, and narcotic use for >2 weeks (9,20). Among the potential cases, two diagnostic definitions were applied:
- Definite NBS: Defined as all of the following–chronic abdominal pain (more than 1 month) (8) in the setting of continued or escalating dosages of narcotics; the pain worsens or incompletely resolves with continued or escalating dosages of narcotics; there is marked worsening of pain when the narcotic dose wanes and improvement when narcotics are reinstituted; there is a progression of the frequency, duration and intensity of pain episodes; and the nature and intensity of the pain is not explained by a current or earlier GI diagnosis (20).
- Probable NBS: Narcotics use (>2 weeks), new abdominal pain leading to any inpatient or outpatient visits, and the nature and intensity of the pain were not explained by a current or earlier GI diagnosis (20).
Survey for GI symptoms in participants taking and not taking narcotics
A total of 2,913 residents of Olmsted County responded to the study questionnaire in 2002–2004. The questionnaires used (Talley bowel disease questionnaires, BDQs) have been shown to be understandable, easily completed, and to have adequate validity (17,18). The BDQ consists of 46 GI symptoms and the somatic symptom checklist. The somatic symptom checklist consists of 12 non-GI illnesses, and participants are instructed to indicate how often each symptom occurred (0 = not a problem to 4 = occurs daily) and how bothersome each was (0 = not a problem to 4 = extremely bothersome when it occurs) during the past year, using separate 5-point scales (21,22). In addition, we asked about any laxative use on the questionnaire.
Charlson comorbidity index
The Charlson comorbidity index (23,24) is a weighted sum of 17 specific chronic diseases adapted by the Rochester Epidemiology Project for use based on the electronic medical index using ICD9-CM codes.
Statistical analysis
We estimated the age- and sex-adjusted prevalence of NBS in the community, adjusting to the distribution of the US population of 2000. The 95% CIs for the prevalence of NBS were estimated, assuming a binomial distribution for cases. For analyzing opioid bowel dysfunction, we identified symptom prevalence on the questionnaire in those with and without chronic narcotic use. Logistic regression models were examined, in which each individual symptom was considered as the dependent variable and chronic narcotic use (as a dummy regression variable) as the primary predictor variable. All P values were two-sided, and P values < 0.05 were considered statistically significant.
RESULTS
A total of 4,954 participants who were originally sampled at random from among all residents of Olmsted County were mailed the questionnaire, and 2,913 returned a completed questionnaire, giving an overall response rate of 59%. The mean age of respondents was 62 years, and 52% were female. Using a logistic regression model for response (no/yes), a weak association with age was detected (odds ratio for responding, per year of age = 1.016 (95% CI: 1.012, 1.020), but no association with gender (odds ratio in females relative to males = 1.1 (95% CI: 0.99, 1.22)).
NBS
Among the 2,913 participants, 117 participants were found to be taking prescription narcotics on a review of their medical charts. The prevalence of chronic narcotic users in the community was 4.0% (95% CI: 3.3, 4.5). Among these 117 participants, 12 participants (10.3 %) were on narcotics for malignant pain, and 107 for non-malignant pain, including musculoskeletal pain, post-surgery pain, vascular pain, pain of connective tissue origin, and other disease (Figure 1).
Figure 1.
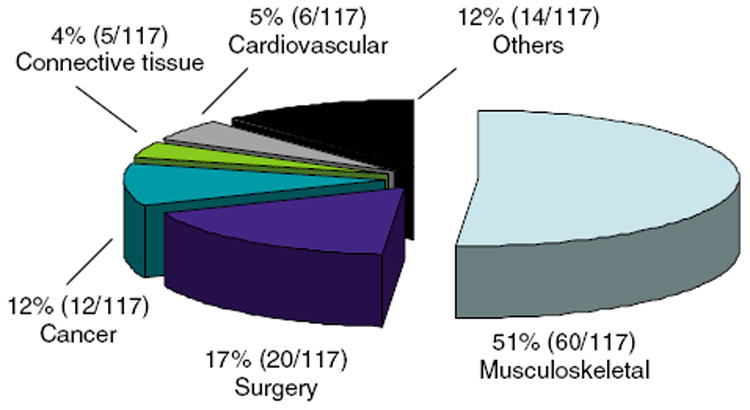
Distribution of the reasons for narcotic use.
Medical records for the 117 participants with any narcotic use were further reviewed to identify whether any of them developed new-onset NBS after the prescription of narcotics. None of the participants met the criteria for definite NBS, but five participants met the criteria for probable NBS, yielding a prevalence of probable NBS of 0.17 % (95% CI: 0.06, 0.40).
Opioid bowel dysfunction
Among the 117 participants with narcotic use, we identified 32 patients who took narcotics for < 5 weeks, and a further 33 patients had begun narcotic use after the survey. Thus, 52 participants who met the criteria for chronic narcotic use before the survey were included in the analysis of opioid bowel dysfunction. Those identified as chronic narcotic users had a mean age of 72 (±9) years, and 71% were female. Among 52 participants with chronic narcotic use, 37 cases (71.2%) had received a daily opioid dose equivalent to ≥10 mg of oral morphine.
Table 1 summarizes the demographic characteristics of participants with and without chronic narcotic use. The participants taking chronic narcotics were older and a greater proportion of them were female, in contrast to participants without chronic narcotic use. In addition, somatic symptom checklist scores and the Charlson comorbidity score were significantly associated with chronic narcotic use (both P < 0.005). Notably, use of laxatives was significantly associated with chronic narcotic use (P < 0.05). However, chronic narcotic users were similar to non-users with respect to smoking history, alcohol history, marital status, educational level, and body mass index (Table 1).
Table 1.
Demographic characteristics of Olmsted County (MN) subjects taking and not taking chronic prescription narcotics
| Chronic narcotics, n=52 (%) | No narcotics, n=2,861 (%) | Overall, n=2,913 (%) | |
|---|---|---|---|
| Age, mean±s.d. | 72±9 | 62±12 | 62±12 |
| Female gender, n (%) | 37 (71%) | 1,491 (52%) | 1,528 (52%) |
| BMI, mean±s.d. | 30.3±8.4 | 29.5±7.5 | 29.5±7.5 |
| SSC score, mean±s.d. | 1.3±0.7 | 0.6±0.5 | 0.6 ±0.5 |
| Smoking (%) | 2 (4%) | 241 (8%) | 243 (8%) |
| Alcohol (%) | 22 (42%) | 1,337 (47%) | 1,359 (47%) |
| Married | 33 (63%) | 2,282 (80%) | 2,315 (79%) |
| Laxative use | 9 (17%) | 221 (8%) | 230 (8%) |
| Charlson Comorbidity Index ≥1 | 39 (75%) | 1,095 (38%) | 1,134 (39%) |
BMI, body mass index; SSC, somatic symptom checklist.
The distribution of individual GI symptoms in participants with or without chronic narcotic use is summarized in Table 2. As expected, symptoms of constipation, including straining, hard stools, and infrequent bowel movements, were more frequently reported by participants taking chronic narcotics. Interestingly, diarrhea-related symptoms including urgency, loose bowel movements, and frequent bowel movements, also were more frequently reported by participants on chronic narcotics. In addition, other GI symptoms, including nausea, heartburn, abdominal pain, and abdominal bloating, were common in participants with chronic narcotic use.
Table 2.
Gastrointestinal symptoms in Olmsted County (MN) subjects taking and not taking chronic prescription narcotics
| Subjects on chronic narcotics (n=52) | Subjects not on narcotics (n=2,861) | Overall, n=2,913 | |
|---|---|---|---|
| Heartburn | 9 (17%) | 434 (15%) | 443 (15%) |
| Acid regurgitation | 9 (17%) | 210 (7%) | 219 (8%) |
| Chest pain | 4 (8%) | 104 (4%) | 108 (4%) |
| Nausea | 5 (10%) | 82 (3%) | 87 (3%) |
| Abdominal bloating | 18 (35%) | 679 (24%) | 697 (24%) |
| Distention | 8 (15%) | 312 (11%) | 320 (11%) |
| Constipation-related symptoms | |||
| Infrequent defecation | 3 (6%) | 95 (3%) | 98 (3%) |
| Straining at stool | 18 (35%) | 529 (18%) | 547 (19%) |
| Hard or lumpy stools | 17 (33%) | 660 (23%) | 677 (23%) |
| Incomplete rectal evacuation | 17 (33%) | 567 (20%) | 584 (20%) |
| Diarrhea-related symptoms | |||
| Urgency | 21 (40%) | 509 (18%) | 530 (18%) |
| Frequent stools (>21 BMs/week) | 4 (8%) | 45 (2%) | 49 (2%) |
| Loose stools | 18 (35%) | 489 (17%) | 507 (17%) |
| Abdominal pain-related symptoms | |||
| Abdominal pain | 26 (50%) | 1074 (38%) | 1100 (38%) |
| Pain relief by a BM | 20 (38%) | 653 (23%) | 673 (23%) |
| More BMs with abdominal pain | 15 (29%) | 465 (16%) | 480 (16%) |
| Pain severity (severe/very severe) | 2 (4%) | 91 (3%) | 93 (3%) |
| Frequent abdominal pain (>1/week) | 13 (25%) | 328 (11%) | 341 (12%) |
BM, bowel movement.
Unadjusted logistic regression analysis showed that acid regurgitation, nausea, abdominal bloating, abdominal pain, straining, feelings of incomplete evacuation, urgency, loose bowel movements, and frequent bowel movements were significantly associated with chronic narcotic use (Table 3). However, after adjusting for age, gender, somatic symptom checklist score, and laxative use, only increased odds for frequent abdominal pain and frequent stools remained significant (Table 3).
Table 3.
Associations between gastrointestinal symptoms and chronic narcotic use
| Symptom | Unadjusted OR (95% CI) | OR (95% CI)a | OR (95% CI)b |
|---|---|---|---|
| Heartburn | 1.2 (0.5, 2.4) | 0.6 (0.3, 1.4) | 0.6 (0.3, 1.4) |
| Acid regurgitation | 2.6 (1.3, 5.4) | 1.1 (0.5, 2.6) | 1.2 (0.5, 2.7) |
| Chest pain | 2.2 (0.8, 6.2) | 0.9 (0.2, 3.1) | 0.8 (0.2, 3.0) |
| Nausea | 3.7 (1.4, 9.4) | 2.2 (0.7, 6.2) | 2.1 (0.7, 6.4) |
| Abdominal bloating | 1.7 (1.0, 3.1) | 0.7 (0.3, 1.3) | 0.6 (0.3, 1.2) |
| Distention | 1.6 (0.7, 3.4) | 0.6 (0.3, 1.6) | 0.6 (0.2, 1.5) |
| Constipation-related symptoms | |||
| Infrequent defecation | 1.8 (0.5, 5.8) | 1.5 (0.4, 5.0) | 1.3 (0.4, 4.6) |
| Straining | 2.4 (1.3, 4.5) | 1.1 (0.5, 2.1) | 1.0 (0.5, 2.0) |
| Hard or lumpy stools | 1.7 (0.9, 3.0) | 0.9 (0.5, 1.8) | 0.8 (0.4, 1.5) |
| Incomplete evacuation | 2.0 (1.1, 3.7) | 0.8 (0.4, 1.6) | 0.7 (0.4, 1.5) |
| Diarrhea-related symptoms | |||
| Urgency | 3.3 (1.9, 5.8) | 1.9 (1.0, 3.6) | 1.8 (0.9, 3.4) |
| Frequent stools (>21 BMs/week) | 5.2 (1.8, 15.2) | 4.5 (1.4, 14.1) | 4.9 (1.6, 15.7) |
| Loose stools | 2.4 (1.2, 4.7) | 1.9 (1.0, 3.6) | 1.8 (0.9, 3.5) |
| Abdominal pain-related symptoms | |||
| Abdominal pain | 1.7 (1.0, 3.0) | 0.9 (0.5, 1.6) | 0.8 (0.4, 1.6) |
| Pain severity (Severe/very severe) | 1.2 (0.30, 5.1) | 0.8 (0.2, 3.5) | 1.7 (0.9, 3.1) |
| Pain relief by a BM | 2.1 (1.2, 3.8) | 1.2 (0.6, 2.3) | 1.1 (0.6, 2.2) |
| Frequent abdominal pain (>1/week) | 2.6 (1.4, 5.0) | 1.1 (0.5, 2.3) | 1.6 (1.1, 2.3) |
BM, bowel movement; CI, confidence interval; OR, odds ratio; SSC, somatic symptom checklist.
Adjusted for age, gender, and SSC.
Adjusted for age, gender, SSC, and laxatives.
DISCUSSION
The possible relationship between narcotic use and symptoms of functional bowel disorders has recently garnered major attention, in part because of a substantial increase in opioid prescription in the United States in recent years (20,25). There have thus been growing concerns about the impact of unintended GI side effects of opiates in the general population, but to our knowledge no data have been available regarding the prevalence of NBS in the community (4). In this study, we found that NBS in the community is probably relatively rare, although those using prescription narcotics chronically report more GI symptoms and use more laxatives. However, we may have missed some cases; as laxative use was two-fold greater in the chronic narcotic use population, it seems logical that some patients simply initiated laxatives for treatment of narcotic-associated constipation without going through an additional outpatient or inpatient visit. A low dose of narcotic may not induce NBS, but among 52 participants prescribed narcotics, most (37 patients or 71%) had received a dosage of narcotics (a daily opioid dose equivalent to >10 mg of oral morphine) that is classified as high in the literature (4). As we carried out a retrospective study based on the medical chart, our application of an arbitrary definition from the literature may have under-estimated the prevalence of NBS because we would not have identified cases that did not present for medical care. On the other hand, as Olmsted County residents almost exclusively have their health care delivered in a system that captures all encounters, and almost all residents see their physician at least once every few years, this bias is unlikely to be very large; any really significant symptomatology is unlikely to go unnoticed and uncharted by the system in place.
Chronic pain may often be poorly managed in clinical practice. Opioids are used extensively for treating moderate to severe pain secondary to malignancy, but narcotic prescription patterns for nonmalignant pain also seem to be increasing. Pletcher et al. (25) reported that opioid prescription for patients making a pain-related visit to the emergency department changed after the national quality improvement initiatives in the late 1990s. Specifically, they observed that opioid prescription for pain-related visits increased from 23% in 1993 to 37% in 2005. We found that 4.0% of the Olmsted County community is taking prescription narcotics, and the majority of participants using narcotics are taking them for non-malignant pain-related reasons; only 8.5% on narcotics were prescribed for malignancy in this study. It is conceivable that narcotic prescription in the community will continue to increase, and therefore we are obliged to understand the impact of these medications on GI health.
Narcotic bowel syndrome was first described by Sandgren et al. (8) in 1984. They observed five patients who apparently had a distinct syndrome comprising chronic abdominal pain, vomiting, weight loss, and features of intestinal pseudo-obstruction associated with prolonged abuse of narcotic analgesics; the symptoms resolved rapidly in all patients when narcotic administration was stopped. In an editorial in 1989, Rogers et al. (9) proposed that NBS be defined as chronic abdominal pain associated with prior narcotic intake in moderate-to-heavy doses over a time period of 2 weeks. It was further suggested that this syndrome is an often overlooked cause of chronic abdominal pain and intestinal pseudo-obstruction (9). In a recent case series, four patients with NBS were identified over a 20-year period; the authors (20) suggested that NBS may now be becoming more prevalent because of increased use of narcotics for chronic non-malignant painful disorders. They further proposed revised diagnostic criteria (20); the review emphasized the presence of chronic or frequently recurring abdominal pain that is treated with increasingly high doses of chronic opiates, creating a viscous cycle in which escalating narcotic use worsens rather than relieves symptoms. However, little other literature exists, with earlier publications comprising only case reports (8,20,26). The lack of epidemiological data may represent poor recognition of the syndrome by clinicians, or the fact that this syndrome has no International Classification of Disease (ICD) code and is not included in the most recent categorization of the functional GI disorders (27). Notably, however, our study identified only five cases (0.17%) of NBS in a community sample of 2,913 participants. Still, we can conservatively calculate on the basis of our results that about 142,587 people in the United States may have NBS (applying the age- and sex-specific proportions of participants identified with NBS in this study to the corresponding age- and sex-specific population totals of US whites in 2000).
The effects of opioids on bowel function have been well documented in patients with cancer pain; the prevalence of opioid-induced constipation has been reported to range from 20 to 40% in patients with malignant or nonmalignant pain (28-30). Among patients being treated with opioids, 8–40% also report nausea or vomiting (3,31,32). A meta-analysis of randomized, placebo-controlled trials in non-cancer patients receiving opioids for pain revealed that ~80% of patients experienced at least one adverse event, with constipation (41%) and nausea (32%) being the most common opioid-related side effects (29). However, it is very difficult to obtain an accurate estimate of the impact of opioid therapy on GI symptoms in the community because of numerous other co-morbidities. The background health status of the patient, including mobility, diet, and other medications, may influence whether or not an opioid induces significant GI side effects. We observed that participants taking narcotics chronically had more GI symptoms compared with those not on this drug class, but we did not detect any significant differences in the prevalence of most GI symptoms once age, gender, and laxative use were taken into account aside from frequent abdominal pain and diarrhea (more frequent stools). The diarrhea reported may actually reflect fecal seepage or incontinence with overflow from fecal impaction, although this could not be discerned from the questionnaire data provided.
The strengths of this study include the investigation of a random community sample. The participants were not necessarily seeking health care for their GI complaints, which should have minimized the selection bias, and this sample provided an excellent opportunity to study the relationship between narcotic use and GI symptoms. A validated questionnaire was applied and a chart review was used to assess prescription narcotic usage.
This study also has a number of important limitations. We were not able to evaluate illicit drug use in this sample although it is probably low. Our data may not be generalizable to the whole US population because the racial composition of this community is predominantly white. The prevalence of GI symptoms may vary by ethnic group, but at a minimum our data are probably generalizable to the US white population. In addition, our study relied on the medical chart to determine the prevalence of NBS; it is conceivable that there is under-reporting of opioid-induced bowel dysfunction, including abdominal pain, by physicians. Although mild GI symptoms may not be recorded on each patient visit, we assume that more severe symptoms would have been charted. Finally, the abdominal pain and bowel dysfunction observed could be due to non-narcotic issues that were not coded. Thus, the underlying disease that the narcotics were being prescribed for could be the explanation for the symptoms recorded. We also cannot exclude the presence of possible functional bowel disorders such as IBS as a cause of the patient’s pain.
We conclude from this careful albeit retrospective population-based study (including a detailed chart review) that NBS in the community is probably relatively rare, although some under-reporting bias cannot be totally excluded. Those with chronic narcotic use appear to report more GI symptoms, but this seems to be explained by other factors, including increased use of laxatives. Further study is required to better define the epidemiology of opioid bowel dysfunction, including NBS, in nonmalignant patients, and clarify the appropriate treatment strategies in the clinical setting.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Narcotic bowel syndrome is thought to be under-recognized, but may be becoming more prevalent because of an increase in the use of narcotics for chronic nonmalignant painful disorders in the United States.
-
✓
The effects of opioids on bowel function have been best studied in patients with cancer pain, but the prevalence of adverse gastrointestinal (GI) effects has not been extensively evaluated in outpatients with chronic nonmalignant pain in the community.
WHAT IS NEW HERE
-
✓
Narcotic bowel syndrome in the community is relatively rare.
-
✓
Those with chronic narcotic use seem to report more GI symptoms, but this is generally explained by other factors, including use of laxatives.
Acknowledgments
Guarantor of the article: Nicholas J. Talley, MD, PhD.
Financial support: This study was made possible in part by the Rochester Epidemiology Project (grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
Footnotes
Specific author contributions: Rok Seon Choung, G. Richard Locke III, Alan R. Zinsmeister, and Nicholas J. Talley participated in the design and analysis of the study and in the writing of the paper. Alan R. Zinsmeister and Cathy D. Schleck provided the statical analysis and assisted in writing the paper.
CONFLICT OF INTEREST Potential competing interests: Talley and the Mayo Clinic have licensed the Talley Bowel Disease Questionnaire.
References
- 1.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–71. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 2.Quality improvement guidelines for the treatment of acute pain and cancer pain. American Pain Society Quality of Care Committee. JAMA. 1995;274:1874–80. doi: 10.1001/jama.1995.03530230060032. [DOI] [PubMed] [Google Scholar]
- 3.Mehendale SR, Yuan CS. Opioid-induced gastrointestinal dysfunction. Dig Dis. 2006;24:105–12. doi: 10.1159/000090314. [DOI] [PubMed] [Google Scholar]
- 4.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:11S–8S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JD, Dello Buono FA. Opioid antagonists in the treatment of opioid-induced constipation and pruritus. Ann Pharmacother. 2001;35:85–91. doi: 10.1345/aph.10121. [DOI] [PubMed] [Google Scholar]
- 6.Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61:1181–7. doi: 10.1111/j.1742-1241.2007.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grond S, Zech D, Diefenbach C, et al. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1,635 cancer patients referred to a pain clinic. J Pain Symptom Manage. 1994;9:372–82. doi: 10.1016/0885-3924(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 8.Sandgren JE, McPhee MS, Greenberger NJ. Narcotic bowel syndrome treated with clonidine. Resolution of abdominal pain and intestinal pseudo-obstruction. Ann Intern Med. 1984;101:331–4. doi: 10.7326/0003-4819-101-3-331. [DOI] [PubMed] [Google Scholar]
- 9.Rogers M, Cerda JJ. The narcotic bowel syndrome. J Clin Gastroenterol. 1989;11:132–5. [PubMed] [Google Scholar]
- 10.IMS. Health National Prescription Audit. 2006 [Google Scholar]
- 11.Sandoval JA, Furlan AD, Mailis-Gagnon A. Oral methadone for chronic noncancer pain: a systematic literature review of reasons for administration, prescription patterns, effectiveness, and side effects. Clin J Pain. 2005;21:503–12. doi: 10.1097/01.ajp.0000146165.15529.50. [DOI] [PubMed] [Google Scholar]
- 12.Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487–9. [PubMed] [Google Scholar]
- 13.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ., III The threat to medical-records research. N Engl J Med. 1997;337:1466–70. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Zinsmeister AR, Melton LJ., III Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. Am J Epidemiol. 1995;142:76–83. doi: 10.1093/oxfordjournals.aje.a117548. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ, Zinsmeister AR, Van Dyke C, et al. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–34. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Phillips SF, Melton J, III, et al. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 18.O’Keefe EA, Talley NJ, Tangalos EG, et al. A bowel symptom questionnaire for the elderly. J Gerontol. 1992;47:M116–21. doi: 10.1093/geronj/47.4.m116. [DOI] [PubMed] [Google Scholar]
- 19.Castillo EJ, Camilleri M, Locke GR, et al. A community-based, controlled study of the epidemiology and pathophysiology of dyspepsia. Clin Gastroenterol Hepatol. 2004;2:985–96. doi: 10.1016/s1542-3565(04)00454-9. [DOI] [PubMed] [Google Scholar]
- 20.Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5:1126–39. doi: 10.1016/j.cgh.2007.06.013. quiz 1121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attanasio V, Andrasik F, Blanchard EB, et al. Psychometric properties of the SUNYA revision of the psychosomatic symptom checklist. J Behav Med. 1984;7:247–57. doi: 10.1007/BF00845390. [DOI] [PubMed] [Google Scholar]
- 22.Locke GR, III, Zinsmeister AR, Talley NJ, et al. Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am J Gastroenterol. 2000;95:157–65. doi: 10.1111/j.1572-0241.2000.01678.x. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 25.Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70–8. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 26.Wong V, Sobala G, Losowsky M. A case of narcotic bowel syndrome successfully treated with clonidine. Postgrad Med J. 1994;70:138–40. doi: 10.1136/pgmj.70.820.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clouse RE, Mayer EA, Aziz Q, et al. Functional abdominal pain syndrome. Gastroenterology. 2006;130:1492–7. doi: 10.1053/j.gastro.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 28.Meuser T, Pietruck C, Radbruch L, et al. Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001;93:247–57. doi: 10.1016/S0304-3959(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 29.Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–80. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–51. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campora E, Merlini L, Pace M, et al. The incidence of narcotic-induced emesis. J Pain Symptom Manage. 1991;6:428–30. doi: 10.1016/0885-3924(91)90041-2. [DOI] [PubMed] [Google Scholar]
- 32.Aparasu R, McCoy RA, Weber C, et al. Opioid-induced emesis among hospitalized nonsurgical patients: effect on pain and quality of life. J Pain Symptom Manage. 1999;18:280–8. doi: 10.1016/s0885-3924(99)00085-8. [DOI] [PubMed] [Google Scholar]


