Abstract
Mesenchymal stem cells can give rise to several cell types, but variations depending on isolation method and tissue source have led to controversies about their usefulness in clinical medicine. Here we show that vascular endothelial cells can transform into multipotent stem-like cells by an ALK2 receptor-dependent mechanism. In lesions from patients with Fibrodysplasia Ossificans Progressiva, a disease where heterotopic ossification occurs as a result of activating ALK2 mutations, or from a mutant ALK2 transgenic mouse model, chondrocytes and osteoblasts express endothelial markers. Tie2-Cre lineage tracing also suggests an endothelial origin of these cells. Expressing mutant ALK2 in endothelial cells, or treatment with the ALK2 ligands TGF-β2 or BMP4, causes endothelial-mesenchymal transition and acquisition of a stem cell-like phenotype. In selective media, these cells differentiate into osteoblasts, chondrocytes, or adipocytes. The process is inhibited by ALK2-specific siRNA. Conversion of endothelial cells to stem-like cells may provide a novel approach to tissue engineering.
INTRODUCTION
Epithelial and endothelial cell plasticity are critical for embryonic development and disease progression1. Transformation of epithelial cells into mesenchymal cells (epithelial-mesenchymal transition, EMT) regulates gastrulation, neural crest and somite dissociation, craniofacial development, wound healing, organ fibrosis, and tumor metastasis2-5. Similarly, endothelial-mesenchymal transition (EndMT) is critical for endocardial cushion formation6-11 and recent studies show that EndMT in the tumor microenvironment generates carcinoma-associated fibroblasts12 and may be essential for cancer progression1. Many fibroblasts formed during cardiac13 and renal14-16 fibrosis are of endothelial origin. EndMT is also implicated in atherosclerosis17, pulmonary hypertension18, and wound healing19. However, whether mature endothelial cells can be induced to differentiate into a variety of cell fates is unknown.
Patients with Fibrodysplasia Ossificans Progressiva (FOP), a disease in which acute inflammation causes heterotopic ossification in soft tissues and formation of an ectopic skeleton20, carry a heterozygous activating mutation (R206H) in the gene encoding the Transforming Growth Factor-beta (TGF-β)/Bone Morphogenetic Protein (BMP) type 1 receptor Activin-like kinase 2 (ALK2)21-23. Heterotopic ossification in FOP lesions begins with a mesenchymal condensation, followed by chondrogenesis and endochondral ossification20. The source of heterotopic cartilage and bone in FOP lesions is unknown.
We show that chondrocytes and osteoblasts from FOP lesions express endothelial markers suggesting that they are derived from vascular endothelial cells. Lineage tracing of heterotopic cartilage and bone also suggests an endothelial origin. Expressing the mutant (R206H) ALK2 in human endothelial cells causes EndMT and acquisition of a multipotent stem cell-like phenotype. EndMT is promoted by treating cells with TGF-β2 or BMP4, and this is prevented by inhibitory ALK2-specific siRNA.
RESULTS
Endothelial origin of heterotopic cartilage and bone
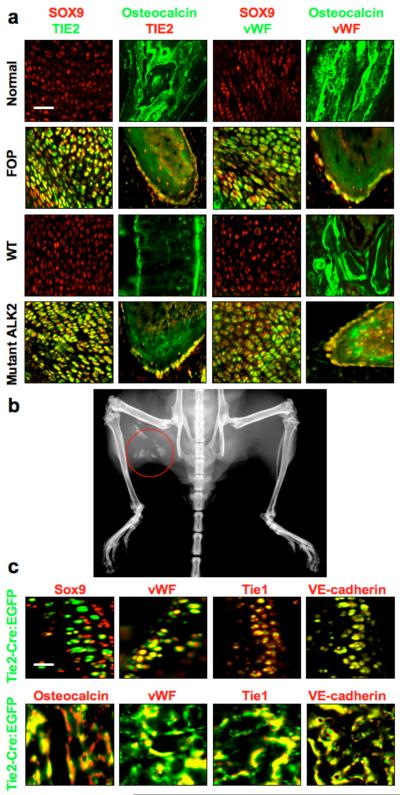
To determine whether formation of heterotopic bone and cartilage in FOP patients could be caused by EndMT, we performed immunohistochemistry on lesional tissue from FOP patients using antibodies specific for the endothelial markers TIE2 and von Willebrand Factor (vWF) and specific for osteocalcin and SOX9 to detect osteoblasts and chondrocytes, respectively. Cells in chondrogenic lesions showed co-expression of TIE2 and vWF with SOX9, whereas osteogenic lesions showed strong co-expression of TIE2 and vWF with osteocalcin in cells lining the calcified tissue. Normal human bone and cartilage from the hip joint showed no evidence of TIE2 or vWF positive chondrocytes or osteoblasts (Fig. 1, Supplementary Fig. 1a, b). In a mouse model of heterotopic ossification induced by a constitutively active ALK2 (caALK2) transgene (Fig. 1b), immunohistochemistry of chondrogenic and osteogenic lesions showed Tie2 and vWF positive chondrocytes and osteoblasts as indicated by co-staining for Tie2 and vWF with Sox9 or Tie2 and vWF with osteocalcin, respectively, suggesting that they arise from endothelial cells. For comparison, bone and cartilage from the knee joints of wild-type mice showed no evidence of Tie2 or vWF positive chondrocytes or osteoblasts (Fig. 1a, Supplementary Fig. 1c, d).
Figure 1.
Endothelial ceΠ differentiation in heterotopic ossification. (a) Immunohistochemistry of chondrogenic (first and third columns from left) and osteogenic (second and forth columns from left) lesions from Fibrodysplasia Ossificans Progressiva (FOP) patients with activating ALK2 mutations and from Cre-dependent constitutively active ALK2 (caALK2) transgenic mice. Chondrogenic lesions show co-expression of the endothelial markers TIE2 and vWF with the chondrocyte marker SOX9. Osteogenic lesions show co-expression of TIE2 and vWF with the osteoblast marker osteocalcin. Normal cartilage and bone from human hip joint (top row) or wild-type (WT) mouse knee joint (third row) show no evidence of TIE2- or vWF-positive chondrocytes or osteoblasts. Scale bar, 40 μm. (b) X-ray image of heterotopic ossification (red circle) in a Cre-induced caALK2 transgenic mouse. (c) Immunohistochemistry of BMP4-induced heterotopic cartilage and bone in Tie2-Cre reporter mice showing EGFP positive chondrocytes (top row) and osteoblasts (bottom row). These EGFP positive cells show expression of endothelial markers vWF, Tie1, and VE-cadherin. Scale bar, 20 μm.
To confirm the endothelial origin of heterotopic cartilage and bone, we used IRG reporter mice crossed with mice carrying a Tie2-Cre transgene for cell lineage tracing. In the offspring, enhanced green fluorescent protein (EGFP) is expressed in cells of Tie2-positive lineage. Staining of the heterotopic cartilage and bone, induced by injecting the ALK2 activating ligand BMP4 intramuscularly, with antibodies specific for Sox9 and osteocalcin, demonstrated that most of the chondrocytes and osteoblasts were EGFP positive. Furthermore, these EGFP positive cells showed co-expression with the endothelial markers vWF, Tie1, and VE-cadherin (Fig. 1c).
Mutation in ALK2 causes endothelial-mesenchymal transition
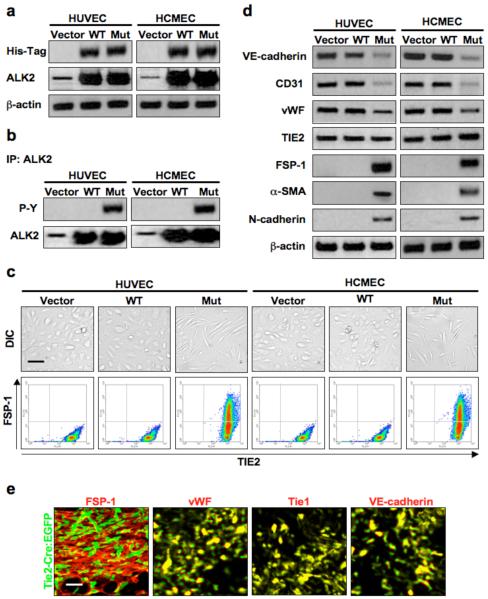
Infecting human umbilical vein endothelial cells (HUVECs) and human cutaneous microvascular endothelial cells (HCMECs) with adenoviral constructs encoding wild-type or mutant (R206H) ALK2 resulted in greatly increased levels of ALK2 (Fig. 2a). Immunoblotting also showed that the mutation induced phosphorylation of ALK2, whereas infection with constructs encoding wild-type ALK2 or vector did not (Fig. 2b). Mutant ALK2 expression caused a change in endothelial cell shape to a mesenchymal morphology and induced co-expression of the mesenchymal marker FSP-1 and the endothelial marker TIE2 (Fig. 2c). EndMT was confirmed by immunoblotting showing that expression of mutant ALK2 reduced levels of endothelial markers VE-cadherin, CD31, and vWF and increased expression of mesenchymal markers FSP-1, α-SMA, and N-cadherin. TIE2 expression did not change (Fig. 2d). Increased expression of EndMT-associated transcription factors24 Snail, Slug, ZEB-1, SIP-1, LEF-1, and Twist in cells expressing mutant ALK2 was confirmed by ELISA (Supplementary Fig. 2a).
Figure 2.
Constitutively active ALK2 promotes endothelial-mesenchymal transition. (a) Immunoblotting showing positive expression of the His-Tag on wild-type (WT) and mutant (Mut) ALK2 in infected endothelial cells, as well as increased expression of total ALK2. β-actin was used as an internal control. (b) Immunoprecipitation demonstrating constitutive tyrosine phosphorylation (P-Y) of the mutant ALK2 receptor. (c) DIC imaging (top row) showing a change in cell morphology in endothelial cells expressing mutant ALK2. Flow cytometry analysis (bottom row) demonstrating co-expression of TIE2 and FSP-1 in cells containing the mutant ALK2 construct. Scale bar, 10 μm. (d) Immunoblotting showing deceased expression of the endothelial markers VE-cadherin, CD31, and vWF and increased expression of the mesenchymal markers FSP-1, α-SMA, and N-cadherin in endothelial cells expressing mutant ALK2 but not in cells infected with vector or wild-type ALK2 adenoviral constructs. TIE2 levels remained constant. β-actin was used as an internal control. (e) Immunohistochemistry of early stage BMP4-induced lesions of heterotopic ossification in Tie2-Cre reporter mice showing EGFP positive mesenchymal cells. Most of these EGFP positive cells also show expression of the endothelial markers vWF, Tie1, and VE-cadherin. Scale bar, 20 μm.
Heterotopic ossification begins with mesenchymal condensation prior to chondrogenesis20. Our Tie2-Cre reporter mice showed EGFP positive mesenchymal cells (co-stained with FSP-1 antibodies) in early lesions of BMP4-induced heterotopic ossification. These mesenchymal cells also expressed endothelial markers vWF, Tie1, and VE-cadherin (Fig. 2e).
EndMT induces a stem cell-like phenotype
To find out whether endothelial cells expressing mutant ALK2 acquire a stem cell-like phenotype, we performed flow cytometry of cells with antibodies specific for the endothelial marker TIE2 and the mesenchymal stem cell marker STRO-1. Mutant ALK2 expressing cells showed co-expression of the two proteins but no STRO-1 expression was found in wild-type ALK2 or vector treated cells (Fig. 3a). Immunoblotting showed that lysates of cells treated with adenoviral mutant ALK2 expressed the stem cell markers25 STRO-1, CD10, CD44, CD71, CD90 and CD117. Lysates of bone marrow-derived mesenchymal stem cells also showed positive expression of all these proteins, but adult human corneal fibroblasts did not (Fig. 3b). In addition, when endothelial cells treated with adenoviral mutant ALK2 were stained with antibodies specific for the His-tagged protein and separated by fluorescence activated cell sorting, only the His-tag positive cell population expressed mesenchymal (FSP-1) and stem cell (STRO-1, CD44, CD90) markers by immunoblotting (Supplementary Fig. 3).
Figure 3.
Formation of endothelial derived multipotent stem-like cells induced by constitutively active ALK2. (a) Flow cytometry analysis showing co-expression of TIE2 and STRO-1 in endothelial cells expressing mutant ALK2. (b) Immunoblotting showing expression of the mesenchymal stem cell markers STRO-1, CD10, CD44, CD71, CD90, and CD117 in endothelial cells expressing mutant ALK2. Human bone marrow derived mesenchymal stem cells (MSC) express these markers, but human corneal fibroblasts (HCF) do not. β-actin was used as an internal control. (c) Immunoblotting showing increased expression of osteoblast (osterix), chondrocyte (SOX9), or adipocyte (PPARγ2) markers in cells treated with mutant ALK2 for 48 h followed by exposure to osteogenic, chondrogenic, or adipogenic culture medium. (d) Positive staining of osteoblast (alkaline phosphatase and alizarin red), chondrocyte (alcian blue), or adipocyte (oil red O) products in endothelial cell cultures treated with mutant ALK2, but not with vector or wild-type ALK2, for 48 h followed by growth in osteogenic, chondrogenic, or adipogenic culture medium, respectively. Scale bar, 100 μm. (e) Positive staining of osteoblast (alizarin red), chondrocyte (alcian blue), or adipocyte (oil red O) products in polylactic acid scaffolds containing endothelial cells transformed by mutant ALK2 implanted into nude mice, followed by local injection of osteogenic, chondrogenic, or adipogenic medium every 72 h for 6 weeks. Scale bar, 100 μm.
Since mesenchymal stem cells are multipotent, we assessed the differentiation capabilities of endothelial cells that undergo EndMT. Endothelial cells were exposed to adenoviral expression constructs for 48 h, and then grown in osteogenic, chondrogenic, or adipogenic media. Immunoblotting using antibodies specific for osteoblast (osterix), chondrocyte (SOX9), and adipocyte (PPARγ2) markers showed that mutant ALK2 increased expression of these markers when cells were grown in their respective differentiation medium (Fig. 3c). Cultures treated with mutant ALK2 showed positive staining for alkaline phosphatase 7 d after osteogenic medium was added, whereas wild-type ALK2 or vector treated cultures showed none. Cells treated with mutant ALK2 and grown in osteogenic medium for 21 d showed high levels of matrix calcification by alizarin red staining. Vector treated cells showed no alizarin red staining. Similar results were found for cells grown in chondrogenic medium for 14 d using the cartilage proteoglycan stain alcian blue, or in adipogenic medium for 7 d using oil red O staining (Fig. 3d). The differentiation potential of endothelial derived mesenchymal stem-like cells was similar to that of bone marrow derived mesenchymal stem cells. Human corneal fibroblasts did not exhibit differentiation activity under the same conditions (Supplementary Fig. 4). We found that differentiation could also occur when polylactic acid sponges, containing HUVECs or HCMECs pretreated with the adenoviral constructs, were implanted into immunodeficient nude mice, followed by local injection of the respective differentiation medium every 72 h for 6 weeks (Fig. 3e).
Ligand-specific induction of EndMT
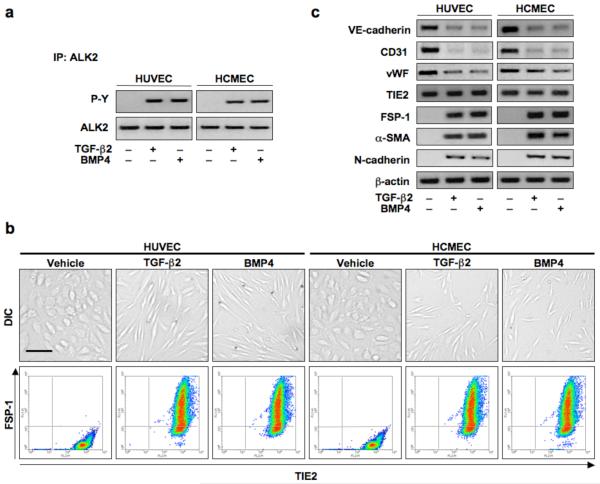
TGF-β2 and BMP4 are ligands known to activate ALK226, 27. We therefore examined receptor phosphorylation in endothelial cells treated with these factors. Immunoprecipitation assays using antibodies specific for ALK2, followed by immunoblotting with phospho-tyrosine (P-Y) specific antibodies indicated that treatment with TGF-β2 or BMP4 for 15 min induced receptor phosphorylation, but treatment with vehicle did not (Fig. 4a). HUVECs and HCMECs treated with recombinant TGF-β2 or BMP4 for 48 h showed a distinct change from cobblestone-like endothelial cell morphology to fibroblast-like morphology. When cells were co-stained using antibodies specific for the endothelial marker TIE2 and the mesenchymal marker FSP-1 and analyzed by flow cytometry, vehicle treated cells were positive for TIE2, but showed no staining for FSP-1. TGF-β2 or BMP4 treated cells showed staining for both (Fig. 4b). Immunoblotting showed that VE-cadherin, CD31, and vWF levels were decreased in cells treated with TGF-β2 or BMP4, while FSP-1, α-SMA, and N-cadherin levels increased. TIE-2 expression levels remained unchanged (Fig. 4c). ELISA analysis of EndMT-associated transcription factors Snail, Slug, ZEB-1, SIP-1, LEF-1, and Twist, revealed that expression of these proteins was much higher in cells treated with TGF-β2 or BMP4 than in control cells (Supplementary Fig. 2b).
Figure 4.
TGF-β2 and BMP4 activate ALK2 and induce endothelial-mesenchymal transition. (a) Immunoblotting of immunoprecipitates confirming phosphorylation of ALK2 by 15 min of TGF-β2 or BMP4 stimulation. (b) DIC imaging, immunocytochemistry and flow cytometry showing a change in cell morphology and co-expression of TIE2 and FSP-1 in endothelial cells treated with TGF-β2 or BMP4 for 48 h. Scale bar, 20 μm. (c) Immunoblotting confirming EndMT with decreased expression of VE-cadherin, CD31, and vWF and increased expression of FSP-1, α-SMA, and N-cadherin in cells treated with TGF-β2 or BMP4. TIE2 levels remained constant.
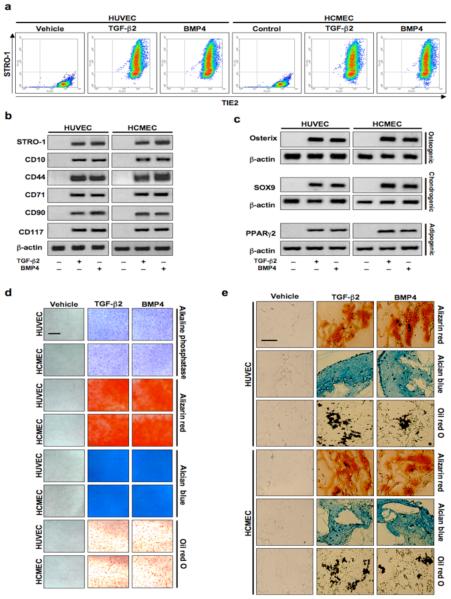
To further assess acquisition of a stem cell-like phenotype, endothelial cells were co-stained with antibodies specific for the endothelial marker TIE2 and the mesenchymal stem cell marker STRO-1. Cells treated with TGF-β2 or BMP4 showed massive co-expression of both markers, but vehicle treated cells showed no expression of STRO-1 (Fig. 5a). Immunoblotting of lysates collected from cells treated under the same experimental conditions showed that STRO-1 and other mesenchymal stem cell markers CD10, CD44, CD71, CD90, and CD117 were not expressed in vehicle treated cells, but were strongly expressed in cells treated with TGF-β2 or BMP4 (Fig. 5b).
Figure 5.
Endothelial cells transformed by treatment with TGF-β2 or BMP4 express mesenchymal stem cell markers and exhibit multipotency. (a) Flow cytometry showing co-expression of TIE2 and STRO-1 in endothelial cells treated with TGF-β2 or BMP4 for 48 h. (b) Immunoblotting confirming increased protein expression of mesenchymal stem cell markers STRO-1, CD10, CD44, CD71, CD90, and CD117 in cells treated with TGF-β2 or BMP4. β-actin was used as an internal control. (c) Immunoblotting showing increased expression of osteoblast (osterix), chondrocyte (SOX9), or adipocyte (PPARγ2) markers in cells treated with TGF-β2 or BMP4 for 48 h followed by exposure to osteogenic, chondrogenic, or adipogenic culture medium, respectively. (d) Positive staining of osteoblast (alkaline phosphatase and alizarin red), chondrocyte (alcian blue), or adipocyte (oil red O) products in endothelial cell cultures treated with TGF-β2 or BMP4 for 48 h followed by growth in osteogenic, chondrogenic, or adipogenic culture medium. Scale bar, 100 μm. (e) Positive staining of osteoblast (alizarin red), chondrocyte (alcian blue), or adipocyte (oil red O) products in polylactic acid scaffolds containing endothelial cells transformed by TGF-β2 or BMP4 subcutaneously implanted into nude mice, followed by local injection of osteogenic, chondrogenic, or adipogenic medium every 72 h for 6 weeks. Scale bar, 100 μm.
To assess differentiation potential, endothelial cells were treated with vehicle, TGF-β2, or BMP4 for 48 h, followed by growth in osteogenic, chondrogenic or adipogenic culture medium. Immunoblotting using lysates from endothelial cells treated with TGF-β2 or BMP4 and then grown in osteogenic medium for 7 d, chondrogenic medium for 14 d or adipogenic medium for 7 d demonstrated increases in protein expression of the osteoblast marker osterix, the chondrocyte marker SOX9, and the adipocyte marker PPARγ2. Vehicle treated cells did not express these markers (Fig. 5c). Cultures treated with TGF-β2 or BMP4 and stained for alkaline phosphatase after 7 d in osteogenic medium, with alizarin red after 21 d in osteogenic medium, alcian blue after 14 d in chondrogenic medium, or oil red O after 7 d in adipogenic medium, differentiated into other cell types, whereas those treated with vehicle did not (Fig. 5d). Multipotency was confirmed in vivo by implanting polylactic acid sponges containing endothelial cells pre-treated with vehicle, TGF-β2, or BMP4 into immunodeficient nude mice, followed by local injection of differentiation medium every 72 h for 6 weeks. Only implants with cells treated with TGF-β2 or BMP4 showed formation of bone, cartilage, or fat (Fig. 5e). In further experiments, HUVECs and HCMECs were pre-labeled with fluorescent quantum dots prior to treatment and implantation. The labeled cells treated with TGF-β2 or BMP4 showed expression of osteocalcin, SOX9, or adiponectin when exposed in vivo to osteogenic, chondrogenic or adipogenic medium, respectively, while vehicle treated cells did not (Supplementary Fig. 5).
Receptor specificity in EndMT
Next we asked whether ALK2 is necessary for acquisition of the multipotent stem cell-like phenotype in endothelial cells. Cells were transfected for 24 h with a siRNA duplex specific for knockdown of ALK2. A scrambled non-specific siRNA duplex was used as a negative control. Immunoblotting of cell lysates showed complete inhibition of ALK2 expression by the ALK2-specific siRNA (Fig. 6a). Transfected cells were subsequently treated with vehicle, TGF-β2, or BMP4 for 48 h, followed by lysis and immunoblotting for the mesenchymal and stem cell markers FSP-1 and STRO-1. TGF-β2 or BMP4 treated cells transfected with control siRNA had much higher expression of FSP-1 and STRO-1 than vehicle-treated cells, whereas ALK2 siRNA treatment blocked these increases (Fig. 6b). These results were confirmed by flow cytometry of cells stained in suspension with antibodies specific for TIE2 and FSP-1. Vehicle treated cells transfected with control siRNA or ALK2 siRNA showed no positive staining for FSP-1. TGF-β2 or BMP4 treated cells transfected with control siRNA showed most cells expressing FSP-1, while cultures transfected with ALK2 siRNA showed very few cells expressing FSP-1 (Fig. 6c). Treatment with the ALK2 inhibitor dorsomorphin also blocked EndMT (Supplementary Fig. 6a).
Figure 6.
ALK2 is necessary for EndMT. (a) Immunoblotting confirming knockdown of ALK2 expression by ALK2 siRNA in HUVEC and HCMEC cultures. (b) Immunoblotting showing increased expression of FSP-1 and STRO-1 in TGF-β2 or BMP4 treated endothelial cells transfected with negative control siRNA, but inhibition of this expression in cells transfected with ALK2 siRNA. β-actin was used as an internal control. (c) Flow cytometry analysis confirming increased numbers of endothelial cells expressing FSP-1 when treated with TGF-β2 or BMP4, and inhibition of such expression in cells treated with ALK2 siRNA. (d) Positive staining of osteoblast (alkaline phosphatase [AP] and alizarin red [AR]), chondrocyte (alcian blue [AB]), or adipocyte (oil red O [OR]) products in cultures transfected with negative control siRNA and treated with TGF-β2 or BMP4 for 48 h, followed by growth in osteogenic, chondrogenic, or adipogenic culture medium. In contrast, expression of ALK2 siRNA prevented the differentiation of these cells. Scale bar, 100 μm.
When cells grown under the same experimental conditions were cultured in osteogenic medium for 7 d, TGF-β2 or BMP4 treated cultures that were transfected with control siRNA showed positive staining for alkaline phosphatase, whereas vehicle treated cultures and cells transfected with ALK2 siRNA showed none. Similar results were found for alizarin red staining after 21 d of incubation in osteogenic medium, chondrocyte proteoglycans by alcian blue staining after 14 d of incubation in chondrogenic medium, and adipocytes by oil red O staining after 7 d of incubation in adipogenic medium (Fig. 6d).
Interestingly, while some ALK2 ligands (TGF-β2, BMP4) stimulate EndMT, BMP7 is an ALK2 activating ligand that inhibits EndMT13(Supplementary Fig. 6b). To determine the differences in signaling induced by different ALK2 ligands, we assessed phosphorylation levels of Smad2 and Smad5. Immunoblotting showed that 1-h of treatment with TGF-β2 or BMP4 promoted phosphorylation of both Smad2 and Smad5, whereas BMP7 stimulated phosphorylation of only Smad5 (Supplementary Fig. 7a). Since ALK2 is commonly associated with Smad5, but not Smad226, we probed for potential interaction of ALK2 with ALK5, which is known to induce phosphorylation of Smad22. Immunoblotting of lysates immunoprecipitated with ALK2 antibodies showed the presence of ALK5 in precipitates from cells treated with TGF-β2 or BMP4. No interaction of ALK2 and ALK5 was found in vehicle or BMP7 treated cells (Supplementary Fig. 7b). We further confirmed this signaling specificity in the case of the mutant (R206H) ALK2. Immunoprecipitation and immunoblotting of lysates from endothelial cells expressing mutant ALK2 showed interaction of ALK2 with ALK5 and phosphorylation of both Smad2 and Smad5, whereas cells treated with wild-type ALK2 or vector did not (Supplementary Fig. 7c, d).
To further define the role of ALK receptors in EndMT, we performed expression knockdown studies using ALK-specific siRNA for all ALK receptors. EndMT-associated decrease in VE-cadherin and increase in CD44 expression induced by TGF-β2 or BMP4 were inhibited by ALK2 siRNA or ALK5 siRNA. Inhibition of ALK1, ALK3, ALK4, ALK6, or ALK7 expression had no effect on EndMT (Supplementary Fig. 8). These data suggest that both ALK2 with ALK5 are necessary for EndMT.
Our experiments show that expression of endothelial markers vWF, TIE1, and VE-cadherin is reduced yet still detectable throughout EndMT. TIE2 levels remain constant through 48 h of treatment with TGF-β2 or BMP4, but a small decrease can be observed after 96 h of treatment (Supplementary Fig. 9a). Therefore, we believe that all these markers can be used to detect endothelial-derived cells that differentiate into other cell types via the endothelial to stem-like cell mechanism. To confirm that osteoblasts, chondrocytes and adipocytes derived from endothelial cells express these markers, we immunoblotted lysates collected from HCMEC cultures treated with vehicle, TGF-β2 or BMP4 for 48 h and grown in osteogenic medium for 7 d, chondrogenic medium for 14 days, or adipogenic medium for 7 d. Osteogenic medium induced expression of the osteoblast marker osteocalcin, chondrogenic medium increased expression of the chondrocyte marker SOX9, and adipogenic medium induced expression of the adipocyte marker PPARγ2. Bone marrow-derived mesenchymal stem cells (MSCs) grown in the same differentiation media showed positive expression of these proteins as well, but cells grown in conventional growth medium did not. Most importantly, MSCs and osteoblasts, chondrocytes, and adipocytes derived from MSCs showed no detectable expression of the endothelial markers TIE2, vWF, VE-cadherin, and TIE1. Primary human osteoblasts, chondrocytes, and adipocytes also showed no expression of these markers. However, osteoblasts, chondrocytes, and adipocytes derived from endothelial cells showed expression of these endothelial markers (Supplementary Fig. 9b). In addition, while hematopoietic stem cells express TIE2 and low levels of TIE1, they do not express the endothelial-specific markers vWF or VE-cadherin (Supplementary Fig. 10).
DISCUSSION
Our findings provide novel insights into the mechanism and potential roles of EndMT. First, the data demonstrate that EndMT results in generation of mesenchymal stem-like cells that can differentiate into multiple cell lineages. The current view is that EndMT produces fibroblastic cells that participate in specific developmental processes, in cancer progression and organ fibrosis1, but the evidence for an endothelial derived stem-like cell broadens the scope for the role of EndMT in normal development, tissue repair, and disease. For example, the observation that chondrocytes and osteoblasts at sites of bone fracture repair stained positive with antibodies specific for endothelial markers28, suggests that EndMT may contribute to the physiological process of fracture repair. Studies of the sources of chondrocytes and osteoblasts in fracture repair and the related process of distraction osteogenesis29 should be of interest.
Second, our data indicate that activation/phosphorylation of ALK2 is necessary and sufficient for EndMT to occur in cells such as HUVECs and HCMECs under the in vitro conditions used in this study. The finding that a major fraction of the chondrocytes and osteoblasts in ectopic ossifying lesions of mice and humans with an activating mutation in ALK2 are likely derived from endothelial cells indicates that the process can occur also in vivo. Our data demonstrate that TGF-β2 and BMP4 are stimulators of EndMT and confirm that BMP7 and vascular endothelial growth factor (VEGF) are inhibitors of EndMT13,30,31(Supplementary Fig. 6b). The negative effect of VEGF on ALK2-mediated EndMT may explain why endochondral and not direct (membranous) bone formation occurs in the lesions of FOP patients. Chondrogenesis occurs in an anti-angiogenic, hypoxic environment32 and this may be the condition that favors ALK2 mediated EndMT and chondrogenesis at lesion sites since hypoxia occurs as a result of inflammation33. In contrast, bone formation requires angiogenesis and osteoblasts produce VEGF34. Factors that cause endothelial-derived cells to convert to chondrocytes in FOP are currently unknown. However, it is likely that inflammatory cytokines play a critical role since the ectopic ossifying lesions are triggered by inflammation20.
Third, our data indicate that FOP, in which the hallmark is pathological bone formation, is a vascular disease based on conversion of endothelial cells into mesenchymal stem-like cells. Accumulation of mesenchymal cells is an early step in the formation of FOP lesions, and this was previously thought to be a result of fibroblast proliferation23. Our data raise the possibility that mesenchymal condensation prior to chondrogenesis is the result of EndMT to stem-like cells that condense and differentiate into chondrocytes by a process that mimicks early steps in skeletal development32. Our data show that the majority of the chondrocytes and osteoblasts found in human FOP lesions, as well as in the lesions of mouse models of FOP, express endothelial markers. The origin of the relatively small fraction of cells that do not express these markers is unknown, but it is possible that these cells arise from mesenchymal stem cells recruited from the bone marrow or surrounding tissues.
METHODS
Cell culture
Human umbilical vein endothelial cells (HUVEC) and human cutaneous microvascular endothelial cells (HCMEC) isolated as previously described35. Cells were tested for purity and found to express no markers for lymphatic endothelial cells or stromal cells (pericytes, smooth muscle cells, etc.)36. This was reconfirmed by flow cytometry analysis (Supplementary Fig. 11a). Clonal populations were isolated with PYREX 8×8 mm cloning cylinders (Corning) and shown to respond in a manner identical to that of the cultures used for our experiments (Supplementary Fig. 11b-d). Cells were grown in culture using EGM-2 medium (Cambrex), containing 10% FBS and 1% Penicillin/Streptomycin, followed by human endothelial serum free medium (Gibco) 24 h prior to all experimental conditions. Bone marrow derived mesenchymal stem cells (ScienCell Research Laboratories) were grown in mesenchymal stem cell medium (ScienCell Research Laboratories). Bone marrow derived hematopoietic stem cells (Lonza) were grown in HPGM medium (Lonza). Human corneal fibroblasts, from a stock provided by Dr. Elizabeth Hay (Harvard Medical School), were grown in RPMI medium, containing 10% FBS and 1% Penicillin/Streptomycin. Primary human osteoblasts, chondrocytes, and adipocytes and their respective growth media were obtained from Cell Applications, Inc. Recombinant TGF-β2, BMP4, and BMP7 proteins (R&D Systems) were added to the serum free culture medium for all relevant experiments at a concentration of 10 ng ml−1. Recombinant VEGF (R&D Systems) was added to cultures at a concentration of 25 ng ml−1. Dorsomorphin (Sigma-Aldrich) was added to cultures at a concentration of 5 μM. All experiments for this study were performed at minimum in triplicate.
Plasmids and adenoviral constructs
Human ALK2 expression constructs were generated by insertion of full-length human ALK2 cDNA (GenBank NW_001105) into the pcDNA 3. 1 D V5-His-TOPO vector (Invitrogen). The ALK2 R206H mutant construct was generated by site-directed mutagenesis of the normal ALK2 sequence using the Gene Tailor Site-Directed Mutagenesis System (Invitrogen). The oligonucleotides used to generate the mutant construct were: forward 5′-GTACAAAGAACAGTGGCTCACCA GATTACACTG-3′; reverse 5′-GTGAGCCACTGTTCTTTGTACCAGAAAAGGAAG-3′. SpeI and SphI sites were used to generate the adenoviral vectors through the Clontech Adeno-X System (University of Pennsylvania Vector Core). Expression from these constructs was confirmed by sequence analysis and immunoblot analysis. Viral constructs were added to cultures at a concentration of 20 pfu ml−1.
Mice
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. Floxed caALK2 transgenic mice37 were a gift from Dr. Yuji Mishina (University of Michigan). To induce expression of caALK2, AV-Cre (University of Pennsylvania Vector Core; 1 × 1011 particles per mouse) was injected into the left hindlimbs of mice at one month of age. The contralateral limb was injected with empty vector as a control. Heterotopic bone and cartilage were detected by X-ray (Senographe DS technology, General Electric Medical Systems) at 14-21 d following A V-Cre injection. Tie2-Cre and IRG reporter mice were obtained from the Jackson Laboratory. Heterotopic ossification was induced in the offspring of crosses between the Tie2-Cre and IRG reporter mice with BMP4 (provided by Genetics Institute; currently Pfizer) injected at a concentration of 0.05 μg μl−1, intramuscularly in growth factor-reduced Matrigel (BD Biosciences) Tissues were recovered at 7 and 14 d after implantation. Tissues were fixed in isopentane (2-methylbutane) as previously described23 and cryosections were cut at 10 μm. Counterstaining of sections from Tie2-Cre IRG reporter mice was performed using blue (shown in red) fluorescent AlexaFluor secondary antibodies (Invitrogen). No leakage or aberrant expression of the reporter was found in any tissues (Supplementary Fig. 12).
Human Tissues
All FOP patient samples were obtained with informed consent and protocols approved by the Investigational Review Board of the University of Pennsylvania. All biopsies were obtained prior to a diagnosis of FOP since tissue trauma in FOP frequently induces episodes of heterotopic ossification. Normal human bone and cartilage tissue from the hip joint was provided by the Department of Pathology at the Beth Israel Deaconess Medical Center and samples were obtained with informed consent and protocols approved by the Investigational Review Board.
Immunoblotting, immunoprecipitation, and immunofluorescence
Immunoassays were performed using the following antibodies at concentrations (and using protocols) recommended by the respective manufacturers: FSP-1 (H00006275-M01; Stressgen), phospho-Smad2 (3101), Smad2 (3122), phospho-Smad5 (9516), Smad5 (9517; Cell Signaling Technology), ALK1 (sc-19547), ALK2 (sc-25449), ALK3 (sc-20736), ALK4 (sc-31297), ALK5 (sc-398), ALK6 (sc-25455), ALK7 (sc-135001), phospho-tyrosine (sc-7020), VE-cadherin (sc-6458), TIE1 (sc-342), TIE2 (sc-324, sc-9026), STRO-1 (sc-47733), CD10 (58939), CD44 (sc-71220), CD71 (sc-32272), CD90 (sc-9163), CD117 (sc-17806), osteocalcin (sc-74495, sc-23790), SOX9 (sc-20095), PPARγ2 (sc-22022; Santa Cruz Biotechnology), osterix (ab22552), adiponectin (ab22554), N-cadherin (ab76057), NG2 (ab83508), vWF (ab68545; Abcam); CD31 (IR610; Dako), His (A00174; GenScript); α-SMA (A5228), β-actin (A1978; Sigma-Aldrich). Samples were run with Criterion precast SDS-PAGE Gels (Bio-Rad). HRP-conjugated IgG TrueBlot reagents (18-8814, 18-8816, 18-8817; eBioscience) were used at a dilution of 1:1000. TrueBlot IgG beads (eBioscience) were used for immunoprecipitation experiments. AlexaFluor secondary antibodies (Invitrogen) were used at a dilution of 1:200. Images were acquired using a Nikon 80i fluorescence microscope.
Flow cytometry
Endothelial cells were stained in suspension using antibodies specific for TIE2, FSP-1, STRO-1, α-SMA, NG2, and His (described above) and the protocols provided by their respective manufacturer. Flow cytometry was performed at the Harvard Medical School, Department of Pathology flow cytometry core facility using a FACSDCalibur (BD Biosciences) cell sorter isolating 30, 000 cells per sample.
Multiplex ELISA
LEF-1 (Cell Signaling Technology), VE-cadherin (sc-6458), CD44 (sc-71220), CD90 (sc-9163), Snail (sc-10433), ZEB-1 (sc-134159; Santa Cruz Biotechnology), FSP-1 (H00006275-M01; Stressgen), SIP-1 (AV33694; Sigma-Aldrich), Slug (ab27568), and Twist (ab49254; Abcam) antibodies were conjugated to Bio-Plex carboxylated beads with unique optical codes using the Bio-Plex Amine Coupling Kit (BioRad). β-actin antibody (A1978; Sigma-Aldrich) was also conjugated to Bio-Plex carboxylated beads to be used as an internal control. Samples were run on a Luminex 200 multiplex testing system (Luminex) using the Universal Cell Signaling Assay Kit and protoco! (Millipore). Experimental values were divided by the β-actin control values to provide normalized data.
CeH differentiation
Cells were grown in StemPro osteogenic, chondrogenic, or adipogenic culture medium (Invitrogen). Alkaline phosphatase staining was performed using the alkaline phosphatase kit and protocol (Sigma-Aldrich) on cultures grown in osteogenic medium for 7 d to detect osteoblasts. Alizarin Red (Sigma-Aldrich) staining was performed for 30 min on cultures grown in osteogenic medium for 21 d to detect matrix calcification. Alcian Blue (Sigma) staining was used to stain chondrocyte proteoglycans for 5 minutes in cultures grown in chondrogenic medium for 14 d. Oil Red O (Sigma-Aldrich) staining was performed for 15 min on cultures grown in adipogenic medium for 7 d. For in vivo analysis, endothelial cells were labeled with fluorescent quantum dots using the Qtracker 525 CeΠ Labeling Kit (Invitrogen). Cells were treated in culture to induce EndMT then absorbed into OPLA polylactic acid scaffolds (BD Biosciences). Scaffolds were surgically implanted subcutaneously into immunodeficient nude mice (Nu/Nu strain; Charles River Laboratories). Local injection of StemPro differentiation medium (described above) was performed in the area of the implants every 72 h for 6 weeks. Scaffolds were cryosectioned and stained with Alizarin Red, Alcian Blue, or Oil Red O as described above. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Harvard Medical School.
RNA interference
siRNA gene expression knockdown studies were performed using the TriFECTa RNAi kit (Integrated DNA Technologies) and corresponding protocoL Each 27mer siRNA duplex was transfected into cells using X-tremeGene siRNA transfection reagent (Roche) following the manufacturer′s guidelines. siRNA was synthesized (Integrated DNA Technologies) with the following sequences: ALK1: 5′-CUGGGCUAUUGAAUCACUUUAGGCUUC-3′; ALK2: 5′-GCAACACUGUCCAUUCUUCUUAACCAG-3′; ALK3: 5′-CAUCUCAUGAAUUCCAAGACAGUAUUA-3′; ALK4: 5′-AUGAGGGAUCUUCCAUGUCCAGUCUCU-3′; ALK5: 5′-CUCAGAAUGUUCUUUAGCUACCACCUC-3′; ALK6: 5′-AUCUGAAUCUGCUUAGCUAUAGUCCUU-3′; ALK7: 5′-ACUUAAAUACUGUACUGUCUUAUCUUU-3′; negative control: 5′-UCACAAGGGAGAGAAAGAGAGGAAGGA-3′.
Statistical analyses
One-way analysis of variance (ANOVA) was performed and confirmed with two-tailed paired student’s t test using GraphPad Prism 4 software. P values less than 0. 05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Xu for technical assistance, J. Bischoff (Children’s Hospital Boston) for providing primary cultures of human endothelial cells, A. Maidment for X-rays, and Y. Mishina (University of Michigan) for providing caALK2 transgenic mice. This work was supported by grants from the National Institutes of Health to B.R.O., R. K., F. S. K., E. M. S., and to the University of Pennsylvania Vector Core. Additional support was from the IFOPA, the Ian Cali and the Weldon Family Endowments, the Penn Center for Musculoskeletal Disorders, the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine, and the Rita Allen Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
D. M. designed and performed experiments, analyzed data, and wrote the manuscript. V. Y. L performed experiments. E. M. S, F. S. K, and R. K. provided research materials, edited the manuscript, and provided technical advice. B.R.O. designed experiments, analyzed data, and wrote the manuscript.
REFERENCES
- 1.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhurst RJ, Derynck R. TGF-beta signaling in cancer - a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 6.Boyer AS, et al. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev. Biol. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- 7.Lai YT, et al. Activin receptor-like kinase 2 can mediate atrioventricular cushion transformation. Dev. Biol. 2000;222:1–11. doi: 10.1006/dbio.2000.9698. [DOI] [PubMed] [Google Scholar]
- 8.Camenisch TD, et al. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, et al. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev. Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okagawa H, Markwald Y, Sugi Y. Functional BMP receptor in endocardial cells is required in atrioventricular cushion mesenchymal cell formation in the chick. Dev. Biol. 2007;306:179–192. doi: 10.1016/j.ydbio.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azhar M, et al. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn. 2009;238:431–442. doi: 10.1002/dvdy.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 13.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 14.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am. J. Pathol. 2009;175:1371–1373. doi: 10.2353/ajpath.2009.090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mironov V, et al. Endothelial-mesenchymal transformation in atherosclerosis: a recapitulation of embryonic heart tissue morphogenesis. Ann. Biomed. Eng. 1995;23:S29A. [Google Scholar]
- 18.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 19.Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp. Eye Res. 2006;83:1309–1316. doi: 10.1016/j.exer.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP) Bone. 2008;43:427–433. doi: 10.1016/j.bone.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan FS, et al. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP) J. Bone Miner. Metab. 2008;26:521–530. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 23.Lounev VY, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 26.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 27.Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- 28.Lewinson D, et al. Expression of vascular antigens by bone cells during bone regeneration in a membranous bone distraction system. Histochem. CeU BioL. 2001;116:381–388. doi: 10.1007/s004180100331. [DOI] [PubMed] [Google Scholar]
- 29.Ilizarov GA. The principles of the Ilizarov method. BulL Hosp. Jt. Dis. Orthop. Inst. 1988;48:1–11. [PubMed] [Google Scholar]
- 30.Paruchuri S, et al. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ. Res. 2006;99:861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc. Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Amarilio R, et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 33.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- 34.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 35.Boye E, et al. Clonality and altered behavior of endothelial cells from hemangiomas. J. Clin. Invest. 2001;107:745–752. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jinnin M, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat. Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuda T, et al. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;44:159–167. doi: 10.1002/dvg.20201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.