Abstract
On the basis of more than a decade of studies on the cellular effects of ethosuximide, currently, the most prudent view is that together with a block of the low threshold, T-type Ca2+ current, a reduction both of the noninactivating Na+ current, and the Ca2+-activated K+ current in thalamic and cortical neurones contribute to the overall therapeutic action of this antiabsence medicine.
rompted by recent findings showing that ethosuximide (ETX) induces a small decrease of the current generated through cloned human T-type Ca2+ channels 1, we briefly review the last decade of studies for and against this mechanism of action and their significance within the present understanding of the pathophysiological processes underlying the generation of spike and wave discharges (SWDs).
The Original Observation
In 1989, work in thalamocortical (TC) neurons (acutely dissociated and in slices) showed that therapeutically relevant concentrations (250 to 750 μM) of ETX decreased the peak amplitude of the low-threshold T-type Ca2+ current (IT) 2, 3, 4. A similar effect was later reported for the IT of neurons in the nucleus reticularis thalami (NRT) 5. This action of ETX was relatively specific (as high voltage-activated Ca2+ currents were unaffected), dose dependent (with a maximal reduction of approximately 32% at 750 μM), and voltage dependent (with a larger reduction for currents evoked between –60 and –40 mV), but there was no effect on the kinetics and steady-state (in)activation properties. ETX was also shown to decrease the Ca2+-activated K+ current [IK(Ca)] in TC neurons 2, 3.
The ability of ETX to reduce IT of thalamic neurons soon gained popularity in clinical and experimental reviews and textbooks as one of the main mechanisms of action of the succinimide class of antiabsence medicines. The rationale of this mechanistic link between ETX and IT simply stemmed from the view that a low-threshold Ca2+ spike (LTS), the main voltage expression of T-type channel activation, underlies the action potential firing that is recorded extracellularly from TC neurones during the characteristic EEG spike and SWDs in some experimental models of absence epilepsy 6, 7 (discussed later here).
The Contradictory Results
During the same period, evidence against this partial block of IT was accumulating. Different groups were unable to detect any action of therapeutically relevant concentrations (250 to 750 μM) of ETX on IT of nonthalamic cells, including human neocortical neurons 8, three classes of rat hippocampal neurons 9, rat dorsal root ganglion (DRG) cells 10, 11, and GH3 pituitary cells 12. In DRG cells, however, a reduction of 91% and 45% of T-type and L-type Ca2+ current, respectively, was observed with 10 μM ETX by Kostyuk et al. 13.
Furthermore, direct attempts to reproduce the block of IT by ETX in TC and NRT neurons from cats and Wistar and Sprague-Dawley rats, as well as from a genetic rat model of absence epilepsy and its nonepileptic control strain, showed either no effect of 0.25 to 1.0 mmol/L ETX 14, 15 or a reduction of 19% at 5 mmol/L 16. In one of these studies 15, two additional actions of ETX on TC neurons were observed: (a) a partial reduction of the noninactivating Na+ current (INaP) (60% at 750 μM) (Fig. 1A1), with no effect on the transient Na+ current, and (b) a partial decrease of IK(Ca) (40% at 500 μM) (Fig. 1B1), confirming previous data 2, 4.
FIGURE 1.
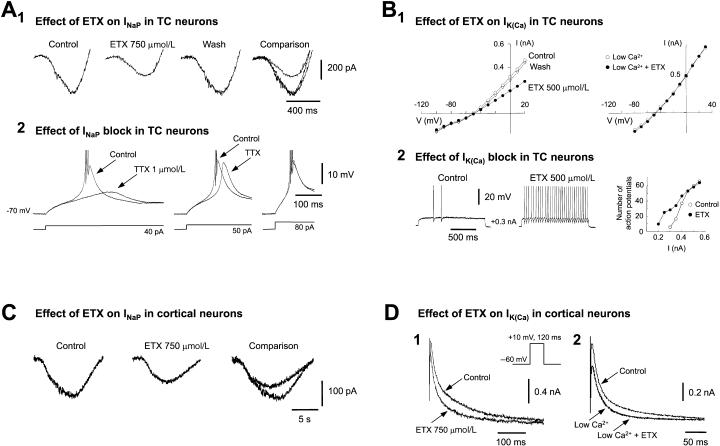
Effect of ethosuximide (ETX) on Na+ and K+ currents of rat thalamocortical (TC) and cortical neurons. (A1.) Whole cell currents elicited by a voltage ramp protocol (from -100 to 50 mV) in a TC neuron show the reversible reduction of the persistent Na+ current (INaP) by 750 μM ETX. A2, LTS, and associated action potential burst firing (top traces) produced by the current steps of increasing amplitude (bottom traces) from a membrane potential of -70 mV, in control conditions and in the presence of 1μM TTX. Note the smaller and delayed low threshold Ca2+ potential in the presence of 1 μM TTX for the two smallest current steps: this is due to the block by TTX of the non-inactivating Na+ current (INaP) underlying the LTS (see ref. 19 for details). Action potentials are truncated for clarity. (B1.) Steady-state current/voltage plot show the reversible reduction by 500μM ETX of the sustained outward whole cell current (left plot). In another TC neuron recorded in a low Ca2+ (0.5mM) – high Mg2+ (8mM) medium (right plot), 500μM ETX has no effect on the sustained current, indicating that its action is on the Ca2+–activated K current (Ik(ca)) component of the sustained outward current. (B2.) Two depolarizing current (0.3nA) pulses show an increased tonic firing in the presence of 500μM ETX.The plot on the right illustrates how this action is restricted to the smallest input currents (0.2–0.4nA). (C.) Whole cell currents elicited by a voltage ramp protocol (from −90 to 50mV) in a layer V cortical pyramidal neuron show the reduction of INaP by 750μM ETX. (D1.) Whole cell currents from another layer V pyramidal neuron show the reduction by 750μM ETX of outward K+ current(s). (D2.) This effect is abolished when ETX is applied in the presence of a low Ca2+ (0.5mM) – high Mg2+ (8mM) medium, indication that ETX is acting on Ik(ca). Panels A and B reproduced with permission from references 15 and 19; © 1998 by Society for Neuroscience.
Data from Cloned T-Type Channels
The small effect of ETX (10% at 1 mmol/L and 15% at 3 mmol/L) detected in mouse 17 and human 18 cloned T-type channels, respectively, had been interpreted as indicating that “both α1G and α1H currents are relatively insensitive to ETX, similar to other reports … in DRG and in thalamic neurons” 18. These findings have been enlarged by Gomora et al. 1, who have shown that 1 mmol/L ETX reduces the current generated through human cloned α1G by 5% and 30% when the holding potential is –100 and –75mV, respectively (Fig 7E in reference 1) (for details, see the previously mentioned commentary). In addition, they observed a preferential reduction (50% at 600 μM) of the steady-state (or “window”) component of the T-type current. Gomora et al.'s interpretation of these data is that they support the notion that native T-type channels are decreased by ETX.
Is the Discrepancy Resolved?
While on the strength of their data one can accept Gomora et al.'s view, the issue remains as to why only one group has so far been able to show a similarly weak block of native thalamic channels by ETX, whereas different groups have failed to do so, particularly as a similar age range, intracellular and extracellular solutions, and types of preparation and thalamic nuclei were used. Gomora et al. 1 suggested that their finding and those of Lacinova et al. 17 provide an explanation to resolve this apparent contradiction, as “studies that reported no effect used very negative holding potentials (–110 mV) where channels are less sensitive to block” 1. This explanation, unfortunately, is not correct, as very negative holding potentials were used in the thalamic studies that did detect an effect of ETX: –100 mV (Figs. 3A and 4 in reference 4), –110 mV (Figs. 1A and 2B in reference 2), –112 mV (Fig 6B, in reference 3), and –120 mV (Fig. 1A in reference 5).
What is striking, instead, is that the steady-state (in)activation curves of the cloned (human and animal) T-type channels are 15 to 25 mV more depolarized 1, 17, 18 than those of the native channels 3, 4, 15. Thus, in contrast to Gomora et al. 1 and Lacinova et al. 17, who tested the ETX block at holding potentials of –60 and –75 mV, respectively, no study on native channels could have correctly used these holding potentials, as even at –75 mV less than 15% of the native current is deinactivated (Fig. 6B in reference 3; Fig. 6b in reference 4; Fig. 3B in reference 15). Although one cannot exclude that a better voltage control is achieved in HEK 293 cells than in acutely dissociated TC neurons, the magnitude of the voltage discrepancy would make this possibility unlikely. Alternatively, the cloned channels might require an auxiliary protein to express similar (in)activation properties as the native channels: In this case, however, it remains to be seen whether cloned channels with more negative (in)activation properties retain a small sensitivity to ETX. Finally, whether caused by the high strength of the charge carrier (5 mmol/L Ca2+ or 10 mmol/L Ba2+), a potential screening effect at the mouth of the channel might have contributed to the block of the recombinant channels by ETX needs further investigation.
Do T-Type Channels Contribute to the Firing of TC and NRT Neurons During SWDs?
In principle, the small block of native IT produced by therapeutically relevant concentrations of ETX would alone affect TC cell excitability by sufficiently decreasing the amplitude of LTSs, thus dampening their ability to evoke action potential bursts 5. A decrease in LTS, however, will also occur because the 50% reduction by ETX of INaP (Fig. 1A1) 15 will remove part of the depolarization required to activate IT, thus resulting in a smaller LTS (Fig. 1A2) 19. As the threshold for activation of IK(Ca) is more depolarized than that of INaP 15, the reduction by ETX of IK(Ca) (Fig. 1B1) becomes physiologically important in the membrane potential region close to the firing threshold. This resulting increase in tonic firing (Fig. 1B2) would contribute to a disruption of TC loop synchronized activity during SWDs.
As there is no established in vitro model that is capable of reproducing SWDs, ETX has only been shown to decrease hypersynchronous thalamic network activities that rely on the presence of LTSs 5, whereas its ability to block the bicuculline-induced thalamic paroxysms 20 has not been tested. In vivo, however, the majority (60%) of cat TC neurons are completely silent during SWDs 21, and in a rat genetic model of absence, no LTSs are recorded in TC neurons during the majority (90%) of SWDs 22, 23. Thus, whether the weak block by ETX of this small T-type component of TC neuron firing during SWDs really represents an important element of its therapeutic action remains questionable. Note that the notion of a small contribution by LTSs to TC neuron firing during SWDs is not in conflict with the recent finding that α1G knockout mice are resistant to pharmacologically induced SWDs 24, as this inability may reflect the concomitant decrease of LTSs in cortical cells.
On the other hand, all NRT neurons in vivo show an LTS (and associated prolonged burst of action potentials) at each cycle of a SWD 25: Thus, the small ETX-induced reduction in the LTSs of NRT neurons (via the small decrease in IT and possibly INaP) would be functionally more important for blocking a paroxysm than an equivalent action in TC neurons. In this respect, it is interesting that ETX evokes a more substantial block of the window component than the peak of IT 1 and that α1I, the most prevalent T-type α subunit in NRT neurons, has the largest window component 26. Furthermore, as IK(Ca) is much larger and functionally more important (for the repolarization that follows an LTS) in NRT than in TC neurons 27, if the ETX block of this current is confirmed in NRT neurons, it is likely that most of the thalamic action of ETX occurs mainly via NRT and not TC neurons. Ultimately, the full therapeutic mechanism of ETX undoubtedly comprises not only these actions on thalamic neurons but also similar effects on cortical cells, which too are endowed with ETX-sensitive INaP and IK(Ca) (Fig. 1C and D) 28 and possibly IT.
References
- 1.Gomora JC, Daud AN, Weiergraber M, Perez-Reyes E. Block of cloned human T-type calcium channels by succinimide antiepileptic drugs. Mol Pharmacol 2001;60:1121–1132. [PubMed] [Google Scholar]
- 2.Coulter DA, Huguenard JR, Prince DA. Specific petit mal anticonvulsants reduce calcium currents in thalamic neurons. Neurosci Lett 1989;98:74–78. [DOI] [PubMed] [Google Scholar]
- 3.Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol 1989;25:582–593. [DOI] [PubMed] [Google Scholar]
- 4.Coulter DA, Huguenard JR, Prince DA. Differential effects of petit mal anticonvulsants and convulsants on thalamic neurones: calcium current reduction. Br J Pharmacol 1990;100:800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci 1994;14:5485–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snead OC, III. Basic mechanisms of generalized absence seizures. Ann Neurol 1995;37:146–157. [DOI] [PubMed] [Google Scholar]
- 7.Seidenbecher T, Staak R, Pape HC. Relations between cortical and thalamic cellular activities during absence seizures in rats. Eur J Neurosci 1998;10:1103–1112. [DOI] [PubMed] [Google Scholar]
- 8.Sayer RJ, Brown AM, Schwindt PC, Crill WE. Calcium currents in acutely isolated human neocortical neurons. J Neurophysiol 1993;69:1596–1606. [DOI] [PubMed] [Google Scholar]
- 9.Thompson SM, Wong RS. Development of calcium current subtypes in isolated rat hippocampal pyramidal cells. J Physiol 1991;439:671–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross RA, Covey DF, Ferrendelli JA. Voltage-dependent calcium channels as targets for convulsant and anti-convulsant alkyl-substituted thiobutyrolactones. J Pharmacol Exp Ther 1997;280:686–694. [PubMed] [Google Scholar]
- 11.Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol 1998;79:240–252. [DOI] [PubMed] [Google Scholar]
- 12.Herrington J, Lingle CJ. Kinetic and pharmacological properties of low voltage-activated Ca2+ current in rat clonal (GH3) pituitary cells. J Neurophysiol 1992;68:213–232. [DOI] [PubMed] [Google Scholar]
- 13.Kostyuk PG, Molokanova EA, Pronchuk NF, Savchenko AN, Verkhratsky AN. Different action of ethosuximide on low- and high-threshold calcium currents in rat sensory neurons. Neuroscience 1992;51:755–758. [DOI] [PubMed] [Google Scholar]
- 14.Pfrieger FW, Veselovsky NS, Gottmann K, Lux HD. Pharmacological characterization of calcium currents and synaptic transmission between thalamic neurons in vitro. J Neurosci 1992;12:4347–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leresche N, Parri HR, Erdemli G, Guyon A, Turner JP, Williams SR, Aprodini E, Crunelli V. On the action of the anti-absence drug ethosuximide in the rat and cat thalamus. J Neurosci 1998;18:4842–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape H-C. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci 1995;15:3110–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacinova l Klugbauer N, Hofmann F. Regulation of the calcium channel α1G subunit by divalent cations and organic blockers. Neuropharmacology 2000;39:1254–1266. 10.1016/s0028-3908(99)00202-6 [DOI] [PubMed] [Google Scholar]
- 18.Todorovic SM, Perez-Reyes E, Lingle CJ. Anticonvulsants but not general anesthetics have differential effects on different T-type current variants. Mol Pharmacol 2000;58:98–108. [DOI] [PubMed] [Google Scholar]
- 19.Parri HR, Crunelli V. Sodium current in rat and cat thalamocortical neurons: role of a non-inactivating component in tonic and burst firing. J Neurosci 1998;18:854–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol 1995;483:641–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci 1995;15:623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinault D, Leresche N, Charpier S, Deniau J-M, Marescaux C, Vergnes M, Crunelli V. Intracellular recordings in thalamic neurones during spontaneous spike and wave discharges in rats with absence epilepsy. J Physiol 1998;509:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slaght SJ, Charpier S, Leresche N, Deniau J-M, Crunelli V. Firing properties of thalamic neurons during spike and wave discharges in the GAERS genetic model of absence epilepsy. Soc Neurosci Abstr 2001;27:969.7. [Google Scholar]
- 24.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron 2001;31:35–45. [DOI] [PubMed] [Google Scholar]
- 25.Slaght SJ, Leresche N, Deniau J-M, Crunelli V, Charpier S. Activity of thalamic reticular neurons during spontaneous genetically determined spike and wave discharges. J Neurosci 2002;22:2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci 1999;19:1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol 1993;468:669–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marfia G, Cope DW, Robinson DR, Eugene D, Crunelli V, Leresche N. Ethosuximide decreases the persistent Na+ current and the Ca2+ activated K+ current of rat cortical pyramidal neurones in vitro. Soc Neurosci Abstr 2000;26:662.1. [Google Scholar]


