Abstract
Purpose of Review
Understanding of the aetiology of tubal ectopic pregnancy (tEP) remains incomplete. We aim to summarize the latest advances in laboratory models of tEP that we believe will, ultimately, contribute to improving the diagnosis and management of the condition.
Recent Findings
Progress in proteome pre-fractionation and multidimensional protein identification technology has proved particularly effective in identifying novel biomarkers of tEP. These, and related global proteomic and genomic approaches, have as yet to be fully exploited in this context but do have substantial potential to inform future hypothesis driven studies. The majority of data generated since 2009 to explain the aetiology of tEP continues to derive from descriptive human ex-vivo studies. In-vitro models of Fallopian tube ciliary and smooth muscle function have improved to a limited degree, on the back of continuing advances in imaging and data acquisition. We believe that the recent development of a primary human Fallopian tube epithelium culture system represents the most significant recent advance in laboratory models for studying ectopic pregnancy. There remain no good animal models of tEP.
Summary
The establishment of a viable animal model of tEP remains the key obstacle to a complete understanding the aetiology of the condition.
Keywords: Ectopic pregnancy, tubal implantation, ciliary beat frequency, contractility
Introduction
The diagnosis and clinical management of ectopic pregnancy remains a significant challenge worldwide. Ectopic pregnancy is the leading cause of maternal death in the first trimester of pregnancy in the developed world [1,2], while an estimated 10% of cases admitted to hospitals in developing countries ultimately die from the condition [3].
An ectopic pregnancy is defined as any pregnancy implanted outside the uterus but the vast majority (>98%) occur in the Fallopian tube [2,4] and the aetiology of tubal implantation is still far from resolved. The bulk of the literature to date supports the hypothesis that it occurs due to a combination of impaired embryo-tubal transport and alterations in the Fallopian tube environment that allow implantation to occur [5]. However, no viable animal model of tubal ectopic pregnancy (tEP) currently exists and the greater part of our knowledge derives from descriptive studies of clinical cases [5,6]. If we are to understand its aetiology and, ultimately, improve its diagnosis by identifying candidate biomarkers and advance clinical management by developing novel medical treatments, there is an absolute requirement for good laboratory models of ectopic pregnancy.
Here we review the existing literature on the current laboratory models and their potential for generating key functional data in the future.
Lessons from descriptive studies
Fallopian tube tissue is readily obtainable, with informed consent, from women undergoing surgery for tEP. However, there is clearly no ethical prerogative for obtaining control Fallopian tube tissue from women with healthy intrauterine pregnancies, and this in-itself makes descriptive studies of gene and/or protein expression in the Fallopian tube of women with ectopic pregnancy challenging. One option is to compare Fallopian tube tissue from women with ectopic pregnancy with Fallopian tube tissue collected during the mid-luteal phase of the menstrual cycle from non-pregnant women undergoing hysterectomy for benign gynaecological conditions [7,8]. Another option is to compare the tissue to that obtained from women in a pseudo-pregnant state, induced by treatment with human chorionic gonadotropin in the days before hysterectomy [9]. Both have merit, but are ultimately open to the possibility that any events observed in the ectopic group may also occur in the Fallopian tube during a healthy intrauterine pregnancy and, therefore, be largely irrelevant. Furthermore, as the data are associative, it is also impossible to ascribe any observed changes to a cause or effect of ectopic pregnancy without following the observation up with appropriate functional studies. Although the latter point may not be relevant to studies aimed purely at identifying diagnostic markers of ectopic pregnancy, it is critical to placing any observation in the context of understanding the aetiology of ectopic pregnancy or identifying risk factors for the condition. Recent studies by our group into the role of prokineticins exemplify this approach, by following up observations that smoking and past Chlamydia trachomatis infection were associated with increased expression of prokineticin receptors −1 (PROKR1) and −2 (PROKR2), respectively, with functional studies that confirmed that the smoking metabolite, cotinine, and C. trachomatis upregulate expression of PROKR1 and PROKR2 in vitro [10,11].
Endometrium can also be obtained with informed consent from women undergoing surgery for a tEP. When an embryo implants in the Fallopian tube, maternal cells at the implantation site usually only display very limited, if any, decidual differentiation, but intrauterine endometrial decidualisation is maintained [12]. Similarities in the cellular composition of decidualised endometrium in ectopic and intrauterine pregnancies have been described [13,14]. However, studies from our group have demonstrated that there are differences at a morphological level, and in the expression of markers of decidualisation (prolactin and IGFBP-1), natural antimicrobial peptides, inhibins/activins and cysteine-rich secretory protein 3 in the decidualised endometrium of ectopic compared to intrauterine pregnancies of similar gestations [15,16,17]. We have proposed these differences as a means of identifying candidate serum biomarkers of ectopic implantation [18].
The availability, ethical considerations permitting, of normal trophoblast/embryo from pregnant women undergoing surgical termination of viable pregnancy and surgical management of miscarriage allows direct comparisons to be made between gestation-matched intrauterine and tubal ectopic pregnancies. This approach lends itself to both aetiology [19,20] and diagnostic biomarker [21] focused studies, but is complicated by the technical difficulties involved in obtaining pure trophoblast samples free of maternal tissue contamination [21].
Biological fluids, such as blood and urine, are much more readily accessible for laboratory studies and are the continuing focus of attempts to identify a biomarker profile for ectopic pregnancy. To date, the vast majority of candidate biomarkers have been selected using hypotheses derived from existing literature on ectopic pregnancy and related pathologies [18,22]. Given recent advances in protein separation and mass spectrometry [23,24], it is perhaps a little surprising that more headway has not been made in identifying novel biological markers of ectopic pregnancy using global proteomic analysis. However, even the most advanced mass spectrometers are limited in their ability to detect differences, no matter how dramatic, in the expression of relatively rare proteins against the backdrop of a small number of highly abundant proteins that is typical of biological samples. This is particularly problematic in the case of human serum, where the 20 most abundant proteins make up 97-99% of the total protein complement [25]. Antibody based affinity columns designed to specifically deplete these proteins from sera [26,27] have recently been deployed to help overcome this obstacle [28]. This comprehensive one-dimensional gel electrophoresis/multidimensional protein identification technology (1-DE MudPIT) study, which also employed cutting-edge label-free quantitative mass spectrometry, identified a total of 70 proteins that appear to be differentially expressed in the serum of women with ectopic pregnancies [28]. Subsequent validation studies have confirmed that at least one of these proteins, ADAM-12, is effective at differentiating between ectopic and viable intrauterine pregnancies [29]. Although antibody based depletion columns make moderately abundant protein identification easier by removing the most abundant proteins, they do not modify the dynamic range present in the remaining proteome. Righetti and Boschetti’s concept of using a library of millions of different multimeric peptide baits to selectively enrich for low abundance proteins [30,31] allowed Sennels et al to extend the number of serum proteins identifiable by standard proteomics methods from 60 [32] to almost 4000 [33] and our initial trials of this technology in concert with a 1-DE MudPIT approach suggest it has considerable promise in the context of ectopic pregnancy biomarker discovery (data not shown).
Lessons from functional studies
Embryo transport through the Fallopian tube is widely accepted to be regulated by a combination of ciliary beat activity and smooth muscle contractions [5]. It is a dynamic process that cannot, at this time, be observed directly in vivo and it is unlikely ever to be open to functional analysis in this context in humans.
Ex vivo models for studying regulation of ciliary beat frequency (CBF) have been developed in animals such as the guinea pig [34] and mouse [35,36], and using human Fallopian tube biopsies [37,38]. In theory, CBF is relatively straightforward to measure using phase contrast or differential contrast in concert with moderately high-speed (≥50 Hz) video [39] or confocal laser scanning [40] microscopy (Fig. 1). This methodology is well suited to agonist/antagonist studies but issues arise from the complex nature of the tissue biopsies, with the potential presence of undefined endogenous agonists/antagonists. In addition, imaging can be more complicated that expected due to the size, mobility and transparency of the biopsies, often requiring sophisticated tissue preparation and presentation [35]. It is also difficult to conduct these experiments in an unbiased manner as dead/immobile cilia are very hard to see and less likely to be recorded. Nevertheless, this methodology has shown that CBF is affected by a variety of factors: it is calcium dependent and requires adenosine triphosphate; and beta-adrenergic stimuli, prostaglandins and angiotensin-II increase CBF, whereas high-dose progesterone decreases it [41,42,43,44]. The methodology has also demonstrated that ciliary activity appears to exhibit cyclical changes [35,45]. Although, findings vary between speicies, with CBF in the fimbrial segment increasing in the luteal phase of the menstrual cycle in humans [45] but decreasing during the estrus phase in mice [35].
Figure 1.
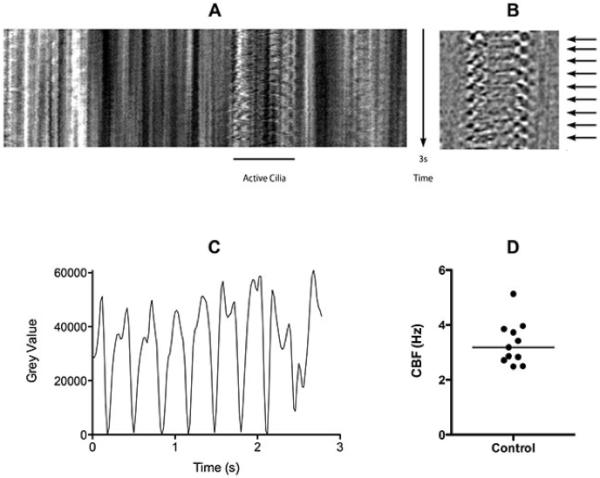
In vitro measurement of cilia beat frequency (CBF) using a confocal laser scanning microscope. Fallopian tube (Lothian research ethics committee 04/S1103/20) was cut into small (2×2 mm) pieces under aseptic conditions and incubated for 48 hrs at 37°C (5% CO2) in RPIM supplemented with 10% FCS and antibiotics. Phase contrast images (512×10 pixels) were collected at 50Hz. A 3s time resolved single line scan through an active cilia is shown (A). Images were subjected to edge enhancement (B) before measuring the fluctuation in grey level over time (C) which corresponds to fluctuations in contrast cause by the movement of the cilia (B: arrows). The CBF of individual cilia was calculated over a 10s period using LabChart software (ADInstruments, Oxford, UK), individual CBF datum and median CBF (3.09 Hz) are shown (D).
Similarly, models of Fallopian tube contractility have been developed using simple methodology involving the isolation of smooth muscle tissue strips or circles from Fallopian tube biopsies under a stereomicroscope. The strips are mounted in chambers under tension and contractions are recorded with a force-displacement transducer and registered on a polygraph or digital data acquisition system (Fig. 2). This approach has demonstrated that the stimulation of alpha adrenergic receptors promotes contraction of the oviductal muscles, while stimulation of beta receptors inhibits contractions [46]. More recent studies have shown that the sex steroid hormones and other factors produced by the oviduct itself, such as nitric oxide [46,47,48], prostacyclin [49] and prostaglandins [49,50,51] may also modulate smooth muscle contraction and play a lead role in embryo transport. This has been complemented by exciting recent studies of murine oviduct smooth muscle contractility that suggest that oviductal pacemaker activity may be damaged by nitric oxide produced in NOS2-expressing macrophages in response to chlamydial infection [52,53].
Figure 2.
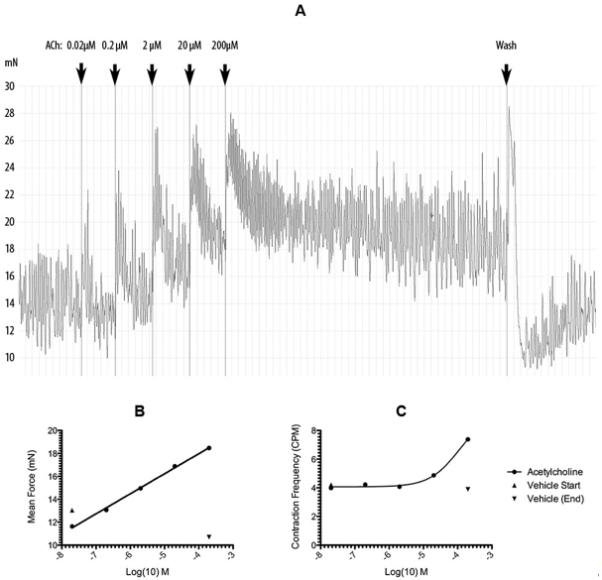
In vitro measurement of Fallopian tube smooth muscle contractility. Human tubal circular smooth muscle rings were be dissected from the ampullo-isthmic junction of Fallopian tube biopsies (Lothian research ethics committee 04/S1103/20) and mounted under 10 mN of tension at 37°C in Kreb’s buffer + Glucose in a organ bath. The strips were attached to isometric transducers and contractility recorded on a data acquisition system using LabChart software (ADInstruments). This allowed fallopian tube smooth muscle contractility to be monitored dynamically in the presence of agonists/antagonists. The effects of adding increasing concentrations of Acetylecholine (Ach) on Fallopian tube smooth muscle contractility are shown over time (A). LabChart software facilitates extraction and analysis of a variety of parameters from the raw transducer data including mean force (B) and contraction frequency (C).
Lessons from cell culture
There are numerous studies that describe human co-culture methods using human embryos and endometrium for the study of endometrial biology [54]. Similar studies using Fallopian tube, designed for further understanding of the aetiology of ectopic pregnancy are lacking due to limited availability of cell lines and problems associated with the use of cells from human Fallopian tubes for co-culture. Only two immortalized oviductal epithelial cell lines have been established [55,56]. The isolation of the epithelial cells from the tubal tissues is technically difficult; the number of cells prepared for co-culture is variable at each opportunity; and tubal epithelial cells, grown in monolayer culture, lose morphological features associated with the epithelium in situ, such as cilia. However, we are optimistic that these problems may be overcome after the recent report of the development of a novel ex vivo primary human Fallopian tube epithelium culture system that faithfully recapitulates the in vivo epithelium, as shown by morphological, ultrastructural and immunophenotypic analyses [57]. Co-culture studies using this model and trophoblast tissue or embryos fertilized in-vitro could be useful in analyzing gene expression changes induced by implantation in the Fallopian tube. The presence of ciliated cells in this system also raises the prospect of a greatly simplified system with which to examine the mechanisms regulating cilia function. Coupled with microarray and proteomic technologies, these studies may prove useful in delineating similarities and differences between tubal and intrauterine implantation.
Lessons from animal models
Several differences between primates (human and non-human) and animals suffering from ectopic pregnancy exist primarily with regard to the site of ectopic implantation [6]. In animals, the abdominal cavity is the most frequent extra-uterine implantation site and only three cases of tubal pregnancy in primates have been reported to date [58,59]. Nevertheless, important information pertaining to the aetiology of tEP has been derived from rodent models [60]. The biological importance of the endocannabinoid receptor, CB1 in the tubal transport process is illustrated by the presence of retained embryos in mice that lack either CB1 or CB1/2, or mice that have been treated with synthetic CB1 antagonist [61]. Embryo retention is also a characteristic of the deletion of Dicer1, ribonuclease III enzyme required for micro-RNA processing. While the complete loss of Dicer1 in mice results in early embryonic lethality [62], studies in female mice carrying a floxed allele of Dicer1 (under the control of antimüllerian hormone receptor 2 promoter-driven Cre recombinase) have revealed that these mice exhibit tubal hypotrophy with the formation of prominent tubal cysts, leading to the disruption of tubal transport [63,64,65]. We believe that future studies should be aimed at inducing tubal implantation in mice to generate useful animal models for ectopic pregnancy. For example, mouse models employing conditional knock-in of specific genes important for implantation in the oviduct (ie. PROKs, COX-2, HOXA10 and LIF) may result in mice in which embryos (fertilized in vivo or in vitro) implant within the oviduct. Treatment of mice with agents aimed at directly disrupting tubal smooth muscle contractility and/or ciliary beat activity, such as PROK, CB1 or NO antagonists may also prove useful in developing an animal model of tubal implantation.
Conclusion
The absence of an animal model of tEP continues to confound attempts to delineate the mechanisms that underlie the condition. The authors are optimistic that a genetically modified mouse model of tEP, or at least implantation, is an achievable goal. In the meantime, workers in the field will have to continue to make the most of clinical samples and the in vitro models of Fallopian tube described in the current review.
Key Points.
Data from human ex-vivo descriptive studies are associative and cannot be ascribed to a cause or effect of ectopic pregnancy without appropriate follow-up functional studies.
The lack of a viable animal model of tubal ectopic pregnancy remains the key obstacle to defining the aetiology of the condition.
Global proteomic and genomic approaches are proving effective in identifying potential biomarkers for ectopic pregnancy.
A new primary human Fallopian tube epithelium culture system represents a significant recent advance in the available laboratory models for studying ectopic pregnancy
Acknowledgements
Andrew Horne is supported by an MRC Clinician Scientist Fellowship.
References
- 1.Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583–591. doi: 10.1016/S0140-6736(05)67103-6. [DOI] [PubMed] [Google Scholar]
- 2.Varma R, Gupta J. Tubal ectopic pregnancy. Clin Evid (Online) 2009:1406. [PMC free article] [PubMed] [Google Scholar]
- 3.Leke RJ, Goyaux N, Matsuda T, Thonneau PF. Ectopic pregnancy in Africa: a population-based study. Obstet Gynecol. 2004;103:692–697. doi: 10.1097/01.AOG.0000120146.48098.f2. [DOI] [PubMed] [Google Scholar]
- 4.Walker JJ. Ectopic pregnancy. Clin Obstet Gynecol. 2007;50:89–99. doi: 10.1097/GRF.0b013e31802f4f79. [DOI] [PubMed] [Google Scholar]
- 5.Shaw JL, Dey SK, Critchley HO, Horne AW. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update. 2010;16:432–444. doi: 10.1093/humupd/dmp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corpa JM. Ectopic pregnancy in animals and humans. Reproduction. 2006;131:631–640. doi: 10.1530/rep.1.00606. [DOI] [PubMed] [Google Scholar]
- 7.Shaw JL, Denison FC, Evans J, et al. Evidence of prokineticin dysregulation in fallopian tube from women with ectopic pregnancy. Fertil Steril. 2010;94:1601–1608. doi: 10.1016/j.fertnstert.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges LE, Horne AW, McDonald SE, et al. Attenuated Tubal and Endometrial Urocortin1 and Corticotropin-Releasing Hormone Receptor Expression in Ectopic Pregnancy. Reprod Sci. 2010 doi: 10.1177/1933719110385132. doi: 10.1177/1933719110385132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Azemi M, Refaat B, Amer S, et al. The expression of inducible nitric oxide synthase in the human fallopian tube during the menstrual cycle and in ectopic pregnancy. Fertil Steril. 2010;94:833–840. doi: 10.1016/j.fertnstert.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JL, Oliver E, Lee KF, et al. Cotinine exposure increases Fallopian tube PROKR1 expression via nicotinic AChRalpha-7: a potential mechanism explaining the link between smoking and tubal ectopic pregnancy. Am J Pathol. 2010;177:2509–2515. doi: 10.2353/ajpath.2010.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw JL, Wills GS, Lee KF, et al. Chlamydia trachomatis infection increases fallopian tube PROKR2 via TLR2 and NFkappaB activation resulting in a microenvironment predisposed to ectopic pregnancy. Am J Pathol. 2011;178:253–260. doi: 10.1016/j.ajpath.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floridon C, Nielsen O, Holund B, et al. Localization and significance of urokinase plasminogen activator and its receptor in placental tissue from intrauterine, ectopic and molar pregnancies. Placenta. 1999;20:711–721. doi: 10.1053/plac.1999.0425. [DOI] [PubMed] [Google Scholar]
- 13.Vassiliadou N, Bulmer JN. Characterization of tubal and decidual leukocyte populations in ectopic pregnancy: evidence that endometrial granulated lymphocytes are absent from the tubal implantation site. Fertil Steril. 1998;69:760–767. doi: 10.1016/s0015-0282(98)00005-3. [DOI] [PubMed] [Google Scholar]
- 14.von Rango U, Classen-Linke I, Kertschanska S, et al. Effects of trophoblast invasion on the distribution of leukocytes in uterine and tubal implantation sites. Fertil Steril. 2001;76:116–124. doi: 10.1016/s0015-0282(01)01859-3. [DOI] [PubMed] [Google Scholar]
- 15.Horne AW, van den Driesche S, King AE, et al. Endometrial inhibin/activin beta-B subunit expression is related to decidualization and is reduced in tubal ectopic pregnancy. J Clin Endocrinol Metab. 2008;93:2375–2382. doi: 10.1210/jc.2008-0136. [DOI] [PubMed] [Google Scholar]
- 16.Horne AW, Duncan WC, King AE, et al. Endometrial cysteine-rich secretory protein 3 is inhibited by human chorionic gonadotrophin, and is increased in the decidua of tubal ectopic pregnancy. Mol Hum Reprod. 2009;15:287–294. doi: 10.1093/molehr/gap019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalgetty DM, Sallenave JM, Critchley HO, et al. Altered secretory leukocyte protease inhibitor expression in the uterine decidua of tubal compared with intrauterine pregnancy. Hum Reprod. 2008;23:1485–1490. doi: 10.1093/humrep/den130. [DOI] [PubMed] [Google Scholar]
- 18.Horne AW, Duncan WC, Critchley HO. The need for serum biomarker development for diagnosing and excluding tubal ectopic pregnancy. Acta Obstet Gynecol Scand. 2010;89:299–301. doi: 10.3109/00016340903568191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp B, Schmitz S, Krusche CA, et al. Dendritic cells are equally distributed in intrauterine and tubal ectopic pregnancies. Fertil Steril. 2011;95:28–32. doi: 10.1016/j.fertnstert.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher A, Brachwitz N, Sohr S, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 21.Horne AW, Shaw JL, Murdoch A, et al. Placental growth factor: a promising diagnostic biomarker for tubal ectopic pregnancy. J Clin Endocrinol Metab. 2011;96:E104–108. doi: 10.1210/jc.2010-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal S, Mercado R, Rivnay B. Ectopic pregnancy early diagnosis markers. Minerva Ginecol. 2010;62:49–62. [PubMed] [Google Scholar]
- 23.Hawkridge AM, Muddiman DC. Mass spectrometry-based biomarker discovery: toward a global proteome index of individuality. Annu Rev Anal Chem (Palo Alto Calif) 2009;2:265–277. doi: 10.1146/annurev.anchem.1.031207.112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian WJ, Jacobs JM, Liu T, et al. Advances and challenges in liquid chromatography-mass spectrometry-based proteomics profiling for clinical applications. Mol Cell Proteomics. 2006;5:1727–1744. doi: 10.1074/mcp.M600162-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 26.Echan LA, Tang HY, Ali-Khan N, et al. Depletion of multiple high-abundance proteins improves protein profiling capacities of human serum and plasma. Proteomics. 2005;5:3292–3303. doi: 10.1002/pmic.200401228. [DOI] [PubMed] [Google Scholar]
- 27.Bjorhall K, Miliotis T, Davidsson P. Comparison of different depletion strategies for improved resolution in proteomic analysis of human serum samples. Proteomics. 2005;5:307–317. doi: 10.1002/pmic.200400900. [DOI] [PubMed] [Google Scholar]
- 28.Beer LA, Tang HY, Sriswasdi S, et al. Systematic Discovery of Ectopic Pregnancy Serum Biomarkers Using 3-D Protein Profiling Coupled with Label-free Quantitation. J Proteome Res. 2011;10:1126–1138. doi: 10.1021/pr1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rausch ME, Beer L, Sammel MD, et al. A disintegrin and metalloprotease protein-12 as a novel marker for the diagnosis of ectopic pregnancy. Fertil Steril. 2011 doi: 10.1016/j.fertnstert.2010.12.040. doi:10.1016/j.fertnstert.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Righetti PG, Boschetti E. The ProteoMiner and the FortyNiners: searching for gold nuggets in the proteomic arena. Mass Spectrom Rev. 2008;27:596–608. doi: 10.1002/mas.20178. [DOI] [PubMed] [Google Scholar]
- 31.Boschetti E, Righetti PG. The ProteoMiner in the proteomic arena: a non-depleting tool for discovering low-abundance species. J Proteomics. 2008;71:255–264. doi: 10.1016/j.jprot.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Hughes GJ, Frutiger S, Paquet N, et al. Plasma protein map: an update by microsequencing. Electrophoresis. 1992;13:707–714. doi: 10.1002/elps.11501301150. [DOI] [PubMed] [Google Scholar]
- 33.Sennels L, Salek M, Lomas L, et al. Proteomic analysis of human blood serum using peptide library beads. J Proteome Res. 2007;6:4055–4062. doi: 10.1021/pr070339l. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura A, Sakuma K, Shimamoto C, et al. Ciliary beat frequency controlled by oestradiol and progesterone during ovarian cycle in guinea-pig Fallopian tube. Exp Physiol. 2010;95:819–828. doi: 10.1113/expphysiol.2010.052555. [DOI] [PubMed] [Google Scholar]
- 35.Shi D, Komatsu K, Uemura T, Fujimori T. Analysis of ciliary beat frequency and ovum transport ability in the mouse oviduct. Genes Cells. 2011;16:282–290. doi: 10.1111/j.1365-2443.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- 36.Bylander A, Nutu M, Wellander R, et al. Rapid effects of progesterone on ciliary beat frequency in the mouse fallopian tube. Reprod Biol Endocrinol. 2010;8:48. doi: 10.1186/1477-7827-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li HW, Liao SB, Chiu PC, et al. Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium. J Clin Endocrinol Metab. 2010;95:E18–25. doi: 10.1210/jc.2010-0273. [DOI] [PubMed] [Google Scholar]
- 38.Papathanasiou A, Djahanbakhch O, Saridogan E, Lyons RA. The effect of interleukin-6 on ciliary beat frequency in the human fallopian tube. Fertil Steril. 2008;90:391–394. doi: 10.1016/j.fertnstert.2007.07.1379. [DOI] [PubMed] [Google Scholar]
- 39.Lyons RA, Saridogan E, Djahanbakhch O. The effect of ovarian follicular fluid and peritoneal fluid on Fallopian tube ciliary beat frequency. Hum Reprod. 2006;21:52–56. doi: 10.1093/humrep/dei306. [DOI] [PubMed] [Google Scholar]
- 40.Doyle RT, Moninger T, Debavalya N, Hsu WH. Use of confocal linescan to document ciliary beat frequency. J Microsc. 2006;223:159–164. doi: 10.1111/j.1365-2818.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- 41.Saridogan E, Djahanbakhch O, Puddefoot JR, et al. Angiotensin II receptors and angiotensin II stimulation of ciliary activity in human fallopian tube. J Clin Endocrinol Metab. 1996;81:2719–2725. doi: 10.1210/jcem.81.7.8675601. [DOI] [PubMed] [Google Scholar]
- 42.Verdugo P. Ca2+-dependent hormonal stimulation of ciliary activity. Nature. 1980;283:764–765. doi: 10.1038/283764a0. [DOI] [PubMed] [Google Scholar]
- 43.Verdugo P, Rumery RE, Tam PY. Hormonal control of oviductal ciliary activity: effect of prostaglandins. Fertil Steril. 1980;33:193–196. doi: 10.1016/s0015-0282(16)44541-3. [DOI] [PubMed] [Google Scholar]
- 44.Villalon M, Verdugo P. Hormonal regulation of ciliary function in the oviduct: the effect of beta-adrenergic agonists. Prog Clin Biol Res. 1982;80:59–65. doi: 10.1002/cm.970020713. [DOI] [PubMed] [Google Scholar]
- 45.Mahmood T, Saridogan E, Smutna S, et al. The effect of ovarian steroids on epithelial ciliary beat frequency in the human Fallopian tube. Hum Reprod. 1998;13:2991–2994. doi: 10.1093/humrep/13.11.2991. [DOI] [PubMed] [Google Scholar]
- 46.Samuelson UE, Sjostrand NO. Myogenic and neurogenic control of electrical and mechanical activity in human oviductal smooth muscle. Acta Physiol Scand. 1986;126:355–363. doi: 10.1111/j.1748-1716.1986.tb07827.x. [DOI] [PubMed] [Google Scholar]
- 47.Ekerhovd E, Brannstrom M, Alexandersson M, Norstrom A. Evidence for nitric oxide mediation of contractile activity in isolated strips of the human Fallopian tube. Hum Reprod. 1997;12:301–305. doi: 10.1093/humrep/12.2.301. [DOI] [PubMed] [Google Scholar]
- 48.Ekerhovd E, Norstrom A. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of fallopian tube contractility. Gynecol Endocrinol. 2004;19:239–246. doi: 10.1080/09513590400019296. [DOI] [PubMed] [Google Scholar]
- 49.Arbab F, Goldsby J, Matijevic-Aleksic N, et al. Prostacyclin is an autocrine regulator in the contraction of oviductal smooth muscle. Hum Reprod. 2002;17:3053–3059. doi: 10.1093/humrep/17.12.3053. [DOI] [PubMed] [Google Scholar]
- 50.Wanggren K, Lalitkumar PG, Hambiliki F, et al. Leukaemia inhibitory factor receptor and gp130 in the human Fallopian tube and endometrium before and after mifepristone treatment and in the human preimplantation embryo. Mol Hum Reprod. 2007;13:391–397. doi: 10.1093/molehr/gam013. [DOI] [PubMed] [Google Scholar]
- 51.Wanggren K, Lalitkumar PG, Stavreus-Evers A, et al. Prostaglandin E2 and F2alpha receptors in the human Fallopian tube before and after mifepristone treatment. Mol Hum Reprod. 2006;12:577–585. doi: 10.1093/molehr/gal058. [DOI] [PubMed] [Google Scholar]
- 52.Dixon RE, Ramsey KH, Schripsema JH, et al. Time-dependent disruption of oviduct pacemaker cells by Chlamydia infection in mice. Biol Reprod. 2010;83:244–253. doi: 10.1095/biolreprod.110.083808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon RE, Hwang SJ, Hennig GW, et al. Chlamydia infection causes loss of pacemaker cells and inhibits oocyte transport in the mouse oviduct. Biol Reprod. 2009;80:665–673. doi: 10.1095/biolreprod.108.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teklenburg G, Macklon NS. Review: in vitro models for the study of early human embryo-endometrium interactions. Reprod Sci. 2009;16:811–818. doi: 10.1177/1933719109334966. [DOI] [PubMed] [Google Scholar]
- 55.Ando H, Kobayashi M, Toda S, et al. Establishment of a ciliated epithelial cell line from human Fallopian tube. Hum Reprod. 2000;15:1597–1603. doi: 10.1093/humrep/15.7.1597. [DOI] [PubMed] [Google Scholar]
- 56.Lee YL, Lee KF, Xu JS, et al. Establishment and characterization of an immortalized human oviductal cell line. Mol Reprod Dev. 2001;59:400–409. doi: 10.1002/mrd.1046. [DOI] [PubMed] [Google Scholar]
- 57.Levanon K, Ng V, Piao HY, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jerome CP, Hendrickx AG. A tubal pregnancy in a rhesus monkey (Macaca mulatta) Vet Pathol. 1982;19:239–245. doi: 10.1177/030098588201900303. [DOI] [PubMed] [Google Scholar]
- 59.Lapin BA, Yakovleva LA. Comparitive Pathology in Monkeys. Edited by Thomas CC. Location of Publisher: Springfield; Illinois: 1963. [Google Scholar]
- 60.Shao R. Understanding the mechanisms of human tubal ectopic pregnancies: new evidence from knockout mouse models. Hum Reprod. 2010;25:584–587. doi: 10.1093/humrep/dep438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Guo Y, Wang D, et al. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- 62.Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 63.Nagaraja AK, Andreu-Vieyra C, Franco HL, et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong X, Luense LJ, McGinnis LK, et al. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology. 2008;149:6207–6212. doi: 10.1210/en.2008-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez G, Behringer RR. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev. 2009;76:678–688. doi: 10.1002/mrd.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]


