Abstract
This review begins with a description of some problems that in recent years have beset an influential circuit model of fear-conditioning and goes on to look at neuroanatomy that might subserve conditioning viewed in a broader perspective, including not only fear, but also appetitive, conditioning. The paper then focuses on basal forebrain functional-anatomical systems, or macrosystems, as they have come to be called, which Lennart Heimer and colleagues described beginning in the 1970’s. Yet more specific attention is then given to the relationships of the dorsal and ventral striatopallidal systems and extended amygdala with the dopaminergic mesotelencephalic projection systems, culminating with the hypothesis that all macrosystems contribute to behavioral conditioning.
There is tremendous current interest in the neurobiological mechanisms underlying conditioned fear stemming in large part from an increasing prevalence in American culture of anxiety and panic disorders, not to mention PTSD1. By 2000, the relevant brain circuitry had seemed to be satisfactorily described2, but a number of serious caveats had been voiced the preceding year3, and an unraveling process accelerated thereafter. Indeed, current theory on the neural substrates of fear conditioning has entered into a state of reassessment4.
The essential elements of fear conditioning are described by the observation that pairing neutral and fear-arousing stimuli causes the neutral one to gain meaning such that it can then drive an organism’s voluntary and involuntary actions. Thus, behaviorally, rats exposed to a brief tone followed immediately by a footshock will soon, frequently after a single trial, come to “freeze” upon hearing the tone. In LeDoux’s2 model of this phenomenon, neuroplasticity reflecting the attachment of “significance” to a neutral stimulus, i.e., heralding the transformation of neutral to conditioned stimulus (CS), occurs in the amygdala, specifically its lateral nucleus (LA). According to LeDoux’s model, LA projects to another part of the amygdala, the central nucleus (CeA), which, in turn sends out divergent descending projections to somatic and autonomic motor effectors in the brainstem, eliciting behavioral freezing and accompanying autonomic responses. Consistent with the model, [1] sensory inputs bearing information about the aversive and neutral (to be conditioned) stimuli converge in LA5, and [2] an increase in the efficacy of CS-related synapses corresponds to conditioning6–8. But, soon it was realized that the CeA consists of two parts, a medial division (CeAm) from which most of its descending projections arise and to which LA does not project, and a lateral one (CeAl) to which it does. While CeAl projects to CeAm and thus might serve as a relay interposed between LA and CeAm, the CeAl to CeAm projection is nearly exclusively inhibitiory (GABAergic) and thus would inhibit rather than activate outputs to brainstem. The model was accordingly adjusted to emphasize instead a projection from LA to amygdaloid “intercalated” nuclei9, which are located between CeAm and CeAl and project to CeAm. This also is a GABAergic projection, however, making it is difficult to conceive how this solves the problem, but because intercalated nuclei comprise several interconnected cell masses, it was reasoned that activation of one would inhibit its neighbor, which in turn would disinhibit the CeAm10. This seems a possible, but precarious, foundation upon which to build such a biologically important function as fear conditioning, and, in any event, more issues came to plague the model (e.g., Ref. 11) Despite this accumulation of complications, the status of the amygdala as a major player in stimulus-consequence associations12–15, seems not to be in jeaopardy (e.g., Refs. 4 and 16), although the underlying brain circuitry and physiological mechanisms appear to require further investigation.
In considering this dilemma, recall that conditioning occurs not only in response to fear-arousing and aversive stimuli, but also to appetitive cues, as in, e.g., conditioned place preference (e.g., Refs. 17 and 18) and postural orienting directed to a CS (e.g., Refs. 19 and 20), and not only in the amygdala. Indeed, appetitive Pavlovian conditioned responses are abolished by lesions in the accumbens territory of the ventral striaum17, which turns out to also contribute to specific forms of aversive conditioning21–24. Moreover, lesions of the CeA not only abolish aversive conditioning, as in freezing to a CS, as described above, but also disrupt orienting to appetitive conditioned cues16, 25. Thus, both structures support Pavlovian responses to fear-arousing and appetitive stimuli, although each may “specialize” in one or the other. This suggests that both structures possess a general capacity to recognize stimulus “significance” and a more specialized capacity to assess the associated adaptive implications in order that a proper Pavlovian response will be mounted. Insofar as function follows structure, it seems reasonable to expect that the neuroanatomical organizations of the CeA and accumbens also should exhibit both similarities and differences and that these might provide some additional insight into the neural mechanisms that underlie conditioning.
This expectation is fulfilled by the concept of basal forebrain functional-anatomical systems or macrosystems, as they came to be called26, 27. Among these, are the dorsal striatopallidal system (basal ganglia as classically described), ventral striatopallidal system (which, relevant to this discussion, includes the accumbens28, 29) and extended amygdala (which includes the CeA30). Structural similarities shared by different macrosystems are reflected in a basic “framework”, essentially that of the basal ganglia30, in which massive projections from the cortical mantle or cortical-like structures31–33, a category that includes LA, terminate densely in subcortical “input” structures consisting predominantly of medium-sized, densely spiny inhibitory (i.e., GABAergic) neurons. Macrosystem input structures, including the CeA and accumbens, also receive massive inputs from the brainstem reticular formation via the midline/intralaminar thalamic nuclei and brainstem monoaminergic cell groups, especially dopaminergic. Medium spiny neurons, in turn, may project out of the macrosystem, as outputs, or massively to structures regarded as part of the macrosystem “intrinsic” circuitry constituting somewhat larger sparsely-spined, GABAergic “pallidal”-like neurons with long radiating aspiny dendrites, such as found in the globus pallidus, ventral pallidum and CeAm. Pallidal-like neurons also may project intrinsically or give rise to outputs. Macrosystem outputs diverge into [1] reentrant pathways to the forebrain, including cortex, via synaptic relays in the thalamus, forebrain and brainstem and [2] descending pathways to somatic and autonomic motor effectors, via relays in the hypothalamus, mesopontine tegmentum and caudal brainstem. Accompanying the host of basic similarities shared by macrosystems are a variety of features that distinguish them, such as the richness and extent of medium spiny neuronal intrinsic axonal arbors, the numbers and transmitter phenotypes of associated large interneurons and the quantity and indentities of neuropeptides, neuropeptide and transmitter receptors, and intracellular signaling cascades utilized30, 34–36.
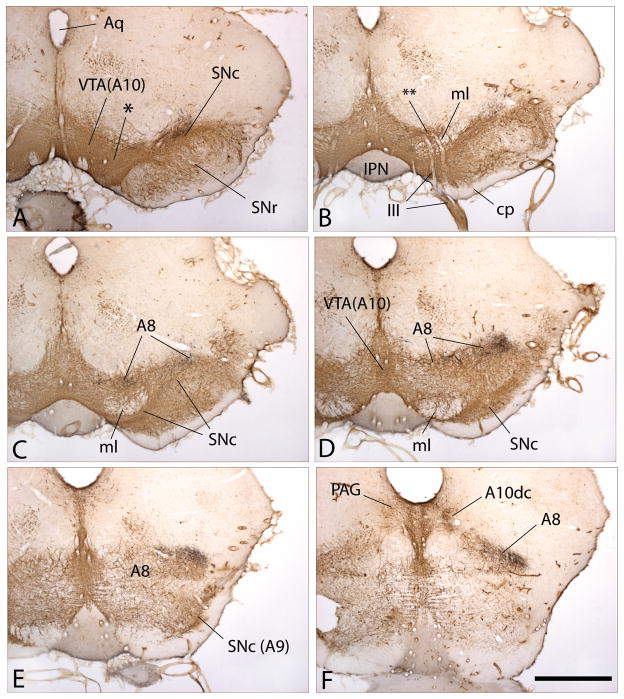
This paper takes a closer look at the dopaminergic innervation of macrosystems. Early pioneering studies revealed with astounding clarity that catecholaminergic and indoleaminergic cell groups embedded in the brainstem provide ascending and descending projections to virtually all parts of the brain and, particularly abundantly, to the basal ganglia and other basal forebrain structures37–41. By distinguishing different fluorescent hues, these investigators discriminated norepinephrine and dopamine (which emit at similar wavelengths and were designated as “A” cell groups and projections) from serotonin (B groups) and epinephrine (C groups). Among the catecholamine-fluorescing cell groups subsequently identified as dopaminergic42, the A8, A9 and A10 groups, occupying, respectively, the midbrain retrorubral field, substantia nigra compacta (SNc) and ventral tegmental area (VTA), are related by connections most strongly to the basal forebrain macrosystems. Although individually designated, A8, A9 and A10 actually comprise a single continuous constellation of dopaminergic neurons (Fig. 1A–F), approximating the form of an ellipsoid encircling the medial lemniscus with A10 (occupying the VTA) lodged in the ventromedial tegmentum and A8 (occupying the retrorubral field) and A9 (occupying the SNc), respectively, extending lateralward above and below the medial lemniscus to meet again in the ventrolateral tegmentum. In addition, an appendage of A8 arches caudomedialward toward the ventrolateral periaqueductal gray (Fig. 1F). Where confluent (e.g., * in Fig. 1A and ** in Fig. 1B), neurons in A8 are indistinguishable from those in A9 or A10, as are those in A9 from those in A10. Nonetheless, A8, A9 and A10 are structurally and functionally differentiated, as is reflected in the relatively distinct, albeit overlapping, topographies of their ascending projections43–50, to be discussed below. Hökfelt et al.51 designated some additional dopaminergic districts, of which only one will be mentioned here - A10dc (dc - dorsal, caudal) is located in the mesopontine periaqueductal gray (PAG) in the vicinity of the dorsal raphe nucleus (Figs. 1F and 2B).
Figure 1.
Figure 1A–F. Photomicrographs illustrating a series of sections through the mesencephalon of the rat shown from A to F in rostrocaudal order. The sections were processed to exhibit immunoreactivity against tyrosine hydroxylase, which marks ventral mesencephalic dopaminergic neurons and axons brown, and thus shows the ventral tegmental area (VTA(A10)), substantial nigra pars compacta (SNc(A9)), and retrorubral field (A8). The juncture of VTA(A10) and SNc(A9) is indicated by * in panel A, as is the zone where VTA(A10) becomes A8 by ** in panel B and continuities between A10, A9 and A8 can be observed in all of the panels. Note in panel A that the dendrites of SNc(A9) dopaminergic neurons extend downward into the substantia nigra pars reticulata (SNr). Panel F illustrates the caudomedial extension of A8 and the tyrosine hydroxylase-immunoreactive (possibly L-DOPA containing) neurons in the periaqueductal gray (PAG), which are designated as A10dc. The black substance in all of the panels marks axons projecting from the central extended amygdala, specifically from the bed nucleus of stria terminalis, that were labeled in the laboratory with a dye. Note that the labeled pathway skirts past the SNc in panels A and B to terminate relatively exclusively within A8. The labeled axons in A8 and A10dc shown panel F are enlarged in Figs. 2A and B, respectively. Other abbreviations: III - oculomotor (3rd cranial) nerve and roots; Aq - cerebral aqueduct; cp - cerebral peduncle; IPN -interpeduncular nucleus; ml - medial lemniscus. Scale bar: 1 mm.
Figure 2.
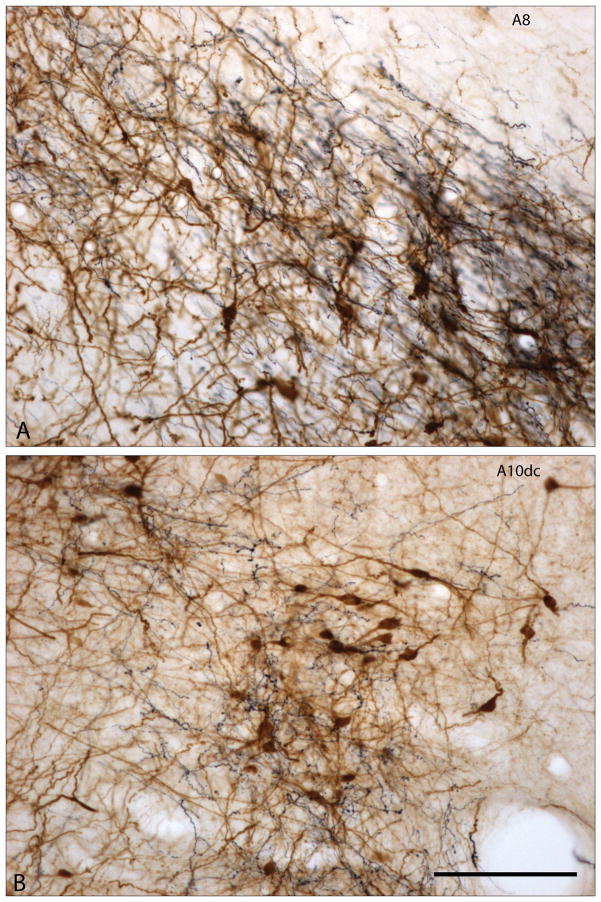
Figure 2A and B. Photomicrographs showing enlargements of areas designated as A8 and A10dc in Fig. 1F. Tyrosine hydroxylase immunoreactive elements are brown and dye-labeled axons projecting from the bed nucleus of stria terminalis are black. The dye-labeled axons form a dense plexus of fibers containing many varicosities suggestive of abundant synaptic contacts. Scale bar: 100 μm.
A9 (the SNc) gives rise to the mesostriatal projection, which, essentially, provides dense dopaminergic innervation to the caudate nucleus and putamen (i.e., the “input” nuclei of the basal ganglia). The caudate-putamen, which also receives massive input from isocortex (neocortex), is involved in the initiation and control of voluntary movements, development and maintenance of motor habits, and possibly the structuring of some cognitive processes52. In turn, the caudate-putamen and other basal ganglia structures, including the globus pallidus and substantia nigra project prominently to A9, which also receives ascending afferents from a number of structures in the brainstem. Mesolimbic projections, from A10 (in the VTA), provide a dense dopaminergic innervation to ventral striatopallidum, and, to a lesser extent, the extended amygdala (CeA, bed nucleus of stria terminalis and associated structures), as well as to a number of other sites in the basal forebrain, such as the septum and preoptic region. All of these structures project back to A10 directly, but this forms but part of the A10 afferent system, which comprises a nearly continuous and extensively interconnected formation of structures extending from the prefrontal cortex to the caudal brainstem53–56. Mesolimbic dopaminergic projections are reported to be involved in a broad range of functions, including locomotor activation (e.g., Ref. 57), reward (e.g., Ref. 58), motivation (e.g., Ref. 59), novelty detection (e.g., Ref. 60), reward prediction and error detection (eg., Ref. 61), and memory and learning (e.g., Ref. 62). Moreover, A10 and its projections, particularly to the accumbens, were identified early on as the primary sites of attack of psychostimulant and opiate drugs of abuse, which were said to “hijack” the reward system. The A10 dopamine-accumbens axis soon became regarded as the target most subject to maladaptative neurochemical, molecular and electrophysiological reorganizations in response to chronic (and acute, as it turns out) administration of such drugs (e.g, Refs. 63 and 64).
In contrast, A8 (the retrorubral field) and its projection system and neural connections, by comparison, have been relatively neglected, having not even been considered in one classic description of the ventral mesencephalic efferents65. Nor did Lindvall and Björkland46 or Fallon and Loughlin47, 48 have much to say about A8 in their respective chapters on central dopamine-containing neuronal systems, and Fallon50 intentionally omitted consideration of A8 in deference to a brief report on it in the same congress49. As it turns out, just as A9 is most closely associated with neostriatum and the somatomotor apparatus, and A10 with the ventral striatopallidum36, A8 appears to be closely related to extended amygdala, which, as noted above, a substantial literature ties closely to behaviors driven by fear and anxiety (see also Refs. 66–68). The paper by Deutch et al.49 sketched out connections of A8 with structures that would in the same year be defined as comprising the central division of the extended amygdala30 and subsequent tract tracing studies have born out this connectional relationship. Thus, A8 is densely innervated by the central nucleus of the amygdala69, 70 and bed nucleus of stria terminalis36, 71. Interestingly, fibers descending from extended amygdaloid structures mainly pass through the VTA (A10) with minimal functional relationship (few axonal varicosities, regarded as sites of synaptic potency) before turning lateralward toward A8 and the lateral part of A9 (Figs. 1A–F and 2A), where many terminal axonal branches and axonal varicosities are observed. This varicosity-rich descending projection of the extended amygdala then continues beyond A8 to enter the periaqueductal gray, where it forms another dense plexus of varicosity-laden terminations among the putatively dopaminergic neurons comprising Hökfelt et al.’s51 A10dc (Figs. 1F and 2B). It has recently been shown that A10dc, which may utilize L-DOPA as a neurotransmitter in place of dopamine52, represents that part of the A8–A10 complex that projects most robustly to the central division of the extended amgydala73, followed in decreasing order by A10 proper, A8, and A9 (Table 1). Thus, it may make sense to group A10dc neurons with A8, in view of their rich connectional relationship with the extended amygdala.
Table 1.
Numbers and % total of tyrosine hydroxylase immunoreactive neurons innervating the central nucleus of the amgydala (CeA) and bed nucleus of stria terminalis (BST)*
| Dahlström and Fuxe (1964) designation | Conventional nomenclature | CeA | % total | BST | % total |
|---|---|---|---|---|---|
| A10dc** | periaqueductal gray | 490 | 45.3 | 574 | 42.9 |
| A10 | ventral tegmental area | 259 | 24.0 | 324 | 24.2 |
| A8 | retrorubral field | 130 | 12.1 | 153 | 11.4 |
| A9 | substantia nigra compacta | 97.0 | 9.0 | 44.0 | 3.3 |
| A12 | hypothalamic arcuate nucleus | 39.7 | 3.7 | 122 | 9.1 |
| A11 | periventricular gray | 28.3 | 2.6 | 29.5 | 2.2 |
| A14 | periventricular hypothalamus | 27.1 | 2.5 | 82.0 | 6.1 |
| A13 | medial zona incerta | 8.4 | 0.8 | 9.5 | 0.7 |
Data were re-calculated from Table 1 in Hasue and Shammah-Lagnado (2002) and reflect the average numbers (CeA [n=7]; BST [n=2]) and percentages (% total) of retrogradely labeled perikarya that were tyrosine-hydroxylase immunoreactive. Perikaryal profiles were counted in every section of a 160 μm-spaced series.
Structures are listed in order of descending % total. A10dc designation is from Hökfelt et al. (1984)
To summarize, fear conditioning is inadequately addressed by a circuit model proposed by Ledoux and colleagues. Conditioned stimuli reflecting fear-arousing and appetitive associations, are best subserved by the amygdala and accumbens, respectively, although the amygdala can modulate the formation of certain forms appetitive, as can the accumbens the formation of certain forms of aversive, associations. In view of this evidence one might hypothesize that the capacity to recognize that a stimulus is significant, a more general aspect of Pavlovian conditioning, is reflected in neuroanatomical organization common to macrosystems, whereas synthesizing an appropriate response to specific stimulus modality, i.e., fear-arousing or appetitive, is reflected in their unique neuroanatomical features. Put more succinctly, the capacity of brain to form neural associations reflecting the interrelationships of various internal and external stimuli is hypothesized to be a property of all basal forebrain macrosystems and to involve their intrinsic and extrinsic connections.
It has been shown herein that shared and unique features also the characterize the dopaminergic connections of macrosystems. Consistent with the striking reciprocity of connections between the SNc (A9) and caudate-putamen, VTA (A10) and accumbens, and A8/A10dc and extended amygdala, lesions and perturbations of dopaminergic innervations in the extended amygdala and accumbens do disrupt fear and appetitive conditioning, respectively74–79 and opposing modulations of the activity of dopaminergic neurons have been correlated with the presentation and omission of appetitive stimuli61. While the precise role played by dopaminergic mechanisms within the macrosystems in the formation and expression of associations underlying conditioning remains to be determined, it seems likely that such associations are an important element in most of the functions that have been attributed to dopamine, such as locomotor activation, reward, motivation, novelty detection, reward prediction, error detection and memory and learning (refs. cited above). The likelihood that dopaminergic actions on association formation play out in several different basal forebrain macrosystems, including the dorsal and ventral striatopallidum, extended amygdala, and the septal-preoptic system30, 33, 36, 80, all acting somewhat differently on the same and different sets of neural associations, suggests that the use of dopaminergic drugs, whether therapeutic or illicit, may have wide ranging behavioral effects.
Acknowledgments
The author is indebted to Beth DeGarmo for outstanding technical assistance. This work was supported by USPHS grants NIH MH-70624, DA-15207 and NS-23805.
Literature Cited
- 1.Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Lowe B. Anxiety disordes in primary care: prevalence, impairment, comorbidity and detection. Ann Int Med. 146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 2.LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 3.Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdal a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- 4.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner BJ, Zimmer J. The architecture and some of the interconnections of the rat’s amygdala and lateral periallocortex. J Comp Neurol. 1984;227:540–557. doi: 10.1002/cne.902270406. [DOI] [PubMed] [Google Scholar]
- 6.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 7.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 8.Collins DR, Paré D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paré D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 11.Koo JW, Han J-S, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiskrantz L. Behavioral changes associated with ablations of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;29:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 13.Kellicut MH, Schwartzbaum JS. Formation of a conditioned emotional response (CER) following lesions of the amygdaloid complex in rats. Psychol Rev. 1963;12:351–358. [Google Scholar]
- 14.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 15.Spevack AA, Campbell CT, Drake L. Effect of amygdalectomy on habituation and CER in rats. Physiol Behav. 1975;15:199–207. doi: 10.1016/0031-9384(75)90236-x. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher M, Holland PC. The amygdala complex: Multiple roles in associative learning and attention. Proc Natl Acad Sci USA. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everitt BJ, Cador M, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: Further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1989;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 18.Everitt BJ, Robbins TW. Amygdala-ventral striatal interactions and reward-related processes. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 401–429. [Google Scholar]
- 19.Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999a;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkinson JA, Willoughby, Robbins TW, Everitt BJ. Disconnection of hte anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: Further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- 21.Riedel G, Harrington NR, Hall G, Macphail EM. Nucleus accumbens lesions impair context, but not cue, conditioning in rats. NeuroReport. 1997;8:2477–2481. doi: 10.1097/00001756-199707280-00013. [DOI] [PubMed] [Google Scholar]
- 22.Parkinson JA, Robbins TW, Everitt BJ. Selective excitotoxic lesions of the nucleus accumbens core and shell differentially affect aversive Pavlovian conditioning to discrete and contextual cues. Psychobiology. 1999b;27:256–266. [Google Scholar]
- 23.Haralambous T, Westbrook RF. An infusion of bupivacaine into the nucleus accubmens disrupts the acquisition, but not the expression, of contextual fear conditioning. Behav Neurosci. 1999;113:925–940. doi: 10.1037//0735-7044.113.5.925. [DOI] [PubMed] [Google Scholar]
- 24.Levita L, Dalley JW, Robbins TW. Disruption of Pavlovian contextual conditioning by excitotoxic lesions of the nucleus accumbens core. Behav Neurosci. 2002;116:539–552. doi: 10.1037//0735-7044.116.4.539. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain: Anatomy to Function. New York: Plenum Press; 1991. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- 27.Heimer L, de Olmos JS, Alheid GF, Zaborszky L. “Perestroika” in the basal forebrain: Opening the border between neurology and psychiatry. Prog Brain Res. 1991;87:109–165. doi: 10.1016/s0079-6123(08)63050-2. [DOI] [PubMed] [Google Scholar]
- 28.Heimer L. The olfactory connections of the diencephalon in the rat. An experimental light- and electron-microscopic study with special emphasis on the problem of terminal degeneration. Brain Behav Evol. 1972;6:484–523. doi: 10.1159/000123728. [DOI] [PubMed] [Google Scholar]
- 29.Heimer L, Wilson RD. The subcortical projections of allocortex: Similarities in the neuronal associations of the hippocampus, the piriform cortex and the neocortex. In: Santini M, editor. Golgi Centennial Symposium Proceedings. New York: Raven Press; 1975. pp. 173–193. [Google Scholar]
- 30.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 31.Carlsen J, Heimer L. The basolateral amygdaloid nucleus as a cortical-like structure. Brain Research. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- 32.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126.147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Heimer L, Van Hoesen GW, Trimble MR, Zahm DS. Anatomy of Neuropsychiatry. Elsevier; Amsterdam: 2008. [Google Scholar]
- 34.Alheid GF. Extended amygdala and basal forebrain. In: Shinnick-Gallagher P, Pitkänen A, Shekhar A, Cahill C, editors. The amygdala in brain function. New York: New York Academy of Sciences; 2003. pp. 185–205. [DOI] [PubMed] [Google Scholar]
- 35.Zahm DS, Grosu S, Irving JC, Williams EA. Discrimination of striatopallidum and extended amygdala in the rat: a role for parvalbumin immunoreactive neurons? Brain Research. 2003;978:141–154. doi: 10.1016/s0006-8993(03)02801-4. [DOI] [PubMed] [Google Scholar]
- 36.Zahm DS. The evolving theory of basal forebrain functional-anatomical “macrosystems. Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurones. Acta Physiol Scan. 1964;62 (Suppl 232):1–55. [PubMed] [Google Scholar]
- 38.Andén NE, Carlsson A, Dahlström A, Fuxe K, Hillarp NÅ, Larsson K. Demonstration and mapping out of nigro-neostriatal dopaminergic neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- 39.Andén NE, Dahlström A, Fuxe K, Larsson K. Mapping out of catecholaminergic and 5-hydroxtryptamine neurons innervating the telencephalon and diencephalon. Life Sci. 1965;4:1275–1279. doi: 10.1016/0024-3205(65)90076-7. [DOI] [PubMed] [Google Scholar]
- 40.Andén NE, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U. Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand. 1966a;67:313–326. [Google Scholar]
- 41.Andén NE, Fuxe K, Hamberger B, Hökfelt T. A quantitative study on the nigro-striatal dopamine neuron system in the rat. Acta Physiol Scand. 1966b;67:306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- 42.Björklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Handbook of Chemical Neuroanatomy. Part I. Vol. 2. Elsevier; Amsterdam: 1984. pp. 55–122. [Google Scholar]
- 43.Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978 Aug 1;180(3):509–32. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- 44.Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–80. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- 45.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunoflourescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 46.Lindvall O, Björkland A. Dopamine- and norepinephrine-containing neuron systems: Their anatomy in the rat brain. In: Emson PC, editor. Chemical Neuroanatomy. Raven Press; New York: 1983. pp. 229–256. [Google Scholar]
- 47.Fallon JH, Loughlin SE. Monoamine innervation of cerebral cortex and a theory of the role of monoamines in cerebral cortex and basal ganglia. Cerebral Cortex. 1985;6:41–127. [Google Scholar]
- 48.Fallon JH, Loughlin SE. Substantia nigra. In: Paxinos G, editor. The Rat Nervous System, Volume 1, Forebrain and Midbrain. 2. Sydney: Academic Press; 1995. pp. 215–237. [Google Scholar]
- 49.Deutch AY, Goldstein M, Baldino F, Jr, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann N Y Acad Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- 50.Fallon JH. Topographic association of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- 51.Höfelt T, Mårtensson R, Björklund A, Kleinau S, Goldstein M. Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Handbook of Chemical Neuroanatomy. Part I. Vol. 2. Elsevier; Amsterdam: 1984. pp. 277–379. [Google Scholar]
- 52.Houk JC, Davis JL, Beiser DG, editors. Models of Information Processing in the Basal Ganglia. MIT Press; Cambridge, MA: 1995. p. 382. [Google Scholar]
- 53.Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979a;187:85–98. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- 54.Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat - anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- 55.Geisler S, Zahm DS. Neurotensinergic afferents of the ventral tegmental area in the rat: [1] re-examination of the origins and [2] responses to acute psychostimulant drug administration. Eur J Neurosci. 2006;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- 56.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 58.Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav. 1980;13(Suppl 1):213–23. doi: 10.1016/s0091-3057(80)80033-5. [DOI] [PubMed] [Google Scholar]
- 59.Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia: Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 60.Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- 61.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 62.Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5:1–12. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 63.Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–63. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 64.Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 65.Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- 66.Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Ann NY Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- 67.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis M, Shi C. The extended amygdala: Are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear and anxiety? Ann NY Acad Sci. 1999;877:292–308. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 69.Krettek JF, Price JL. Amygdaloid projections to subcortical structures within the forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 70.Zahm DS, Jensen S, Williams EA, Martin JR., III Direct comparison of projections from the central nucleus of the amygdala and nucleus accumbens shell. Eur J Neurosci. 1999;11:1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 71.Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 72.Misu Y, Goshima Y, Ueda H, Okamura H. Neurobiology of L-DOPA systems. Prog Neurobiol. 1996;49:415–454. doi: 10.1016/0301-0082(96)00025-1. [DOI] [PubMed] [Google Scholar]
- 73.Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- 74.White NM, Carr GD. The conditioned place preference is affected by two independent reinforcement processes. Phamacol Biochem Behav. 1985;23:591–618. doi: 10.1016/0091-3057(85)90127-3. [DOI] [PubMed] [Google Scholar]
- 75.White NM, Messier C, Carr GD. Operationalizing and measuring the organizing influence of drugs on behaviour. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer Verlag; New York: 1985. pp. 275–290. [Google Scholar]
- 76.Phillips AG, Fibiger HC. Anatomical and neurochemical substrates of drug reward. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer Verlag; New York: 1987. pp. 275–290. [Google Scholar]
- 77.Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Research. 1998;795:128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 78.Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Research. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- 79.Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist onPavlovian fear conditioning. Behav Neurosci. 2000;114:647–51. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- 80.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]