Abstract
This study examined the impact of emotion on opiate withdrawal induced hyperalgesia to determine whether emotional states modulate the magnitude of hyperalgesia. One hundred Hispanic males were recruited into one of three groups: heroin withdrawal, long-term heroin abstinence, and controls. Participants were presented with pictures to induce neutral, positive and negative emotional states. Affective valence, arousal, pain threshold and tolerance to ischemic pain were measured. When pain threshold and tolerance were compared, the withdrawal group displayed significant heightened pain sensitivity when negative affect was induced. We also found that former heroin addicts showed heightened pain sensitivity following months of abstinence.
Keywords: Emotion, Hyperalgesia, Opiates, Pain Modulation, Withdrawal
1. Introduction
Classically, pain has been defined as “an unpleasant sensory and emotional experience associated with tissue damage or described in terms of such damage”1. However, only recently has the emotional component of pain been recognized as not just an accessory part of the painful experience but also as an important modulator of pain itself; decreasing it when positive emotions or memories are elicited 2-3, or increasing subjective pain intensity and decreasing pain tolerance when negative emotions (like fear or anger) are elicited 2,4-6. Data supporting the fact that perception of painful stimuli is profoundly influenced by emotional variables is based not only on behavioral studies but also on anatomical7 and physiological research8.
The emotional modulation of pain has been studied primarily in healthy persons2,4,9 and in some clinical populations, including patients with rheumatoid arthritis10 and psychiatric problems such as depression and post-traumatic stress disorder11. However, relatively little research has investigated the role of emotion in modulating the enhanced pain sensitivity observed during opiate withdrawal and abstinence12,13 in heron dependent individuals (as defined in the fourth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 1994), even though shared neuroanatomical and neuropharmacological mechanisms are involved in the negative emotional-motivational effects of drug withdrawal14. Given the high incidence of psychiatric co-morbidities associated with opiate use15,16, increased anxiety states experienced by this group17, and the proposed links between negative affect with compulsive use of opiates18 and craving19, a better understanding of the influence of affective states on pain perception is of particular importance.
Hyperalgesia (increased sensitivity to noxious stimulation) resulting from long-term opiate administration has been previously observed in humans18,19,20 and animal models21-23. It is known that, when compared to other drug users, heroin addicts display a particularly severe hyperalgesic state24, which constitutes a strong negative reinforcement for continued use of drugs because use immediately alleviates the hyperalgesia25. Despite advances in the understanding of the physiopathology of opiate withdrawal-induced hyperalgesia, the contribution of stressful and emotional factors to the hyperalgesic state remains relatively unexplored. This is surprising because negative affect increases drug craving and is associated with an increased risk of relapse in opiate addicts26. Craving is a multi-dimensional construct that includes both reward craving as well as relief craving designed to escape or avoid withdrawal-induced hyperalgesia19. Recent evidence suggests that negative affect is the strongest predictor of both reward and relief craving and subsequent relapse27,28. We hypothesize that negative affect may intensify relief craving by heightening withdrawal-induced hyperalgesia. Consistent with this view, recent evidence suggests that cue-induced craving is associated with enhanced pain sensitivity in abstinent opiate addicts13. Although no one has directly examined the impact of negative affect on heroin withdrawal-induced hyperalgesia, these findings suggest that negative affect may increase opiate withdrawal-induced hyperalgesia, which may contribute to increased relief craving and continued drug use among heroin addicts.
The purpose of this study was to examine the impact of emotion on hyperalgesic states triggered by opiate withdrawal by determining whether emotional states modulate the magnitude of hyperalgesia observed during heroin withdrawal and abstinence. This was accomplished by examining ischemic pain threshold and tolerance during emotional picture-viewing in Hispanic male heroin-dependents during withdrawal and abstinence periods compared to controls. We hypothesized that negative affect would heighten and positive affect would attenuate the magnitude opiate withdrawal induced hyperalgesia. Because negative affect has been shown to be the most powerful predictor of relief craving28 we further hypothesized that negative affect would cause greater increases in pain sensitivity in heroin addicts compared to healthy controls.
2. Material and Methods
2.1 Participants
One hundred Hispanic males were recruited into one of three groups: acute heroin withdrawal, long-term heroin abstinence, and healthy controls. The acute withdrawal group (60 volunteers) was recruited from several rehabilitation centers in Ciudad Juarez, Chih., Mexico through counselors’ notification during a pretreatment interview that described the study. Acute withdrawal patients met the following criteria: 1) a history of opiate dependence during the last month as defined in the DSM-IV, 2) within 24 to 72 h of their last heroin dose29, which is a period of intense withdrawal-induced hyperalgesia (opiate withdrawal signs –rhinorrhoea, piloerection, perspiration, lacrimation, tremor, mydriasis, hot and cold flushes, restlessness, vomiting, abdominal cramps, anxiety, bones and muscles ache-) were assessed by clinical examination30, 3) were not taking any kind of medications (testing was concluded prior to formal treatment), 4) no circulatory, cardiac or neurological problems and 5) no depression or psychiatric problems other than drug addiction. A second group (ex-users group) was formed from 20 healthy former heroin users (mean length of opioid abstinence 30. 4 months) recruited from volunteers working in rehabilitation centers and through rehabilitation follow up appointments. The third group (non-users group) consisted of 20 healthy non-user volunteers without a history of heroin use with similar socio-demographic characteristics as the other two groups. Participants in non-users and ex-users groups were required to meet the following criteria: 1) gender and age similar to subjects in the withdrawal group, 2) a negative history of illicit substance use of any kind during the last 10 months (for the ex-users group) or never in lifetime (for the non users group), 3) absence of acute and chronic pain of any type, and 4) no medication use. Divergence in the number of volunteers among groups is a consequence of implementing the study in detoxification centers where the number of active heroin users was high and volunteers for the other two groups were scarce. All procedures in the study protocol were approved by the Texas A&M Institutional Review Board and all participants provided informed consent.
2.2 Ischemic Pain Procedure
The submaximal effort tourniquet procedure was used to induce ischemic pain31. The tourniquet task involves the use of a standard blood pressure cuff to occlude circulation to the arm. Prior to inflating the tourniquet cuff to 200 mmHg, the subject’s arm was raised for 30 s to promote venous drainage, and then the cuff was inflated, the experimenter’s stopwatch started, and the arm returned to the side. To promote forearm ischemia, subjects completed 20 handgrip exercises with an intersqueeze interval of 2 s. 32. Participants were instructed to report when they first felt pain (pain threshold) and when the pain became intolerable (pain tolerance limit). The time required to reach these two endpoints was recorded. The procedure was terminated at the point of tolerance or after 20 min, whichever came first. After the participants reached their pain tolerance limit and the cuff was deflated, they were asked to rate the perceived intensity and unpleasantness of their ischemic pain using visual analogue scales (VAS). This was done to determine whether the participants used the same subjective criteria to determine their pain tolerance limit.
2.3 Visual Stimulus Materials
The International Affective Picture System (IAPS) is a set of emotionally-evocative, color photographs of diverse stimuli designed to provide standardized affective stimuli33. These pictures were collected from different sources and classified into affective categories by large groups of participants using average ratings of affective valence (pleasantness) and arousal using the Self-Assessment Manikin34. The mean ratings of valence and arousal for these materials are highly internally consistent and have shown stability and concordance with emotional behaviors and physiological events such as heart rate and skin conductance response35,5. During viewing the participant was seated 2 feet from a 10′′ high for 12″ wide projected image. Pictures’ slide shows were presented for 4 s each. The order of presentation of the pictures was randomized to avoid order effects.
Pictures for this study were selected according to three levels of affective valence (in a 1 to 10 scale): pleasant or “positive” pictures (mean valence 6.80), neutral (mean valence 5.49) and unpleasant or “negative” pictures (mean valence 3.68), and also to minimize differences in arousal level across affective category (mean arousal for the positive, neutral and negative pictures was 3.76, 3.15 and 3.79, respectively). For each condition a set of 30 different pictures was selected a.
2.4 Manipulation checks
2.4.1 VAS
Using Visual Analogue Scale (VAS), participants were asked to rate the intensity and unpleasantness of their pain during the tourniquet test using two VAS. Participants rated their pain intensity on a 100 mm VAS ranging from 0 (no pain) to 100 (the most intense pain you can imagine), whereas pain unpleasantness was rated on a VAS from 0 (not at all unpleasant) to 100 (most unpleasant pain imaginable). The VAS score is determined by measuring the distance (in millimeters) between the 0 point and the patient’s mark36.
2.4.2 CES-D
The Center for Epidemiological Studies-Depression Scale (CES-D), a 20-item questionnaire of depression symptoms was administered in order to detect preexisting emotional distress. Participants rated their symptoms on a 0 to 3 scale with total scores ranging from 0 to 60. Mild depressive symptomatology is indicated by a score of 16 and higher scores indicate greater depressive symptoms37.
2.4.3 SAM
The Self-Assessment Manikin (SAM) is a non-verbal pictorial assessment technique that measures affective valence and arousal34. For each affect dimension, the subjects were instructed to place an “X” on or between a series of 5 cartoon pictographs depicting different levels of affective valence (1 “unhappy” to 9 “happy”) or arousal (1 “calm” to 9 “excited”). This yielded ratings ranging between 0 and 9 for each dimension. Using the SAM, participants rated subjective valence and arousal following exposure to the IAPS slides as a way of determining whether the participants experienced the targeted emotional state. The SAM was administered immediately after the tourniquet test rather than after the slide show to minimize possible demand or expectation effects38.
2.5 Procedure
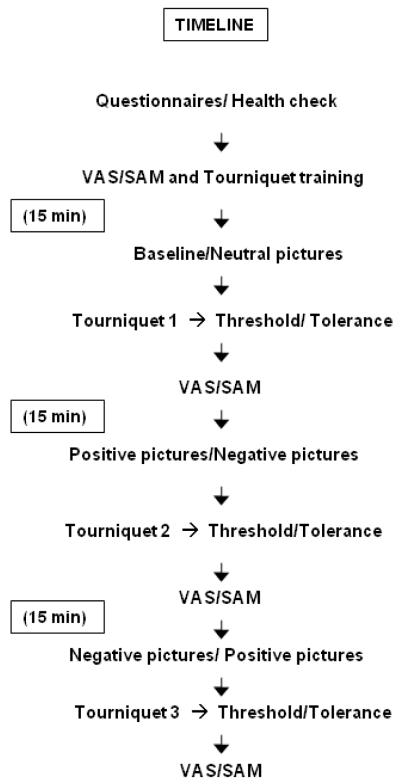
Figure 1 illustrates the experimental procedure. The experiment was conducted in a quiet room maintained between 22-24°C. After obtaining informed consent, subjects were acclimated for 15 min while they completed the demographics, health status, and CES-D questionnaires. Next, the participants practiced using the SAM and VAS measures by rating hypothetical stimuli. Participants were then instructed on the tourniquet procedures. A tourniquet cuff was positioned on the subject’s arm and the arm placed on a table to the side. Following the pictures slide-show pain threshold and tolerance limit were measured as described in the Ischemic Pain Procedure section. Immediately after the procedure, the subjects were asked to indicate their maximal pain intensity and pain unpleasantness on a VAS. Participants completed a total of 3 tourniquet tests spaced 15 minutes apart. Neutral slides were viewed before the baseline tourniquet test. Fifteen minutes following the termination of the baseline tourniquet test, participants were randomly assigned to view either a positive or negative slide show prior to each of the two remaining tourniquet tests. The order of the positive and negative sideshows was counterbalanced across and within subjects. After each tourniquet test, the subjects completed the VAS and SAM scales.
Figure 1.
Experimental Procedure (Timeline).
2.6 Statistical analysis
Analysis of variance (ANOVA) and trend analyses were used to assess differences across slide show and drug history conditions. Post-hoc mean comparisons were conducted using a Tamhane test. Data are presented as mean ± SEM.
3. Results
3.1 Participant Characteristics
Table 1 presents participant characteristics by group. No significant differences of age between groups were found. Twenty-five other participants (originally in the withdrawal group) were excluded for presenting with abscesses on their arms, concomitant heavy use of other drugs (cocaine, marihuana and/or alcohol) during one week previous to the study or clinical depression determined by scoring16 points or more in the CES-D scale. Additionally, the “Ex-users” group averaged a 30.4 months of abstinence ( SD 29.3) ranging from 10 to 120 months with a median of 15 months (SEM=6.75); 70% of the individuals in this group (n=14) reported 24 months or less of abstinence and the other 30% (n=6) reported abstinence times ranging from 3 to 10 years.
Table 1.
Participants characteristics by group
| Withdrawal Group | Ex-users Group | Non-users Group | |
|---|---|---|---|
| Sample | 60 | 20 | 20 |
| Age mean (SD) | 30.6 years (8.3) | 35.1 years (7.1) | 29.7 years (5.1) |
| Ethnicity (n) | |||
| Hispanic | 60 | 20 | 20 |
| Time of Drug Use (Mean) | 8.7 years | 10 years | 0 |
| Co-morbid substance use | |||
| Cocaine (n) | 21 | 0 | 0 |
| Alcohol/ Marihuana (n) | 25 | 0 | 0 |
3.2 Manipulation Checks
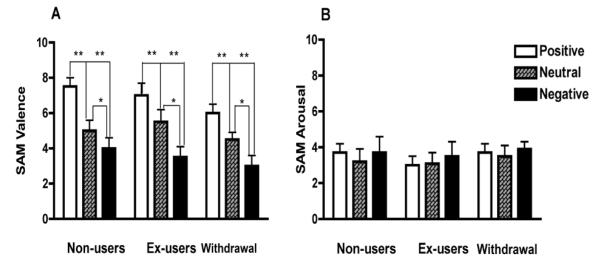
Figure 2 depicts the effects of the negative, neutral and positive IAPS slide conditions on SAM valence (2A) and arousal ratings (2B). An ANOVA conducted on the affective valence scores showed a significant main effect of slide category on valence ratings, F (2, 97) = 80.175, p< 0.001 plus a significant linear trend (F (1, 97) = 168.58, p<0.001, η2 = .63) with the negative pictures inducing the lowest score and positive pictures inducing the highest score. No interaction between slide categories and drug history group was found, p > 0.05. There was also a significant effect of drug history group on valence ratings, F (2, 97) = 7.67, p< 0.001 (Fig.2A). An ANOVA conducted on the affective arousal scores shown no differences across drug history groups, F (2, 97) = 0.511, p > 0.05, and slide conditions, F (2, 97) = 2.918, p > 0.05 (Fig. 2B).
Figure 2.
Mean valence (2A) and arousal (2B) scores of the experimental groups after viewing positive, neutral and negative pictures. Error bars indicate SEM. (*) Asterisk p<0.05, (**) Asterisks p<0.01.
3.3 Pain Intensity and Unpleasantness Scores
There were no effects of the affect and drug history conditions on VAS ratings of pain intensity and pain unpleasantness following the termination of the tourniquet test as determined by ANOVA analysis. This suggests that subjects used consistent criteria to determine pain tolerance across the affect and drug history conditions. Thus, changes observed in tolerance latency in the affect and drug history conditions should reflect a change in pain sensitivity, rather than a shift in response bias.
3.4 Ischemic Pain Threshold and Tolerance
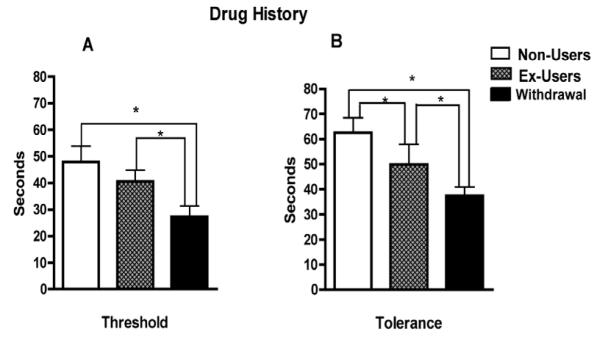
Figure 3 depicts the effect of drug history on ischemic pain threshold and tolerance of the experimental groups (non-user, ex-user, and withdrawal). Analysis on pain threshold (Fig.3A) revealed a significant main effect of drug history, F (2, 97) = 18.09, p < 0.001. Post-hoc analysis reveals that the withdrawal group exhibited the lower pain thresholds when compared to the non-users and ex-users, p < 0.001. The main effect of drug history on pain tolerance (Fig. 3B) was also significant, F (2, 97) = 16.770, p < 0.001. Post hoc comparisons for tolerance revealed a marginally significant effect between control and ex-users (p < 0.05), and significant differences between the ex-user and withdrawal groups, as well as between the control and withdrawal groups (p < 0.001). There were no significant interactions between picture category and drug history for either the threshold or the tolerance (p = 0.300).
Figure 3.
Effects of drug history on ischemic pain threshold (3A) and tolerance (3B) of the experimental groups. Error bars indicate SEM. (*) Asterisk p<0.05.
Figure 4 depicts the effects of picture content (negative, neutral and positive) on ischemic pain threshold and tolerance of the experimental groups (non-user, ex-user, and withdrawal). For each dependent variable, a mixed ANOVA was conducted entering picture content (positive, neutral, negative) as the within-subject variable and drug history (nonuser, ex-user, withdrawal) as the between group variable.
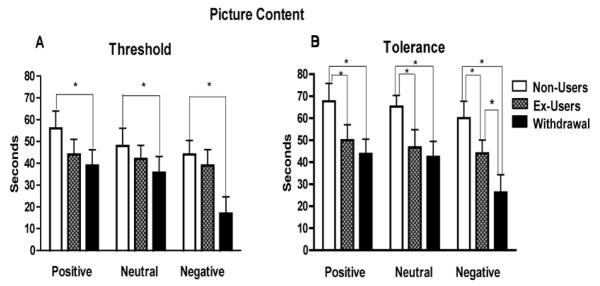
Figure 4.
Effects of picture content on ischemic pain threshold (4A) and tolerance (4B) of the experimental groups. Error bars indicate SEM. (*) Asterisk p<0.05.
Data analysis revealed a significant main effect of picture content on pain threshold and tolerance. The analysis on pain threshold (Fig.4A) found a significant main effect of picture content, F (2, 97) = 5.65, p < 0.005, along with a significant linear trend, F (1, 97) = 11.951, p < 0.001, η2 = .110. Post-hoc mean comparisons indicated that the negative slides resulted in the lowest thresholds when compared to positive and neutral slides in the withdrawal group, p < 0.001. No significant differences in pain threshold between neutral and positive pictures were found within any of the groups, p > 0.05. Examination of the ischemic pain tolerance data (Fig.4B) indicated a significant main effect of picture content, F (2, 97) = 5.149, p < 0.001, with a significant linear trend for picture content, F (1, 97) = 9.571, p < 0.005. Post-hoc mean comparisons indicated that the negative slides resulted in the lowest tolerance compared to the neutral and positive slides, p < 0.001.
The interaction between picture content and drug group was not significant for pain threshold, (F (2, 97) = 1.972, p > 0.05), or pain tolerance, F (2, 97) = 1.228, p= 0.300, when all affective categories were included. However, analyses focusing on the negative and neutral affect slides revealed a significant interaction between picture content and drug history for pain threshold, F (1, 97) = 3.73, p < 0.05, and pain tolerance, F (1, 97) = 3.16, p < 0.05, indicating that impact of the negative slides depended on drug history. Post-hoc mean comparisons revealed that this interaction was driven by the stronger reductions in pain threshold and tolerance observed in the withdrawal group when compared to the non-users, p < 0.05.
4. Discussion
The goal of this study was to examine the influence of emotional states on opiate-induced hyperalgesia. As expected, individuals in the heroin withdrawal group showed strong reductions of pain thresholds and pain tolerance when compared to the non-users, indicating opioid withdrawal-induced hyperalgesia. Additionally, ex-heroin users exhibited heightened pain sensitivity after an extended period of abstinence. We also found that individuals in all groups (non-users, ex-users and withdrawal) showed reduced pain tolerance after viewing negative pictures when compared to the tolerance latencies observed after viewing positive and neutral pictures. Consistent with our hypothesis, negative affect produced greater reductions in pain threshold and tolerance in the acute heroin withdrawal subjects compared to the healthy control subjects. This is important because it suggests that negative affect induction produces greater increases in pain sensitivity in heroin addicts undergoing acute withdraw, and consequently, this greater vulnerability to negative affect may contribute to increased relief craving and risk of relapse. Additionally, the results showed that ex-heroin users continued to show heightened pain sensitivity after an extended period of abstinence.
Previous research conducted in healthy subjects suggests that negative affect increases pain sensitivity by amplifying pain processing and enhancing attention to noxious stimuli2,5,9,39,40. Similar mechanisms may be responsible for amplifying pain sensitivity during heroin withdrawal. In addition, it is known that during withdrawal, opiate users experience considerable mood disturbance12,38, which may interact with negative affective modulation and contribute to the exacerbation of the observed hyperalgesia. Consistent with this view, our results show that negative affect modulation was greater in the heroin withdrawal group. It is also possible that the neural systems involved in negative affect modulation may be altered by brain stress systems that attempt to adapt to the chronic presence of the opiate drug and restore equilibrium. Brain stress systems mediated by corticotropin-release factor (CRF) are disrupted by chronic use of illicit drugs by triggering a common response of elevated adrenocorticotropic hormone, corticosterone, and amygdala CRF during acute withdrawal, as showed in animal experimentation41-44. Moreover, acute withdrawal from these drugs produces an anxiety-like state that can be reversed by CRF antagonists45. Additional evidence suggests that the extended amygdala may provide a common anatomical substrate integrating brain stress systems with hedonic processing systems 46 to produce the between-system opponent process described by Koob45. Importantly, the extended amygdala is not only thought to play a major role in fear conditioning47 but also in the emotional component of pain processing as has been showed in humans48 and animals49.
Contrary to our expectations, viewing of positive pictures did not increase pain thresholds and tolerance. There are different potential reasons why viewing positive pictures did not result in decreased pain sensitivity relative to neutral pictures in any of the groups, particularly the withdrawal group. One possible explanation is that the intensity of the positive affect elicited by the positive pictures was not sufficient enough to have an effect on pain modulation. This speculation is particularly relevant for the withdrawal group, whose members, as corroborated by manipulation checks reported amplified negative affect. Thus, while the pain modulatory mechanisms activated following negative affect induction may be able to amplify the intensity of hyperalgesia, positive affect induction may not be able to attenuate it. However, it remains possible that the induction of more intense positive affective states may be able to attenuate pain in this population. Additionally, overlapping neuroanatomical regions involved in emotion and pain processing 18 which are associated with the defensive and appetitive systems, have been implicated in affective pain modulation50,11. Thus, prior activation of these systems by affective stimuli alters the processing of subsequent pain signals. Because emotion and pain processes share a common neural substrate51-53, emotional states may prime the pain system to be either more or less responsive to incoming noxious stimuli. In agreement with this hypothesis, it is likely that in the present study, the negative slides activated the aversive motivational system heightening heroin withdrawal-induced hyperalgesia. Moreover, the defensive and appetitive systems are believed to be mutually exclusive54, an idea supported by findings showing that such systems have distinct neural bases55,56. Thus, the withdrawal group may have been unresponsive to the positive slides (primers of the appetitive system) because their defensive system had been activated prior to the introduction of the experimental manipulation (the withdrawal group reported high levels of negative affect).
The second major result in our work was the fact that the ex-users group also showed evidence of a hyperalgesic response as indicated by their increased sensitivity to ischemic pain with respect to non-users. This response manifested only on tolerance assessments. This finding has two main implications: First, the results suggest that hyperalgesia may persist in former heroin-users even after years of abstinence. Second, the hyperalgesia observed in the withdrawal group may be due to a combined effect of heroin withdrawal side-effects and either long-term changes in pain sensitivity produced by a long history of heroin use, or by pre-existing differences between nonusers and ex-users such as genetic factors. These mechanisms are not clear, even though it is believed to result from neuroplastic adaptations in the nervous systems that cause sensitization of pronociceptive pathways57. Advances in this field suggest that quantitative differences in pain sensitivity are influenced by both environmental and genetic factors58. However, even though several genes associated with pain sensitivity have been studied in animals, only a few genes in humans have been identified59,60,61. Although these advances promise to identify genetic modulators of pain, ultimately, variation in pain perception caused by genetic variations is only part of complex system involving different levels of neuronal processing60.
The finding that long-term heroin use could cause an exacerbation of pain after the drug’s withdrawal it is not new; however innovative emerging theories can explain the phenomenon by further developing the molecular mechanisms of the opponent-process theory62. According to this theory pleasant or aversive affective states derived from drug use would be automatically opposed by centrally mediated mechanisms reducing the intensity of these states63. In the case of the effect of opiates on pain, pain sensitivity is determined by the interaction or summation effect of two mutually opposing processes, one anti-nociceptive and the other pro-nociceptive64. Among the last group (pro-nociceptive processes) fundamental in the development of hyperalgesia, the follow mechanisms, derived from studies with animals, are proposed: 1) descending facilitation (facilitation of synaptic transmission in dorsal horn neurons)65, 2) activation of adenylcyklase, 3) increase of proteinkinase C (involved in opioid receptor-desensitation)66 , 4) NMDA-receptor activation64 and 5) release of peptides with opioid-antagonistic properties such as cholecystokinin (CCK)67, neuropeptide FF (NPFF) and nociceptin (orphanin FQ)68.
An important finding in the present study was the fact that greater differences between groups were observed for pain tolerance when compared to pain threshold. Interestingly, existing reports in the literature present different results: Liebmann et al. reported a significant increment in pain thresholds in former opiate users (observable as reduced pain sensitivity)69. In contrast with this finding, studies involving active and former users found that active users displays lower pain tolerances and former users fluctuate from normality to pain intolerance70. Other studies found that ex-users of heroin under methadone treatment are clearly hyperalgesics24. Differences in population samples and methodology aside, variation in results are evident.
In concordance with the last group of studies, our results revealed reduced pain tolerance in the ex-users group manifested by decreased pain tolerance. Although the exact mechanisms remain to be elucidated, higher neuronal affective circuits are thought to play a major role in determining pain tolerance (e.g. descending control)71 compared to pain thresholds70. Thus, centrally mediated processes, involved in the opponent-processes mechanisms, should have a larger impact on pain tolerance than on pain thresholds as reflected in this and other studies.
5. Limitations
Although the present study brings some design advantages over previous research, it is necessary to acknowledge that the majority of individuals in the withdrawal group and ex-users were polysubstance users. That is, we cannot be absolutely certain of the extent to which previous use of other drugs influence these results. Given that only few studies have examined the impact of drug-use history on pain perception, and with mixed findings70,72, further studies are needed to clarify this issue.
A potential limitation associated with using pictures from the International Affective Picture System (IAPS) to induce different mood states, is that they may not generate affective states that fully reflect the moods and events normally experienced during opiate withdrawal. While this is a limitation inherent in using IAPS, there are several advantages as well. The primary advantage is that they provide well-characterized and standardized pictures, which have been used in numerous studies to manipulate mood valence across a wide variety of populations across countries, cultures, age groups, in general and disorder-specific populations (e.g., smokers, eating disorders, personality disorders) and for which there is substantial self-report and psychophysiological validation data. Norms are available for the IAPS pictures for arousal-producing quality and valence on a strongly positive to strongly negative scale. Skin conductance responses provoked by these pictures have been found to correlate with the self-report arousal dimension. Moreover, the valence of the pictures has been shown to systematically modulate the magnitude of the acoustic startle response, with negative pictures amplifying the startle response and positive pictures inhibiting the startle response. Thus, the IAPS pictures provided a well-validated stimulus set for modulating mood. Whereas using images that may have been specifically relevant to the context of this population would be valuable, we would first need to conduct a series of validation studies to determine whether the pictures induce the targeted emotional and psychophysiological responses. Since this was our first study, we wanted to avoid some potential drawbacks of using not standardized materials. However, future studies could investigate whether more pronounced effects are observed using negative affect inducing pictures that are more relevant to this population to determine whether the same pattern of results might be seen with more naturalistic stimuli.
6. Conclusions
We have previously shown that the induction of negative emotion increases pain sensitivity in healthy adults5. The present findings extend this line of work by demonstrating that negative emotion not only exacerbates heroin withdrawal-induced hyperalgesia, it causes greater increases in pain sensitivity in the heroin withdrawal group compared to healthy controls. This suggests that opiate-derived hyperalgesia is influenced by negative affect and thus interventions designed to decrease negative affect could be effective in attenuating pain during withdrawal and risk of relapse. It is shown also that ex-heroin users continued to show heightened pain sensitivity following months of abstinence. Future research will be needed to determine whether the heightened pain sensitivity observed in abstinent ex-heroin addicts is a consequence of long-term opiate use or of a pre-morbid condition.
Acknowledgments
This study was supported by NIH grant T32-MH65728, and financial aid from MALRC (Mexican American and U.S. Latino Research). NIH and MALRC had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
The IAPS identification numbers are, for positive conditions: 1500, 1600, 1601, 1603, 1604, 1930, 2000, 2058, 2070, 2170, 2208, 2209, 2222, 2260, 2332, 2530, 2540, 2560, 2589, 2600, 2660, 2791, 4614, 5010, 5020, 5030, 5594, 5760, 5764, 5779; for negative conditions: 2205, 2206, 2276, 2321, 2375.1, 2399, 2490, 2590, 2750, 6010, 7234, 7235, 7700, 7705, 9001, 9008, 9010, 9045, 9046, 9090, 9101, 9102, 9110, 9190, 9220, 9331, 9341, 9360, 9373 9390; and for neutral conditions 2200, 2210, 2214, 2215, 2250, 2260, 2270, 2299, 2304, 2310, 2320, 2358, 2393, 2440, 2480, 2495, 2512, 2570, 2580, 2630, 2840, 5510, 5720, 5731, 7000, 7006, 7250, 7280, 7281, and 7282.
References
- 1.Merskey Editorial The need of a taxonomy. Pain. 1979;6:247–252. [Google Scholar]
- 2.de Wied M, Verbaten MN. Affective pictures processing, attention, and pain tolerance. Pain. 2001;90:163–172. doi: 10.1016/s0304-3959(00)00400-0. [DOI] [PubMed] [Google Scholar]
- 3.Gelkopf M, Kreitler S. Is humor only fun, and alternative cure or magic? The cognitive therapeutic potential of humor. J Cognit Psychother. 1996;10:235–254. [Google Scholar]
- 4.Godinho F, Magnin M, Frot M, Perchet C, Garcia-Larrea L. Emotional modulation of pain: Is It the Sensation or What We Recall. The Journal of Neuroscience. 2006;26(44):11454–11461. doi: 10.1523/JNEUROSCI.2260-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: effects of affective picture modulation. Psychosom Med. 2001;63:79–90. doi: 10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Wunsch A, Philippot P, Plaghki L. Affective associative learning modifies the sensory perception of nociceptive stimuli without participant’s awareness. Pain. 2003;102:27–38. doi: 10.1016/s0304-3959(02)00331-7. [DOI] [PubMed] [Google Scholar]
- 7.Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. NeuroImage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Larrea L, Bastuji H, Mauguiere F. Mapping study of selective attentional effects on somatosensory evoked potentials. Electroencephal Clin Neurophysiol. 1991;82:101–114. doi: 10.1016/0168-5597(91)90122-e. [DOI] [PubMed] [Google Scholar]
- 9.Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry. 2001;14:241–245. [Google Scholar]
- 10.Hamilton NA, Zautra AJ, Reich J. Individual differences in emotional processing and reactivity to pain among older women with rheumatoid arthritis. Clin J Pain. 2007;23(2):165–172. doi: 10.1097/AJP.0b013e31802b4f58. [DOI] [PubMed] [Google Scholar]
- 11.Klossika I, Flor H, Kamping S, Bleichhardt G, Trautmann N, Treede RD, Bohus M, Schmahl C. Emotional modulation of pain: A clinical perspective. Pain. 2006;124(3):264–268. doi: 10.1016/j.pain.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Liebmann PM, Lehofer UM, Moser M, Legl T, Pernhaupt G, Schauenstein K. Nervousness and pain sensitivity: II. Changed relation in ex-addicts as a predictor for early relapse. Psychiatry Research. 1998;79:55–58. doi: 10.1016/s0165-1781(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 13.Ren ZY, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204(3):423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Llera A, Salvany D, Brugal MT, Sánchez-Niubó SM, Torrens M, the ITINERE Investigators Psychiatric comorbidity in young heroin users. Drug and Alcohol Dependence. 2006;84(1):48–55. doi: 10.1016/j.drugalcdep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clinical Psychology Review. 2000;20(2):235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 17.Sareen J, Chartier M, Paulus MP, Stein MB. Illicit drug use and anxiety disorders, Findings from two community surveys. Psychiatry Research. 2006;142:11–17. doi: 10.1016/j.psychres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 18.White JM. Pleasure into pain: The consequences of long-term opioid use. Addictive Behaviors. 2004;29:1311–1324. doi: 10.1016/j.addbeh.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Tiffany ST, Carter BL, Singleton EG. Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction. 2000;95(Suppl 2):S177–87. doi: 10.1080/09652140050111753. [DOI] [PubMed] [Google Scholar]
- 18.Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;3:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Compton P, Athanasos P, Elashoff D. Withdrawal hyperalgesia after acute opioid physical dependence in nonaddicted humans: a preliminary study. The Journal of Pain. 2003;4(9):511–519. doi: 10.1016/j.jpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Lipman JJ, Blumenkopf BJ. Comparison of subjective and objective analgesic effects of intravenous and intrathecal morphine in chronic pain patients by heat beam dolorimetry. Pain. 1989;39(3):249–256. doi: 10.1016/0304-3959(89)90037-7. [DOI] [PubMed] [Google Scholar]
- 21.Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Re. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 22.Sweitzer SM, Allen CP, Zissen MH, Kendig JJ. Mechanical allodynia and thermal hyperalgesia upon acute opioid withdrawal in the neonatal rat. Pain. 2004;110(1-2):269–280. doi: 10.1016/j.pain.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Zissen MH, Zhang G, McKelvy A, Propst JT, Kendig JJ, Sweitzer SM. Tolerance, opioid-induced allodynia and withdrawal associated allodynia in infant and young rats. Neuroscience. 2007;144(1):247–262. doi: 10.1016/j.neuroscience.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koppert W. Opioid-induced hyperalgesia pathophysiology and clinical relevance. Acute Pain. 2007;9:21–34. [Google Scholar]
- 25.Koob GF, Le Moal M. Drug Addiction, Dysregulation of Reward, and Allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 26.El SE, Bashir TZ Sheikh. High-risk relapse situations and self-efficacy: Comparison between alcoholics and heroin addicts. Addictive Behaviors. 2004;29(4):753–758. doi: 10.1016/j.addbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Ling JL, Preston KL. Real-Time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66(1):88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grüsser SM, Mörsen CP, Wölfling K, Flor H. The Relationship of Stress, Coping, Effect Expectancies and Craving. Eur Addict Res. 2007;13:31–38. doi: 10.1159/000095813. [DOI] [PubMed] [Google Scholar]
- 29.Covington HE, III, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. European Journal of Pharmacology. 2003;467(1-3):1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- 30.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two New Rating Scales for Opiate Withdrawal. American Journal of Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 31.Moore PA, Duncan GH, Scott DS, Gregg JM, Ghia JN. The submaximal effort tourniquet test: Its use in evaluating experimental and chronic pain. Pain. 1979;6:375–382. doi: 10.1016/0304-3959(79)90055-1. [DOI] [PubMed] [Google Scholar]
- 32.Maixner W, Gracely RH, Zuniga JR, Humphrey CB, Bloodworth GR. Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol. 1990;259:1156–1163. doi: 10.1152/ajpregu.1990.259.6.R1156. [DOI] [PubMed] [Google Scholar]
- 33.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): digitized photographs, instruction manual and affective ratings. University of Florida; Gainesville, FL: 2005. Technical Report A-6. [Google Scholar]
- 34.Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 35.Lang PJ. The emotion probe: studies of motivation and attention. Am Psychol. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- 36.Lund L, Lunderber T, Sandrberg L, Svensson E. Lack of interchangeability between visual analogue and verbal rating pain scales: a cross sectional description of pain etiology groups. BMC Medical Research Methodology. 2005;5(31):1–27. doi: 10.1186/1471-2288-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 38.Polivy J, Doyle C. Laboratory induction of mood states through reading of self-referent mood statements: affective changes or demand characteristics? J Abnorm Psychol. 1980;89:386–390. doi: 10.1037//0021-843x.89.2.286. [DOI] [PubMed] [Google Scholar]
- 39.Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 40.Wunsch A, Philippot P, Plaghki L. Affective associative learning modifies the sensory perception of nociceptive stimuli without participant’s awareness. Pain. 2003;102:27–38. doi: 10.1016/s0304-3959(02)00331-7. [DOI] [PubMed] [Google Scholar]
- 41.Koob GF, Heinrichs SC, Menzaghi F, Pich EM, Britton KT. Corticotropin releasing factor, stress and behavior. Seminars in the Neurosciences. 1994;6:221–229. [Google Scholar]
- 42.Koob GF, Le Moal M. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 43.Merlo-Pich E, Lorang M, Yeganeh M, de Fonseca F Rodriguez, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitaryadrenal axis in the rat: role of corticotropin-releasing factor (CRF) Journal of Pharmacology and Experimental Therapeutics. 1984;229:127–131. [PubMed] [Google Scholar]
- 45.Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. Advances in Experimental Medicine and Biology. 1995;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- 47.Le Doux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 48.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. The Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 49.Crown ED, King TE, Meagher MW, Grau JW. Shock-induced hyperalgesia: III. Role of the bed nuclus of the stria terminalis and amygdaloid nuclei. Behav Neurosci. 2000;114:561–573. [PubMed] [Google Scholar]
- 50.Duquette M, Roy M, Lepore F, Peretz I, Rainville P. Cerebral mechanisms involved in the interaction between pain and emotion. Rev Neurol. 2007;163(2):169–179. doi: 10.1016/s0035-3787(07)90388-4. [DOI] [PubMed] [Google Scholar]
- 51.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;4:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 52.King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005;14(4):194–205. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- 53.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;2:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 54.Greenwald MK, Cook EW, Lang PJ. Affective judgment and psychophysiological response: dimensional covariation in the evaluation of pictorial stimuli. J Psychophysiol. 1989;3:51–64. [Google Scholar]
- 55.Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 56.Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol. 2007;98(3):1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- 57.Chu LF, Angst MS, Clark D. Opioid-induced Hyperalgesia in Humans Molecular Mechanisms and Clinical Considerations. Clinical Journal of Pain. 2008;24(6):479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 58.Foulkes T, Wood JN. Pain genes. PLoS Genet. 2008;4(7) doi: 10.1371/journal.pgen.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Hum Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Mol Genet. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. 1. [DOI] [PubMed] [Google Scholar]
- 60.Lötsch J, Geisslinger G. Current evidence for a modulation of nociception by human genetic polymorphisms. Pain. 2007;132(1-2):18–22. doi: 10.1016/j.pain.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 61.Tegeder I, Adolph J, Schmidt H, Woolf CJ, Geisslinger G, Lötsch J. Reduced hyperalgesia in homozygous carriers of a GTP cyclohydrolase 1 haplotype. European Journal of Pain. 2008;12(8):1069–1077. doi: 10.1016/j.ejpain.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Célèrier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: A sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laulin JP, Celerier E, Larcher A, LeMoal M, Simonnet G. Opiate tolerance to daily heroin administration: An apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631–36. doi: 10.1016/s0306-4522(98)00652-6. [DOI] [PubMed] [Google Scholar]
- 64.Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain. Neuroreport. 2003;14:1–7. doi: 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- 65.Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Re. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 66.Mao J, Price D, Mayer D. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 67.Maier SF, Wiertelak EP, Watkins LR. Endogenous pain facilitory systems Antianalgesia and hyperalgesia. APS Journal. 1992;1(3):191–198. [Google Scholar]
- 68.Harrison LM, Grandy DK. Opiate modulating properties of nociceptin/orphanin FQ. Peptides. 2000;21(1):151–172. doi: 10.1016/s0196-9781(99)00185-0. [DOI] [PubMed] [Google Scholar]
- 69.Liebmann PM, Lehofer M, Schonauer-Cejpek M, Legl T, Pernhaupt G, Moser M, Schauenstein K. Pain sensitivity in former opioid addicts. Lancet. 1994;344:1031–32. doi: 10.1016/s0140-6736(94)91697-7. [DOI] [PubMed] [Google Scholar]
- 70.Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: correlates of drug type and use status. Journal of Pain and Symptom Management. 1994;9(7):462–473. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 71.Birklein F, Depmeier C, Rolke R, Hansen C, Rautenstrauss B, Prawitt D, Magerl W. A family-based investigation of cold pain tolerance. Pain. 2008;138:111–118. doi: 10.1016/j.pain.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Rhudy JL, Dubbert PM, Parker JD, Burke RS, Williams AE. Affective modulation of pain in substance-dependent veterans. Pain Medicine. 2006;7(6):483–500. doi: 10.1111/j.1526-4637.2006.00237.x. [DOI] [PubMed] [Google Scholar]