Abstract
The leukotriene A4 hydrolase enzyme is a dual functioning enzyme with the following two catalytic activities: an epoxide hydrolase function that transforms the lipid metabolite leukotriene A4 to leukotriene B4 and an aminopeptidase function that hydrolyzes short peptides. To date, all drug discovery efforts have focused on the epoxide hydrolase activity of the enzyme, because of extensive biological characterization of the pro- inflammatory properties of its metabolite, leukotriene B4. Herein, we have designed a small molecule, 4-methoxydiphenylmethane, as a pharmacological agent that is bioavailable and augments the aminopeptidase activity of the leukotriene A4 hydrolase enzyme. Pre-clinical evaluation of our drug showed protection against intranasal elastase-induced pulmonary emphysema in murine models.
Keywords: Emphysema, COPD, LTB4, aminopeptidase, leukotriene A4 hydrolase, murine model
The leukotriene A4 hydrolase (LTA4H) enzyme has been a target for drug discovery, because of its role in the biosynthesis of the lipid metabolite leukotriene B4 (LTB4). LTB4 is a potent neutrophil chemo-attractant1-4 and has been described in the setting of several human pathologies to include the following: sepsis, 5-8 shock,9, 10 cystic fibrosis,11-15 coronary artery disease,16-18 connective tissue disease,2, 19-23 and chronic obstructive pulmonary disease (COPD).24-26 Studies on COPD and LTB4 have also demonstrated significant association between LTB4 and emphysematous COPD. 14, 24, 27-29 Despite the relevance of LTB4 in several diseases, clinical trials in humans have failed to show similar beneficial effects in inhibiting this metabolite for several diseases such as rheumatoid arthritis, cystic fibrosis, and inflammatory bowel diseases.30-34
Recent work suggests that the LTA4H enzyme may also exert protective properties in the inflammatory process. 35-37 The LTA4H enzyme is a dual functioning hydrolase that catalyzes the addition of water to the epoxy lipid LTA4 to give rise to LTB4. However, in the presence of short peptides, LTA4H acts as an aminopeptidase that cleaves the N-terminus of the peptide.38 All previous drug discovery efforts targeting the LTA4H enzyme have focused on the inhibition of LTB4 biosynthesis without regard to the aminopeptidase activity of the enzyme.
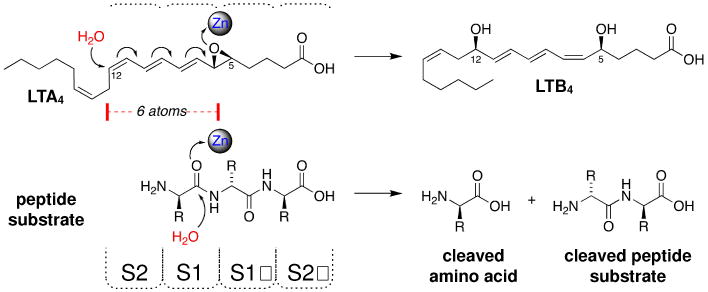
The two reactions catalyzed by the LTA4H enzyme involve the hydrolysis of their corresponding substrate. Haeggstrom and co-workers have shown that a carboxylic acid group that participates in hydrogen-bonding interactions with Arg563 of the enzyme is essential for catalytic hydrolysis.39 When the LTA4 substrate and a peptide substrate are aligned at their carboxylic acid moieties, a simple two-dimensional analysis reveals that the addition of water likely occurs at two different regions of the enzyme (Figure 1). Peptide cleavage at the P1 site (the peptide bond of the N-terminal amino acid residue) occurs in the S1 pocket of the peptidase as designated using the nomenclature of Schechter and Berger.40 This would place the hydrolytic site of the LTA4 lipid metabolite in approximately the S2 pocket, which is positioned 6 atoms away from the hydrolytic site of a peptide substrate (Figure 1). Since the hydrolytic site of the two substrates occur at very different regions of the enzyme, a pharmacological approach was envisioned to uncouple these two actions of the enzyme.
Figure 1. Two-Dimensional Analysis of LTA4H-mediated Hydrolysis.
In the course of our studies Lai group reported that 4-methoxyphenoxybenzene upregulates the aminopeptidase activity of the LTA4H enzyme.41 We confirmed Lai's in vitro results showing upregulation of LTA4H aminopeptidase activity in the presence of 4-methoxyphenoxybenzene. Subsequently, we tested this compound in our intranasal (IN) elastase-induced murine model of pulmonary emphysema. Surprisingly, our study resulted in >90% mortality within 10 days. A chemical stability study revealed that 4-methoxyphenoxybenzene decomposes rapidly (within minutes) in pH6.8 buffer solution to give two peaks by HPLC of presumably toxic products. We exchanged the central oxygen atom to a methylene group to afford a chemically stable entity, which retained the in vitro activity of the lead compound and was suitable to test our hypothesis that the aminopeptidase activity of the LTA4H enzyme is protective.
Chemical design and synthesis
On the basis of Lai's molecular model of 4-methoxyphenoxybenzene bound to the LTA4H enzyme, we deduced that the central oxygen atom of the drug did not participate in binding. Since this oxygen atom is the likely site for cleavage to give the toxic degradation products, our strategy involved exchange of the ether linkage to a methylene bridge to the two aryl groups. The compound 4-methoxydiphenylmethane (4-MDM), shown in Figure 2, was synthesized in one step in >90% yield from commercially available materials.
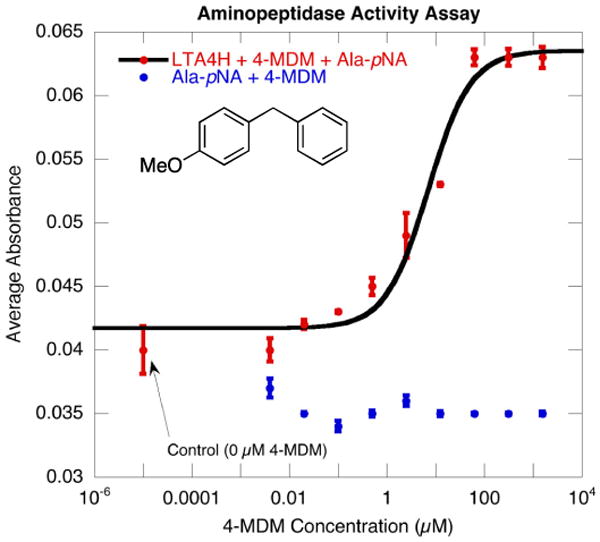
Figure 2. In Vitro Aminopeptidase Assay of the LTA4H enzyme.
In vitro characterizations
The compound 4-MDM is a close analog to the LTA4H augmentor reported by Lai and co-workers, and we expected it to have increased chemical stability and in turn better bioavailability. First, we conducted an in vitro aminopeptidase assay to confirm the effect of the drug on augmenting the aminopeptidase activity of the LTA4H enzyme. In order to measure the aminopeptidase activity of the enzyme, L-alanine p-nitroanilide (Ala-p-NA) was used as the reporter group. Cleavage of the peptide bond of the Ala-p-NA resulted in an observable change in wavelength absorption at 430 nm. 41
As shown in Figure 2, the aminopeptidase activity assay of the LTA4H enzyme consisted of two control experiments. First, the aminopeptidase activity experiment was carried out in the absence of 4-MDM to determine the base line activity of the enzyme at the time of our measurement. Second, the experiment was carried out in the absence of the LTA4H enzyme to determine any inherent instability of the reporter group in the presence of 4-MDM. As expected, the assay containing both the enzyme and the drug showed a dose-responsive increase in the average absorbance measured at 430 nm, which indicates cleavage of the Ala-p-NA reporter group. The estimated concentration for 50% activation (AC50) is 50 μM.
Effect of 4-MDM on elastase-induced murine emphysema
To assess the effect of 4-MDM on the pathogenesis of pulmonary emphysema, 4-MDM was formulated in peanut oil. Mice were then treated with escalating doses of 4-MDM via IP injection daily. The maximally tolerated dose of 4-MDM was 30 mg/kg mouse weight. Subsequently, mice were treated daily with either 30 mg of 4-MDM/kg of mouse weight or vehicle (peanut oil) via IP injection starting on day −1. The emphysema in wild type mice was induced by IN administration of 0.75 μg of elastase per kilogram of mouse weight in 100 μL of PBS or PBS alone as vehicle on day 0.36 Multiple modalities were applied to reliably assess the severity of pulmonary emphysema in these animals. Lung compliance was measured by the Flexivent 28 days post IN elastase or IN vehicle exposure. Pre mortem lung compliance measured by this method is expected to increase with worsening of emphysema. As shown in Figure 3A, animals post IN elastase exposure show higher compliances as compared to the animals post IN vehicle exposure. However, the animals that received daily IP injections of 4-MDM showed significantly reduced lung compliances post IN elastase exposure as compared to the animals that received IP vehicle post IN elastase exposure. Post mortem lung volumes were measured by volume displacement technique after harvesting the lungs en block, inflating the lungs with a uniform 25cm water pressure, then fixing them in paraformaldehyde for 18 hours (Figure 3B & 3C). Lung volume is expected to increase with the worsening of emphysema. As shown in Figure 3B & 3C, animals post IN elastase exposure showed larger lung volumes as compared to the animals post IN vehicle exposure. The animals that received daily IP injections of 4-MDM show significantly reduced lung volumes post IN elastase exposure as compared to the animals that received IP vehicle post IN elastase exposure. Post mortem chord length is indicative of alveolar sizes, and therefore larger chord lengths suggest more severe emphysema (Figure 3D & 3E). Again, as shown in Figure 3D & 3E, animals post IN elastase exposure showed longer chord lengths (larger alveolar sizes in the lung histology) as compared to the animals post IN vehicle exposure. The animals that received daily IP injections of 4-MDM showed significantly shorter chord lengths post IN elastase exposure as compared to the animals that received IP vehicle post IN elastase exposure. Levels of LTB4 in the BALF were assessed by R&D ELISA kit (Cat# KGE006). The 4-MDM IP treatment did not alter the levels of LTB4 in these animals (E/V = 93.1 pg/mL ± 26.1 vs E/D = 124.2 pg/mL ± 47.1, p-value = not significant, where E=IN elastase; V=IP peanut oil drug vehicle; D=IP 4-MDM).
Figure 3. In Vivo Characterizations of 4-MDM in Murine Intra-nasal Elastase-induced Pulmonary Emphysema.
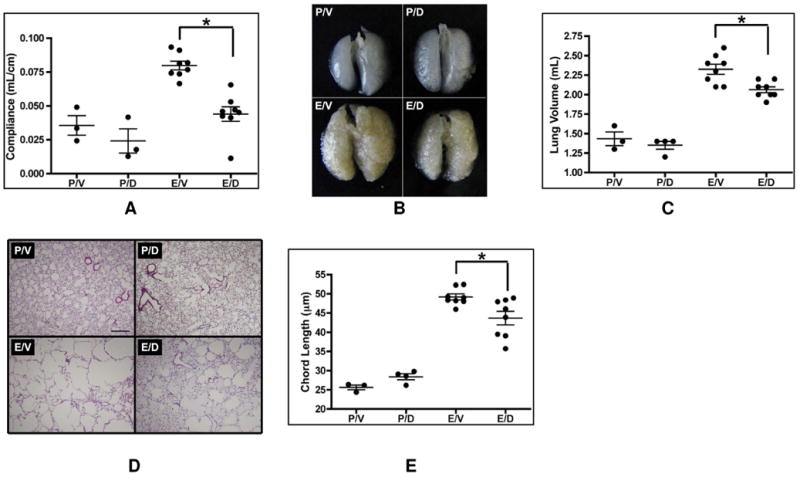
Mouse lungs were assessed 28 days after either intra-nasal (IN) elastase or vehicle exposure with either daily intra-peritoneal (IP) injection of 4-MDM or vehicle treatment in WT mice at the ages of 6-8 weeks. All mice were sacrificed at the ages of 10-12 weeks. P=IN phosphate buffered saline vehicle, E=IN elastase; V=IP peanut oil drug vehicle; D=IP 4-MDM; horizontal line is the mean of each group; vertical bars are +/- SEM; and * indicates p < 0.05.
(A) Pre mortem lung compliance measurement with a Scireq Flexivent. As emphysema worsens, compliance increases.
(B) Representative pictures of the gross mouse lungs after harvested en block, inflated at a uniform 25cm water pressure, and fixed in paraformaldehyde for 18 hours. These are a representative pictures of the lungs used for lung volume measurement.
(C) Post mortem lung size measurement by volume displacement technique after inflating the lungs with a uniform 25cm water pressure. As emphysema worsens, the sizes of the lungs increase.
(D) Representative H&E histology pictures of the mice lungs used for the chord length measurement. Large holes are emphysematous alveoli. These pictures were taken at 5× magnification.
(E) Post mortem computerized measurement of average alveolar sizes. As emphysema worsens, lung loses elasticity, and therefore, alveolar sizes increase when inflated with a uniform 25 cm water pressure.
These data show that elastase treatment results in damaged emphysematous alveoli as measured by lung compliance, gross lung inspection, lung volume, and chord length. Our results further show that the IP administration of 4-MDM formulated in peanut oil resulted in a significant decrease (p < 0.05) in emphysematous lung damages by multiple modalities of assessment without any effect on in vivo LTB4 production. Overall, 4-MDM was found to effectively protect murine lungs from emphysematous destruction.
The LTB4 metabolite is a pro-inflammatory lipid derived from the 5-lipoxygenase pathways and biosynthesized by the LTA4H enzyme. The targeting of the LTA4H enzyme has been the subject of numerous drug discovery efforts over the past 20 years. 42-47 Despite the tremendous amount of data suggesting the implications of this enzyme in numerous diseases, a viable clinical agent has not yet come to market. Our speculation is that the second function of this enzyme, that is, its aminopeptidase activity, is important in the resolution phase of inflammation, and that indiscriminate inhibition may be responsible for the divergent outcomes encountered when targeting this enzyme.
Only recently has an endogenous peptide substrate for the LTA4H enzyme been identified. Blalock and co-workers reported that the proline-glycine-proline tripeptide (PGP) is a ligand for the LTA4H enzyme, and processing of this peptide by LTA4H was found to be essential for timely resolution of neutrophilic inflammation in a murine model for influenza.37 However, Orning and co-workers have termed the LTA4H aminopeptidase as an arginine aminopeptidase, because of its high specificity and efficiency in cleaving tripeptides that contain an N-terminal arginine residue.38 It is not clear if cleavage of a single peptide, such as PGP, or cleavage of a combination of different peptides is responsible for the protective properties of the LTA4H enzyme. Overall, these findings suggest that the biology of the LTA4H enzyme is much more complex than previously appreciated.
We chose to use the Ala-p-NA to measure the aminopeptidase activity of the LTA4H enzyme. Haeggstrom and co-workers have shown that Ala-p-NA is a good mimetic for peptide substrates of the LTA4H enzyme on the basis of a series of aminopeptidase assays, wherein the LTA4H enzyme was found to perform comparably with the Ala-p-NA reporter system.48 Haeggstrom also concluded that the Ala-p-NA molecule is likely to bind in a similar orientation as a peptide substrate to the LTA4H enzyme on the basis of a comparative analysis of the crystal structure of the LTA4H enzyme and a molecular model of Ala-p-NA in the binding pocket of the enzyme.48 Additionally, the aminopeptidase assay using the Ala-p-NA reporter group is operationally simple to carry out and results can be obtained rapidly.
Our previous work suggested a potential protective mechanism associated with the LTA4H enzyme and neutrophilic inflammation.36 In that report, we showed that intranasal exposure to elastase in the wild type mice resulted in accumulation of LTB4 and neutrophils in the lungs. However, the LTA4H -/- mice unexpectedly showed a delayed influx of neutrophils during the resolution phase of the inflammation. Even though the complete knockout of the LTA4H loci was protective against emphysema, we interpreted this result as suggesting that the lack of LTA4H function paradoxically promoted the influx of neutrophils by non-LTB4-associated mechanisms. In conjunction with the report by Blalock and co-workers, we rationally hypothesized that the aminopeptidase activity of the LTA4H enzyme was responsible for the persistent neutrophilic inflammation in our murine model.
Conventional experimental approaches (i.e. transgenic knock-in, knock-out or available pharmacological agents) are incapable of uncoupling the complex dual activities of the LTA4H enzyme. Therefore, they are inherently inadequate to investigate the individual enzymatic activities of the LTA4H enzyme. In order to test our hypothesis, we identified 4-MDM as a small molecule that upregulates the aminopeptidase activity of the LTA4H enzyme. This pharmacological agent was found to have the following properties: 1) the compound 4-MDM is chemically stable in a pH6.8 buffer system, which is the typical pH of the sites of inflammation; 2) 4-MDM upregulates LTA4H aminopeptidase activity in vitro with an AC50 of approximately 50 μM; and, 3) multi-dimensional evaluations in an elastase-induced murine model of pulmonary emphysema showed 4-MDM to be protective against emphysematous damage. In combination, these results support our hypothesis that the aminopeptidase activity of the LTA4H enzyme is an important function of the enzyme and may be responsible for the protective properties associated with the enzyme.
Overall, we have shown for the first time that a small molecule augmentor of the aminopeptidase activity of the LTA4H enzyme protects murine lungs from IN elastase-induced pulmonary emphysema. In light of recent developments in the understanding of LTA4H biology, it is plausible that apparent protective properties of the LTA4H enzyme noted in our in vivo study may reside in its aminopeptidase activity. The dichotomous nature of this enzyme represents an intriguing problem for drug discovery efforts, which we attempted to address with a small molecule as a pharmacological agent. Taken together, our results suggest that the aminopeptidase activity of the LTA4H enzyme can be targeted as a new strategy for treating inflammatory diseases.
Acknowledgments
This work was supported by the Flight Attendant Medical Research Institute (YMS), by National Institutes of Health Grants K08-HL-91127 (YMS), The Wellcome Trust (082727/Z/07/Z) (RJS), and the Lombardi Comprehensive Cancer Center at Georgetown University (MP).
Abbreviations
- 4-MDM
4-methoxydiphenylmethane
- AC50
concentration at 50% enzyme augmentation
- Ala-p-NA
L-alanine p-nitroanilide
- COPD
chronic obstructive pulmonary disease
- IN
intranasal
- LTA4
leukotriene A4
- LTA4H
leukotriene A4 hydrolase
- LTB4
leukotriene B4
- PGP
proline-glycine-proline
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shin EH, Lee HY, Bae YS. Biochem Biophys Res Commun. 2006;348:606. doi: 10.1016/j.bbrc.2006.07.084. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RI, Benincaso AI, Anderson RJ, Coblyn JS, Austen KF, Weinblatt ME. Arthritis Rheum. 1992;35:376. doi: 10.1002/art.1780350403. [DOI] [PubMed] [Google Scholar]
- 3.Martin TR, Altman LC, Albert RK, Henderson WR. Am Rev Respir Dis. 1984;129:106. doi: 10.1164/arrd.1984.129.1.106. [DOI] [PubMed] [Google Scholar]
- 4.Czarnetzki B. Clin Exp Immunol. 1983;54:486. [PMC free article] [PubMed] [Google Scholar]
- 5.Tavares-Murta BM, Zaparoli M, Ferreira RB, Silva-Vergara ML, Oliveira CH, Murta EF, Ferreira SH, Cunha FQ. Crit Care Med. 2002;30:1056. doi: 10.1097/00003246-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Roos D, Kuijpers TW, Mascart-Lemone F, Koenderman L, de Boer M, van Zwieten R, Verhoeven AJ. Blood. 1993;81:2735. [PubMed] [Google Scholar]
- 7.Doi F, Goya T, Torisu M. Heptology. 1993;17:1086. [PubMed] [Google Scholar]
- 8.Hartiala KT, Langlois L, Goldstein IM, Rosenbaum JT. Infect Immun. 1985;50:527. doi: 10.1128/iai.50.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Roller BH. J Immunol. 1999;163:6810. [PubMed] [Google Scholar]
- 10.Stojadinovic A, Kiang J, Smallridge R, Galloway R, Shea-Donohue T. Gastroenterology. 1995;109:505. doi: 10.1016/0016-5085(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 11.Carpagnano GE, Barnes PJ, Geddes DM, Hodson ME, Kharitonov SA. Am J Respir Crit Care Med. 2003;167:1109. doi: 10.1164/rccm.200203-179OC. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence R, Sorrell T. Lancet. 1993;342:465. doi: 10.1016/0140-6736(93)91594-c. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence RH, Sorrelli TC. Clin Exp Immunol. 1992;89:321. doi: 10.1111/j.1365-2249.1992.tb06953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Driscoll BR, Cromwell O, Kay AB. Clin Exp Immunol. 1984;55:397. [PMC free article] [PubMed] [Google Scholar]
- 15.Cromwell O, Walport MJ, Taylor GW, Morris HR, O'Driscoll BR, Kay AB. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:251. [PubMed] [Google Scholar]
- 16.Maznyczka A, Mangino M, Whittaker A, Braund P, Palmer T, Tobin M, Goodall AH, Bradding P, Samani NJ. Clin Sci (Lond) 2007;112:411. doi: 10.1042/CS20060271. [DOI] [PubMed] [Google Scholar]
- 17.Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M, Manolescu A, Arnar DO, Andersen K, Sigurdsson A, Thorgeirsson G, Jonsson A, Agnarsson U, Bjornsdottir H, Gottskalksson G, Einarsson A, Gudmundsdottir H, Adalsteinsdottir AE, Gudmundsson K, Kristjansson K, Hardarson T, Kristinsson A, Topol EJ, Gulcher J, Kong A, Gurney M, Thorgeirsson G, Stefansson K. Jama. 2005;293:2245. doi: 10.1001/jama.293.18.2245. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi S. Kurume Med J. 2000;47:63. doi: 10.2739/kurumemedj.47.63. [DOI] [PubMed] [Google Scholar]
- 19.Grespan R, Fukada SY, Lemos HP, Vieira SM, Napimoga MH, Teixeira MM, Fraser AR, Liew FY, McInnes IB, Cunha FQ. Arthritis Rheum. 2008;58:2030. doi: 10.1002/art.23597. [DOI] [PubMed] [Google Scholar]
- 20.Kowal-Bielecka O, Distler O, Kowal K, Siergiejko Z, Chwiecko J, Sulik A, Gay RE, Lukaszyk AB, Gay S, Sierakowski S. Arthritis Rheum. 2003;48:1639. doi: 10.1002/art.11042. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara T, Takada S, Miyamoto M, Nomura M, Kato M. Inflammation. 1996;20:43. doi: 10.1007/BF01487744. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths RJ, Pettipher ER, Koch K, Farrell CA, Breslow R, Conklyn MJ, Smith MA, Hackman BC, Wimberly DJ, Milici AJ, et al. Proc Natl Acad Sci USA. 1995;92:517. doi: 10.1073/pnas.92.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperling RI, Coblyn JS, Larkin JK, Benincaso AI, Austen KF, Weinblatt ME. Arthritis Rheum. 1990;33:1149. doi: 10.1002/art.1780330815. [DOI] [PubMed] [Google Scholar]
- 24.Izquierdo JL, Almonacid C, Parra T, Perez J. Arch Bronconeumol. 2006;42:332. doi: 10.1016/s1579-2129(06)60542-9. [DOI] [PubMed] [Google Scholar]
- 25.Stockley RA, Bayley DL, Unsal I, Dowson LJ. Am J Respir Crit Care Med. 2002;165:1494. doi: 10.1164/rccm.2109013. [DOI] [PubMed] [Google Scholar]
- 26.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Am J Respir Crit Care Med. 1999;160:1968. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]
- 27.Profita M, Giorgi RD, Sala A, Bonanno A, Riccobono L, Mirabella F, Gjomarkaj M, Bonsignore G, Bousquet J, Vignola AM. Allergy. 2005;60:1361. doi: 10.1111/j.1398-9995.2005.00892.x. [DOI] [PubMed] [Google Scholar]
- 28.Hubbard RC, Fells G, Gadek J, Pacholok S, Humes J, Crystal RG. J Clin Invest. 1991;88:891. doi: 10.1172/JCI115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanno Y, Kakuta Y, Aikawa T, Shindoh Y, Ohno I, Takishima T. Am J Chin Med. 1988;16:145. doi: 10.1142/S0192415X88000212. [DOI] [PubMed] [Google Scholar]
- 30.Usery JB, Self TH, Muthiah MP, Finch CK. Pharmacotherapy. 2008;28:1183. doi: 10.1592/phco.28.9.1183. [DOI] [PubMed] [Google Scholar]
- 31.Konstan MW. Pediatr Pulmonol. 2005;28S:125. [Google Scholar]
- 32.Hawkey CJ, Dube LM, Rountree LV, Linnen PJ, Lancaster JF. Gastroenterology. 1997;112:718. doi: 10.1053/gast.1997.v112.pm9041232. [DOI] [PubMed] [Google Scholar]
- 33.Roberts WG, Simon TJ, Berlin RG, Haggitt RC, Snyder ES, Stenson WF, Hanauer SB, Reagan JE, Cagliola A, Tanaka WK, Simon S, Berger ML. Gastroenterology. 1997;112:725. doi: 10.1053/gast.1997.v112.pm9041233. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Gonzalez F, Alten RH, Bensen WG, Brown JP, Sibley JT, Dougados M, Bombardieri S, Durez P, Ortiz P, de-Miquel G, Staab A, Sigmund R, Salin L, Leledy C, Polmar SH. Ann Rheum Dis. 2007;66:628. doi: 10.1136/ard.2006.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim YM, Zhu Z, Zheng T, Lee CG, Homer RJ, Ma B, Elias JA. J Immunol. 2006;177:1918. doi: 10.4049/jimmunol.177.3.1918. [DOI] [PubMed] [Google Scholar]
- 36.Shim YM, Paige M, Hanna H, Kim SH, Burdick MD, Strieter RM. American journal of physiology Lung cellular and molecular physiology. 2010;299:L749. doi: 10.1152/ajplung.00116.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE. Science. 2010;330:90. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orning L, Gierse JK, Fitzpatrick FA. J Biol Chem. 1994;269:11269. [PubMed] [Google Scholar]
- 39.Rudberg PC, Tholander F, Andberg M, Thunnissen MM, Haeggstrom JZ. The Journal of biological chemistry. 2004;279:27376. doi: 10.1074/jbc.M401031200. [DOI] [PubMed] [Google Scholar]
- 40.Schechter I, Berger A. Biochem Biophys Res Commun. 1967;27:157. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 41.Jiang X, Zhou L, Wei D, Meng H, Liu Y, Lai L. Bioorg Med Chem Lett. 2008;18:6549. doi: 10.1016/j.bmcl.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 42.Yuan W, Munoz B, Wong CH, Haeggstrom JZ, Wetterholm A, Samuelsson B. J Med Chem. 1993;36:211. doi: 10.1021/jm00054a004. [DOI] [PubMed] [Google Scholar]
- 43.Penning TD, Chandrakumar NS, Chen BB, Chen HY, Desai BN, Djuric SW, Docter SH, Gasiecki AF, Haack RA, Miyashiro JM, Russell MA, Yu SS, Corley DG, Durley RC, Kilpatrick BF, Parnas BL, Askonas LJ, Gierse JK, Harding EI, Highkin MK, Kachur JF, Kim SH, Krivi GG, Villani-Price D, Pyla EY, Smith WG. J Med Chem. 2000;43:721. doi: 10.1021/jm990496z. [DOI] [PubMed] [Google Scholar]
- 44.Penning TD, Russell MA, Chen BB, Chen HY, Liang CD, Mahoney MW, Malecha JW, Miyashiro JM, Yu SS, Askonas LJ, Gierse JK, Harding EI, Highkin MK, Kachur JF, Kim SH, Villani-Price D, Pyla EY, Ghoreishi-Haack NS, Smith WG. J Med Chem. 2002;45:3482. doi: 10.1021/jm0200916. [DOI] [PubMed] [Google Scholar]
- 45.Davies DR, Mamat B, Magnusson OT, Christensen J, Haraldsson MH, Mishra R, Pease B, Hansen E, Singh J, Zembower D, Kim H, Kiselyov AS, Burgin AB, Gurney ME, Stewart LJ. J Med Chem. 2009;52:4694. doi: 10.1021/jm900259h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandanayaka V, Mamat B, Mishra RK, Winger J, Krohn M, Zhou LM, Keyvan M, Enache L, Sullins D, Onua E, Zhang J, Halldorsdottir G, Sigthorsdottir H, Thorlaksdottir A, Sigthorsson G, Thorsteinnsdottir M, Davies DR, Stewart LJ, Zembower DE, Andresson T, Kiselyov AS, Singh J, Gurney ME. J Med Chem. 2010;53:573. doi: 10.1021/jm900838g. [DOI] [PubMed] [Google Scholar]
- 47.Grice CA, Tays KL, Savall BM, Wei J, Butler CR, Axe FU, Bembenek SD, Fourie AM, Dunford PJ, Lundeen K, Coles F, Xue X, Riley JP, Williams KN, Karlsson L, Edwards JP. J Med Chem. 2008;51:4150. doi: 10.1021/jm701575k. [DOI] [PubMed] [Google Scholar]
- 48.Rudberg PC, Tholander F, Thunnissen MM, Haeggstrom JZ. The Journal of biological chemistry. 2002;277:1398. doi: 10.1074/jbc.M106577200. [DOI] [PubMed] [Google Scholar]


