Abstract
Extinction learning refers to the phenomenon that a previously learned response to an environmental stimulus, for example, the expression of an aversive behaviour upon exposure to a specific context, is reduced when the stimulus is repeatedly presented in the absence of a previously paired aversive event. Extinction of fear memories has been implicated with the treatment of anxiety disease but the molecular processes that underlie fear extinction are only beginning to emerge. Here, we show that fear extinction initiates upregulation of hippocampal insulin-growth factor 2 (Igf2) and downregulation of insulin-growth factor binding protein 7 (Igfbp7). In line with this observation, we demonstrate that IGF2 facilitates fear extinction, while IGFBP7 impairs fear extinction in an IGF2-dependent manner. Furthermore, we identify one cellular substrate of altered IGF2 signalling during fear extinction. To this end, we show that fear extinction-induced IGF2/IGFBP7 signalling promotes the survival of 17–19-day-old newborn hippocampal neurons. In conclusion, our data suggest that therapeutic strategies that enhance IGF2 signalling and adult neurogenesis might be suitable to treat disease linked to excessive fear memory.
Keywords: fear extinction, gene expression, hippocampus, learning and memory, mouse models
Introduction
The precise molecular clockwork that regulates memory function is far from being understood. Such research is, however, of great relevance since memory disturbances are key features of various neurological disorders. Extinction of fear memory is a particular form of cognitive function, that is of special interest because of its involvement in the treatment of anxiety and mood disorders (Myers and Davis, 2007; Fischer and Tsai, 2008; Quirk and Mueller, 2008; Pape and Pare, 2010; Radulovic and Tronson, 2010). Here, extinction training is central as a therapeutic strategy to inhibit excess fear. Fear is normally a protective mechanisms aimed to promote an adaptive response to danger. However, extreme extrinsic events can force the system beyond its limits. Such a homeostasis breakdown often leads to excessive fear and mental illnesses like anxiety and mood disorders, which severely affect the life of patients and are an increasing burden to our societies (Sotres-Bayon et al, 2006; Bremner et al, 2008; Fischer and Tsai, 2008; Hartley and Phelps, 2010). During extinction training, fear inhibition is achieved by repeated re-exposure to the fear-eliciting stimulus in the absence of any aversive event. This normally leads to a gradual reduction of fear, namely the extinction of fear memories. To understand the molecular mechanisms underlying fear extinction is, therefore, of great clinical importance.
In the laboratory, fear extinction is often studied using the Pavlovian fear conditioning (FC) paradigm. In the contextual version of this paradigm, a single exposure of rodents to a novel context followed by an electric foot-shock elicits the acquisition of conditioned fear. On the basis of associative learning, the animals display an inborn aversive freezing behaviour upon re-exposure to the conditioned context. This form of contextual FC is hippocampus dependent and leads to a long-lasting fear memory (Fischer et al, 2004, 2007; Frankland et al, 2006; Matynia et al, 2008). During extinction training, animals are repeatedly re-exposed to the conditioned context without receiving the foot-shock again (extinction trial, E), which eventually results in the decline of the aversive freezing behaviour (Sananbenesi et al, 2007).
Experimental data obtained across species demonstrated that the amygdala, the medial prefrontal cortex and the hippocampus are critically involved in fear extinction (Phelps and LeDoux, 2005; Myers and Davis, 2007; Bremner et al, 2008; Fischer and Tsai, 2008; Quirk and Mueller, 2008; Ponomarev et al, 2010; Shin and Liberzon, 2010). The most common theories suggest that fear extinction involves a combination of associative processes such as new learning and also non-associative mechanisms involving decreased responsiveness to the fear-eliciting stimulus (Myers and Davis, 2007). The molecular processes that underlie fear extinction are only beginning to emerge.
In this study, we observe that contextual fear extinction training induced the upregulation of hippocampal insulin-growth factor 2 (IGF2) and downregulation of insulin-growth factor binding protein 7 (IGFBP7), which attenuates the biological function of IGFs. We demonstrate that IGF2 facilitates fear extinction, while IGFBP7 impairs fear extinction in an IGF2-dependent manner. Our data, furthermore, suggest that one important cellular substrate of altered IGF2 signalling during fear extinction is adult neurogenesis. In conclusion, our data indicate that therapeutic strategies to enhance IGF2 signalling and the survival of newborn hippocampal neurons could be suitable therapeutic avenue to treat neuropsychiatric disorders that involve excessive fear memory.
Results
Fear extinction regulates hippocampal Igf2 and Igfbp7 levels
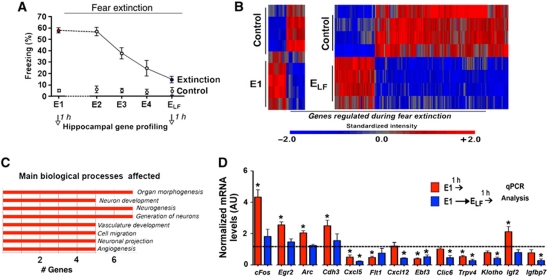
We employed mice to investigate fear extinction in the hippocampus-dependent contextual FC paradigm (Sananbenesi et al, 2007). To this end, male C57BL/6J mice were exposed to the FC box (context) followed by an electric foot-shock, which elicits the acquisition of conditioned contextual fear. For extinction training, animals were repeatedly re-exposed to the conditioned context on consecutive days (24 h interval) without receiving the foot-shock again (extinction trial, E). This procedure eventually results in the decline of the aversive freezing behaviour (Figure 1A; Sananbenesi et al, 2007). Mice that were exposed to the conditioning context without receiving FC training served as control groups. Since pilot experiments showed that the expression of cFos do not significantly differ among mice that were exposed to the context either twice or five times this control group was pooled for gene array analysis and in subsequent experiments. To gain a better understanding of the molecular processes underlying fear extinction, we performed a genome-wide analysis of the hippocampal transcriptome during fear extinction. In the employed paradigm, fear extinction is a gradual process. To capture the longitudinal course of fear extinction, we decided to perform hippocampal microarray analysis at two time points: (1) after the first extinction trial (E1) when animals display high levels of aversive freezing behaviour and (2) at the extinction trial on which the freezing behaviour was significantly reduced when compared to E1. This extinction trial, in the case of this experiment E5, we termed ‘extinction trial low freezing’ (ELF) (Figure 1A). Please note that we will use the term ELF only for experiments in which we need to define a time point for molecular analysis. Mice that were exposed to the conditioning context without receiving FC training served as control groups. Previous data showed that the immediate early gene FBJ osteosarcoma oncogene (cFos), a marker for neuronal activity, was transiently upregulated at the beginning but towards the end of extinction training (Tronson et al, 2009). Therefore, we decided to perform the microarray at the time point 1 h after E1 and ELF. This experimental approach is obviously a compromise and does not capture all critical time windows potentially implicated with contextual fear extinction. We are aware that microarray studies are prone to false positives and especially data obtained from complex biological systems need to be interpreted cautiously. Yet, we reasoned that our approach might provide a realistic chance to identify genes that may have an important role in fear extinction and thus open up new avenues for further experiments.
Figure 1.
Fear extinction regulates hippocampal gene expression. (A) Experimental design. Mice (n=5/group) were subjected to contextual fear conditioning (FC) followed by extinction training on consecutive days. Hippocampal tissue was prepared for gene array analysis 1 h after E1 and ELF. Mice that were subjected to the conditioning context but did not undergo fear extinction served as control groups. (B) Heat map showing differential gene expression during fear extinction. (C) GO-term analysis of genes regulated during fear extinction. (D) The expression of selected genes upon fear extinction was analysed via qPCR. *P<0.05 versus control; E, extinction trial; ELF, extinction trial low freezing; (n=5/group); error bars indicate s.e.m.
We found that 137 genes, implicated with biological processes such as cell proliferation, neuronal projection or neurogenesis, were differentially expressed during fear extinction (Figure 1B and C; Supplementary Table S1). To confirm the gene array data, we analysed hippocampal expression of genes that were chosen to represent signalling cascades affected during fear extinction by quantitative real-time PCR (qPCR) using the same mRNA samples used for the microarray (Supplementary Figure S1) and in samples from an independent experiment (Figure 1D). Hippocampal lysates obtained 1 h after mice were exposed to FC training served as an additional control group (Supplementary Figure S2). Consistent with our microarray results, we observed a strong upregulation of cFos expression 1 h after FC and E1. Notably, cFos induction significantly decreased upon ELF exposure, when compared to E1 (Figure 1D). A similar trend was observed for other immediate early genes such as early growth response 2 (Egr2) and activity-regulated cytoskeleton-associated protein (Arc) as well as chromodomain helicase DNA binding protein 3 (Chd3) (Figure 1D). We also confirmed differential expression for a number of genes that were linked to insulin-growth factor (Igf) signalling. To this end, the Igf2, Igfbp7 and Klotho genes were exclusively regulated upon fear extinction (Figure 1D).
While our microarray data point to a number of interesting signalling cascades that certainly merit further investigation in the present study we decided to focus on hippocampal IGF2 signalling. This decision was based on the fact that two genes of this pathway were regulated in an opposite manner. Namely, Igf2 was upregulated after E1 and Igfbp7 was downregulated after ELF. However, since IGFBPs attenuate the function of IGFs (Baxter, 1991), such a regulation would ultimately result in increased hippocampal IGF signalling. Especially IGF1 (Trejo et al, 2008; Llorens-Martín et al, 2009, 2010), but most recently also IGF2 (Chen et al, 2011) have been implicated with cognitive function, but its role in fear extinction has not been investigated previously. In summary, these data strongly suggested a role of Igf2 and Igfbp7 in fear extinction that merit further investigation.
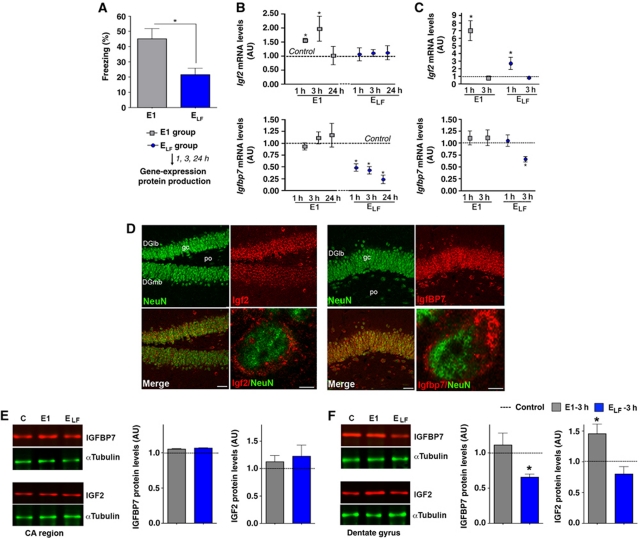
First, we decided to investigate the regulation of Igf2 and Igfbp7 during fear extinction in greater detail. We subjected additional groups of mice to our fear extinction paradigm and analysed hippocampal Igf2 and Igfbp7 expression 1, 3 and 24 h after exposure to E1 and ELF (Figure 2A). We observed that Igf2 expression was significantly increased 1 and 3 h after exposure to E1 but did not change upon exposure to ELF (Figure 2B). Elevated Igf2 levels after E1 exposure are not due to the FC procedure (Supplementary Figure S3). The expression of Igfbp7 did not change after E1 but was strongly downregulated after exposure to ELF (Figure 2B). This effect was not simply due to the passing of time because Igfbp7 expression did not change in mice that were exposed to FC and E1 but sacrificed 4 days later without further extinction training (Supplementary Figure S3). In an additional experiment, we also analysed the expression of Igf2 and Igfbp7 in specific hippocampal subregions and found that the differential expression of Igf2 and Igfbp7 was most prominent in the hippocampal dentate gyrus (Figure 2C; Supplementary Figure S4) where significant levels of IGF2 and IGFBP7 protein are produced (Figure 2D). The expression of other key molecules of the hippocampal IGF signalling pathway including Igf1, Igf1 receptor, Igfbp4 and Igfbp5 was not altered during extinction training (Supplementary Figure S5). In conclusion, these data establish that fear extinction training regulates hippocampal Igf2 and Igfbp7 levels. However, changes in mRNA level do not always translate into altered protein amount. Without a corresponding change in IGF2 and IGFBP7 protein levels such a pathway may, however, not have any biological relevance in contextual fear extinction. To address this question, we analysed the levels of IGF2 and IGFBP7 protein after fear extinction via quantitative immunoblot. Mice were exposed to our fear extinction paradigm and lysates from the hippocampal cornu ammonis (CA) and dentate gyrus regions were prepared 3 h after E1 and ELF. We chose the time point 3 h since changes in protein levels usually follow changes in mRNA expression. In line with the gene expression data, we observed that IGF2 protein levels increased 3 h after E1 exposure in the hippocampal dentate gyrus but not in the CA region (Figure 2E and F). The levels of IGFBP7 decreased 3 h after ELF in the dentate gyrus but not in the CA region (Figure 2E and F). In conclusion, our data suggest that the extinction of contextual fear memory involves altered hippocampal IGF2/IGFBP7 function.
Figure 2.
Fear extinction regulates hippocampal Igf2/Igfbp7 expression. (A) Mice were subjected to fear extinction. Behavioural analysis confirmed that freezing behaviour was significantly reduced upon ELF exposure, when compared to E1. Mice that were subjected to the conditioning context but not subjected to fear extinction served as control (*P<0.05 versus E1; n=15/group). (B) qPCR analysis of Igf2 and Igfbp7 expression in the total hippocampus at different time points after fear extinction training (*P<0.05 versus control; n=5/group). (C) qPCR analysis of Igf2 and Igfbp7 expression in the dentate gyrus (*P<0.05 versus control; n=5/group). (D) Representative images showing immunostaining for IGF2 and IGFBP7 protein levels in the dentate gyrus. Scale bars low magnification images: 50 μm; high magnification: 2 μm. (E) Left panel: Representative immunoblot analysis of IGF2 and IGFBP7 protein levels in the CA region 3 h after exposure to E1 or ELF. Right panel. Quantification (n=5/group). (F) Left panel: Representative immunoblot analysis of IGF2 and IGFBP7 protein levels in the dentate gyrus region 3 h after exposure to E1 or ELF. Right panel. Quantification (*P<0.05 versus control, n=5/group). DGlb, dentate gyrus lateral blade; DGmb, dentate gyrus medial blade; E, extinction trial; ELF, extinction trial low freezing; gc, granular cell layer of the dentate gyrus; Po, polymorph layer of the dentate gyrus; error bars indicate s.e.m.
IGF2 and IGFBP7 regulate fear extinction
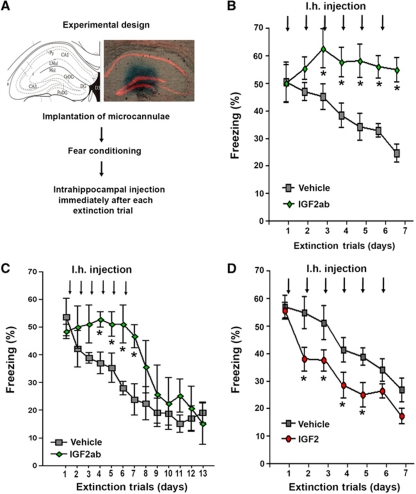
On the basis of the above-described data, we decided to further investigate the role of IGF2 and IGFBP7 during fear extinction. IGF2 and IGFBP7 are secreted proteins that act in an autocrine or paracrine manner (Broughton and Partridge, 2009). As such, we reasoned that a suitable approach to study the role of those proteins would be to manipulate their levels during fear extinction via intrahippocampal injection of recombinant proteins or blocking antibodies. Therefore, microcannulae were implanted into the dentate gyrus of mice. After all mice had recovered from surgery they were subjected to FC followed by extinction training (Figure 3A). To test whether decreasing physiological IGF2 levels in the hippocampus would affect fear extinction mice were injected with an IGF2 blocking antibody (IGF2ab; Ostrovsky et al, 2009) immediately after each extinction trial. When compared to the vehicle group, fear extinction was severely impaired in IGF2ab-treated mice (Figure 3B). Extinction training was stopped when the vehicle group reached ELF criteria. Importantly, mice were able to extinct fear memories once IGF2ab treatment was stopped (Figure 3C). Moreover, prolonged IGF2ab treatment did not cause obvious hippocampal damage or long-lasting memory impairment (Supplementary Figure S6). Next, we analysed if increasing hippocampal IGF2 levels would affect fear extinction. The experiment was performed as described above but instead of IGF2ab, mice were injected with IGF2 while the other group received vehicle solution. Fear extinction was significantly facilitated in IGF2-treated mice (Figure 3D). In conclusion, those results show that IGF2 is required for successful fear extinction. Notably, inhibition of physiological IGF1 signalling via intrahippocampal injection of IGF1 blocking antibody (IGF1ab) did not affect fear extinction. Similarly, administration of IGF1 had no effect on fear extinction and did not rescue IGFBP7-mediated impairment of fear extinction (Supplementary Figure S7). These data point to a specific role of IGF2 signalling in fear extinction and also suggest that injection procedure itself does not affect fear extinction.
Figure 3.
Hippocampal IGF2 signalling regulates fear extinction. (A) Experimental design. The image shows the position of the injection cannulae as visualized by methylene blue injection. (B) Fear extinction was significantly impaired in mice that were injected immediately after each extinction trial with IGF2ab when compared to the vehicle group (*P<0.05 versus vehicle, n=10/group). (C) In an independent experiment, we found that IGF2ab-treated mice were able to successfully extinct fear memories once IGF2ab treatment was stopped. (D) Fear extinction was significantly enhanced in mice that were injected immediately after each extinction trial with IGF2 when compared to the vehicle group (*P<0.05 versus vehicle, n=10/group). Py, pyramidal cell layer; rad, stratum radiatum; Lmol, lacunosum moleculare; CA1, subfield CA1; CA3, subfield CA3; DG, dentate gyrus. Error bars indicate s.e.m.
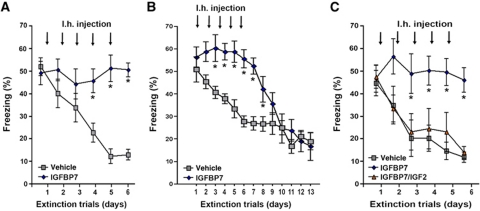
Next, we employed the same experimental strategy to analyse the role of IGFBP7 in fear extinction. Microcannulae were implanted into the dentate gyrus of mice. Immediately after each extinction trial, mice were injected with IGFBP7 or vehicle. Animals that were treated with IGFBP7 displayed severely impaired fear extinction when compared to the vehicle group (Figure 4A). Once IGFBP7 treatment was stopped, mice were able to extinct fear memories (Figure 4B). Prolonged IGFBP7 treatment did not cause obvious hippocampal damage or long-lasting memory impairment (Supplementary Figure S6). Next, we employed an additional experimental group in which IGFBP7 was co-injected with IGF2. While in agreement with our previous results, IGFBP7-treated mice showed severely impaired fear extinction, this effect was completely reversed in mice that were co-injected with IGFBP7 and IGF2 indicating that administration of additional IGF2 could overcome the effect of IGFBP7 (Figure 4C). Notably, administration of IGF1 was not able to rescue the effect of IGFBP7 (Supplementary Figure S7). None of the treatments affected locomotor activity (Supplementary Figure S8). Although we cannot exclude that IGF2 and IGFBP7 affect fear extinction also via independent mechanisms, our data strongly support the view that a hippocampal IGF2/IGFBP7 signalling pathway mediates fear extinction.
Figure 4.
Hippocampal IGFBP7 signalling regulates fear extinction. (A) Fear extinction was significantly impaired in mice that were injected immediately after each extinction trial with IGFBP7 when compared to the vehicle group (*P<0.05 versus vehicle) (n=10/group). (B) In an independent experiment, we observed that once IGFBP7 treatment was stopped, those mice were able to successfully extinct fear memories. (C) Mice were subjected to fear extinction and subsequently injected with IGFBP7, IGFBP7 and IGF2 or vehicle. IGF2 injection rescued the effect of IGFBP7 (*P<0.05 versus vehicle) (n=10/group). Error bars indicate s.e.m.
Fear extinction promotes the survival of 17–19-day-old newborn hippocampal neurons
Having established that a hippocampal IGF2/IGFBP7 pathway regulates the extinction of fear memories we wondered about the cellular substrate targeted by this pathways. Since IGF signalling is implicated with various cellular functions (Broughton and Partridge, 2009) it is likely that multiple cellular mechanisms account for the observed effect of IGF2 and IGFBP7 on fear extinction. We hypothesized that one candidate mechanism by which IGF2/IGFBP7 signalling affects the extinction of fear memories could be the regulation of adult neurogenesis. This is based on three findings: (1) on the fact data mining using GO-term analysis identified neurogenesis as a key biological process affected by fear extinction in our gene array analysis (Figure 1C); (2) although little is known on the specific role of IGF2 (Lehtinen et al, 2011) and IGFBP7, IGF signalling has been implicated with cell proliferation and adult neurogenesis (Leventhal et al, 1995; Aberg et al, 2003; Bateman and McNeill, 2006; Broughton and Partridge, 2009; Lee and Son, 2009); and (3) a recent study demonstrated that adult hippocampal neurogenesis is required for fear extinction (Deng et al, 2009).
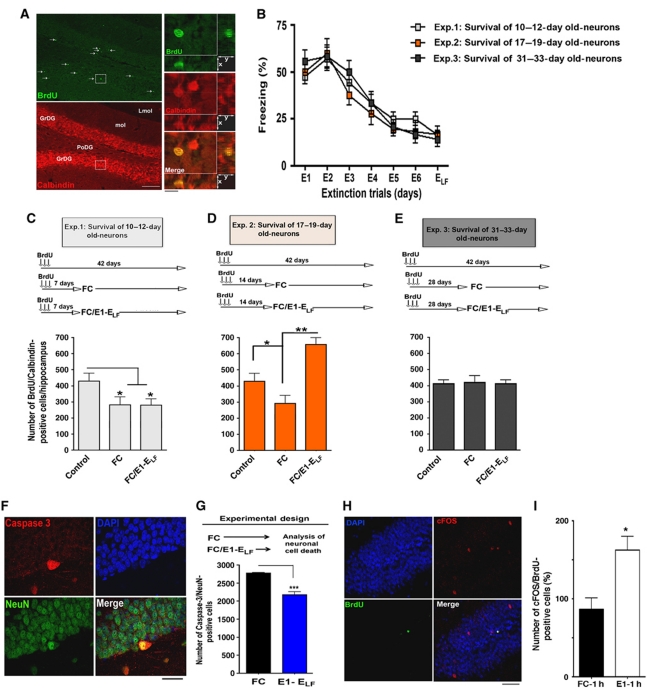
Adult neurogenesis is a complex process that consists of three main phases: the proliferation of neuroprogenitor cells, differentiation into neurons and the survival of the newly generated neurons (Kempermann et al, 2004; Jessberger et al, 2008; Zhao et al, 2008). In the hippocampus, newborn cells originate from proliferating neuronal progenitor cells in the subgranular zone. The rate of proliferation can be measured by labelling dividing cells with the base analogue Bromodeoxyuridine (BrdU) that can subsequently be detected by immunostaining. However, we found that exposure of mice to our extinction paradigm did not affect the proliferation of hippocampal neuroprogenitor cells (Supplementary Figure S9). Therefore, we designed experiments to test whether fear extinction affects the survival of newly generated neurons. Within 1–4 weeks after birth, adult neurons undergo a critical period of maturation in which they display greater plasticity than fully mature neurons (Schmidt-Hieber et al, 2004; Tashiro et al, 2007; Deng et al, 2010). During this phase, the survival of those immature cells can be affected by environmental factors (Kempermann et al, 2004; Zhao et al, 2008; Lucassen et al, 2010). To investigate the effect of fear extinction on neuronal survival, we labelled dividing cells by BrdU injection. All experimental groups were sacrificed 42 days after the first BrdU injection and the number of surviving neurons was analysed by co-immunostaining of BrdU and Calbindin, a well-established marker for mature granule cells (Figure 5A). After BrdU injection, mice were separated into three experimental groups that underwent fear extinction and reached ELF criteria (Figure 5B). Importantly, the groups of mice were exposed to fear extinction at different time points after BrdU injection. To this end, groups were exposed to the first extinction trial when labelled newborn cells were either 10–12 days (Figure 5C), 17–19 days (Figure 5D) or 31–33 days (Figure 5E) old. The survival of newly generated adult neurons was analysed by counting the number BrdU/Calbindin-positive cells in comparison with a control group that did not underwent FC or extinction training. Mice that were exposed to FC but did not underwent fear extinction served as additional control groups (Figure 5C–E).
Figure 5.
Fear extinction promotes the survival of immature newly generated neurons. (A) Representative images showing BrdU/Calbindin-positive cells that indicate maturation of newborn hippocampal neurons. Arrows indicate BrdU-positive cells. Scale bar low magnification images: 100 μm. Scale bar high magnification images: 20 μm. (B) Fear extinction, as indicated by the gradual decline of aversive freezing behaviour throughout extinction trials, was similar among the experimental groups that were used to analyse the survival of newly generated hippocampal neurons during fear extinction (n=8/group). (C–E) Upper panels show the experimental design to analyse the effect of fear extinction on 10–12-day-old (C), 17–19-day-old (D) or 31–33-day-old (E) hippocampal neurons. Lower panels display the quantification of BrdU/Calbindin-positive cells under each experimental condition. Note that successful fear extinction significantly enhanced the survival of 17–19-day-old newly generated neurons when compared to the control (*P<0.05) or fear conditioning group (**P<0.01). (F) Representative images showing active caspase-3-positive cells, indicative of apoptotic cell death, in the hippocampal dentate gyrus region. Scale bar: 20 μm. (G) Mice were subjected to fear conditioning or fear extinction training. The number of active caspase-3-positive cells in the dentate gyrus was significantly reduced in mice that underwent extinction training when compared to mice that were only exposed to fear conditioning (***P<0.0001). (H) Representative image showing a cFOS-positive 17–19-day-old neuron in the hippocampus of a mouse 1 h after exposure to E1. Scale bar: 50 μm. (I) Mice were subjected to fear conditioning or fear extinction training. The number of cFOS/BrdU-positive cells was analysed 1 h after exposure to E1 or 1 h after FC. The data are normalized to the FC group (P=0.0154). GrDG, granular cell layer of the dentate gyrus; Lmol, lacunosum moleculare. Error bars indicate s.e.m.
Interestingly, FC significantly decreased the survival of 10–12-day-old newly generated neurons and this effect was not affected by extinction training (Figure 5C). While adult neurogenesis has been implicated with various types of memory formation (Zhao et al, 2008), it has been reported that depending on the experimental paradigm FC can impair hippocampal neurogenesis (Pham et al, 2005). Similarly, FC decreased the survival rate 17–19-day-old neurons. In striking contrast, the number of surviving BrdU/Calbindin-positive cells was significantly increased in mice that underwent fear extinction (Figure 5D). Similar data were obtained when BrdU/Calretinin-positive cells were analysed immediately after the end of fear extinction training (Supplementary Figure S10). Notably, neither FC nor fear extinction had any effect on 31–33-day-old adult-generated neurons (Figure 5E). In line with these data, we found that the number of active caspase-3-positive neurons, indicating apoptotic cell death, was significantly lower in the dentate gyrus of mice that underwent extinction training and reached ELF criteria when compared to mice subjected to FC without subsequent extinction training (Figure 5F and G). These data suggest that fear extinction specifically enhances the survival of 17–19-day-old hippocampal neurons. At first, it may appear counterintuitive that those immature neurons should contribute to extinction learning. However, previous findings suggesting that such immature newborn neurons can participate in neuronal plasticity (Schmidt-Hieber et al, 2004; Tashiro et al, 2007; Deng et al, 2009, 2010). To provide further evidence that in our experimental settings 17–19-day-old newborn neurons are able to participate in neuronal network plasticity we analysed if those cells can induce cFOS levels during fear extinction. cFOS is a well-established marker for neuronal activity, that is upregulated after exposure to E1 and has been used previously to assay neuronal plasticity in immature newborn neurons (Jessberger and Kempermann, 2003). Notably, we were to show that 17–19-day-old newborn display increased cFOS expression upon exposure to fear extinction (Figure 5H and I) further supporting the view that those neurons are able to participate in neuronal activity related to extinction learning. In conclusion, our data establish that fear extinction initiates a signalling pathway that promotes survival of immature 17–19-day-old hippocampal neurons.
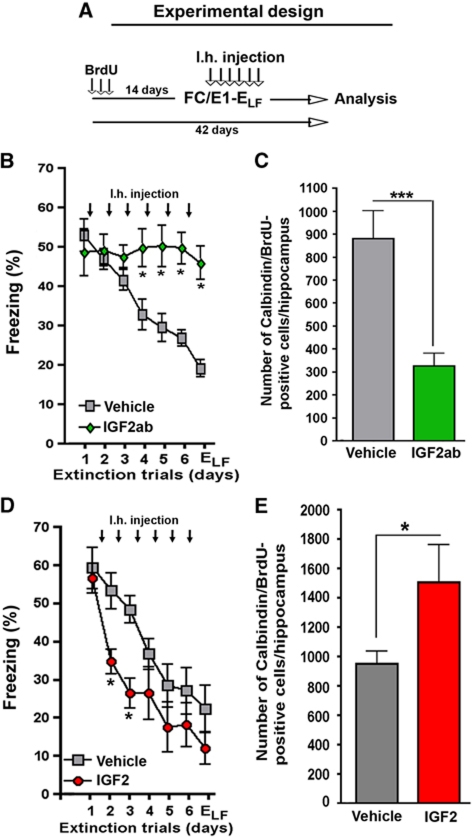
On the basis of these data, we were able to design experiments that allowed us to investigate the role of IGF2 and IGFBP7 on neuronal survival during fear extinction. Although an autocrine mode of action cannot be excluded, our finding that fear extinction can be manipulated by intrahippocampal injection of recombinant IGF2, IGF2ab or IGFBP7 (see Figures 3 and 4) indicates that IGF2 and IGFBP7 mediate this effect in a paracrine manner. This is in line with the fact that IGF2 and IGFBP7 are secreted proteins and our finding that 17–19-day-old newborn neurons express insulin-growth factor 1 receptor (IGF1r) (Supplementary Figure S11) the main receptor through which IGF2 mediates biological function, since IGF2 receptor normally attenuates IGF2 signalling (Brown et al, 2009). However, IGF2r has recently been implicated with memory consolidation (Chen et al, 2011). Interestingly, in the context of fear extinction inhibition of IGF1r impaired fear extinction while inhibition of IGF2r had no effect (Supplementary Figure S11). To this end, mice were injected with BrdU and subjected to FC followed by extinction training 14 days later. One group of mice was injected immediately after each extinction trial with IGF2ab while a control group received vehicle solution (Figure 6A). As shown before, administration of IGF2ab severely impaired fear extinction (Figure 6B). In the same group of mice, neuronal survival was analysed 42 days after the first BrdU injection. Notably, neuronal survival was significantly reduced in IGF2ab-treated mice when compared to the vehicle group (Figure 6C). Importantly, the injection procedure itself did not seem to severely affect neuronal survival, as indicated by the fact that similar number of newborn cells were detected in the FC/E1-ELF group (Figure 5D) and the vehicle groups shown in Figure 6C and E.
Figure 6.
IGF2 promotes the survival of immature neurons during fear extinction. (A) Experimental design. Mice were injected with BrdU on three consecutive days and subjected to fear extinction training 14 days later. Immediately after each extinction trial, mice were injected with vehicle IGF2 or IGF2ab. Neuronal survival was analysed 42 days after the first BrdU injection by counting BrdU/Calbindin-positive cells. (B) Fear extinction was significantly impaired in mice injected intrahippocampally with an IGF2ab (*P<0.05). (C) Survival of 17–19-day-old neurons was impaired in IGF2ab-treated mice (***P<0.0001 versus vehicle). (D) Fear extinction was significantly facilitated in the IGF2-treated mice (*P<0.05 versus vehicle). (E) Survival of 17–19-day-old neurons was enhanced in IGF2-treated mice (*P<0.05; **P<0.01). n=8/group; Error bars indicate s.e.m.
Next, we analysed the effect of elevated IGF2 levels on the survival of 17–19-day-old newborn neurons during fear extinction. Similar to our previous findings, fear extinction was significantly enhanced in the IGF2-treated group (Figure 6D). Notably, when compared to vehicle-treated mice the survival of 17–19-day-old adult-generated neurons was significantly enhanced in the IGF2 group (Figure 6E). In conclusion, these data suggest that during fear extinction IGF2 signalling contributes to the survival of 17–19-day-old newborn hippocampal neurons.
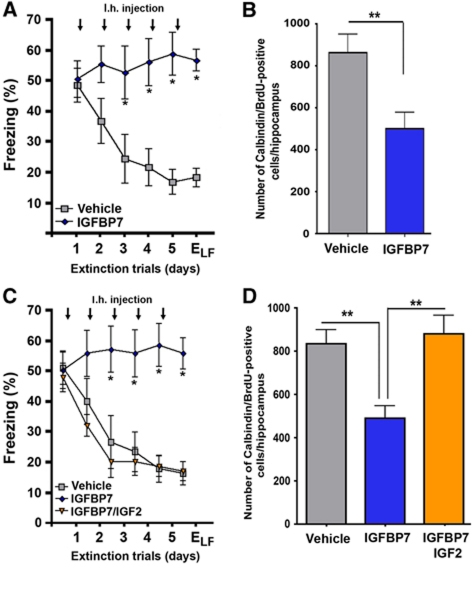
Next, we analysed the role of IGFBP7 on neuronal survival during fear extinction. Mice were injected with BrdU and subjected to fear extinction 14 days after the first BrdU injection. Immediately after each extinction trial, mice were injected with IGFBP7 or vehicle. As shown previously, mice that were treated with IGFBP7 displayed severely impaired fear extinction (Figure 7A). In comparison with the vehicle group, IGFBP7-treated mice displayed reduced survival of 17–19-day-old neurons (Figure 7B). Interestingly, IGFBP7-mediated impairment of fear extinction (Figure 7C) and the survival of 17–19-day-old newborn neurons (Figure 7D) were reversed in mice that were co-injected with IGFBP7 and IGF2.
Figure 7.
IGFBP7 regulates the survival of immature neurons during fear extinction in an IGF2-dependent manner. (A) Using the same experimental approach as shown in Figure 6A, we found that fear extinction was significantly impaired in mice injected intrahippocampally with IGFBP7 (*P<0.05 versus vehicle). (B) Survival of 17–19 days was impaired in IGFBP7-treated mice (**P<0.001 versus vehicle). (C) Mice were injected with either vehicle, IGFBP7 or co-injected with IGFBP7 and IGF2. While fear extinction was impaired in IGFBP7-injected mice, co-injection with IGF2 was able to rescue this effect. (D) IGF2 rescued IGFBP7-mediated impairment of neuronal survival during fear extinction (**P<0.001 versus Igfbp7/vehicle). n=8/group; Error bars indicate s.e.m.
In conclusion, our data show that fear extinction critically requires elevated hippocampal IGF2 signalling. Under physiological conditions, this is achieved by upregulation of Igf2 and downregulation of Igfbp7. We furthermore provide evidence that one of the cellular substrates affected by altered IGF2/IGFBP7 signalling during fear extinction is adult neurogenesis.
Discussion
In this study, we employ an unbiased, genome-wide approach to investigate hippocampal gene expression during fear extinction. Our aim was to identify interesting novel pathways for further analysis of the mechanisms that underlie contextual fear extinction. As such, the experimental design of our microarray study is certainly a compromise that did not allow us to detect all possible transcriptome changes associated with fear extinction, neither was it designed to dissect mechanisms of fear extinction from those linked to reconsolidation (Monfils et al, 2009). A more detailed analysis of the gene expression after different extinction trials and additional time points will certainly reveal further important candidate pathways. Nevertheless, among the identified genes that showed differentially expression, genes involved with insulin signalling were of particular interest. This is because we found that the Igf2 gene was transiently upregulated after exposure to E1, whereas the Igfbp7 mRNA decreased after ELF. Since IGFBPs attenuate the function of IGFs (Oh et al, 1996; Yamanaka et al, 1997; Wang et al, 2008; Suzuki et al, 2010) these data suggested that, despite the fact that Igf2 and Igfbp7 are regulated in opposite directions, fear extinction might involve elevated hippocampal IGF2 signalling. Moreover, while IGF2 signalling has been implicated with cognitive function (Chen et al, 2011), to the best of our knowledge its role in fear extinctions has not been addressed so far. In addition, previous data showed that Igf2 and Igfbp7 can be regulated in response to environmental stimuli (Jiang et al, 2008; Tomimaru et al, 2009) and established that both proteins are present in hippocampal neurons (Rodriguez et al, 2007; Wajapeyee et al, 2008; Csoregh et al, 2009). In summary, those considerations prompted us to select Igf2 and Igfbp7 for further analysis. The role of other interesting target genes identified in our microarray study is currently ongoing. A more detailed analysis of Igf2 and Igfbp7 expression during fear extinction revealed that Igf2 upregulation and Igfbp7 downregulation were most prominent in the hippocampal dentate gyrus region. In line with increased gene expression during fear extinction, we detected a significant upregulation of IGF2 protein levels in the dentate gyrus but not in the CA regions. Similarly, quantitative immunoblot analysis showed extinction-dependent downregulation of IGBP7 protein levels in the dentate gyrus but not in the CA region of mice exposed to extinction training. While the precise mechanisms by which Igf2 and Igfbp7 are regulated remain to be investigated, our data suggested the possibility that hippocampal IGF2/IGFBP7 signalling could have an important role in contextual fear extinction.
To test a role for IGF2 signalling during fear extinction directly, we designed experiments to either decrease or enhance hippocampal IGF2 signalling. We found that inhibiting physiological IGF2 signalling by intrahippocampal injection of an IGF2 blocking antibody (Ostrovsky et al, 2009) impaired fear extinction. Conversely, administration of recombinant IGF2 protein into the dentate gyrus of mice facilitated fear extinction. These data are in agreement with the upregulation of IGF2 during fear extinction. Notably, mice that received intrahippocampal IGFBP7 injection displayed severely impaired fear extinction, which is in line with the fact that during successful fear extinction IGFBP7 levels decrease. Importantly, the effect of IGFBP7 administration on fear extinction was rescued by IGF2 injection, suggesting that both proteins act in the same pathway. In line with the fact that IGF2 can mediate its biological action via GF1 receptor, blocking IGF1 receptor impaired fear extinction. Interestingly, IGF1 had no effect on fear extinction and also failed to rescue the effect of IGFBP7, suggesting that under physiological conditions the action of hippocampal IGF1 might be tightly controlled.
The fact that we were able to manipulate fear extinction by direct injection of IGF2, IGF2ab or IGFBP7 into the hippocampus of mice is in line with the paracrine action of those proteins (Bateman and McNeill, 2006). As such, our data suggest a scenario in which the fine-tuning of hippocampal IGF2 signalling, that achieved via upregulation of Igf2 and downregulation of Igfbp7, is required for successful fear extinction.
IGF signalling has been implicated with a number of cellular substrates, such as cell morphology or survival, that could potentially contribute to the observed effect on fear extinction (Leventhal et al, 1995; Aberg et al, 2003; Bateman and McNeill, 2006; Rodriguez et al, 2007; Chao and D’Amore, 2008; Scolnick et al, 2008; Broughton and Partridge, 2009; Lee and Son, 2009). In search for a cellular substrate of altered IGF2 signalling during fear extinction, we decided to focus initially on the role of adult neurogenesis. This was based on the fact, that GO-term analysis of the genes identified in our microarray study pointed to neurogenesis as a key biological process affected by fear extinction. Notably, this is in line with a recent study showing that the specific elimination of immature adult-born hippocampal neurons impairs contextual fear extinction in mice (Deng et al, 2009). In this study, it was found that the depletion of immature 1–4-week-old newborn neurons significantly impaired fear extinction. While this finding at first may appear surprising, it is of note that within 1–4 weeks after birth newly born hippocampal neurons undergo a critical period of maturation (Zhao et al, 2008). Importantly, such immature neurons are known to display enhanced plasticity (Schmidt-Hieber et al, 2004) and their survival can be affected by environmental stimuli (Kempermann et al, 1997; van Praag et al, 1999; Ge et al, 2005; Zhao et al, 2008; Lucassen et al, 2010). In line with these data, we found that fear extinction training specifically promotes the survival of 17–19-day-old hippocampal neurons while the proliferation of neuroprogenitor cells was not affected. Together with the Deng et al study, these data strongly suggest that contextual fear extinction critically involves the survival of immature newborn hippocampal neurons which can have an immediate role in data processing within the hippocampal circuitry. To this end, one of the generally accepted functions of the dentate gyrus is its role in pattern separation (Kempermann et al, 2004; McHugh et al, 2007; Zhao et al, 2008; Clelland et al, 2009). It has been suggested that a new event activates a subset of dentate gyrus neurons which affects a specific set of CA3 cells creating a specific CA3 pattern and that this procedure can be modulated by the recruitment of newborn neurons (Kempermann et al, 2004; Aimone et al, 2006, 2009). Therefore, it is intriguing to speculate that fear extinction specifically recruits 17–19-day-old adult-generated neurons to create a novel pattern that is, however, still linked to the original fear memory trace. As such, the specific recruitment of immature newly generated neurons into the original fear memory trace may affect the way this memory trace is retrieved (Supplementary Figure S12).
Notably, we found that the survival of 17–19-day-old neurons is regulated by hippocampal IGF2/IGFBP7 signalling during fear extinction. To this end, facilitation of fear extinction via intrahippocampal IGF2 injection correlated with enhanced survival of 17–19-day-old newly generated neurons. In turn, inhibition of physiological IGF2 signalling by intrahippocampal injection of IGF2ab, which impaired fear extinction, also impaired the survival of 17–19-day-old neurons. These data are in line with the fact that IGF2 can promote cell survival (Chao and D’Amore, 2008; Lehtinen et al, 2011). In contrast to the action of IGF1 (Llorens-Martín et al, 2009), a role for IGF2 in adult neurogenesis has not been reported before suggesting that IGF2 mediates fine-tuning of the IGF system under physiological conditions. This is in line with the fact that IGF1 but not IGF2 function is critical for proper brain development (Bateman and McNeill, 2006; Broughton and Partridge, 2009). In addition, mice that were treated with IGFBP7 displayed decreased neuronal survival and severely impaired fear extinction. IGFBP7 also attenuates the action of IGF1 and VEGF signalling (Tamura et al, 2009) which promotes adult neurogenesis (Cao et al, 2004; Heine et al, 2005). As such, the effect of IGFBP7 on fear extinction-dependent neuronal survival might be mediated via multiple targets. Nevertheless, the effect of IGFBP7 administration on fear extinction and neuronal survival was rescued by IGF2 injection, suggesting that IGFBP7 mediates the observed actions, at least in part, in an IGF2-dependent manner.
It is likely that in addition to neuronal survival hippocampal IGF2/IGFBP7 signalling regulates fear extinction also via other cellular substrates, a possibility that remains to be investigated. Nevertheless, in support of the data presented in this study a role for adult neurogenesis in hippocampal memory erasure (Feng et al, 2001) and fear extinction (Deng et al, 2009) has been demonstrated recently. Moreover, chronic administration of Fluoxetine, a drug that increases neurogenesis and is used to treat anxiety and mood disorders including PTSD (Santarelli et al, 2003; Bremner et al, 2008; Pollak et al, 2008; Takemura and Kato, 2008), also facilitates contextual fear extinction (Siegmund and Wotjak, 2007).
In conclusion, our data show that fear extinction critically requires elevated hippocampal IGF2 signalling. Under physiological conditions, this is achieved by upregulation of Igf2 and downregulation of Igfbp7. We furthermore provide evidence that one of the cellular substrates affected by altered IGF2/IGFBP7 signalling during fear extinction is adult neurogenesis. To this end, fear extinction specifically increases the survival of 17–19-day-old neurons in an IGF2/IGFBP7-dependent manner. We suggest, that strategies that enhance IGF2 signalling, potentially via inhibition of IGFBP7, could be a suitable therapeutic avenue to promote extinction in the context of anxiety diseases.
Materials and methods
Animals
Adult male C57Bl/6 wild-type mice (3 months of age) were housed under standard conditions with free access to food and water. All experiments were carried out in accordance with the animal protection law and were approved by the District Government of Germany.
Contextual fear extinction
Behaviour testing was performed by an experimenter that was blind to the treatment as described previously (Fischer et al, 2004; Sananbenesi et al, 2007) using TSE Systems apparatuses and software as well as Freeze Scan software (Clever Systems). In brief, FC was carried out with a computerized fear conditioning system (TSE, Bad Homburg, Germany) using a computer connected to a control unit containing a shock and a tone generator. Animals were allowed to explore the training cage for 3 min followed by a mild electric shock (2 s, 0.7 mA). Context-dependent freezing, defined as the absence of movements other than those required for breathing, was assessed 24 h later. Extinction of contextual fear was performed on consecutive days (24 h intervals) consisting of re-exposure to the training context in a non-reinforced manner for 3 min. In all experiments, this extinction training protocol led to a significant reduction of freezing behaviour. However, as described before the dynamics of fear extinction can vary among experiments (Sananbenesi and Fischer, 2009) and thus treatment groups should only be compared to the given control group.
Locomotor activity
Mice were subjected to a 3-min exposure to a context. The distance travelled (in cm) during this 3-min context exposure is used as a measurement of the locomotor activity (Supplementary Figure S8).
Cannulation and drug injection
Microcannulae were inserted into the dorsal hippocampus as previously described (Sananbenesi et al, 2007). In brief, bilateral microcannulae were inserted into the dentate gyrus region of the hippocampus: anteroposterior −1.70 mm relative to bregma; lateral ±1 mm; dorsoventral 2 mm from skull. Stock solutions were IGF2 (Sigma, Munich, Germany; 1 μg/μl in 0.1% BSA in sterile PBS), IGF2 blocking antibody (R&D Systems, 1 μg/μl in PBS), Anti-IGF2 receptor antibody (R&D Systems, 20 ng/μl in PBS), IGF1 (Sigma, 1 μg/μl in 0.1% BSA in sterile PBS), IGF1 blocking antibody (R&D Systems, 1 μg/μl in PBS), IGF1 receptor antagonist JB1 (Bachem Biosciences, 80 ng/μl in PBS), IGFBP7 (R&D Systems, 0.5 μg/μl in 0.1% BSA in sterile PBS). Drugs were injected bilaterally into the hippocampus (0.25 μl, 0.25 μl/min). As such, the total amount of these drugs injected in the brain was 250 ng (IGF2), 250 ng (IGF2 blocking antibody), 5 ng (Anti-IGF2 receptor antibody), 250 ng (IGF1), 250 ng (IGF1 blocking antibody), 20 ng (IGF1 receptor antagonist JB1) and 125 ng (IGFBP7).
BrdU injections and staining
BrdU (Sigma) was prepared as a stock solution of 5 mg/ml in sterile filtered 0.9% NaCl. Mice received four intraperitoneal injections during the same day (every 2 h) of BrdU each at 50 mg/kg of body weight at the desired time point to evaluate neuronal proliferation. Mice received during three consecutive days three intraperitoneal injections of BrdU per day, every 4 h, each at 50 mg/kg of body weight to assess neuronal survival. Animals were sacrificed either 24 h after the last injection to evaluate neuronal proliferation (Doublecortin/BrdU), 19 days after the last injection to check neuronal differentiation (Calretinin/BrdU), 42 days after the first BrdU injection to assess neuronal survival (Calbindin/BrdU) or 19 days after the first injection to check neuronal activity (cFos/BrdU). Double labelling of BrdU together with doublecortin (proliferation), Calretinin (differentiation), Calbindin (survival) and cFOS (neuronal activity) was assessed with a fluorescence microscope and a Leica confocal laser-scanning microscope (510 LSM; lasers HENE 543 NM and ARGON 458/488 NM). Sections were coded for confocal imaging that was performed by a rater blind to the experimental conditions.
Immunohistochemistry
Immunostaining was performed as described previously (Fischer et al, 2004; Sananbenesi et al, 2007; Peleg et al, 2010) and analysed using a Leica SP2 confocal microscope. Antibodies were used in the following concentrations: BrdU (mouse monoclonal diluted 1:1000; Millipore, Billerica, USA), NeuN (mouse monoclonal diluted 1:1000; Chemicon International, USA), active Caspase 3 (rabbit polyclonal 1:300; Abcam, Cambridge, UK); IGF2 (rabbit polyclonal 1:200; Abcam, Cambridge, UK); IGFBP7 (rabbit polyclonal 1:200; Abcam); IGF1r (rabbit polyclonal 1:300; Abcam); doublecortin (rabbit polyclonal 1:1000; Abcam); Calretinin (rabbit polyclonal 1:500; Abcam); Calbindin D-28k (rabbit polyclonal 1:300; Swant, Bellinzona, Switzerland); cFOS (sc-52) (rabbit polyclonal 1:2000; Santa Cruz Biotechnology, USA); Map-2 (rabbit polyclonal 1:1000; Synaptic Systems, Göttingen, Germany); Synaptophysin (clone SVP-38) (rabbit polyclonal 1:1000; Sigma); Cy3 labelled (goat anti-rabbit 1:500; Jackson ImmunoResearch, USA) and Alexa488 labelled (donkey anti-mouse 1:500; Invitrogen, USA).
Quantification of cell numbers
Stereological analysis of the number of cells was performed on one-in-nine series of 40 μm free-floating coronal sections which were analysed by confocal microscopy to count cells expressing the indicated marker throughout the whole rostrocaudal extent of the dentate gyrus, as described (Gould et al, 1999; Lie et al, 2005; Taupin, 2007). We calculated the total estimated number of cells within the dentate gyrus by multiplying the average number of positive cells for each marker per section by the total number of 40 μm sections comprising the entire dentate gyrus. We performed the quantifications for Fos+, BrdU+ and BrdU+/Fos+ similar to Kee et al (2007). In brief, BrdU/cFos double labelling was performed using a confocal microscope with a × 40 objective to quantify the phenotype Fos+, BrdU+ and BrdU+/Fos+ cells through the entire (one-in-nine series of 40 μm free-floating coronal sections) anterior–posterior extent of the dentate gyrus. We quantify the different cell phenotypes in the granule cell layer and including the subgranular zone.
Immunoblotting
Lysates for immunoblotting were prepared as previously described (Fischer et al, 2007). Brain tissue was homogenized in TX-buffer (50 mM Tris–HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 1% NP-40, 0.1% SDS), subjected to the Bioruptor (Diagenode) for 15 min (High, 30 s ON, 30 s OFF) before centrifugation at 12 000 r.p.m. for 10 min. The supernatant was used for immunoblotting. Immunoblots were performed using fluorescent secondary antibodies and data were quantified using an Odyssey Imager (Licor). It was made sure that responses were within the linear range. Antibodies were used in the following concentrations: for IGF2 (rabbit polyclonal 1:1000; Abcam), for IGFBP7 (rabbit polyclonal 1:1000; Abcam) and for α-Tubulin (clone B-5-1-2) (mouse monoclonal 1:1000, Sigma).
Quantitative real-time PCR
Tissue from total hippocampus was dissected and prepared for qPCR analysis as described before (Peleg et al, 2010). The dissection of dentate gyrus and CA regions was performed under a stereomicroscope (Motic) as described previously (Hagihara et al, 2009). qPCR was performed using a Roche 480 light cycler. cDNA was generated using the Transcriptor High Fidelity cDNA Synthesis kit (Roche) and qPCR for individual genes was performed using the Roche Universal probe library (UPL). Data were normalized to the housekeeping gene hypoxanthine phosphoribosyltransferase (Hprt).
DNA microarray
The microarray study was carried out as mono-colour experiment. Total RNA was isolated from mice 1 h after exposure to FC and 1 h after exposure to ELF. Mice that were subjected to the conditioning context without receiving the foot-shock served as control group. Total RNA was Cy3 labelled according to Agilent's Low RNA Input Fluorescent Linear Amplification Kit and hybridized to Agilent Whole Mouse Genome 4 × 44K G4122F microarrays according to the manufacturer’s protocol. Quantity and Cy-dye incorporation rates of the generated target material were assessed using a NanoDrop ND-100. Post processing washes were done according to the Agilent Technologies SSPE protocol (v2.1), replacing wash solution 3 by acetonitril, followed by immediate scanning using an Agilent G2505B scanner. Intensity data were extracted using Agilent’s Feature Extraction (FE) software, version 9.5.3.1 and analysed using the Limma (Smyth, 2004) package of Bioconductor (Gentleman et al, 2004). The microarray data analysis consists of the following steps: (1) between-array normalization, (2) fitting the data to a linear model and (3) detection of differential gene expression. VSN normalization (Huber et al, 2002) was applied to the intensity values as a method for between-array normalization, to assure that the intensities had similar distributions across arrays. To estimate the average group values for each gene and assess differential gene expression, a simple linear model was fit to the data, and group-value averages and standard deviations for each gene were obtained. To find genes with significant expression changes between groups, empirical Bayes statistics were applied to the data by moderating the standard errors of the estimated values (Smyth, 2004). P-values were obtained from the moderated t-statistic and corrected for multiple testing with the Benjamini–Hochberg method (Benjamini and Hochberg, 1995). For each gene, the null hypothesis, that there is no differential expression between degradation levels, was rejected when its adjusted P-value was <0.05. GO association analysis was carried out using the topGO package of Bioconductor (Alexa et al, 2006).
Statistical analysis
The data were analysed by unpaired Student's t-test and one-way or two-way ANOVA (Analysis Of Variance). Errors are displayed as standard error of the mean (s.e.m.).
Supplementary Material
Acknowledgments
We thank Professor S Jessberger and Dr D Lie for helpful comments. This work was partially supported the EUYRI award of the European Science Foundation to AF. The work was furthermore supported by the German-Research-Foundation (DFG) grant SA1050/2-1 to FS. RCAB is supported by an EMBO Long-term fellowship and GN is supported by a PhD fellowship of the Hans and Ilse Breuer Foundation. The European Neuroscience Institute is jointly funded by the University Medicine Göttingen and the Max Planck Society.
Author contributions: RCAB performed most of the behavioural experiments and the analysis of adult neurogenesis. He analysed the gene array data and contributed all figures and supplementary material. DAD and JW performed the gene array including validation of differential gene expression shown in Figure 1 and Supplementary Figures S1 and S2. DAD contributed to the initial analysis of newborn neurons shown in Supplementary Figure S9. KB contributed to the experiments shown in Figures 5 and 6. SB contributed to all qPCR experiments. UH contributed to the experiments shown in Supplementary Figure S6. NG, SB, HYA and AZ performed some of the qPCR analysis shown in Supplementary Figures S4 and S5. GSR and LO provided the bioinformatic analysis of the gene array shown in Figure 1. FS contributed to the experiment shown in Supplementary Figure S3. RCAB, FS and AF conceived the experiments and AF wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS (2003) IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci 24: 23–40 [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH (2006) Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9: 723–727 [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH (2009) Computational influence of adult neurogenesis on memory encoding. Neuron 61: 187–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexa A, Rahnenfuhrer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607 [DOI] [PubMed] [Google Scholar]
- Bateman JM, McNeill H (2006) Insulin/IGF signalling in neurogenesis. Cell Mol Life Sci 63: 1701–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC (1991) Insulin-like growth factor (IGF) binding proteins: the role of serum IGFBPs in regulating IGF availability. Acta Paediatr Scand Suppl 372: 107–114; discussion 115 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300 [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E (2008) Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res 167: 171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S, Partridge L (2009) Insulin/IGF-like signalling, the central nervous system and aging. Biochem J 418: 1–12 [DOI] [PubMed] [Google Scholar]
- Brown J, Jones EY, Forbes BE (2009) Keeping IGF-II under control: lessons from the IGF-II-IGF2R crystal structure. Trends Biochem Sci 34: 612–619 [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36: 827–835 [DOI] [PubMed] [Google Scholar]
- Chao W, D’Amore PA (2008) IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev 19: 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM (2011) A critical role for IGF-II in memory consolidation and enhancement. Nature 469: 491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325: 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoregh L, Andersson E, Fried G (2009) Transcriptional analysis of estrogen effects in human embryonic neurons and glial cells. Neuroendocrinology 89: 171–186 [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH (2009) Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci 29: 13532–13542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ (2001) Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32: 911–926 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J (2004) Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci 24: 1962–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning & memory after neuronal loss is associated with chromatin remodeling. Nature 447: 178–182 [DOI] [PubMed] [Google Scholar]
- Fischer A, Tsai LH (2008) Counteracting molecular pathways regulating the reduction of fear: implications for the treatment of anxiety diseases. In Post Traumatic Stress Disorders Basic Science and Clinical Disorder, Shiromani PJ, Keane TM, LeDouxand JE (eds). New York: Humana Press [Google Scholar]
- Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ (2006) Stability of recent and remote contextual fear memory. Learn Mem 13: 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H (2005) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Lacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265 [DOI] [PubMed] [Google Scholar]
- Hagihara H, Toyama K, Yamasaki N, Miyakawa T (2009) Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp 17; pii: 1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA (2010) Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35: 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ (2005) Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 21: 1304–1314 [DOI] [PubMed] [Google Scholar]
- Huber W, von Heydebreck A, Sültmann H, Poustka A, Vingron M (2002) Variance stabilization applied to microarray data calibration and to the quantification of differential expression. ISMB 18: 96–104 [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G (2003) Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci 18: 2702–2712 [DOI] [PubMed] [Google Scholar]
- Jessberger S, Toni N, Clemenson GD Jr, Ray J, Gage FH (2008) Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci 11: 888–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Kumar SD, Loh WT, Manikandan J, Ling EA, Tay SS, Dheen ST (2008) Global gene expression analysis of cranial neural tubes in embryos of diabetic mice. J Neurosci Res 86: 3481–3493 [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10: 355–362 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386: 493–495 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH (2004) Functional significance of adult neurogenesis. Curr Opin Neurobiol 14: 186–191 [DOI] [PubMed] [Google Scholar]
- Lee E, Son H (2009) Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep 42: 239–244 [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D’Ercole AJ, Wong ET, LaMantia AS, Walsh CA (2011) The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69: 893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal PS, Randolph AE, Vesbit TE, Schenone A, Windebank A, Feldman EL (1995) Insulin-like growth factor-II as a paracrine growth factor in human neuroblastoma cells. Exp Cell Res 221: 179–186 [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437: 1370–1375 [DOI] [PubMed] [Google Scholar]
- Llorens-Martín M, Torres-Alemán I, Trejo JL (2009) Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist 15: 134–148 [DOI] [PubMed] [Google Scholar]
- Llorens-Martín M, Torres-Alemán I, Trejo JL (2010) Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol Cell Neurosci 15: 109–117 [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czeh B (2010) Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol 20: 1–17 [DOI] [PubMed] [Google Scholar]
- Matynia A, Anagnostaras SG, Wiltgen BJ, Lacuesta M, Fanselow MS, Silva AJ (2008) A high through-put reverse genetic screen identifies two genes involved in remote memory in mice. PLoS One 3: e2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S (2007) Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317: 94–99 [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324: 951–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M (2007) Mechanisms of fear extinction. Mol Psychiatry 12: 120–150 [DOI] [PubMed] [Google Scholar]
- Oh Y, Nagalla SR, Yamanaka Y, Kim HS, Wilson E, Rosenfeld RG (1996) Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-I and -II. J Biol Chem 271: 30322–30325 [DOI] [PubMed] [Google Scholar]
- Ostrovsky O, Ahmed NT, Argon Y (2009) The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol Biol Cell 20: 1855–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Java S, Agis-Balboa RC, Cota P, Wittnam J, Gogul-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, KAng H, Farinelli L, Chen W, Fischer A (2010) Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328: 753–756 [DOI] [PubMed] [Google Scholar]
- Pham K, McEwen BS, Ledoux JE, Nader K (2005) Fear learning transiently impairs hippocampal cell proliferation. Neuroscience 130: 17–24 [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187 [DOI] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER (2008) An animal model of a behavioral intervention for depression. Neuron 2008: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Rau V, Eger EI, Harris RA, Fanselow MS (2010) Amygdala transcriptome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology 35: 1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC (2010) Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci 21: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S, Gaunt TR, Day IN (2007) Molecular genetics of human growth hormone, insulin-like growth factors and their pathways in common disease. Hum Genet 122: 1–21 [DOI] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A (2009) The epigenetic bottleneck of neurodegenerative and psychiatric diseases. Biol Chem 390: 1145–1153 [DOI] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, Tsai LH (2007) A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci 10: 1012–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809 [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429: 184–187 [DOI] [PubMed] [Google Scholar]
- Scolnick JA, Cui K, Duggan CD, Xuan S, Yuan XB, Efstratiadis A, Ngai J (2008) Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron 57: 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I (2010) The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35: 169–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT (2007) A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res 41: 848–860 [DOI] [PubMed] [Google Scholar]
- Smyth GK (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: article3 [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE (2006) Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry 60: 329–336 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Igarashi S, Nojima M, Maruyama R, Yamamoto E, Kai M, Akashi H, Watanabe Y, Yamamoto H, Sasaki Y, Itoh F, Imai K, Sugai T, Shen L, Issa JP, Shinomura Y, Tokino T, Toyota M (2010) IGFBP7 is a p53 responsive gene specifically silenced in colorectal cancer with CpG Island methylator phenotype. Carcinogenesis 31: 342–349 [DOI] [PubMed] [Google Scholar]
- Takemura NU, Kato N (2008) Adult neurogenesis and systemic adaptation: animal experiments and clinical perspectives for PTSD. Prog Brain Res 167: 99–109 [DOI] [PubMed] [Google Scholar]
- Tamura K, Hashimoto K, Suzuki K, Yoshie M, Kutsukake M, Sakurai T (2009) Insulin-like growth factor binding protein-7 (IGFBP7) blocks vascular endothelial cell growth factor (VEGF)-induced angiogenesis in human vascular endothelial cells. Eur J Pharmacol 610: 61–67 [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH (2007) Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci 27: 3252–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P (2007) BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev 53: 198–214 [DOI] [PubMed] [Google Scholar]
- Tomimaru Y, Eguchi H, Wada H, Noda T, Murakami M, Kobayashi S, Marubashi S, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M, Nagano H (2009) Insulin-like growth factor-binding protein 7 alters the sensitivity to interferon-based anticancer therapy in hepatocellular carcinoma cells. Br J Cancer 102: 1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I (2008) The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci 37: 402–411 [DOI] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J (2009) Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J Neurosci 29: 3387–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270 [DOI] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR (2008) Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132: 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K (2008) Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40: 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Wilson EM, Rosenfeld RG, Oh Y (1997) Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J Biol Chem 272: 30729–30734 [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132: 645–660 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.