Abstract
The activation of the Rac1 GTPase during cell signalling entails its translocation from the cytosol to membranes, release from sequestering Rho GDP dissociation inhibitors (RhoGDI), and GDP/GTP exchange. In addition to those steps, we show here that optimal Rac1 activation during cell signalling requires the engagement of a downstream, cytoskeletal-based feedback loop nucleated around the cytoskeletal protein coronin 1A and the Rac1 exchange factor ArhGEF7. These two proteins form a cytosolic complex that, upon Rac1-driven F-actin polymerization, translocates to juxtamembrane areas where it expands the pool of activated, membrane-bound Rac1. Such activity requires the formation of an F-actin/ArhGEF7-dependent physical complex of coronin 1A with Pak1 and RhoGDIα that, once assembled, promotes the Pak1-dependent dissociation of Rac1 from the Rac1/RhoGDIα complex and subsequent Rac1 activation. Genetic evidence demonstrates that this relay circuit is essential for generating sustained Rac1 activation levels during cell signalling.
Keywords: ArhGEF7, F-actin, Pak, RhoGDI, Rho/Rac GTPases
Introduction
The Rac1 GTPase controls general processes such as cytoskeletal dynamics, cell-cycle entry, cell size, and gene transcription (Jaffe and Hall, 2005; Bustelo et al, 2007; Saci et al, 2011). It also regulates cell type-specific processes that include, among others, antigen-triggered immune responses, antibacterial neutrophil functions, macrophage phagocytosis, and axon migration/guidance (Jaffe and Hall, 2005; Bustelo et al, 2007). To trigger those functions, Rac1 interacts with downstream effectors working as protein kinases, lipid-related enzymes, adaptor/scaffolding proteins, and/or direct regulators of actin filament growth, stabilization, and branching (Jaffe and Hall, 2005; Bustelo et al, 2007). For example, the regulation of the F-actin cytoskeleton by Rac1 entails the participation of several downstream elements, including Pak family serine/threonine kinases, phosphatidylinositol-4 phosphate-5 kinase, and F-actin regulators such as Baiap2/IRSp53 or Wasf/Wave (Jaffe and Hall, 2005; Bustelo et al, 2007).
To assemble properly balanced biological responses, the activity of Rac1 is controlled at different levels. One of them is the modulation of the transition between the inactive GDP-bound state and the active GTP-bound conformation. This step is controlled by Rac1 GDP/GTP exchange factors (GEFs) and GTPase activating proteins. GEFs promote the activation step by catalysing the release of the GTPase-bound GDP molecules. GTPase activating proteins facilitate the transition to the inactive Rac1 state by favouring the hydrolysis of GTPase-bound GTP molecules (Bos et al, 2007). Another regulatory layer consists of the dynamic segregation of the inactive and active Rac1 pools in different subcellular localizations. The best-known regulators in charge of this process are Rho GDP dissociation inhibitors (RhoGDIs), a group of proteins that sequester GDP–Rac1 in the cytosol and, in addition, retrieve Rac1 from the plasma membrane (DerMardirossian and Bokoch, 2005). The strength of the Rac1/RhoGDI interaction is negatively modulated by many signals, including the Src-, protein kinase C (PKC)-, and Pak-dependent phosphorylation of RhoGDIs (Price et al, 2003; DerMardirossian et al, 2004, 2006) and by interactions with phospholipids (Chuang et al, 1993; Tolias et al, 1998; Chae et al, 2008; Abramovici et al, 2009). However, the shuttling of Rac1 between subcellular compartments can be also achieved by alternative mechanisms. For example, active Rac1 can be stored in intracellular endocytic vesicles that move back to the plasma membrane via Rab5/clathrin-dependent delivery routes upon cell re-stimulation (Palamidessi et al, 2008). Finally, a third regulatory layer is based on intrinsic properties of the plasma membrane that affect either its ‘receptivity’ for the docking of Rac1 or the stability of membrane-bound Rac1. This layer is regulated by specific membrane lipids that bind to the hydrophilic Rac1 C-terminal tail (Heo et al, 2006; Yeung et al, 2008), lipid rafts (del Pozo et al, 2004, 2005; Balasubramanian et al, 2007), integrin activation status (del Pozo et al, 2004, 2005), and/or the presence of Rac1 docking proteins such as ArhGEF7 (ten Klooster et al, 2006), an Rac1 GEF also known as β-Pix and Cool1 (Rosenberger and Kutsche, 2006). Despite this progress, it is still unclear how all these regulatory layers are coordinated to ensure the engagement of Rac1-dependent routes in an efficient and physiologically coherent manner.
To identify new human proteins involved in the regulation of the translocation of Rac1 to the plasma membrane, we have recently conducted a genome-wide functional screen using an HEK293 T-cell line expressing a cytosolic Rac1 bioreporter. This approach led to the identification of coronin 1A as one of the proteins whose overexpression induced the translocation of Rac1 to the plasma membrane. This protein belongs to a large family of β-propeller, WD40 domain containing proteins that control diverse aspects of the F-actin polymerization and branching cycle (Rybakin and Clemen, 2005; Uetrecht and Bear, 2006). Coronins play important roles in cell signalling, cell migration, phagocytosis, and vesicle trafficking (Rybakin and Clemen, 2005; Uetrecht and Bear, 2006). In particular, coronin 1A has been shown to be specifically involved in T-lymphocyte migration and survival, as determined by the identification of Coro1A gene mutations in T-cell immunodeficiencies and the analysis of the phenotypes of Coro1A−/− mice (Foger et al, 2006; Haraldsson et al, 2008; Mueller et al, 2008, 2011; Mugnier et al, 2008; Shiow et al, 2008, 2009). However, it is still controversial whether the phenotypes seen in Coro1A−/− T cells are linked to the cytoskeletal regulatory properties of this protein (Mueller et al, 2011). The identification of coronin 1A in our cellomic screen suggested that this protein, either per se or in a concerted manner with the F-actin cytoskeleton, could actively regulate the plasma membrane localization of Rac1. The investigation of this hypothesis led us to discover a coronin 1A-driven cytoskeletal feedback loop that contributes to the amplification of the membrane pool of activated Rac1 during cell signalling.
Results
Coronin 1A promotes the translocation of a subset of Rho/Rac family proteins
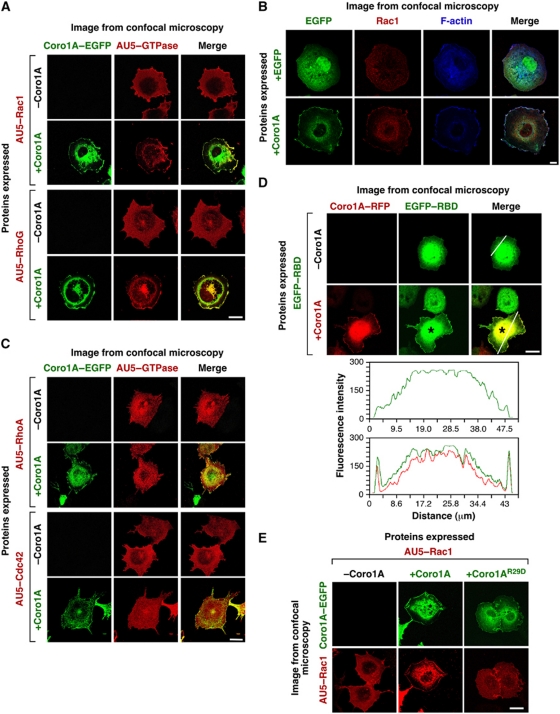
We isolated coronin 1A (Coro1A) in a recent genome-wide cellomic screening aimed at identifying proteins that promoted Rac1 translocation in vivo (see Supplementary data). To confirm the implication of Coro1A in this process, we first performed confocal microscopy analysis to verify whether the ectopic expression of an enhanced green fluorescent protein (EGFP)-tagged Coro1A affected the subcellular localization of AU5-tagged versions of Rac1 and the structurally related RhoG GTPase. These experiments demonstrated that Coro1A–EGFP does promote the translocation of those two GTPases to the plasma membrane in COS1 cells (Figure 1A). We also verified that Coro1A–EGFP induces the translocation of endogenous Rac1 in the same cell type (Figure 1B). Co-staining with Alexa Fluor 635-phalloidin, a toxin that binds to polymerized actin (Wulf et al, 1979), indicated that Rac1 is tethered to F-actin rich juxtamembrane areas of cells rather than to the overall plasma membrane (Figure 1B), suggesting a functional connection with the cytoskeletal-related functions of Coro1A. Three independent immunofluorescence-based experiments confirmed that this translocation step was specific. First, we found that Coro1A–EGFP does not induce the translocation of more distantly related Rho/Rac family members such as AU5–RhoA and AU5–Cdc42 (Figure 1C). Second, we observed that Coro1A–EGFP cannot translocate Rac1C189S (Supplementary Figure S1), a mutant protein that cannot become associated with membranes due to defective C-terminal prenylation and processing (Bustelo et al, 2007). Finally, we demonstrated that the ectopic expression of other proteins involved in F-actin binding and regulation, such as coronin 1B (Coro1B), cortactin or Baiap2, do not induce the translocation of Rac1 (Supplementary Figure S2).
Figure 1.
Coro1A translocates and activates Rac1 in an F-actin-dependent manner. (A, C) COS1 cells transiently expressing the indicated proteins (left) were subjected to confocal microscopy analysis to visualize EGFP (left panels, green colour) and AU5-tagged GTPases (middle panels, red colour). Areas of colocalization between Coro1A and GTPases are shown in yellow (right panels). (B) COS1 cells expressing either EGFP or Coro1A–EGFP were fixed, incubated with anti-Rac1 antibodies, stained with Alexa Fluor 635-phalloidin, and then analysed by confocal microscopy to visualize the subcellular distribution of EGFPs (left panels, green colour), endogenous Rac1 (second column of panels from left, red colour), and F-actin (third column of panels from left, dark blue colour). Areas of colocalization between Coro1A, Rac1, and F-actin are shown in white (right panels). (D) COS1 cells expressing the indicated proteins (left) were subjected to confocal analysis to detect the subcellular localization of Coro1A–RFP (left panels, red colour) and EGFP–RBD (middle panels, green colour). The areas of colocalization of both proteins are shown in yellow (right panels). The asterisk marks a cell co-expressing Coro1A–RFP and EGFP–RBD. The white lanes present on cells (right panels) highlight the cell sections selected in each case to measure the distribution of fluorescence intensities for EGFP–RBD (green lanes) and Coro1A–RFP (red lanes) in the graphs present at the bottom of this panel. (E) COS1 cells expressing the indicated proteins (top) were analysed by confocal microscopy to visualize the subcellular distribution of Rac1 (bottom panels, red colour) and the Coro1A–EGFPs (upper panels, green colour). Scale bars, 20 μm (A, C–E) and 10 μm (B).
To test whether the Coro1A-induced translocation of Rac1 was associated with the activation of the GTPase, we next used a bioreporter designed to detect GTP-bound Rac1 in vivo. This molecule, which is composed of the Rac1 binding domain (RBD) of Pak1 fused to the EGFP, can be used as a biosensor to track the Rac1 activation state due to the high affinity of the RBD moiety towards active, GTP-bound Rac1 (Manser et al, 1994; Srinivasan et al, 2003; Phee et al, 2005). To this end, this biosensor was expressed transiently in COS1 cells either in the absence or in the presence of a Coro1A–red fluorescent protein (RFP) and its subcellular localization tracked down by confocal microscopy. Whereas the EGFP–Pak1 RBD protein was present in the nucleus and perinuclear cytoplasm when expressed alone (Figure 1D), it became enriched at the plasma membrane when co-expressed with Coro1A–RFP (Figure 1D); thus, demonstrating that the overexpression of Coro1A leads to the generation of a membrane-localized pool of activated Rac1 molecules.
To investigate whether the translocation of Rac1 was linked to the F-actin binding properties of Coro1A, we repeated the above experiments by expressing a Coro1A–EGFP containing an R to D missense mutation at position 29. This mutation, which is analogous to the R30D mutation previously described in Coro1B (Cai et al, 2007), cannot interact with F-actin as determined by total internal reflection fluorescence (IN-L, OM, and MAdP; data not shown) and proteomic experiments (VO and XRB, unpublished). This mutant protein shows an entirely cytosolic localization and cannot trigger Rac1 translocation (Figure 1E). Taken together, these results indicate that Coro1A promotes the translocation of Rac1 to juxtamembrane plasma membrane areas in an F-actin-dependent manner.
Coronin 1A favours Rac1 translocation and activation in vivo
To confirm the involvement of Coro1A in Rac1 regulation using more quantitative and cell population-based approaches, we generated three COS1 cell lines stably expressing a non-chimeric EGFP, Coro1A–EGFP, or Coro1AR29D–EGFP. These cell lines will be designated hereafter as ACC1-1, ACC1-2, and VOS1-3 cells, respectively. Using membrane fractionation experiments, we found that Coro1A–EGFP-expressing cells contained two-fold more endogenous Rac1 distributed within membrane-enriched fractions than control cells (Supplementary Figure S3A, upper panel). In contrast, the membrane pool of endogenous RhoA (Supplementary Figure S3A, second panel from top) and the transmembrane CD98 protein (Supplementary Figure S3A, third panel from top) underwent no significant changes in control and Coro1A–EGFP-expressing cells. We also evaluated the relative levels of active Rac1 during epidermal growth factor (EGF)- and integrin-mediated signalling using pull-down experiments with a glutathione S-transferase (GST)–Pak1 RBD fusion protein as bait. Coro1A–EGFP-expressing cells showed higher levels of activated endogenous Rac1 than control cells under both serum starvation and EGF-stimulated conditions (Supplementary Figure S3B, upper panel). This was not due to enhanced signalling from the EGF receptor, because control and Coro1A–EGFP-expressing cells displayed similar increases in the stimulation of Erk upon EGF stimulation (Supplementary Figure S3B, third panel from top). Those cells also displayed higher amounts of activated endogenous Rac1 than control cells upon plating onto fibronectin-coated dishes (Supplementary Figure S3C, upper panel), an experimental condition that triggers integrin-mediated Rac1 activation (del Pozo et al, 2000). Coro1AR29D–EGFP-expressing cells behave as control cells in terms of integrin-mediated Rac1 activation (Supplementary Figure S3C, upper panel).
We next performed loss-of-function studies to investigate if the expression of endogenous Coro1A was important for proper Rac1 activation kinetics in COS1 cells. For that purpose, we generated two independent Coro1A-deficient cell lines (ACC2-2 and ACC2-4) by transducing CORO1A shRNA-encoding lentiviruses into parental COS1 cells. As negative control, we used a third stable cell line (ACC2-1) harbouring a different CORO1A-directed shRNA that could not block Coro1A expression. As additional control, we generated using a similar approach both control (VOS7-1) and Coro1B-deficient (VOS7-3) COS1 cells. Immunoblot analysis confirmed the effective elimination of Coro1A (Figure 2A and Supplementary Figure S4A, seventh and fifth panels from top, respectively) and Coro1B (Figure 2C, bottom panel) in the appropriate cell lines. Pull-down experiments carried out with the GST–Pak1 RBD indicated that the two Coro1A-deficient cells triggered very low activation levels of endogenous Rac1 upon EGF stimulation when compared with control cells (Figure 2A and Supplementary Figure S4A, upper panels; Figure 2B). This was not due to a general defective signalling response, because those cells displayed normal phosphorylation kinetics of both the EGF receptor (Figure 2A, third panel from top) and Erk (Figure 2A and Supplementary Figure S4A, fifth and third panels from top, respectively). Also, we could restore normal levels of Rac1 activation in Coro1A-deficient cells by transfecting an shRNA-insensitive CORO1A transcript mutant (Supplementary Figure S4B, upper panel), further demonstrating that the defective activation of endogenous Rac1 is a direct consequence of the Coro1A depletion in those cells. Our two Coro1A-deficient cell lines were also defective in the activation of Rac1 during fibronectin-mediated adhesion conditions (Supplementary Figure S5, upper panel), indicating that the implication of Coro1A in Rac1 activation is not limited to the EGF receptor route. The Coro1B deficiency did not affect Rac1 activation in COS1 cells (Figure 2C, upper panel). To verify the implication of Coro1A in Rac1 activation in a different cell type, we carried out similar experiments using control (VOS6-1) and Coro1A-deficient (VOS6-3) HEK293 T cells. The Coro1A deficiency also led to reduced Rac1 activation levels in those cells during EGF signalling (Figure 2D, upper panel).
Figure 2.
Endogenous Coro1A is important for Rac1 activation in COS1 and HEK293 T cells. (A, B) Serum-starved control (ACC2-1) and Coro1A-deficient (ACC2-2) COS1 cells were stimulated with EGF and GTP–Rac1 levels determined (A, top panel). Aliquots of the same cell lysates were used in western blot (WB) experiments with the indicated antibodies (A, right) to determine the levels of expression or phosphorylation of the proteins under study. p, phospho. Data obtained in this and three additional independent experiments were used to quantify (B) GTP–Rac1 levels obtained in each condition. **P⩽0.01 compared with the appropriate wild-type time point. (C) Serum-starved control (VOS7-1) and Coro1B-deficient (VOS7-3) COS1 cells were stimulated with EGF and GTP–Rac1 levels determined (top panel). Aliquots of the same cell lysates were used in WB experiments with the indicated antibodies (right) to determine the levels of expression of the proteins under study (n=2 independent experiments). (D) Quiescent control (VOS6-1) and Coro1A-deficient (VOS6-3) HEK293 T cells were treated with EGF for the indicated periods of time and GTP–Rac1 levels determined by pull-down experiments (top panel). Samples of same cell extracts were used in WB experiments with the indicated antibodies (right) to determine the levels of expression or phosphorylation of the proteins under study. Data obtained in three independent experiments were used to quantify GTP–Rac1 levels obtained in each condition. (E) Serum-starved control (ACC2-1) and Coro1A-deficient (ACC2-2) COS1 cells transiently expressing EGFP–Rac1 were either left non-stimulated (−EGF) or stimulated with EGF (+EGF) for 10 min and subjected to confocal microscopy to visualize the subcellular localization of EGFP–Rac1 (green colour) and F-actin (red colour). Areas of colocalization between Rac1 and F-actin are shown in yellow (right panels). Scale bar, 20 μm. The Rac1 translocation index (TI) is indicated on the right of each cell lane experiments. **P⩽0.01 compared with non-stimulated wild-type cells.
Since the GST–Pak1 RBD pull-down experiments are not informative about the subcellular localization where the activation of Rac1 takes place, we decided to monitor by confocal microscopy the migration of ectopic EGFP–Rac1 protein in control and Coro1A-deficient cells during EGF stimulation conditions. As expected, EGFP–Rac1 displayed a homogeneous cytosolic distribution in serum-starved cells independently of their Coro1A expression status (Figure 2E). Upon EGF stimulation, the translocation of EGFP–Rac1 to the plasma membrane took place efficiently in control but not so in Coro1A-deficient cells (Figure 2E). The latter cells also generated smaller membrane ruffles upon EGF stimulation (Figure 2E). This latter phenotype is possibly due to the direct effects of Coro1A on Rac1 translocation (this work) and on its direct regulation of F-actin filament dynamics (Rybakin and Clemen, 2005; Uetrecht and Bear, 2006). In fact, all Coro1A-deficient and Coro1AR29D-expressing stable COS1 cell lines exhibit basal cytoskeletal defects that are associated with increased actomyosin contractility, enlargement of focal adhesions, and smaller cell areas regardless of the stimulation status. A milder version of this phenotype is also observed in Coro1B-deficient COS1 cells (Figure 2E; VO and XRB, unpublished observations). Collectively, these results indicate that Coro1A is a bona fide regulator of the subcellular localization and activation of Rac1 during EGF- and integrin-mediated signalling.
The Coro1A-dependent translocation of Rac1 requires Pak and upstream Rac1 activity
We next used a pharmacological approach to get insights on the mechanism that mediates the translocation of Rac1 induced by Coro1A. To this end, COS1 cells expressing transiently AU5–Rac1 alone or in combination with Coro1A–EGFP were treated with drugs previously shown to interfere with the Rac1 pathway. Those included β-methyl-cyclodextrin (βMCD), cytochalasin D (CytD), GF109203X, and Tat-Pak18. βMCD promotes Rac1 internalization by disrupting lipid rafts (Klein et al, 1995; Simons and Toomre, 2000). CytD is a mycotoxin that disrupts the F-actin cytoskeleton (Schliwa, 1982). GF109203X is a pan-specific PKC inhibitor that interferes with the Ca2+- and PKC-dependent dissociation of Rac1 from RhoGDIs (Toullec et al, 1991; Price et al, 2003). Tat-Pak18 is a cell permeable peptide that eliminates the ArhGEF7/Pak1 interaction (Maruta et al, 2002). After those treatments, cells were fixed and the subcellular localizations of both AU5–Rac1 and Coro1A–EGFP were determined by confocal microscopy. These experiments revealed that the disruption of lipid rafts and the F-actin cytoskeleton eliminated the juxtamembrane subcellular localization of both Coro1A–EGFP and AU5–Rac1 (Supplementary Figure S6, compare second with third and fourth rows of panels, respectively). The inhibition of PKC did not have any effect in the localization of those two proteins (Supplementary Figure S6, compare second and bottom row of panels). By contrast, we found that the inhibition of Pak1 eliminated the Coro1A–EGFP-induced translocation of AU5–Rac1 to the plasma membrane without affecting the juxtamembrane localization of Coro1A–EGFP (Supplementary Figure S7, compare second and third row of panels). The inactive version of the Tak-Pak peptide (Tat-PakR192A) did not alter the Coro1A–EGFP-dependent translocation of Rac1 (Supplementary Figure S7, compare second and fourth row of panels). These results indicate that Pak1 is acting downstream of Coro1A in this biological process.
Since activated Rac1 promotes F-actin cytoskeletal change and lipid rafts, we decided to investigate whether signalling from this GTPase could be also important for the localization of Coro1A in juxtamembrane areas. To this end, we monitored the subcellular pattern of Coro1A–EGFP upon expression of the Rac1T17N dominant-negative mutant in COS1 cells. As in the case of the treatments with βMCD and CytD, the expression of the Rac1 dominant-negative mutant eliminated the juxtamembrane localization of the co-transfected Coro1A–EGFP (Supplementary Figure S8, compare top and bottom row of panels). Similar results were obtained when the endogenous Rac1 was eliminated using siRNAs (Supplementary Figure S9). These results suggest that Coro1A requires basal upstream Rac1 activity to engage its Rac1 translocation pathway.
RhoGDIα and Pak1 are involved in the Coro1A-dependent translocation of Rac1
It has been recently shown that Pak family kinases contribute to the stimulation of Rac1-dependent pathways by facilitating the release of the GTPase from Rac1/RhoGDI complexes in a phosphorylation-dependent manner (DerMardirossian et al, 2004). This step weakens the stability of the RhoGDI complexes formed with Rac1 but not of those established with either RhoA or Cdc42 (DerMardirossian et al, 2004), a functional selectivity reminiscent of that found with Coro1A and Rho/Rac family GTPases (see above, Figure 1A–C). Since we observed that the Tat-Pak18 peptide inhibited the translocation of Rac1 induced by Coro1A, we evaluated whether a similar mechanism could be involved in this process. To this end, we compared the amount of Rac1 that co-immunoprecipitates with RhoGDIα as a read-out for measuring the relative stability of the endogenous RhoGDIα/Rac1 complex in EGF-stimulated control and Coro1A-deficient cells. As shown in Figure 3A (upper panel), the dissociation of Rac1 from RhoGDIα takes place much less efficiently in Coro1A-deficient cells. Normal dissociation levels of Rac1 from RhoGDIα were rescued by re-expressing wild-type Coro1A in Coro1A-deficient cells (Figure 3B, upper panel), demonstrating that dissociation defect present in those cells is a direct consequence of the CORO1A mRNA knockdown. Importantly, this rescue activity is not reproduced when Coro1A-deficient cells are transfected with the Coro1AR29D mutant version that cannot bind F-actin (Supplementary Figure S10, upper panel). Consistent with a potential role of Pak1 in the Rac1/RhoGDIα dissociation process mediated by Coro1A, we observed that the co-expression of a catalytically inactive Pak1K299R mutant abolished the rescue of the dissociation of Rac1 from RhoGDIα induced by Coro1A re-expression in Coro1A-deficient cells (Figure 3B, upper panel).
Figure 3.
Pak1 is involved in the Coro1A-dependent activation of Rac1. (A, B) Control (ACC2-1) and Coro1A-deficient (ACC2-2) COS1 cells expressing the indicated EGFP (A, B; top) and Myc–Pak1K229R (B, top) were either left non-stimulated or stimulated with EGF (top), immunoprecipitated (IP) with anti-RhoGDIα antibodies, and subjected to WB (two top panels) with the antibodies indicated on the right. Aliquots of the same cell lysates were analysed by WB to detect the indicated phosphoproteins and proteins (four (A) and five (B) bottom panels). In (A), the normalized Rac1 levels co-immunoprecipitating with RhoGDI in each condition are indicated (n=3). *P⩽0.05; ***P⩽0.001 compared with the indicated experimental pairs (brackets). In (B), n=2 so no statistical analysis was performed. (C) Anti-EGFP (top subset of panels) and anti-RhoGDIα (middle subset of panels) immunoprecipitates obtained from cell lysates containing the indicated combinations of ectopically expressed proteins (top) were subjected to WB analysis with indicated antibodies (right). Aliquots of the same cell lysates (bottom panels) were analysed by WB using the indicated antibodies (right). (D) Schematic representation of the pathway proposed according to this work and previous publications (DerMardirossian et al, 2004). Activation steps and inactivation steps are shown as arrows and blunted lanes, respectively.
Coro1A forms a trimeric complex with Pak1 and RhoGDIα
To further explore the functional relationship among Coro1A, Pak1, and RhoGDIα, we investigated whether they could form heteromolecular complexes in vivo. To this end, we used co-immunoprecipitation experiments in COS1 cells expressing the indicated combinations of Coro1A–EGFP, Coro1AR29D–EGFP, and Myc–Pak1. These analyses demonstrated that Pak1 associates with Coro1A–EGFP but not with Coro1AR29D–EGFP (Figure 3C, upper panel; see also Supplementary Figure S11A below). Endogenous RhoGDIα could also interact with Coro1A–EGFP, but not with Coro1AR29D–EGFP, when Pak1 was simultaneously co-expressed in cells (Figure 3C, second panel from top). The interaction of those three proteins was also demonstrated in reverse co-immunoprecipitation experiments performed with anti-RhoGDIα antibodies (Figure 3C, fifth and sixth panels from top; Supplementary Figure S11B). Proper expression of all proteins analysed in these experiments was demonstrated by western blot analyses (Figure 3C, four bottom panels; Supplementary Figure S11C).
Further experiments indicated that these interactions were affected by the same drugs and protein mutants previously shown to interfere with the translocation of Rac1 by Coro1A. Thus, we found that the co-immunoprecipitation of Myc–Pak1 and endogenous RhoGDIα with Coro1A–EGFP was eliminated upon the treatment of cells with the Tat-Pak18 peptide (Supplementary Figure S11D, two top panels), βMCD (Supplementary Figure S12A, two top panels), and CytD (Supplementary Figure S12D, two top panels). This interaction was also blocked by dominant-negative Rac1T17N in cells (Supplementary Figure S11G, two top panels), further indicating that Coro1A requires some basal upstream signalling from Rac1 to assemble this biological response. By contrast, the co-immunoprecipitation of both Myc–Pak1 and endogenous RhoGDIα with Coro1A–EGFP was not disrupted when cells were pre-cultured with the inactive Tak-PakR192A peptide (Supplementary Figure S11D, two top panels) or the PKC inhibitor (Supplementary Figure S12D, two top panels). Similar data were obtained when we investigated the presence of Myc–Pak1 and Coro1A–EGFP in anti-RhoGDIα immunoprecipitates (Supplementary Figure S11B, E and H and Supplementary Figure S12B and E; two top panels). The similarity in the results obtained with the co-immunoprecipitation and the single cell-based translocation experiments previously shown in Supplementary Figures S6–S8 suggests that the formation of the Coro1A/Pak1/RhoGDIα complex and the Coro1A-induced Rac1 translocation are functionally intertwined processes. Taken together, these results indicate that Coro1A forms a heteromolecular complex with Pak1 and RhoGDIα that is important to promote the instability of the RhoGDIα/Rac1 pair during cell stimulation conditions (Figure 3D).
ArhGEF7 is essential for the interaction of Coro1A with Pak1 and RhoGDIα as well as for Rac1 activation
Given that Tat-Pak18, a cell permeable peptide that works as an antagonist of the Pak1/ArhGEF7 binding site (Maruta et al, 2002), blocked the formation of Coro1A complexes (Supplementary Figure S11D and E) and Coro1A-mediated Rac1 translocation (Supplementary Figure S7), we decided to investigate whether ArhGEF7 could play a role in those processes. To this end, we expressed ArhGEF7 in combination with non-chimeric EGFP, Coro1A–EGFP, or Coro1AR29D–EGFP in COS1 cells and possible interactions were monitored using co-immunoprecipitation experiments. ArhGEF7 did co-immunoprecipitate with both Coro1A–EGFP and Coro1AR29D–EGFP (Figure 4A, upper panel), indicating that this interaction does not require the F-actin binding properties of Coro1A. Confirming the specificity of the interaction, ArhGEF7 did not co-immunoprecipitate with the non-chimeric EGFP (Figure 4A, upper panel). Despite the above data, we observed using confocal microscopy analyses that only wild-type Coro1A can induce the translocation of ArhGEF7 from the cytosol to juxtamembrane areas (Figure 4B, compare second and third row of panels), demonstrating that the Coro1A-driven translocation of ArhGEF7 to the plasma membrane requires the association of Coro1A with F-actin filaments.
Figure 4.
Coro1A and ArhGEF7 participate in the formation of the Rac1 translocation complex. (A) Anti-EGFP immunoprecipitates (three top panels) and total cellular lysates (three bottom panels) obtained from COS1 cells transiently expressing the indicated combination of proteins (top) were subjected to immunoblot analysis with the specified antibodies (right). (B) COS1 cells expressing the indicated proteins (left) were analysed by confocal microscopy to visualize the subcellular distribution of Coro1A proteins (left panels, green colour) and Myc–ArhGEF7 (middle panels, red colour). Areas of colocalization of those two proteins are shown in yellow (right panels). Scale bar, 20 μm. The Myc–ArhGEF7 TI in each condition is shown on the right of each experimental condition. **P⩽0.01 compared with cells expressing Myc–ArhGEF7 without Coro1A (n=3). (C–E) Anti-EGFP (C) and anti-RhoGDI (D) immunoprecipitates derived from exponentially growing ACC3-1 (control) cells and ACC3-2 cells (lacking expression of ArhGEF7 but ectopically expressing Coro1A–EGFP) were analysed by WB with the indicated antibodies (right) to detect the interaction of Coro1A with Pak1 and RhoGDIα (C) and the association of RhoGDIα with Pak1 and Coro1A (D) in each cell lane. Total cell lysates were analysed in parallel by WB analysis to monitor the expression levels of proteins under study (E).
To investigate whether ArhGEF7 could play a role in the formation of the Coro1A/Pak1/RhoGDIα complex, we transduced shRNA-encoding lentivirus into Coro1A-deficient ACC1-2 cells to generate stable ArhGEF7-deficient (referred to as ACC3-2) and control (designated as ACC3-1) cell lines. Co-immunoprecipitation experiments indicated that the interaction of both Myc–Pak1 and endogenous RhoGDIα with Coro1A–EGFP was abolished in the absence of ArhGEF7 (Figure 4C, first and third panels from top). Similar results were obtained when the presence of co-immunoprecipitated Myc–Pak1 and Coro1A–EGFP was monitored in immunocomplexes obtained with anti-RhoGDIα antibodies (Figure 4D, three top panels). A small percentage of endogenous ArhGEF7 was also detected in control cells (Figure 4C and D; second panel from top). All tested proteins were properly expressed, as demonstrated by immunoblot analysis of total cellular lysates (Figure 4E). These results indicate that ArhGEF7 is essential for the formation of the Coro1A/Pak1/RhoGDIα complex.
To further substantiate the participation of ArhGEF7 in the Coro1A-dependent translocation of Rac1, we checked whether the elimination of ArhGEF7 could mimic some of the signalling defects previously observed in Coro1A-deficient COS1 cells. We observed that the ARHGEF7 knockdown decreased the basal levels of GTP–Rac1 present in quiescent cells and, in addition, hampered the EGF-triggered activation of Rac1 (Figure 5A and B). However, the reduction in ArhGEF7 protein levels did not affect other EGF-dependent responses such as the autophosphorylation of the EGF receptor (Figure 5A, third panel from top) or the engagement of the downstream Ras/Erk route (Figure 5A, fifth panel from top). As in the case of Coro1A-deficient cells, we finally observed that the dissociation of Rac1 from RhoGDIα triggered by EGF stimulation takes place much less efficiently in ArhGEF7-deficient than in control cells (Figure 5C, upper panel). Normal EGF-mediated dissociation rates of Rac1 were restored when wild-type ArhGEF7 was expressed in ArhGEF7-deficient cells (Figure 5C, upper panel). Such rescue did not occur when an ArhGEF7 protein containing an inactive DH domain was expressed in ArhGEF7-deficient cells (Figure 5C, upper panel). Proper stimulation of cells in each condition was demonstrated by the detection of the phosphorylation state of Erk (Figure 5C, fifth panel from top).
Figure 5.
Endogenous ArhGEF7 is essential for Rac1 activation in COS1 cells. (A, B) The indicated, serum-starved ACC3-1 (control) and ACC3-2 (lacking expression of ArhGEF7 but ectopically expressing Coro1A–EGFP) cell lines (top) were either left non-stimulated or stimulated with EGF for 10 min (top) and GTP–Rac1 levels detected by pull-down experiments (A) and quantified (B) by densitometry. *P⩽0.05; **P⩽0.01 compared with non-stimulated control or between the indicated conditions (brackets) (n=3). In parallel, cell lysates were used in immunoblot analysis to verify the protein/phosphorylation levels of the indicated proteins (A, six bottom panels). (C) ACC3-1 (control) and ACC3-2 (lacking expression of ArhGEF7 but ectopically expressing Coro1A–EGFP) cells expressing the indicated proteins (top) were either left non-stimulated or stimulated with EGF (top), immunoprecipitated with anti-RhoGDIα antibodies, and subjected to WB with the indicated antibodies (right) to visualize the amount of Rac1 (top panel) present in the anti-RhoGDIα immunoprecipitates (second panel from top). Aliquots of the same cell lysates were analysed by WB with the indicated antibodies (right) to detect the phosphoproteins and proteins under analysis (five bottom panels). The normalized Rac1 levels co-immunoprecipitating with RhoGDIα in each condition are indicated below the second top panel. *P⩽0.05 compared with the indicated experimental pair (brackets, n=3).
Coro1A and ArhGEF7 are important for Rac1 activation in T lymphocytes
Since Coro1A and ArhGEF7 are highly expressed in lymphocytes (Okumura et al, 1998; Nal et al, 2004; Missy et al, 2008), we investigated whether the above regulatory network was conserved in the Jurkat T-cell line. It has been shown before that Coro1A plays roles in those cells (Mueller et al, 2011). Likewise, ArhGEF7 is the only GEF involved in Pak1 activation in T-cell receptor (TCR)-stimulated Jurkat cells (Ku et al, 2001; Phee et al, 2005). With the exception of the previously reported AhrGEF7/Pak1 interaction present in those cells (Ku et al, 2001; Phee et al, 2005), we could not detect any stable interaction of endogenous Coro1A with AhrGEF7, Pak1, or RhoGDI using standard co-immunoprecipitation experiments (VO and XRB, data not shown). To circumvent this problem, we decided to detect these potential interactions in vivo using the recently described in situ proximity ligation assay. This technique relies on the use of fixed cells instead of cell lysates, thus avoiding the disassembly of complexes due to, for example, the disruption of the cytoskeleton that takes place using standard immunoprecipitation protocols (Soderberg et al, 2006). If proteins are in close physical proximity (<30–40 nm), this method generates intracellular fluorescent spots that can be visualized by standard confocal microscopy. Using this method, we observed that Coro1A was in close proximity with ArhGEF7, Pak1, and RhoGDIα in the cytosol and plasma membrane patches (Figure 6A, upper panels). This pattern did not change upon stimulation of the TCR with anti-CD3 antibodies (Figure 6A, bottom panels). The use of controls confirmed that LPA signals were specific (Figure 6B). Since this technique is not optimal to detect subcellular localization areas, we performed immunofluorescence experiments to investigate whether endogenous Coro1A and ArhGEF6 were present in the immune synapse, an F-actin-enriched structure implicated in T-cell activation (Vicente-Manzanares and Sanchez-Madrid, 2004). To this end, we induced the formation of this structure by mixing Jurkat T cells with Staphylococcal enterotoxin E-loaded Raji B cells. Whereas these two proteins were seen in the cytosol in non-conjugated T and B cells, we observed that they were both enriched in specific subareas of the immune synapse formed by conjugated T cells (Figure 6C). No protein polarization was seen in conjugated B cells (Figure 6C). The individual detection of Coro1A and ArhGEF7 in the immune synapse of T cells has been reported before by others (Phee et al, 2005; Haraldsson et al, 2008).
Figure 6.
Endogenous Coro1A is involved in Rac1 activation in T cells. (A) In situ LPA to detect physical proximity of Coro1A with ArhGEF (left panels), Pak1 (middle panels), and RhoGDIα (right panels) in non-stimulated (top panels) and anti-CD3 stimulated (lower panels) Jurkat cells. Areas of colocalization are seen as red spots. Nuclei were stained with DAPI (blue colour). (B) Negative controls of the experiment shown in (A). None: cells stained with secondary antibodies alone; α-Coro1A, cells stained with anti-Coro1A antibodies plus the two secondary antibodies; α-Coro1A+α-CD31 and α-Coro1A+α-H3, cells stained according to the standard protocol using the mouse monoclonal antibody to Coro1A and rabbit polyclonal antibodies to CD31 and histone H3 (H3), respectively. Scale bar, 5 μm. (C) Non-conjugated (right panel) and conjugated (rest of panels) Jurkat and superantigen-loaded Raji B cells were fixed, stained with antibodies to Coro1A and ArhGEF7 and subjected to confocal analyses. Signals derived from Coro1A and ArhGEF7 are shown in green and red colour, respectively. Areas of colocalization are shown in yellow (third and fourth panels from left). The blue colour also seen in those two panels corresponds to the 7-amino-4-chloromethylcoumarin biotracker used to label B cells prior to conjugation. B, B cell. Scale bar, 5 μm. (D) Protein expression of endogenous ArhGEF7 and Coro1A using immunoblot analysis of total lysates from the indicated cell lines (top). WT, wild type. (E, F) Indicated Jurkat clones (top) were stimulated with anti-CD3 antibodies for the indicated periods of time (top) and then subjected to pull-down experiments (E) or immunoblot analyses (F) to detect the levels of Rac1 GTP (E, top panel), phospho-Erks (E, lower panel), and phospho-Jnks (F, top panel) (n=2).
Next, we generated ArhGEF7- (VOS3-2) and Coro1A-deficient (VOS2-1) Jurkat cells (Figure 6D) to investigate whether these proteins contributed to Rac1 activation during TCR stimulation conditions. We observed that both the ArhGEF7 and the Coro1A deficiency affected the TCR-mediated activation of endogenous Rac1 (Figure 6E, upper panel). This was not due to intrinsic signalling defects of the TCR, because ArhGEF7- and Coro1A-deficient cells triggered normal Erk activation levels upon stimulation with anti-CD3 antibodies (Figure 6E, lower panel). To corroborate these observations using an independent technique, we investigated the activation level of c-Jun N-terminal kinase (Jnk), a downstream effector of Rac1 (Lopez-Lago et al, 2000; Kaminuma et al, 2001). The kinetics of phosphorylation of this serine/threonine kinase upon TCR engagement were significantly delayed in Coro1A-depleted cells compared with control cells (Figure 6F, upper panel). Additional immunoblots confirmed equal loading of cell extracts in all samples (Figure 6F, lower panel).
Coro1A triggers signalling routes in T cells in an ArhGEF7-dependent manner
The above experiments confirmed that Coro1A and ArhGEF7 were important for the TCR-mediated induction of Rac1 signals. However, they could not distinguish whether Coro1A had an active part in this process and, if so, whether such action was mediated by ArhGEF7. To approach those issues, we investigated whether the overexpression of Coro1A per se was capable of inducing the activation of Jnk using luciferase-based reporter assays. In addition, we also investigated whether Coro1A could induce the stimulation of the nuclear factor of stimulated T cells (NF-AT), a transcriptional factor essential for T-cell proliferation and cytokine production (Macian, 2005). We decided to use this second functional read-out because (i) it is partially dependent on Rac1 activity (Woodrow et al, 1993; Kaminuma et al, 2001; Zugaza et al, 2002); (ii) previous reports indicated that NF-AT activity levels are defective in stimulated Coro1A−/− T cells (Mueller et al, 2008, 2011); (iii) AhrGEF7 participates in this response in a Pak1-dependent manner (Phee et al, 2005). To approach this issue, we electroporated Jurkat cells with the appropriate reporter plasmids plus Coro1A–EGFP-, Coro1AR29D–EGFP-, or EGFP–Coro1B-encoding expression vectors. As negative control, we co-transfected vectors encoding the non-chimeric EGFP. As positive control, we co-electroporated expression plasmids expressing wild-type Vav1, a phosphorylation-dependent Rac1 GEF known to activate Jnk and NF-AT in T cells (Wu et al, 1995; Bustelo, 2000; Lopez-Lago et al, 2000; Zugaza et al, 2002). Upon transfection, Jurkat cells were either left non-stimulated or stimulated with anti-CD3 antibodies and luciferase activities determined. As expected, we observed that Vav1 gave high levels of activation of both Jnk (Figure 7A, left panel) and NF-AT (Figure 7B, left panel) in non-stimulated cells and, to a much larger extent, in stimulated Jurkat cells. The overexpression of Coro1A–EGFP led to a significant increase in those two activities in non-stimulated cells and, at much higher levels, in anti-CD3-stimulated Jurkat cells (Figure 7A and B, left panels). By contrast, we observed much lower activities of both Jnk and NF-AT when either Coro1AR29D–EGFP (Figure 7A and B) or EGFP–Coro1B (Supplementary Figure S13A) was used in these reporter assays. Immunoblot experiments demonstrated, however, that the latter proteins were expressed at levels comparable to the Coro1A–EGFP protein (Supplementary Figures S13B and S14). Interestingly, the Coro1A-driven Jnk and NF-AT activities were severely reduced when experiments were conducted in ArhGEF7-deficient Jurkat cells (Figure 7A and B; compare right and left panels). This was not a general signalling defect in those cells, because the stimulation of Jnk and NF-AT activities induced by Vav1 did not change significantly in function of the ArhGEF7 expression status (Figure 7A and B, compare right and left panels). These results indicate that Coro1A has a TCR-dependent active role in the activation of these two biological pathways and, in addition, provide further support for the downstream role of ArhGEF7 and Rac1 in its signal transduction route.
Figure 7.
Coro1A stimulates NF-AT and Jnk activities in an ArhGEF7-dependent manner. (A, B) Wild-type (left panels) and ArhGEF7-deficient (VOS3-2, right panels) cells expressing the indicated EGFP-tagged proteins (bottom) were subjected to Jnk (A) and NF-AT (B) luciferase assays. Data show the mean and s.d. of a representative experiment performed in independent triplicates. Expression levels of proteins used are shown in Supplementary Figure S14 online. Similar results were obtained in an independent experiment also performed in triplicate. (C) Schematic representation of the regulatory model for the Coro1A-mediated translocation and activation of Rac1. The Coro1A-based relay mechanism involved in the amplification of Rac1 signals reported here is shown in green colours. The first stimulus triggering Rac1 activation is shown in blue colour. Elements common to both phases are shown in black. Inhibitors shown to block some of these steps are shown in red. Hypothetical steps are shown as a broken lane.
Discussion
The results shown here indicate that Coro1A forms a complex with ArhGEF7, Pak1, and RhoGDIα that favours dissociation of the inhibitory RhoGDIα/Rac1 complex during cell signalling in a Pak1- and F-actin-dependent manner (Figure 7C). The latter property is interesting, since it indicates that this new route is probably involved in a relay mechanism that ensures the amplification of Rac1 signals upon the stimulus-induced activation of an initial small pool of Rac1 (Figure 7C). Alternatively, this route can be set in motion by the induction of F-actin polymerization via the stimulation of other GTPases (i.e., Cdc42, RhoG, and Arf6), integrins, or the establishment of cell-to-cell contacts. Using shRNA-mediated knockdown approaches with endogenous Coro1A and ArhGEF7, we have demonstrated that this new translocation/activation mechanism is indeed essential for the proper activation of Rac1 in a number of cell types and stimulation conditions, including EGF-stimulated COS1 and HEK293 T cells, fibronectin-stimulated COS1 cells, and anti-CD3-stimulated Jurkat T cells. This latter activity is particularly interesting, since it is known that Coro1A plays critical roles in T lymphocytes (Foger et al, 2006; Haraldsson et al, 2008; Mueller et al, 2008, 2011; Mugnier et al, 2008; Shiow et al, 2008, 2009). Interestingly, defective Rac1 activation has been reported before in Coro1A−/−-deficient T cells stimulated by the CXCL12 chemokine (Foger et al, 2006). Despite the above results, it is important to note that Coro1A and ArhGEF7 are not ubiquitously expressed, so it is unlikely that the Rac1 translocation/activation mechanism reported here will be utilized by all cell types.
The use of mutant versions and inhibitors has allowed us to establish the relative hierarchical position of each of the members of the Coro1A-nucleated complex. Thus, the observation that ArhGEF7 can interact with Coro1AR29D, a mutant version of Coro1A that cannot associate with F-actin, indicates that the Coro1A/ArhGEF7 represents the complex core from which the rest of components build upon (Figure 7C). The assembly of the second layer of proteins onto this core is mechanistically complex, since it requires the presence of both ArhGEF7 and the F-actin cytoskeleton. The implication of ArhGEF7 in the formation of this complex is mediated by its direct interaction with Pak1 (Manser et al, 1998; Ku et al, 2001; Phee et al, 2005), since the addition of a cell permeable competitor peptide to the ArhGEF7/Pak1 interface (Tat-Pak19) completely eliminates the formation of the Coro1A/Pak1/RhoGDIα complex. Likewise, we have observed that the use of a Pak1 mutant lacking the proline-rich region recognized by ArhGEF7 cannot associate with Coro1A or RhoGDIα (ACC and XRB, unpublished data). Despite this, the requirement of F-actin for the formation of the entire Coro1A/Pak1/RhoGDIα complex indicates that additional ancillary partners and/or signals must also contribute to this process. Whether these extra elements are proteins, membrane lipids, or other upstream signals remains to be determined. Finally, our data demonstrate that the formation of this complex is important to ensure proper dissociation rates of GDP–Rac1 from RhoGDIα, a Pak1-dependent function that favours both the docking of Rac1 onto the plasma membrane and its subsequent activation by Rho/Rac-specific GEFs present in the cytoskeleton and/or plasma membrane (Figure 7C). One of such GEFs can be in fact ArhGEF7 itself, since its presence in the Coro1A complex will ensure its close proximity with the released Rac1 molecules (Figure 7C). Although the presence of a catalytic activity in the ArhGEF7 DH domain is controversial (Rosenberger and Kutsche, 2006), it has been recently shown that it can promote Rac1 activation via its association with smgGDS (Shin et al, 2006).
It is possible that alternative or parallel mechanisms similar to the one reported here may exist in cells. For example, it has been shown that the Cdc42-specific GEFs FGD1 and Itsn1 associate with the F-actin regulatory proteins cortactin and N-Wasp, respectively (Hussain et al, 2001; Hou et al, 2003; Kim et al, 2004). However, the mechanism(s) by which those cytoskeletal proteins mediate the Cdc42 activation in vivo is not fully known. We surmise that this type of connections, if occurring in the same cell type, could be important to allow the spatiotemporal segregation of Cdc42 and Rac1 activation in some biological contexts. For example, the utilization of Cdc42 GEFs by Arp2/3 activating/stabilizing proteins such as cortactin or N-Wasp will ensure that Cdc42 will be recruited first to the Arp2/3-positive actin filament branching points while, at the same time, will set the basis for the next targeting of Coro1A proteins to those structures. The subsequent replacement of Arp2/3 by Coro1A molecules at the F-actin branching points (Cai et al, 2008) will shut off Cdc42 activation while favouring the recruitment of Rac1 molecules. This stepwise mechanism could be of interest, for example, for the sequential generation of filopodia and lamellipodia in the leading edge of migrating cells, two processes that are under the regulation and Cdc42 and Rac1, respectively (Nobes and Hall, 1995; Etienne-Manneville and Hall, 2002).
Surprisingly, we have seen that the function of Coro1A in the Rac1 route is not shared by Coro1B, another highly related protein belonging to the coronin family. Although the basis of this functional specificity is currently unclear, it is known that Coro1A and Coro1B differ in specific amino-acid stretches within the β-propeller domain, in the C-terminal tails located downstream of the β-propeller region, and in the number of PKC-dependent phosphorylation sites (Cai et al, 2005; Rybakin and Clemen, 2005; Uetrecht and Bear, 2006; Oku et al, 2008). Specific and even antagonistic functions have been described before for the two coronin family members of D. discoideum (Shina et al, 2011). Whether other coronin family members have a Coro1A-like function on Rac1 pathway regulation remains to be investigated. In any case, it is worth noting that we have isolated in our screening a second β-propeller-containing protein (WDR26), suggesting that other proteins with coronin-like structures may participate in the amplification of Rac1 signals in either independent or concerted manners with Coro1A.
Materials and methods
Immunofluorescence techniques
To monitor the distribution of the proteins under analysis, transfected cells were fixed in 3.7% formaldehyde in a phosphate-buffered saline solution for 15 min. Cells were then permeabilized in TBS-T (25 mM Tris–HCl (pH 8.0), 150 mM NaCl, 0.1% Tween-20) for 10 min, washed thrice with TBS-T, blocked in a 2% bovine serum albumin in TBS-T for 30 min, and stained using the appropriate stepwise addition of primary and secondary antibodies diluted in blocking solution for 1 h each. To visualize the F-actin cytoskeleton, the above preparations were subsequently incubated with either rhodamine- or Alexa Fluor 635-labelled phalloidin diluted 1:200 in blocking solution for 20 min. To detect lipid rafts, an Alexa Fluor 647-conjugated CTxB was added to cultured cells (0.5 μg/ml) for 2 min prior fixation. Stained preparations were mounted onto microscope slides using Mowiol (Calbiochem) and analysed using a Zeiss LSM510 confocal microscope and a × 63 objective (Zeiss). Microscope images were exported and processed for presentation using the Canvas 8 software (8.0.5 version, Deneba Systems). Imports of confocal images and profiles of fluorescence intensity along cells were made using the LSM510 software (Zeiss).
When indicated, the Rac1 translocation index obtained in each experimental condition was calculated as previously described (Del Pozo et al, 2002). To this end, AU5-, EGFP-, or mCherry-positive cells were selected from confocal images obtained under the indicated experimental conditions and scored 0 for no detectable Rac1 in the plasma membrane, 1 for weak Rac1 plasma membrane staining, and 2 for high Rac1 localization in the plasma membrane. The Rac1 translocation index to the plasma membrane was calculated as: (y+2z)/(x+y+z), where x, y, and z are the number of cells that were scored as 0, 1, or 2, respectively. The same approach was used to calculate the ArhGEF7 translocation index.
Statistical analyses
Data from at least three independent experiments were analysed using the Student's t-test. In single cell-based experiments, each of those experiments involved the analysis of at least 50 independent, randomly picked cells. P-values ⩽0.05 were considered as statistically significant.
Supplementary Material
Acknowledgments
XRB work is supported by grants from the NIH (5R01-CA73735-13), the Spanish Ministry of Science and Innovation (SMSI) (SAF2009-07172 and RD06/0020/0001), the Castilla y León Autonomous Government (GR97), and the 7th Framework European Union Program (FP7-HEALTH-2007-A-201862). MAdP was supported by SMSI grants SAF2008-02100 and RD06/0020/1033, the European Science Foundation Young Investigator Award, and by the EMBO Young Investigator Program. FXP was supported by SMSI (SAF2008-00350), Castilla y León Autonomous Government (CSIC001A10-2) and Fundación Solórzano grants.
Author contributions: AC-C carried out the cellomic screening and the work shown in Figures 1A, C, D, E, 2A, E, 3–5 and Supplementary Figures S3A, B, S4, S6–S8, and S10–S12. VO performed experiments shown in Figures 1B, 2C, D and 6 and Supplementary Figures S1, S2, and S9. MB generated data of Figure 7 and Supplementary Figures S13 and 14. VS and FXP generated reagents for the cellomic screening and performed the first screening rounds. IN-L, OM, and MAdP performed experiments related to Supplementary Figures S3C and S5. JRC generated vectors and mutants used in these experiments. XRB directed the work and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abramovici H, Mojtabaie P, Parks RJ, Zhong XP, Koretzky GA, Topham MK, Gee SH (2009) Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell 20: 2049–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA (2007) Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol 9: 1381–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129: 865–877 [DOI] [PubMed] [Google Scholar]
- Bustelo XR (2000) Regulatory and signaling properties of the Vav family. Mol Cell Biol 20: 1461–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustelo XR, Sauzeau V, Berenjeno IM (2007) GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 29: 356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Holoweckyj N, Schaller MD, Bear JE (2005) Phosphorylation of coronin 1B by protein kinase C regulates interaction with Arp2/3 and cell motility. J Biol Chem 280: 31913–31923 [DOI] [PubMed] [Google Scholar]
- Cai L, Makhov AM, Bear JE (2007) F-actin binding is essential for coronin 1B function in vivo. J Cell Sci 120(Part 10): 1779–1790 [DOI] [PubMed] [Google Scholar]
- Cai L, Makhov AM, Schafer DA, Bear JE (2008) Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell 134: 828–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YC, Kim JH, Kim KL, Kim HW, Lee HY, Heo WD, Meyer T, Suh PG, Ryu SH (2008) Phospholipase D activity regulates integrin-mediated cell spreading and migration by inducing GTP-Rac translocation to the plasma membrane. Mol Biol Cell 19: 3111–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TH, Bohl BP, Bokoch GM (1993) Biologically active lipids are regulators of Rac-GDI complexation. J Biol Chem 268: 26206–26211 [PubMed] [Google Scholar]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA (2004) Integrins regulate Rac targeting by internalization of membrane domains. Science 303: 839–842 [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA (2005) Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol 7: 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA (2002) Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol 4: 232–239 [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA (2000) Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J 19: 2008–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM (2005) GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 15: 356–363 [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Rocklin G, Seo JY, Bokoch GM (2006) Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell 17: 4760–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerMardirossian C, Schnelzer A, Bokoch GM (2004) Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell 15: 117–127 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Foger N, Rangell L, Danilenko DM, Chan AC (2006) Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science 313: 839–842 [DOI] [PubMed] [Google Scholar]
- Haraldsson MK, Louis-Dit-Sully CA, Lawson BR, Sternik G, Santiago-Raber ML, Gascoigne NR, Theofilopoulos AN, Kono DH (2008) The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity 28: 40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T (2006) PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314: 1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Estrada L, Kinley AW, Parsons JT, Vojtek AB, Gorski JL (2003) Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum Mol Gen 12: 1981–1993 [DOI] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, McPherson PS (2001) Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol 3: 927–932 [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269 [DOI] [PubMed] [Google Scholar]
- Kaminuma O, Deckert M, Elly C, Liu YC, Altman A (2001) Vav-Rac1-mediated activation of the c-Jun N-terminal kinase/c-Jun/AP-1 pathway plays a major role in stimulation of the distal NFAT site in the interleukin-2 gene promoter. Mol Cell Biol 21: 3126–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Hou P, Gorski JL, Cooper JA (2004) Effect of Fgd1 on cortactin in Arp2/3 complex-mediated actin assembly. Biochemistry 43: 2422–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Gimpl G, Fahrenholz F (1995) Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34: 13784–13793 [DOI] [PubMed] [Google Scholar]
- Ku GM, Yablonski D, Manser E, Lim L, Weiss A (2001) A PAK1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J 20: 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lago M, Lee H, Cruz C, Movilla N, Bustelo XR (2000) Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol Cell Biol 20: 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F (2005) NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 5: 472–484 [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367: 40–46 [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192 [DOI] [PubMed] [Google Scholar]
- Maruta H, He H, Nheu T (2002) Interfering with Ras signaling using membrane-permeable peptides or drugs. Methods Mol Biol 189: 75–85 [DOI] [PubMed] [Google Scholar]
- Missy K, Hu B, Schilling K, Harenberg A, Sakk V, Kuchenbecker K, Kutsche K, Fischer KD (2008) AlphaPIX Rho GTPase guanine nucleotide exchange factor regulates lymphocyte functions and antigen receptor signaling. Mol Cell Biol 28: 3776–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P, Liu X, Pieters J (2011) Migration and homeostasis of naive T cells depends on coronin 1-mediated prosurvival signals and not on coronin 1-dependent filamentous actin modulation. J Immunol 186: 4039–4050 [DOI] [PubMed] [Google Scholar]
- Mueller P, Massner J, Jayachandran R, Combaluzier B, Albrecht I, Gatfield J, Blum C, Ceredig R, Rodewald HR, Rolink AG, Pieters J (2008) Regulation of T cell survival through coronin-1-mediated generation of inositol-1,4,5-trisphosphate and calcium mobilization after T cell receptor triggering. Nat Immunol 9: 424–431 [DOI] [PubMed] [Google Scholar]
- Mugnier B, Nal B, Verthuy C, Boyer C, Lam D, Chasson L, Nieoullon V, Chazal G, Guo XJ, He HT, Rueff-Juy D, Alcover A, Ferrier P (2008) Coronin-1A links cytoskeleton dynamics to TCR alpha beta-induced cell signaling. PLoS ONE 3: e3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nal B, Carroll P, Mohr E, Verthuy C, Da Silva MI, Gayet O, Guo XJ, He HT, Alcover A, Ferrier P (2004) Coronin-1 expression in T lymphocytes: insights into protein function during T cell development and activation. Int Immunol 16: 231–240 [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81: 53–62 [DOI] [PubMed] [Google Scholar]
- Oku T, Kaneko Y, Murofushi K, Seyama Y, Toyoshima S, Tsuji T (2008) Phorbol ester-dependent phosphorylation regulates the association of p57/coronin-1 with the actin cytoskeleton. J Biol Chem 283: 28918–28925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M, Kung C, Wong S, Rodgers M, Thomas ML (1998) Definition of family of coronin-related proteins conserved between humans and mice: close genetic linkage between coronin-2 and CD45-associated protein. DNA Cell Biol 17: 779–787 [DOI] [PubMed] [Google Scholar]
- Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP (2008) Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134: 135–147 [DOI] [PubMed] [Google Scholar]
- Phee H, Abraham RT, Weiss A (2005) Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat Immunol 6: 608–617 [DOI] [PubMed] [Google Scholar]
- Price LS, Langeslag M, ten Klooster JP, Hordijk PL, Jalink K, Collard JG (2003) Calcium signaling regulates translocation and activation of Rac. J Biol Chem 278: 39413–39421 [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Kutsche K (2006) AlphaPIX and betaPIX and their role in focal adhesion formation. Eur J Cell Biol 85: 265–274 [DOI] [PubMed] [Google Scholar]
- Rybakin V, Clemen CS (2005) Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking. Bioessays 27: 625–632 [DOI] [PubMed] [Google Scholar]
- Saci A, Cantley LC, Carpenter CL (2011) Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell 42: 50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M (1982) Action of cytochalasin D on cytoskeletal networks. J Cell Biol 92: 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EY, Lee CS, Cho TG, Kim YG, Song S, Juhnn YS, Park SC, Manser E, Kim EG (2006) betaPak-interacting exchange factor-mediated Rac1 activation requires smgGDS guanine nucleotide exchange factor in basic fibroblast growth factor-induced neurite outgrowth. J Biol Chem 281: 35954–35964 [DOI] [PubMed] [Google Scholar]
- Shina MC, Muller-Taubenberger A, Unal C, Schleicher M, Steinert M, Eichinger L, Muller R, Blau-Wasser R, Glockner G, Noegel AA (2011) Redundant and unique roles of coronin proteins in Dictyostelium. Cell Mol Life Sci 68: 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM (2009) Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a Coronin-1A mutation and a chromosome 16p11.2 deletion. Clin Immunol 131: 24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, Xu Y, Jenne CN, Foger N, Sorensen RU, Goodnow CC, Bear JE, Puck JM, Cyster JG (2008) The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol 9: 1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 3: 995–1000 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR (2003) Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 160: 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL (2006) Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol 172: 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Couvillon AD, Cantley LC, Carpenter CL (1998) Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol Cell Biol 18: 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J (1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781 [PubMed] [Google Scholar]
- Uetrecht AC, Bear JE (2006) Coronins: the return of the crown. Trends Cell Biol 16: 421–426 [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Sanchez-Madrid F (2004) Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol 4: 110–122 [DOI] [PubMed] [Google Scholar]
- Woodrow M, Clipstone NA, Cantrell D (1993) p21ras and calcineurin synergize to regulate the nuclear factor of activated T cells. J Exp Med 178: 1517–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Katzav S, Weiss A (1995) A functional T-cell receptor signaling pathway is required for p95vav activity. Mol Cell Biol 15: 4337–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T (1979) Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA 76: 4498–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319: 210–213 [DOI] [PubMed] [Google Scholar]
- Zugaza JL, Lopez-Lago MA, Caloca MJ, Dosil M, Movilla N, Bustelo XR (2002) Structural determinants for the biological activity of Vav proteins. J Biol Chem 277: 45377–45392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.