Abstract
Cortisol concentration in both serum and saliva sharply increases and reaches a peak within the first hour after waking in the morning. This phenomenon is known as the cortisol awakening response (CAR) and is used as an index of hypothalamus–pituitary–adrenal (HPA) axis function. We examined whether ovarian steroid concentrations increased after awakening as with the CAR in the HPA axis. To do this, cortisol, estradiol-17β (E2), and progesterone (P4) concentrations were determined in saliva samples collected immediately upon awakening and 30 and 60 min after awakening in women with regular menstrual cycles and postmenopausal women. We found that both E2 and P4 concentrations increased during the post-awakening period in women with regular menstrual cycles, but these phenomena were not seen in any postmenopausal women. The area under the E2 and P4 curve from the time interval immediately after awakening to 60 min after awakening (i.e. E2auc and P4auc) in women with regular menstrual cycles were greater than those in the postmenopausal women. E2 and P4 secretory activity during the post-awakening period was influenced by the phase of the menstrual cycle. E2auc in the peri-ovulatory phase and P4auc in the early to mid-luteal phase were greater than in the menstrual phase. Meanwhile, cortisol secretory activity during the post-awakening period was not influenced by menstrual status or the phase of menstrual cycle. These findings indicate that, as with the CAR in the HPA axis function, ovarian steroidogenic activity increased after awakening and is closely associated with menstrual status and phase of menstrual cycle.
Introduction
The measurement of steroid concentrations in saliva has become more common over the past several years. The collection of saliva is easier than a venipuncture and can readily be repeated at frequent intervals. These advantages provide for a better assessment of the diurnal rhythm-mediated secretion of cortisol and sex steroids.
The diurnal pattern of cortisol secretory activity is well characterized. Cortisol reaches a zenith around the time of awakening in the morning and a nadir after the onset of sleep. The most distinctive feature of the cortisol secretory rhythm is a sharp increase in cortisol levels in serum and in saliva within the first 30–45 min after awakening from nocturnal sleep (Pruessner et al. 1997, Wilhelm et al. 2007). This increase in the cortisol level after awakening is termed the cortisol awakening response (CAR; Federenko et al. 2004). An ACTH peak occurs prior to the CAR (Wilhelm et al. 2007) and the occurrence of the CAR is dependent on the waking timing (Federenko et al. 2004, Dettenborn et al. 2007). Therefore, researchers believe that the CAR is a neuroendocrine activation of the hypothalamus–pituitary–adrenal (HPA) axis in response to awakening and the suprachiasmatic nucleus (SCN), which is the master pacemaker controlling circadian rhythmicity, is involved in the occurrence of the CAR (Fries et al. 2009, Clow et al. 2010). The CAR is used as a reliable index of the HPA axis function in various clinical research areas, and an altered CAR is known to be associated with health issues, such as fatigue, depression, and anxiety, and the prognosis of patients with a severe disease (Sephton et al. 2000, Chida & Steptoe 2009).
The timing of the preovulatory LH surge in rodents and humans has provided evidence that the SCN is also involved in regulating the function of the hypothalamus–pituitary–ovary (HPO) axis. The preovulatory LH surge occurs around the time of lights-off in nocturnal laboratory rodents (Legan & Karsch 1975, Seegal & Goldman 1975) and around lights-on in diurnal murid rodent (McElhinny et al. 1999). Although a higher estradiol-17β (E2) concentration is an absolute prerequisite for generating the LH surge, the occurrence of a preovulatory LH surge is gated by a circadian oscillator in the SCN. Studies exploring the effect of the SCN in animals have shown that bilateral lesions of the SCN result in abolition of the preovulatory LH surge, induction of persistent estrus, and polyfollicular ovaries in gonadally intact female rats (Brown-Grant & Raisman 1977, Gray et al. 1978) and the elimination of daily afternoon surges of LH in estrogen-treated ovariectomized rats (Kawakami et al. 1980). In women, two studies have reported a possible role for the SCN in determining the preovulatory LH surge. One study reported that the preovulatory LH surge began at 0800 h in most women (80% of cases) who had normal menstrual cycles (Kerdelhue et al. 2002). Another study examining the timing of the LH surge found that the surges occurred between 0400 and 0800 h in 48% of cases and between 0000 and 0400 h in 37% of cases (Cahill et al. 1998).
Meanwhile, it is known that hormones produced in the HPA axis exert inhibitory effects on hormone secretion at multiple levels in the HPO axis under stress conditions (Chrousos et al. 1998, Kalantaridou et al. 2004). However, the relationship between these two endocrine systems is by no means unidirectional. Some studies have shown the temporal relationship between the HPA and HPO axes. For example, corticosterone and ACTH reach peak levels at the end of the light phase in female rat (Atkinson & Waddell 1997), and the preovulatory LH surge also occurs at the end of the light phase in the proestrus female rat and mice (Naftolin et al. 1972, Bronson & Vom Saal 1979). In humans, Kerdelhue et al. (2002) have reported that plasma cortisol reaches a peak when the preovulatory LH surge begins, e.g. at 0400 h when the LH surge begins at 0400 or 0800 h when it begins at 0800 h.
There is diurnal variation in the pattern of pulsatile LH secretion and ovarian E2 and progesterone (P4) secretion in women. Studies on the LH profiles obtained by repeated blood sampling from adult women of a reproductive age have shown that the interpulse interval and pulse amplitude of LH are greater during nocturnal sleep than in day time and that LH concentrations increase sharply after awakening from nocturnal sleep (Kapen et al. 1981, Pincus et al. 1998, Hall et al. 2005). Although the acrophase (i.e. time of maximal concentration) of the E2 diurnal rhythm is modulated by menstrual phases (Bao et al. 2003), studies on sex steroid secretion in normal cyclic women have suggested that E2 and P4 secretion exhibit a diurnal variation (Delfs et al. 1994, Bao et al. 2004).
The SCN receives direct light information via the optic tract (retinohypothalamic tract), translates this information into a daily pattern of activity, and transmits information about the time of day to hypophysiotropic corticotropin-releasing hormone (CRH)-synthesizing neurons in the paraventricular nucleus (PVN; Buijs et al. 2003) and to GnRH-synthesizing neurons in the preoptic area (POA; de la Iglesia et al. 1995). Transmission of the time-of-day signal from the SCN to CRH- and GnRH-synthesizing neurons is thought to be involved in the entrainment of the circadian rhythm governing the activities of the HPA and HPO axes respectively (Kalsbeek & Buijs 2002).
As for the HPA axis function, the endocrine rhythm of the HPO axis is also regulated by the SCN. It could thus be speculated that increases in the E2 and P4 concentrations occur after an awakening from nocturnal sleep, similar to the CAR in the HPA axis function, if each component of the HPO axis is functioning normally. The menstrual cycle is regulated by the coordinated action between the ovaries and the hypothalamic–pituitary axis; the ovaries secrete E2 and P4 in response to gonadotropins and send feedback messages to the hypothalamic–pituitary axis (Buffet et al. 1998). Therefore, we hypothesized that any increases in E2 and P4 concentrations after the awakening period would occur in women with regular menstrual cycles. To test this hypothesis, this study examined whether the E2 and P4 concentrations increased within the first hour after awakening in women with a regular menstrual cycle. In addition, we examined whether the post-awakening increases in sex steroid concentrations differed throughout the menstrual cycle and whether these patterns differed between women with a normal menstrual cycle and those who have undergone menopause.
Materials and Methods
Subjects
National health insurance companies within Korea support periodical physical examinations for individuals who have not experienced hospitalization within 2 years after the previous health examination. Among the subjects receiving this periodical physical examination, healthy women aged 20–60 years were recruited between April 2009 and January 2011 from the Honam Medical Center (HMC). Information regarding medical history and menstrual cycle regularity, length, and history was obtained by face-to-face interview, and weight and height were measured at presentation. Exclusion and inclusion criteria were principally adopted from previous studies (Hall et al. 2000, Bao et al. 2003, 2004). Volunteers were excluded from the study if they met any one of the following criteria: 1) women who previously had a regular menstrual cycle but had experienced an irregular menstrual cycle two or more times in the 6 months preceding study entry and were aged 20–50 years (i.e. women with irregular menstrual cycles or perimenopausal women); 2) women undergoing hormone replacement therapy, taking oral contraceptives, tamoxifen, or other estrogen-, P4-, or glucocorticoid-containing drugs within the last 6 months; 3) women who were pregnant, lactating, showed evidence of infertility, or presence of sexually transmitted diseases or had been diagnosed with ovarian dysfunction; 4) night-shift workers, women diagnosed with chronic illness or abnormal body mass index (BMI ≤18 or ≥25); or 5) women taking antidepressant drugs or smokers. Subjects who last used oral contraceptives or steroid-containing drugs more than 6 months prior to this study were eligible.
Population-based studies have reported that menopause typically occurs in the late 40s (mean age, 48·5–49·4 years) in Korean women (Park et al. 2002, Ku et al. 2004). Women were classified into two groups based on their current menstrual status and age as follows: 1) women with a natural and regular menstrual cycle, with periods of 27–30 days for the 3 months preceding study entry, and age 20–40 years (women with regular cycles); and 2) women with no menses for more than 12 consecutive months preceding study entry, without other medical reasons for menses to stop, and age 50–60 years (postmenopausal women).
Because the subjects in the regular cyclic group were not synchronized in the phases of their menstrual cycles, they were divided into subgroups based on their menstrual phase on the day of saliva sample collection, as described previously (Bao et al. 2003): phase 1 subgroup, 2–3 days after the start of the menstrual cycle (menstrual phase; n=5); phase 2 subgroup, 12–14 days (late follicular, peri-ovulatory phase; n=16); phase 3 subgroup, 18–23 days (early to mid-luteal phase; n=14); phase 4 subgroup, 25–28 days (late luteal phase; n=13).
Saliva collection
One hundred and twenty-four women with regular menstrual cycles and 91 postmenopausal women were initially recruited in this study. Participants were asked to collect their saliva samples immediately upon awakening and 30 and 60 min after awakening for a single day, with a minimum volume of 1·5 ml saliva at each time point. They were asked not to alter their routine sleep–wake cycle. A saliva-collecting pack containing three collecting tubes, a zipper bag, and instructions was distributed or delivered to each subject.
Participants were instructed to collect saliva samples at the designated times (<5 min after awakening, 30±5 and 60±5 min after awakening) on workdays (Kunz-Ebrecht et al. 2004, Kim et al. 2010) and to note each collection time on the marking area of the collecting tube (Simport, Inc., Beloeil, QC, Canada). Saliva was collected without external stimulation but with muscle movement and expectoration into a collecting tube. All participants were asked not to drink alcohol on the previous night. During the sample collection period, patients were asked to refrain from eating food, drinking fluids, and brushing their teeth and to rinse their mouths with water before each sample collection. Steroid concentrations in saliva are stable after storage of saliva at −20 °C or −80 °C for up to 1 year, and freezing and thawing of samples up to four times before analysis does not affect measured concentrations (Groschl et al. 2001). Therefore, participants were asked to keep saliva samples in their own domestic freezers before sample submission.
Samples contaminated with blood, as determined by visual inspection, were excluded from the study. Samples collected outside of the designated times and those lacking a time or date on the marking area of the tube were also excluded, but this sample exclusion procedure was not informed to participants in order to increase the veracity of subject's self-reports. After inspection procedures, saliva samples collected from 184 subjects (women with regular cycles: n=98; postmenopausal women: n=86) and accepted for the cortisol assay procedures.
The typical CAR was defined as an increase in cortisol levels at least 2·5 nmol/l above the individual's baseline in compliant subjects (Wust et al. 2000, Kunz-Ebrecht et al. 2004). It is known that collecting the first sample with a delay of more than 10 min after awakening (i.e. non-compliant subject) in healthy subjects produces an absence of cortisol increase after awakening period (Kudielka et al. 2003). The typical CAR was not found and cortisol concentrations immediately upon awakening and 30 min after awakening in some subjects (n=28) were 16·4±3·8, 15·0±3·2, and 10·2±2·8 nmol/l respectively. We considered these as non-compliant subjects. They were asked again to collect another batch of saliva samples and not to delay in taking the first saliva sample after awakening. Cortisol concentrations immediately upon awakening and 30 and 60 min after awakening were 9·8±4·6, 18·3±7·5, and 13·9±7·6 nmol/l (n=19) respectively in the second batch of saliva samples. Other subjects (n=9) did not participate in collection of the second batch of saliva samples. They were considered non-responders or non-compliant subjects, and their samples were excluded from this study.
Ultimately, the data obtained from saliva samples collected from 93 women with regular cycles (mean age, 32·1±5·5 years; BMI, 21·5±2·1) and 82 postmenopausal women (mean age, 55·6±3·6 years; BMI, 22·2±1·8) were included in this study. Women with regular cycles woke up 0646±33 min (range 0525–0737) and postmenopausal women woke up 0701±29 min (range 0615–0750).
The collected samples were stored at −70 °C after removal of debris. To precipitate mucins, the samples were thawed and centrifuged (10 000 g, 15 min, 4 °C; Gozansky et al. 2005). The supernatant was collected and stored at −70 °C until the assay was performed. All participants gave their consent, and they were given information regarding their hormonal concentrations. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Hospital's Institutional Review Board (HMC). All saliva samples were analyzed at the Hormone Research Center at Chonnam National University.
Salivary steroid measurements
Steroid concentrations in saliva were determined using RIA as described previously (Ahn et al. 2007). Iodine-125-labeled cortisol (cortisol-3-(O-carboxymethyl oximino)-2-125I-iodohistamine) and estradiol (16α-(125I)-iodo-3,estradiol) were obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA, USA). Iodine-125-labeled progesterone (progesterone-11α-hemisuccinyl-(2-125I)-iodotyrosine methyl ester) was obtained from Institute of Isotopes Company (Budapest, Hungary).
The reference standards for cortisol, E2, and P4 were obtained from Sigma–Aldrich. A standard stock solution (10 mmol/l) of cortisol, E2, or P4 was prepared in ethanol, and working standards were made by diluting the stock solution using 0·1% gelatin containing 50 mM PBS (pH=7·2).
Cortisol and E2 antisera were purchased from AbD Serotec (Oxford, UK). Cortisol antiserum cross-reacts with aldosterone, 11-deoxycorticosterone, 11-deoxycortisol, 21-deoxycortisol, corticosterone, and other steroids, with cross-reactions of 0·001, 4·1, 5·7, 0·5, 1·2, and <0·01% respectively. E2 antiserum cross-reacts with estrone, estriol, dihydrotestosterone, and other steroids, with cross-reactions of 0·4, 0·1, 0·05, and <0·01% respectively. P4 antiserum was purchased from Abcam. P4 antiserum cross-reacts with 17α-hydroxyprogesterone, E2, corticosterone, 11α-hydroxyprogesterone, and other steroids, with cross-reactions of 2·7, <0·1, <0·1, 15·9, and <0·01% respectively.
Exogenously added 5·5 and 22·0 nmol/l cortisol in charcoal-stripped saliva was determined to be 5·4±0·7 nmol/l (n=20) and 22·9±1·9 nmol/l (n=20) respectively. The inter-assay coefficients of variation (CV) as assessed from quality controls with mean cortisol concentrations of 3·6 and 10·9 nmol/l were 7·4 and 8·5% respectively (n=26). The analytical sensitivity for cortisol was 0·4 nmol/l. Exogenously added 10·0 and 50·0 pmol/l E2 in charcoal-stripped saliva was determined to be 11·3±1·5 pmol/l (n=20) and 52·4±4·5 pmol/l (n=20) respectively. The inter-assay CV as assessed from quality controls with mean E2 concentrations of 18·4 and 183·6 pmol/l were 11·5 and 13·2% respectively (n=26). The analytical sensitivity for E2 was 3·7 pmol/l. Exogenously added 50·0 and 250·0 pmol/l P4 in charcoal-stripped saliva was determined to be 51·1±4·7 pmol/l (n=20) and 258·1·4±8·3 pmol/l (n=20) respectively. The inter-assay CV as assessed from quality controls with mean P4 concentrations of 159·0 and 795·0 pmol/l were 12·5 and 10·1% respectively (n=26). The analytical sensitivity for P4 was 31·8 pmol/l.
Data analysis
Two types of auxiliary index (i.e. mean increase in steroid concentrations and integrated steroid concentrations) were adapted to this study to analyze steroid secretory activity during the post-awakening period. After determination of cortisol, E2, and P4 concentrations, the mean increases in steroid concentrations after awakening (MnInc; AS30 min+AS60 min)/2)−AS0 min, where AS is awakening steroid concentrations) and the relative mean increases in steroid concentrations after awakening (MnInc %; AS30 min+AS60 min)/2)/AS0 min×100) were calculated for each subject after modification of the previously proposed formula (Wust et al. 2000). Total cortisol, E2, or P4 secretion during the post-awakening period was calculated as area under the curve (auc) with respect to ground from the time interval immediately after awakening to 60 min after awakening by using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA).
The Shapiro–Wilk normality test revealed that the variables, i.e. steroid concentrations at each examined time point, and auxiliary indices were not normally distributed. Differences in steroid concentrations among examined time points were analyzed using the Kruskal–Wallis test. Differences in auxiliary index (MnInc % and AUC) throughout four phases of the menstrual cycles were analyzed using the Kruskal–Wallis test, and when the one-way ANOVA test indicated a significant difference (P<0·05), Dunn's multiple comparisons test was performed to locate specific group differences. Between-group differences in the observed steroid concentrations at each time point and each auxiliary index (MnInc % or AUC) were analyzed using the Mann–Whitney test. The hormone values were square root transformed to normalize the sample distributions for a one-way repeated measure ANOVA. Statistical calculations were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA), and a P value <0·05 was considered significant.
Results
Cortisol secretory activity within the first hour after awakening
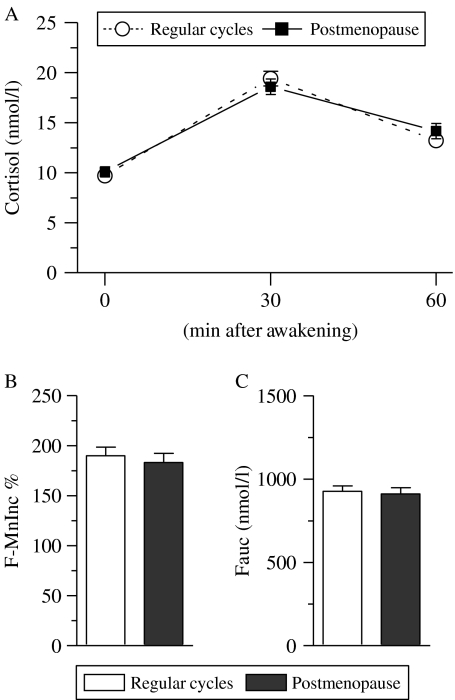
Cortisol profiles within the first 1 h after awakening in women with regular cycles and postmenopausal women are presented in Fig. 1A, and indices for cortisol secretory activity during post-awakening period (F-MnInc % and Fauc) in each examined group are presented in Fig. 1B. Cortisol levels increased after awakening and reached a peak level 30 min after awakening in both groups of women (Fig. 1A). A one-way repeated measures ANOVA (group (regular cycles, postmenopause)×time (three different time points)) with cortisol concentration as the dependent variable revealed that there was no significant group effect (F1, 519=0·11, P=0·74), which reflected similar cortisol levels in examined groups. In addition, there was no significant interaction effect (F2, 519=0·99, P=0·37), which indicated same patterns of cortisol secretion in both groups. We also observed an effect of time (F2, 519=101·9, P<0·001), which reflected the changes in cortisol levels after awakening (Fig. 1A). The Mann–Whitney test revealed that there was no significant difference between two groups in cortisol concentration immediately upon awakening (P=0·48), 30 min (P=0·22), and 60 min (P=0·71; Fig. 1A).
Figure 1.
Changes in cortisol concentration within the first hour after awakening in women with regular menstrual cycles and postmenopausal women. Cortisol concentration at each examined time point is depicted in (A). The relative mean increase in cortisol concentration 30 and 60 min after awakening over the individual baseline (F-MnInc %) and the integrated cortisol concentration ranging from the time immediately upon awakening to 60 min after awakening (i.e. area under the cortisol curve with respect to baseline, Fauc) in each examined group are depicted in (B and C) respectively. Each data point in (A) and each bar in (B and C) represents mean±s.e.m.
No difference was found in the relative mean increases in cortisol concentrations after awakening (F-MnInc %) and the integrated cortisol concentration ranging from the time immediately upon awakening to 60 min after awakening (i.e. area under the cortisol curve with respect to baseline, Fauc) between both groups (P>0·05 for all analyses by the Mann–Whitney test; Fig. 1B and C).
E2 secretory activity within the first hour after awakening
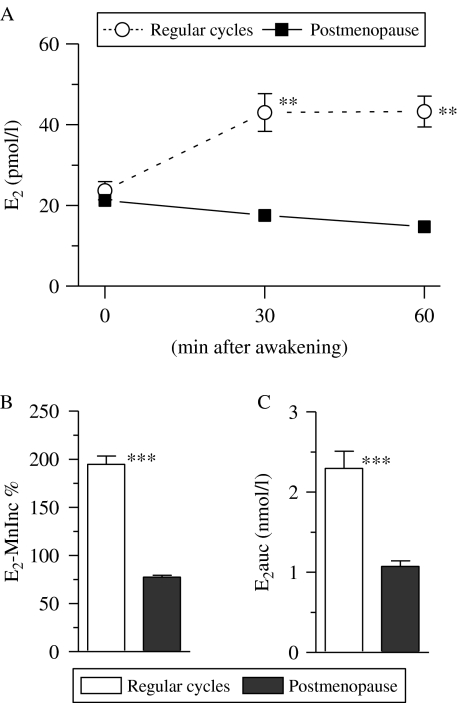
E2 concentration was determined in the same saliva samples that were used for determining cortisol concentration. E2 profiles within the first 1 h after awakening in women with regular cycles and postmenopausal women are presented in Fig. 2A. The E2 profiles in women with regular cycles and postmenopausal women were analyzed using one-way repeated measures ANOVA (group (regular cycles, postmenopause)×time (three different time points)) with E2 concentration as the dependent variable. We found that there was a significant group effect (F1, 519=63·1, P<0·001), with higher E2 levels in the women with regular cycle than in postmenopausal women. In addition, there was a significant interaction effect (F2, 519=12·1, P<0·001), which indicated differential patterns of E2 secretion between both groups. We also observed an effect of time (F2, 519=101·9, P<0·001), which reflected the changes in E2 levels post-awakening period (Fig. 2A).
Figure 2.
Difference in estradiol-17β (E2) concentrations within the first hour after awakening in women with regular menstrual cycles and postmenopausal women. E2 concentration at each examined time point is depicted in (A). The relative mean increase in E2 concentration 30 and 60 min after awakening over the individual baseline (E2-MnInc %) and integrated E2 concentration ranging from the time immediately upon awakening to 60 min after awakening (i.e. area under the E2 curve with respect to baseline, E2auc) are depicted in (B and C) respectively. Each data point in (A) and each bar in (B and C) represents mean±s.e.m. Asterisks denote the level of significance between both groups: **P<0·01; ***P<0·001 (by the Mann–Whitney test).
In women with regular cycles, E2 concentrations 30 and 60 min after awakening were significantly higher than that immediately upon awakening (P<0·001 for all analyses by the Mann–Whitney test; Fig. 2A). However, E2 concentrations at all the time point examined were similar to each other in postmenopausal women (P=0·08 by the Kruskal–Wallis test; Fig. 2A). E2 concentration immediately upon awakening was comparable in both groups (P=0·86 by the Mann–Whitney test), but E2 concentrations 30 and 60 min after awakening in women with regular cycle were significantly higher than those in the postmenopausal women (P<0·001 for all analyses by the Mann–Whitney test; Fig. 2A).
All women with regular menstrual cycles showed a positive E2-MnInc (range 0·4–93·0 pmol/l), but none of the postmenopausal women showed a positive E2-MnInc. The Mann–Whitney test revealed that the E2-MnInc % and E2auc in women with regular cycles were significantly higher than those in the postmenopausal women (P<0·01 for all analyses; Fig. 2B and C).
P4 secretory activity within the first hour after awakening
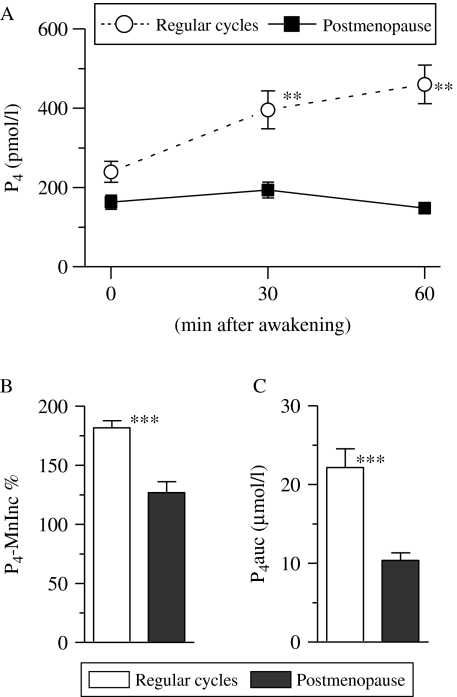
P4 concentration was also determined in the same saliva samples that were used for determining cortisol and E2 concentrations. The P4 profiles within the first 1 h after awakening in women with regular cycles and postmenopausal women are presented in Fig. 3A. The P4 profiles in women with regular cycles and postmenopausal women were analyzed using one-way repeated measures ANOVA (group (regular cycles, postmenopause)×time (three different time points)) with P4 concentration as the dependent variable. We found that there was a significant group effect (F1, 519=50·6, P<0·0001), with higher P4 levels in the women with regular cycles than in the postmenopausal women. In addition, there was a significant interaction effect (F2, 519=6·04, P<0·01), which indicated differential patterns of P4 secretion between both groups. We also observed an effect of time (F2, 519=101·9, P<0·001), which reflected the changes in P4 levels post-awakening period (Fig. 2A).
Figure 3.
Difference in progesterone (P4) concentration within the first hour after awakening in women with regular menstrual cycles and postmenopausal women. P4 concentration at each examined time point is depicted in (A). The relative mean increase in P4 concentration 30 and 60 min after awakening over the individual baseline (P4-MnInc %) and integrated P4 concentration ranging from the time immediately upon awakening to 60 min after awakening (i.e. area under the P4 curve with respect to baseline, P4auc) are depicted in (A and B) respectively. Each data point in (A) and each bar in (B and C) represents mean±s.e.m. Asterisks denote the level of significance between both groups: **P<0·01; ***P<0·001 (by the Mann–Whitney test).
In women with regular cycles, P4 concentrations 30 and 60 min after awakening were significantly higher than concentrations immediately upon awakening (P<0·001 for all analyses by the Mann–Whitney test). However, the Kruskal–Wallis test revealed that P4 concentrations at all the time points examined were comparable in women in menopause (P=0·32; Fig. 3A). P4 concentration immediately upon awakening was compared between both groups (P>0·05 by the Mann–Whitney test), but the concentrations 30 and 60 min after awakening in women with regular cycles were significantly higher than those in the postmenopausal women (P<0·001 for all analyses by the Mann–Whitney test; Fig. 3A).
All women with regular menstrual cycles showed a positive P4-MnInc (range 6·24–1148·6 pmol/l), but 52% (n=39) postmenopausal women showed a positive P4-MnInc (range 3·1–242·1 pmol/l). The Mann–Whitney test revealed that P4-MnInc % and P4auc in women with regular cycles were significantly higher than those in the postmenopausal women (P<0·001; Fig. 3B and C).
Variation of cortisol, E2, and P4 secretory activities within the first hour after awakening throughout four phases of the menstrual cycle
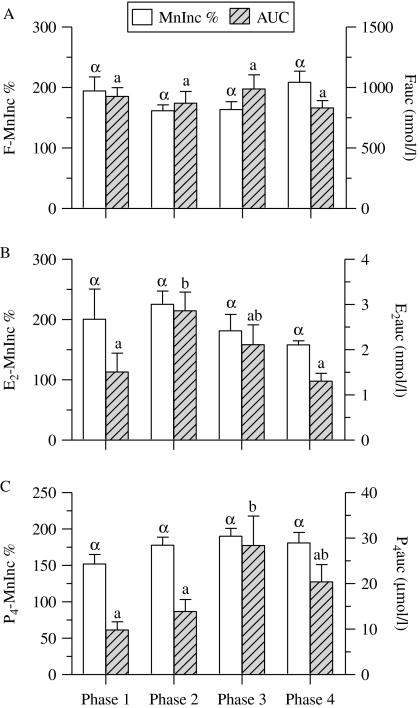
The variation of cortisol, E2, and P4 secretory activities within the first hour after awakening according to the phase of the menstrual cycle in women with regular cycles were analyzed using auxiliary indices for steroid secretory activity (MnInc % and AUC; Fig. 4).
Figure 4.
Changes in cortisol, estradiol-17β, and progesterone (P4) secretory activity throughout phases of the menstrual cycles in women with regular menstrual cycles. Cortisol (F), estradiol-17β (E2), and P4 secretory activity in each phase is presented in panels A–C respectively. The relative mean increases in steroid concentrations 30 and 60 min after awakening over the individual baseline (MnInc %) and the integrated steroid concentrations ranging from the time immediately upon awakening to 60 min after awakening (i.e. area under the curve (AUC) with respect to baseline) are depicted as mean±s.e.m. Bars with different Greek or Latin letters in each panel are significantly different from each other (P<0·05) by the Kruskal–Wallis test with Dunn's post-hoc test.
The F-MnInc % was similar at each phase of the menstrual cycle (range 161·6–208·5%), as was the Fauc at each phase (range 831·4–988·7 nmol/l; P>0·05 for all analyses by the Kruskal–Wallis test; Fig. 4A).
The E2-MnInc % in phase 2 of the menstrual cycle (peri-ovulatory phase) was slightly higher than in other phases, but this difference was not significant (P>0·05 by the Kruskal–Wallis test; Fig. 4B). Changes in the E2auc throughout the phases of the menstrual cycle were observed. Dunn's post-hoc tests revealed that the E2auc in phase 2 was significantly greater than that in phases 1 (menstrual phase) and 4 (late luteal phase; P<0·05), but significant differences in the E2auc were not found between other phases (Fig. 4B).
The P4-MnInc % was similar in each phase of the menstrual cycle (P>0·05 for all analyses by the Kruskal–Wallis test; Fig. 4C). Dunn's post-hoc test revealed that the P4auc in phase 3 (early to mid-luteal phase) was significantly greater than that in phases 1 and 2 (P<0·05 for all analyses), but significant differences in the P4auc were not found between other phases (Fig. 4C).
Discussion
This study examined ovarian sex steroid and adrenocortical cortisol secretory activities within the first hour after awakening in women with regular menstrual cycles and postmenopausal women. We found that both the E2 and P4 concentrations were simultaneously increased after awakening in women with regular menstrual cycles. However, E2 and P4 concentrations did not increase in any of the postmenopausal women. The E2auc and P4auc in women with regular menstrual cycles were greater than in postmenopausal women. The sex steroid secretory activity after awakening was different throughout the menstrual phases; the E2auc at phase 2 (periovulatory phase) and the P4auc at phase 3 (early to mid-luteal phase) of the menstrual cycle were significantly greater than in the menstruation phase. However, the effect of the menstrual status and menstrual phase on the cortisol secretory activity in the awakening period was negligible. The Fauc and F-MnInc % were comparable between both groups and throughout the phases of menstrual cycle.
The E2 or P4 concentration in saliva can be used as an index for ovarian function. Previous studies on salivary E2 and P4 concentrations have mainly focused on their fluctuations during the menstrual cycle using single or multiple time point samples (Choe et al. 1983, Bao et al. 2003, 2004, Celec et al. 2009). In this study, we determined the E2 and P4 concentrations in the post-awakening period to examine whether these sex steroid concentrations increased in response to awakening. This assessment was based on a method for determining the CAR (Pruessner et al. 1997), and adrenocortical and ovarian steroid secretory activities were examined together by the determination of cortisol, E2, and P4 concentrations from the same saliva sample. This study found a simultaneous increase in cortisol, E2, and P4 concentrations after women with regular menstrual cycles. This result implies that both the HPA axis and the HPO axis functions are activated together in response to awakening in women with regular menstrual cycles. Some studies have reported similar observations in rats and humans with respect to the simultaneous occurrence of a preovulatory LH surge and the acrophase of the circadian corticosterone or cortisol rhythm (Raps et al. 1971, Chiappa & Fink 1977, Kerdelhue et al. 2002). Because the hormonal secretory rhythms in the HPA and HPO axes are coordinated by a circadian system, the hypothalamic-SCN system (Kriegsfeld & Silver 2006), a simultaneous increase in cortisol, E2, and P4 concentrations after awakening is considered a result of an SCN-mediated co-activation of the HPA and HPO axes functions in women with regular menstrual cycles.
The increases in both E2 and P4 in women with regular menstrual cycles were absent in postmenopausal women. Because a dramatic loss of ovarian function starts during the perimenopausal period, one can postulate that the phenomenon in menopausal women could result from the lowered ovarian functionality. Indeed, postmenopausal women's ovaries are characterized by a high rate of ovarian follicle atresia, a depletion of non-growing follicles and a deficiency in early-growing follicles (Gosden 1987, Gougeon et al. 1994, Faddy 2000, Hansen et al. 2008). Therefore, it is reasonable to propose that a deficiency of functionally active ovarian follicles (i.e. gonadotropin-responsive follicles) in postmenopausal women was a primary cause of the absence of an increase in either E2 or P4 concentrations after awakening.
There were differences in the E2 and P4 secretory activities throughout the phases of the menstrual cycle. The E2- and P4-MnInc % exhibited a rhythmic pattern throughout the menstrual cycle, but phase-specific increases in these indices were not found. The E2auc and P4auc also exhibited a rhythmic pattern throughout the menstrual cycle. The E2auc at the periovulatory phase and the P4auc at the early to mid-luteal phase were higher than in the menstrual phase. Throughout the menstrual cycle, the E2 and P4 secretory activities after awakening were similar to the previously published E2 and P4 secretory profiles determined in daily-collected saliva samples (Gann et al. 2001, Gandara et al. 2007). This result implies that the E2 and P4 secretory activities after awakening are influenced by the phase of the menstrual cycle.
In contrast to ovarian steroids, the F-MnInc % and Fauc did not significantly change with the menstrual status or phase. This result coincides well with previous studies on the changes in the HPA axis function throughout menstrual phases, which have shown that the CAR is not reduced or heightened by the phase of the menstrual cycle (Kudielka & Kirschbaum 2003) and that the secretion of ACTH and cortisol in response to stress is also not influenced by the phase of the menstrual cycle (Kirschbaum et al. 1999). Thus, we considered that the effect of ovarian steroids on the CAR may be negligible.
The CAR is initiated only after morning awakening from nocturnal sleep, not after awakening in the night or after a nap in the early night (Federenko et al. 2004, Dettenborn et al. 2007). This time dependency suggests the involvement of the SCN in mounting the CAR. Although the regulatory mechanism of the CAR is not fully understood, the transmission pathway of the circadian signal from the SCN to the adrenal glands in animals provides a clue in understanding the CAR in humans. In rodents, it is well documented that the SCN communicates with neuroendocrine neurons via neurotransmitters, such as arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP; Buijs et al. 2003). The rhythmicity of AVP release from SCN terminals exerts a controlling effect on the rhythmic secretion of CRH and ACTH (Buijs et al. 2003, Perreau-Lenz et al. 2004, Kalsbeek et al. 2006). At the same time, the SCN transmits its circadian information to the adrenal gland via the intermediolateral column of the spinal cord and sympathetic outflow, which modulates adrenal sensitivity to ACTH (Buijs et al. 1999, Ishida et al. 2005, Ulrich-Lai et al. 2006). Because SCN-related neural pathways are similarly organized in rodents and humans (Buijs et al. 2003), it has been suggested that the SCN-mediated activation of the neuroendocrine pathway, which stimulates ACTH secretion, and the sympathetic nervous system, which modulates adrenal sensitivity for ACTH, are involved in the initiation of the CAR (Fries et al. 2009, Clow et al. 2010).
The SCN transmits information about the time of the day, which is involved in the regulation of estrus, via direct synaptic contacts with GnRH and estrogen receptor-α (ERα)-containing neurons (de la Iglesia et al. 1995, Watson et al. 1995). It is known that the majority of GnRH neurons and ER-containing neurons contacted by SCN efferents are located in the POA in rats and hamsters (de la Iglesia et al. 1995, Watson et al. 1995, van der Beek et al. 1997) and that the SCN coordinates the timing of the GnRH/LH secretion via a rhythmic release of VIP and AVP (van der Beek et al. 1997, Kalsbeek et al. 2002).
Although no direct neuronal connection between the SCN neurons and the ovaries has been reported, the connections between the SCN and the preautonomic neurons of the PVN, which are involved in the autonomic innervation of the ovary, indicate that the SCN also uses the autonomic nervous system to control the circadian activity of the HPO axis (Gerendai et al. 1998, 2002). On the basis of these similarities between SCN-related neural and neuroendocrine pathways in the HPA and HPO axes, it can be said that the increases in E2 and P4 levels after awakening are a phenomenon analogous to the CAR in the HPA axis.
The menstrual cycle is strictly regulated by a coordinated action between the ovaries and the hypothalamus–pituitary axis. Gonadotropins act on the ovaries to stimulate follicular development and steroid secretion, but the secretion of hormones from the hypothalamus–pituitary axis is modulated by ovarian steroids (Buffet et al. 1998). The ovarian hormones E2 and P4 are known to be potent feedback modulators of GnRH and gonadotropin secretion (Yamaji et al. 1972). Because E2 exerts feedback action on the pituitary to alter the number of GnRH receptors and ERs (Shupnik et al. 1989, Kaiser et al. 1993) and because E2 also directly influences neurotransmitters in the brain that controls the pattern of the pulsatile secretion of GnRH (Smith & Jennes 2001), it seems likely that increased E2 and P4 levels after awakening provide feedback to the hypothalamus–pituitary axis to ensure the coordination of hormone secretion by the HPO axis and the ovarian cycle.
It is known that the CAR is influenced by various factors, such as stress-related factors (job stress and life stress), sleep-related factors (sleep duration and sleep quality), and awakening factors (forced awakening, light intensity during awakening, and wake-up time; Fries et al. 2009). Most of these factors have been shown to influence the CAR, which may be blunted or enhanced, but the CAR does not occur after taking a nap in the early evening (awakening: 1845–2030 h) or after nightly awakenings (Federenko et al. 2004, Dettenborn et al. 2007). It is known that bright light has a greater impact on the circadian rhythm of cortisol and the body temperature than other periodic behavioral stimuli, such as the sleep–wake schedule and social contact (Czeisler et al. 1986, Duffy et al. 1996). Similarly, early morning bright light exposure (0500–0800 h) was shown to result in an immediate inhibition of melatonin secretion and a robust increase in cortisol secretion in subjects who were kept in a state of continuous wakefulness for 36 h in dim light conditions, but bright afternoon light exposure (1300–1600 h) did not have any effect on hormone secretion (Leproult et al. 2001). The results of these studies suggest that the sensitivity of the SCN to bright light is time gated, but information regarding the minimum sleep duration required and time range of the responsiveness of the SCN to bright light is limited. If we accept the similarity in the transmission of time-of-day information from the SCN to the HPA axis and to the HPO axis, it could be speculated that an increase in the E2 and P4 levels after awakening would also be influenced by various factors, such as sleep- and awakening-related factors.
Taken together, hormone secretion is regulated not only by feedback loops (i.e. concentration signals) but also by the SCN (i.e. temporal signals; Kriegsfeld & Silver 2006). The CAR is considered to be an SCN-mediated activation of the HPA axis function (Fries et al. 2009, Clow et al. 2010). We found that, as with the CAR in the HPA axis, waking up may be a potential stimulator of the HPO axis and may result in an increase in sex steroid levels within the first hour after awakening. However, unlike the situation for the HPA axis, the increases in the sex steroid levels after awakening were dependent on the woman's menstrual status, as such increases were not observed in any of the postmenopausal women. These results suggest that the measurement of sex steroid levels during the post-awakening period could provide information regarding the HPO axis response to awakening. The question whether altered (heightened or reduced) increases or the absence of increases in E2 and/or P4 after the awakening period is associated with menstrual disruption remains to be elucidated.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0013356). This work was also supported by the Grant of the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Biohousing Research Institute).
Author contribution statement
R S A developed the idea for the paper. J H K and B C C participated in the study design and execution. J H K participated in the collection of saliva samples and interview of subjects. J H C determined steroid concentrations in saliva. S H L undertook the statistical analyses. R S A, B C C, and S S S participated in the analysis and interpretation of data and wrote the manuscript. All authors have approved the final version of the manuscript.
References
- Ahn RS, Lee YJ, Choi JY, Kwon HB, Chun SI. Salivary cortisol and DHEA levels in the Korean population: age-related differences, diurnal rhythm, and correlations with serum levels. Yonsei Medical Journal. 2007;48:379–388. doi: 10.3349/ymj.2007.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/en.138.9.3842. [DOI] [PubMed] [Google Scholar]
- Bao AM, Liu RY, van Someren EJ, Hofman MA, Cao YX, Zhou JN. Diurnal rhythm of free estradiol during the menstrual cycle. European Journal of Endocrinology. 2003;148:227–232. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- Bao AM, Ji YF, Van Someren EJ, Hofman MA, Liu RY, Zhou JN. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Hormones and Behavior. 2004;45:93–102. doi: 10.1016/j.yhbeh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, van den Hurk R, Buijs RM. Synaptic contacts between gonadotropin-releasing hormone-containing fibers and neurons in the suprachiasmatic nucleus and perichiasmatic area: an anatomical substrate for feedback regulation? Brain Research. 1997;755:101–111. doi: 10.1016/S0006-8993(97)00086-3. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Vom Saal FS. The preovulatory surge of luteinizing hormone secretion in mice: variation in magnitude due to ambient light intensity. Biology of Reproduction. 1979;20:1005–1008. doi: 10.1095/biolreprod20.5.1005. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proceedings of the Royal Society of London, Series B: Biological Sciences. 1977;198:279–296. doi: 10.1098/rspb.1977.0098. [DOI] [PubMed] [Google Scholar]
- Buffet NC, Djakoure C, Maitre SC, Bouchard P. Regulation of the human menstrual cycle. Frontiers in Neuroendocrinology. 1998;19:151–186. doi: 10.1006/frne.1998.0167. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. European Journal of Neuroscience. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. Journal of Endocrinology. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertility and Sterility. 1998;70:56–59. doi: 10.1016/S0015-0282(98)00113-7. [DOI] [PubMed] [Google Scholar]
- Celec P, Ostanikova D, Skoknova M, Hodosy J, Putz Z, Kudela M. Salivary sex hormones during the menstrual cycle. Endocrine Journal. 2009;56:521–523. doi: 10.1507/endocrj.K09E-020. [DOI] [PubMed] [Google Scholar]
- Chiappa SA, Fink G. Hypothalamic luteinizing hormone releasing factor and corticotrophin releasing activity in relation to pituitary and plasma hormone levels in male and female rats. Journal of Endocrinology. 1977;72:195–210. doi: 10.1677/joe.0.0720195. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Choe JK, Khan-Dawood FS, Dawood MY. Progesterone and estradiol in the saliva and plasma during the menstrual cycle. American Journal of Obstetrics and Gynecology. 1983;147:557–562. doi: 10.1016/0002-9378(83)90016-9. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Annals of Internal Medicine. 1998;129:229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep–wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- Delfs TM, Klein S, Fottrell P, Naether OG, Leidenberger FA, Zimmermann RC. 24-Hour profiles of salivary progesterone. Fertility and Sterility. 1994;62:960–966. doi: 10.1016/s0015-0282(16)57058-7. [DOI] [PubMed] [Google Scholar]
- Dettenborn L, Rosenloecher F, Kirschbaum C. No effects of repeated forced wakings during three consecutive nights on morning cortisol awakening responses (CAR): a preliminary study. Psychoneuroendocrinology. 2007;32:915–921. doi: 10.1016/j.psyneuen.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. Journal of Physiology. 1996;495:289–297. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy MJ. Follicle dynamics during ovarian ageing. Molecular and Cellular Endocrinology. 2000;163:43–48. doi: 10.1016/S0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- Federenko I, Wust S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/S0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gandara BK, Leresche L, Mancl L. Patterns of salivary estradiol and progesterone across the menstrual cycle. Annals of the New York Academy of Sciences. 2007;1098:446–450. doi: 10.1196/annals.1384.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann PH, Giovanazzi S, Van Horn L, Branning A, Chatterton RT., Jr Saliva as a medium for investigating intra- and interindividual differences in sex hormone levels in premenopausal women. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:59–64. [PubMed] [Google Scholar]
- Gerendai I, Toth IE, Boldogkoi Z, Medveczky I, Halasz B. Neuronal labeling in the rat brain and spinal cord from the ovary using viral transneuronal tracing technique. Neuroendocrinology. 1998;68:244–256. doi: 10.1159/000054372. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Kocsis K, Halasz B. Supraspinal connections of the ovary: structural and functional aspects. Microscopy Research and Technique. 2002;59:474–483. doi: 10.1002/jemt.10225. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Follicular status at the menopause. Human Reproduction. 1987;2:617–621. doi: 10.1093/oxfordjournals.humrep.a136601. [DOI] [PubMed] [Google Scholar]
- Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biology of Reproduction. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- Gozansky WS, Lynn JS, Laudenslage ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic–pituitary–adrenal axis activity. Clinical Endocrinology. 2005;63:336–341. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- Gray GD, Soderstein P, Tallentire D, Davidson JM. Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology. 1978;25:174–191. doi: 10.1159/000122739. [DOI] [PubMed] [Google Scholar]
- Groschl M, Wagner R, Rauh M, Dorr HG. Stability of salivary steroids: the influences of storage, food and dental care. Steroids. 2001;66:737–741. doi: 10.1016/S0039-128X(01)00111-8. [DOI] [PubMed] [Google Scholar]
- Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2000;85:1794–1800. doi: 10.1210/jc.85.5.1794. [DOI] [PubMed] [Google Scholar]
- Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. Journal of Clinical Endocrinology and Metabolism. 2005;90:2050–2055. doi: 10.1210/jc.2004-2033. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Human Reproduction. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport. 1995;6:1715–1722. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metabolism. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133:931–934. doi: 10.1210/en.133.2.931. [DOI] [PubMed] [Google Scholar]
- Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Stress and the female reproductive system. Journal of Reproductive Immunology. 2004;62:61–68. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell and Tissue Research. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, Buijs RM. Central vasopressin systems and steroid hormones. Progress in Brain Research. 2002;139:57–73. doi: 10.1016/s0079-6123(02)39007-1. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Perreau-Lenz S, Buijs RM. A network of (autonomic) clock outputs. Chronobiology International. 2006;23:521–535. doi: 10.1080/07420520600651073. [DOI] [PubMed] [Google Scholar]
- Kapen S, Sternthal E, Braverman L. A pubertal 24-hour luteinizing hormone (LH) secretory pattern following weight loss in the absence of anorexia nervosa. Psychosomatic Medicine. 1981;43:177–182. doi: 10.1097/00006842-198104000-00009. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Arita J, Yoshioka E. Loss of estrogen-induced daily surges of prolactin and gonadotropins by suprachiasmatic nucleus lesions in ovariectomized rats. Endocrinology. 1980;106:1087–1092. doi: 10.1210/endo-106-4-1087. [DOI] [PubMed] [Google Scholar]
- Kerdelhue B, Brown S, Lenoir V, Queenan JT, Jr, Jones GS, Scholler R, Jones HW., Jr Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology. 2002;75:158–163. doi: 10.1159/000048233. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee YJ, Ahn RS. Day-to-day differences in cortisol levels and molar cortisol-to-DHEA ratios among working individuals. Yonsei Medical Journal. 2010;51:212–218. doi: 10.3349/ymj.2010.51.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Hormones and Behavior. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SY, Kang JW, Kim H, Ku PS, Lee SH, Suh CS, Kim SH, Choi YM, Kim JG, Moon SY. Regional differences in age at menopause between Korean–Korean and Korean–Chinese. Menopause. 2004;11:569–574. doi: 10.1097/01.gme.0000142913.70089.a1. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/S0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1097/01.PSY.0000058374.50240.BF. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/S0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, L'Hermite-Balériaux M, Van Cauter E. Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. Journal of Clinical Endocrinology and Metabolism. 2001;86:151–157. doi: 10.1210/jc.86.1.151. [DOI] [PubMed] [Google Scholar]
- McElhinny TL, Sisk CL, Holekamp KE, Smale L. A morning surge in plasma luteinizing hormone coincides with elevated Fos expression in gonadotropin-releasing hormone-immunoreactive neurons in the diurnal rodent, Arvicanthis niloticus. Biology of Reproduction. 1999;61:1115–1122. doi: 10.1095/biolreprod61.4.1115. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Brown-Grant K, Corker CS. Plasma and pituitary luteinizing hormone and peripheral plasma oestradiol concentrations in the normal oestrous cycle of the rat and after experimental manipulation of the cycle. Journal of Endocrinology. 1972;53:17–30. doi: 10.1677/joe.0.0530017. [DOI] [PubMed] [Google Scholar]
- Park YJ, Kim HS, Kang HC. The age at menopause and related factors in Korean women. Journal of Korean Academy of Nursing. 2002;32:1024–1031. [Google Scholar]
- Perreau-Lenz S, Pevet P, Buijs RM, Kalsbeek A. The biological clock: the bodyguard of temporal homeostasis. Chronobiology International. 2004;21:1–25. doi: 10.1081/CBI-120027984. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Padmanabhan V, Lemon W, Randolph J, Rees Midgley A. Follicle-stimulating hormone is secreted more irregularly than luteinizing hormone in both humans and sheep. Journal of Clinical Investigation. 1998;101:1318–1324. doi: 10.1172/JCI985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J, Wolf O, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/S0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Raps D, Barthe PL, Desaulles PA. Plasma and adrenal corticosterone levels during the different phases of the sexual cycle in normal female rats. Experientia. 1971;27:339–440. doi: 10.1007/BF02138184. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Goldman BD. Effects of photoperiod on cyclicity and serum gonadotropins in the Syrian hamster. Biology of Reproduction. 1975;12:223–231. doi: 10.1095/biolreprod12.2.223. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shupnik MA, Gordon MS, Chin WW. Tissue-specific regulation of rat estrogen receptor mRNAs. Molecular Endocrinology. 1989;3:660–665. doi: 10.1210/mend-3-4-660. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction. 2001;122:1–10. doi: 10.1530/rep.0.1220001. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;290:R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Langub MC, Jr, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Research. 1995;689:254–264. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response – normal values and confounds. Noise & Health. 2000;7:79–88. [PubMed] [Google Scholar]
- Yamaji T, Dierschke DJ, Bhattacharya AN, Knobil E. The negative feedback control by estradiol and progesterone of LH secretion in the ovariectomized rhesus monkey. Endocrinology. 1972;90:771–777. doi: 10.1210/endo-90-3-771. [DOI] [PubMed] [Google Scholar]