Abstract
The prefrontal cortex is considered essential for learning to perform cognitive tasks though little is known about how the representation of stimulus properties is altered by learning. To address this issue, we recorded neuronal activity in monkeys before and after training on a task that required visual working memory. After the subjects learned to perform the task, we observed activation of more prefrontal neurons and increased activity during working memory maintenance. The working memory–related increase in firing rate was due mostly to regular-spiking putative pyramidal neurons. Unexpectedly, the selectivity of neurons for stimulus properties and the ability of neurons to discriminate between stimuli decreased as the information about stimulus properties was apparently present in neural firing prior to training and neuronal selectivity degraded after training in the task. The effect was robust and could not be accounted for by differences in sampling sites, selection of neurons, level of performance, or merely the elapse of time. The results indicate that, in contrast to the effects of perceptual learning, mastery of a cognitive task degrades the apparent stimulus selectivity as neurons represent more abstract information related to the task. This effect is countered by the recruitment of more neurons after training.
Keywords: learning, monkey, neurophysiology, principal sulcus
Introduction
The prefrontal cortex (PFC) is known to play an important role in higher cognitive functions (Miller and Cohen 2001). Prefrontal lesions in humans cause profound deficits in the ability to represent information in memory and to plan future actions; prefrontal dysfunction has also been implicated in a number of mental illnesses, most notably schizophrenia (Goldman-Rakic 1994). Accordingly, neural correlates of working memory (Fuster and Alexander 1971; Funahashi et al. 1989) and a wide range of other cognitive functions such as representation of abstract rules, decisions, categories, numerical quantities, conflicting choices, and sequences of actions have been observed in neurophysiological studies of animals trained to perform behavioral tasks (Kim and Shadlen 1999; Freedman et al. 2001; Wallis et al. 2001; Averbeck et al. 2002; Nieder et al. 2002; Barraclough et al. 2004; Mansouri et al. 2007; Sigala et al. 2008). It has also been recognized that prefrontal responses to the same operant stimuli may differ as a function of the task the animal has been trained to perform (White and Wise 1999; Asaad et al. 2000; Wallis et al. 2001) and prefrontal lesions and inactivation disrupt cognitive tasks that depend on working memory (Chafee and Goldman-Rakic 2000; Hoshi et al. 2000; Buckley et al. 2009). However, the impact of learning to perform a task itself has not been examined directly. Little is known about what types of neuronal changes are associated with learning to perform a working memory task per se and how training in such a task alters neuronal responses.
The impact of training on brain activation has received much attention in human studies recently, since training in working memory tasks has proved effective as a remediating intervention in cases of brain injury and mental disorders (Wexler et al. 2000; Klingberg et al. 2002; Westerberg et al. 2007). Human functional magnetic resonance imaging (fMRI) studies generally reveal increased blood oxygen level–dependent (BOLD) activation in the prefrontal cortex after training to perform working memory (Hempel et al. 2004; Olesen et al. 2004; Moore et al. 2006; Dahlin et al. 2008; McNab et al. 2009) and other cognitive tasks (Fletcher et al. 1999; Nyberg et al. 2003). However, several studies have also revealed decreased BOLD activation after training in tasks that require working memory (Garavan et al. 2000; Jansma et al. 2001; Milham et al. 2003; Landau et al. 2004; Sayala et al. 2006), possibly as a consequence of improved strategies in the task, increasing efficiency (Klingberg 2010). Even an unequivocal change in brain activation after learning to perform a working memory task cannot resolve the nature of underlying neuronal changes. In principle, increased activation could be the result of a larger cortical population being recruited by the task or increased firing of the same population of cortical neurons. Individual neuronal responses may also become more selective for the properties of the operant stimuli; this is the case in perceptual learning, which is associated with the emergence of neurons highly selective for the properties of stimuli that subjects are trained to recognize and discriminate, both in the prefrontal cortex and in the other cortical areas (Kobatake et al. 1998; Rainer and Miller 2000; Yang and Maunsell 2004; Gilbert et al. 2009).
To understand the effects of learning, we recorded single-neuron discharges elicited by visual stimuli in the prefrontal cortex before and after monkeys were trained to perform tasks that required visual working memory. Rather than testing how responses to new stimuli change as subjects improve in recognizing and distinguishing them from each other, we presented the same highly discriminable stimuli before and after training and examined the changes in single-unit and population responses as the stimuli became incorporated into a cognitive task.
Materials and Methods
Three male, rhesus monkeys (Macaca mulatta) with no prior experimentation experience and weighing 5–12 kg were used in these experiments. All animal experiments were performed in compliance with the guidelines set forth by the National Institutes of Health, as reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee.
Experimental Setup
The monkeys sat in a primate chair with their head restrained under dim illumination and viewed a computer monitor positioned 68 cm away. The monkeys were trained to hold their gaze on a white 0.2° fixation target. While maintaining fixation, visual stimuli were presented on the screen. Eye position was monitored using an infrared eye position tracking system (ISCAN). Eye position was sampled at 240 Hz, digitized, and recorded. Breaks in fixation exceeding a 2° window terminated the trial. Correct completion of a trial resulted in delivery of a liquid reward (fruit juice). Software developed in-house (Meyer and Constantinidis 2005) controlled the visual stimulus presentation, online monitoring of eye position, and synchronization of stimuli with neurophysiological data. The system was implemented in MATLAB (Mathworks), using the Psychophysics Toolbox (Brainard 1997; Pelli 1997).
Stimulus Presentation in Pretraining Phase
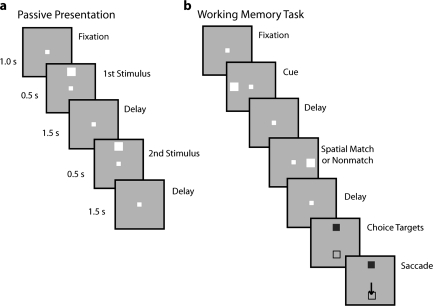
Methods for the pretraining phase have been described previously (Meyer et al. 2007). Briefly, monkeys were required to fixate, while stimuli were displayed on a screen. The monkeys received a liquid reward (fruit juice) for maintaining fixation throughout the trial. The stimulus set involved a white 2° square that appeared randomly in 1 of 9 locations arranged on a 3 x 3 grid of 10° spacing between adjacent stimuli. The stimulus appeared for 500 ms and was followed by a “delay period” that typically lasted for 1.5 s (Fig. 1a). After the delay period, a second white square appeared either at the same location or a different (typically diametric) location for 500 ms. This was followed by a second 1.5-s delay period. The stimuli were presented for thousands of trials over a period of a few weeks prior to beginning of the experiments to ensure that monkeys were familiar with them before any of the recordings were obtained.
Figure 1.
Behavioral task. Successive frames represent sequence of stimulus presentation on the screen. (a) Passive presentation. Stimuli were presented passively, while the monkey was simply required to fixate. (b) Working memory task. The monkey was required to remember the spatial location of the first stimulus and saccade to a green choice target (shown as filled square) if the second stimulus appeared at a matching location. If the 2 stimuli appeared at different locations, the monkey was required to the blue target, instead (shown as an open square). The relative position of the 2 choice targets varied randomly from trial to trial.
Working Memory Task
We trained the same monkeys to perform a behavioral task that required working memory for spatial locations. The task (Fig. 1b) required animals to remember the spatial location of a stimulus flashed briefly on the screen, to observe a second stimulus, and to indicate whether the 2 stimuli appeared at the same or different locations by making a saccade toward a green or blue target, respectively, which appeared after a second delay period. The stimuli and timing of presentations were identical to those in the pretraining task, except for the choice targets. The targets appeared at locations orthogonal to the stimuli and the location of the blue and green target varied randomly from trial to trial. One monkey was trained in a different type of spatial task, which was a variant of the delayed response task (Funahashi et al. 1989). The overall structure of the trial was identical to the first 5 frames of Figure 1b with the only difference that the second stimulus was always a match and appeared at the same location as the cue. After the end of the second delay period, no choice targets appeared and the animal was trained to saccade toward the location of the remembered visual stimulus. The animals were additionally trained in a working memory task that required them to remember the features of stimuli and the combination of locations and features (data not shown).
Surgery and Neurophysiology
A 20-mm diameter craniotomy was performed over the prefrontal cortex and a recording cylinder was implanted. The location of cylinders was assessed with anatomical MRI imaging. Neural recordings were carried out in areas 8, 9, 12, 45, and 46 of the lateral prefrontal cortex with either single or multiple microelectrodes. We used glass-coated Tungsten electrodes of 250 μm diameter, with an impedance of 1 MΩ at 1 kHz (Alpha-Omega Engineering) and epoxylite-coated Tungsten electrodes with a diameter of 125 μm and an impedance of 4 MΩ at 1 KHz (FHC). Arrays of up to 8-microelectrodes spaced 0.2–1.5 mm apart were advanced into the cortex through the dura with a microdrive system (EPS drive, Alpha-Omega Engineering). The electrical signal from each electrode was amplified, band-pass filtered between 500 and 8 kHz, and recorded with a modular data acquisition system (APM system, FHC). Waveforms that exceeded a user-defined threshold were sampled at 25-μs resolution, digitized and stored. During experiments, electrodes were advanced into the cortex, while the monkey sat quietly without any stimuli being displayed. Typically data from 20 stimulus presentations were collected for each cue location. After data collection was complete, a second set of neurons was sometimes sampled by advancing electrodes further, in the same fashion as at the beginning of the experiment. We recorded from all neurons that we isolated without any attempt to select neurons based on their response properties.
Anatomical Localization
Recordings were performed from both the dorsal and the ventral prefrontal cortex. Our recordings sampled the caudal half of the principal sulcus and areas dorsal and ventral to it. We defined the dorsal prefrontal cortex as the region comprising the 2 banks of the principal sulcus (≤2 mm from the center of the principal sulcus), the extension of this zone posterior to the principal sulcus as far as the arcuate sulcus, and the superior convexity of the prefrontal cortex dorsal to the principal sulcus (>2 mm from the center of the principal sulcus). This region incorporates parts of areas 46, 9 and 8a (Preuss and Goldman-Rakic 1991). Ventral prefrontal cortex was defined as the cortical area in the inferior convexity of the prefrontal cortex lateral to the principal sulcus (>2 mm from the center of the principal sulcus). This region incorporates parts of areas 12 and 45.
Data Analysis
All data analysis was performed using the MATLAB computational environment (Mathworks). Action potential waveforms recorded from microelectrodes were sorted into separate units using an automated cluster analysis method based on the KlustaKwik algorithm (Harris et al. 2000). The method relied on principal component analysis of the waveforms, implemented in MATLAB. We also performed analysis on multiunit records, which were created by pooling all sorted spikes.
To ensure that the changes in neuronal firing and discriminability that we detected were not the result of systematic differences in the inherent properties of neurons sampled, we compared the signal-to-noise ratio (SNR) of neuronal recordings before and after training (Joshua et al. 2007). For each neuron, we defined SNR as the ratio between the peak-to-trough magnitude of the mean action potential, divided by the standard deviation of the noise. The latter was computed from the baseline of each waveform, derived from the first 10 data points (corresponding to 0.25 ms) of each sample. SNR does not have a simple relationship with action potential size as it depends on a number of factors (Nelson et al. 2008), most importantly the effective impedance of the electrode in the brain medium (which is affected by the thickness of the exposed dura) and the distance of the tip of the electrode from the recorded neuron (which is adjusted interactively during experiments). Nevertheless, SNR provides an overall measure of recording quality.
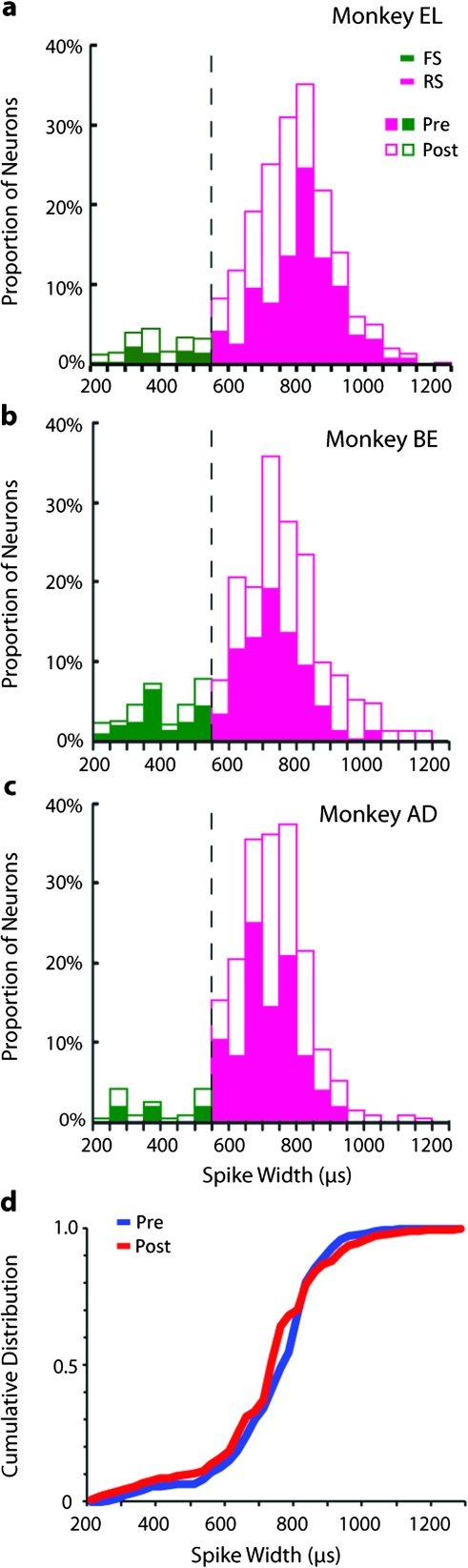
A neuron's spike width was determined by calculating the distance between the 2 troughs of the average waveform. We distinguished between fast-spiking (FS—putative interneurons) and regular-spiking (RS—putative pyramidal) neurons based on previous analysis (Constantinidis and Goldman-Rakic 2002) which determined the criterion width to lie between 540 and 570 μs (relying on a 30-μs sampling period, instead of the 25 μs used here). We used the center of this interval as our criterion so that units were classified as FS if they exhibited as spike width of ≤550 μs and RS if they exhibited a spike width of ≥575 μs (Fig. 2). Errors in classification may only dilute the differences between the 2 populations reported here.
Figure 2.
Distribution of spike waveforms. (a–c) Histogram of neuronal spike widths before (closed bars) and after training (open bars) for the 3 monkeys included in the study. FS and RS units were identified based on spike width. (d) Cumulative distribution of spike widths before and after training for all units.
Firing rate of units was then determined for each of the task epochs. We identified neurons that responded to the visual stimuli, evidenced by significantly elevated firing rate in the 500-ms interval of a stimulus presentation, compared with the 1-s interval of fixation (paired t-test, P < 0.05). Neurons with elevated activity in other task epochs were similarly identified. We also identified neurons with significant decrease of firing rate in a task epoch, in the absence of elevated firing rate in response to another stimulus presentation during the same task epoch. Only trials from correct behavioral responses to the task are presented in the paper.
To ensure that neuronal responses remained stable during the data set analyzed, we identified recordings in which a significant effect of trial presentation sequence was evident in the baseline firing rate (analysis of variance [ANOVA],P<0.05), for example, due to a neuron disappearing or appearing during a run, as we were collecting data from multiple electrodes. Data from these sessions were truncated so that analysis was performed on a range of trials with stable firing rate. Approximately 10% of neuronal recordings were processed in this fashion.
The spatial selectivity of visually responsive neurons was assessed by comparing the discharge rates during the presentation of the first stimulus at the 9 grid locations. Neurons with significantly different responses to the 9 stimulus locations (ANOVA,P<0.05) were considered spatially selective. Average firing rates recorded for each of the stimulus set were rotated around the center so that the best location was plotted in location 5 (Fig. 5).
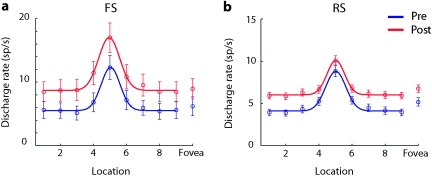
Figure 5.
Stimulus selectivity. (a) Average discharge rate of FS neurons with significantly elevated responses are plotted before (N = 25) and after training (N = 44). The arrangement of spatial locations has been rotated so that the best response is at location 5 for every neuron; responses at the foveal location are plotted separately. Line represents Gaussian fit. Points 1 and 9 represent the same location. Error bars represent standard errors. (b) Average discharge rate of RS neurons prior to (N = 290) and after training (N = 381).
Receiver operating characteristic (ROC) analysis was performed by comparing the distributions of firing rates of a neuron with 2 stimulus conditions as described previously (Constantinidis et al. 2001). The area under the ROC curve represents the probability that an ideal observer can discriminate between the 2 stimuli based on their firing rate in each trial (Tolhurst et al. 1983). For each neuron, we compared responses at the location that elicited the best responses and at its diametric location. The analysis was performed in a time-resolved fashion, comparing responses in a 100-ms long moving window, computed in 50-ms steps.
Population firing rate maps (Fig. 6) were created by averaging responses from all neurons active in the task at each of the 9 stimulus locations tested. Responses at intermediate points were estimated using spline interpolation. Results are plotted so that positive values in the horizontal axis represent the contralateral visual field (responses from neurons recorded from the right hemisphere have been reflected across the vertical meridian).
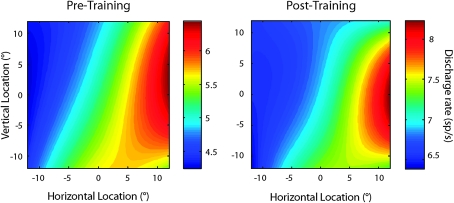
Figure 6.
Population firing rate maps. The color map indicates population discharge rate at each spatial location by averaging responses from all neurons with significantly elevated responses recorded before (N = 315) and after (N = 425) training. The contralateral location is depicted as positive horizontal location; responses from the right hemisphere have been reflected.
Results
Data were collected from 3 monkeys that had no prior training on any laboratory task. During the initial phase of data collection, the subjects were required only to maintain fixation, while stimuli were presented on a screen. A trial consisted of the sequential presentation of 2 visual stimuli separated by a “delay” interval during which only the fixation stimulus remained on the screen. A total of 1324 neurons were recorded during this phase using an unbiased sampling strategy in which any neuron encountered by our microelectrodes was studied. A second phase of data collection commenced after monkeys were trained to perform a visual working memory task that required subjects to remember the locations of the stimuli. Critically, the stimuli and timing of presentation were identical to those for the initial passive presentation phase (Fig. 1). Neurophysiological recordings were repeated in the same cortical areas, while the monkey executed the working memory task. A total of 1351 neurons were recorded from these animals. To ensure that we sampled the same types of neurons before and after training, we compared the distributions of spike widths recorded (Fig. 2). The 2 samples exhibited no significant difference in terms of their means (t-test, P > 0.1) or in the shape of their distributions (Kolmogorov–Smirnov test, P > 0.5).
Discharge Rate
In the context of a working memory task, a typical prefrontal neuron responds transiently to the appearance of a visual stimulus and sustains its discharge after the stimulus is extinguished (Fuster and Alexander 1971; Funahashi et al. 1989). We identified neurons activated during the stimulus presentation and delay periods, exhibiting significant elevation of firing rate compared with the baseline fixation period that preceded stimulus presentation (paired t-test, P < 0.05). A significant increase in the percentage of neurons activated during the stimulus (χ2-test, P < 10−10) and the delay period (χ2-test, P < 10−20) was observed in the prefrontal cortex after training (Fig. 3). Significantly elevated activity in the delay period compared with the baseline fixation period after presentation of a stimulus is often equated with active memory maintenance, however, we should note that individual neurons with significantly elevated firing rate during the delay period have been previously observed even in naïve animals (Meyer et al. 2007).
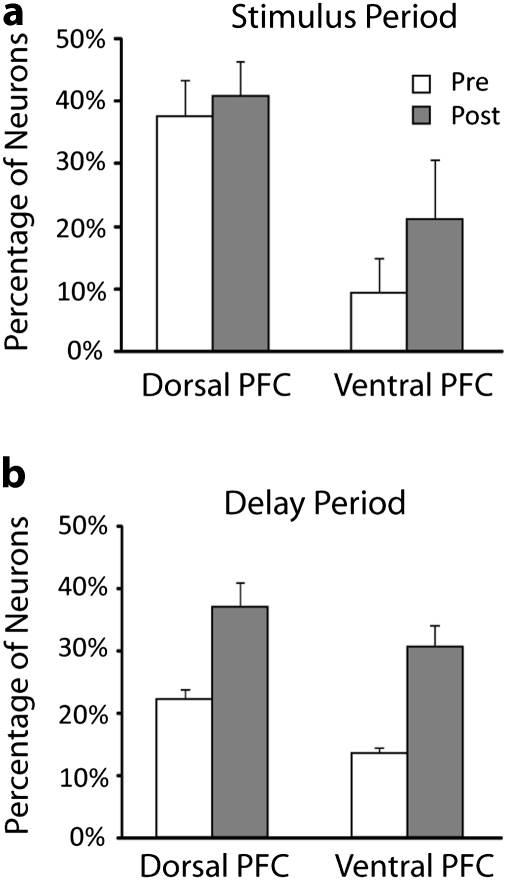
Figure 3.
Percentage of activated neurons before and after training. (a) Percentage of neurons with significantly elevated firing rate during the presentation of visual stimuli before and after training. Average percentages are shown for results pooled across all monkeys. Error bars denote standard error of percentages observed between monkeys. Responses from dorsal and ventral prefrontal regions are shown separately. (b) Percentage of neurons active during the delay period after the stimulus presentation. N = 735 and 851 for dorsal PFC, pretraining, and posttraining, respectively. N = 589 and 500 for ventral PFC, pretraining, and posttraining, respectively.
To ensure that sampling differences could not account for the increase in the proportion of prefrontal neurons that were active during the stimulus or delay period, we performed a bootstrap analysis. For each electrode penetration conducted prior to training, we calculated the proportion of neurons active during the stimulus or delay period that were encountered in that penetration. We then determined the number of neurons recorded in each penetration after training and assigned to each penetration the expected number of responsive neurons drawn randomly from the pretraining distribution (with replacement). We repeated this randomization test 1 000 000 times, separately for the dorsal and ventral prefrontal cortex and estimated the distribution of the expected number of neurons active after training, if their properties had not changed due to training. We thus determined the probability that an artifact of sampling could produce an increase in the proportion of neurons with elevated responses that we obtained after training. The difference between expected and observed results was significant at the α = 0.01 level for the dorsal PFC neurons with responses during the stimulus presentation period and at the α = 10−6 level for all other comparisons, confirming that activation after training could not be accounted by sampling effects.
Neurons in the dorsal and ventral subdivision of the prefrontal cortex differed in their percentage of neurons activated by the task (Fig. 3). However, the subset of neurons activated during the stimulus presentation or delay period in both prefrontal subdivisions exhibited similar effects of training in terms of average firing rate and selectivity for stimuli. Data therefore were pooled from the entire prefrontal cortex for subsequent analyses. Our data set included 315 activated neurons recorded before and 425 activated neurons recorded after training. Among those, we identified 69 FS (putative interneurons) and 671 RS (putative pyramidal) neurons (see Materials and Methods). A similar increase in the percentage of FS and RS units activated by the task was observed before and after training (data not shown).
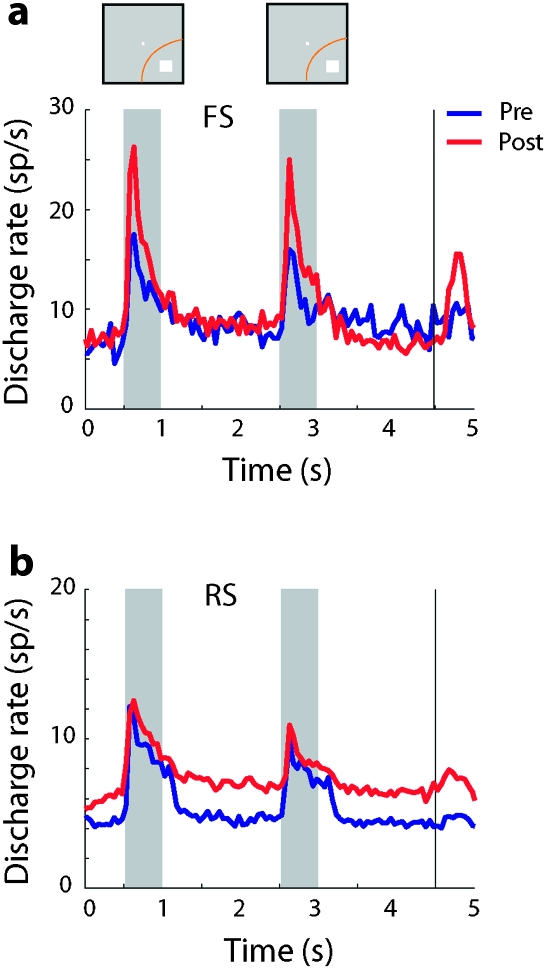
Neurons active during the trial exhibited an overall higher firing rate after training (Fig. 4). Combined with the effect of an increased proportion of neurons being activated in a trial (Fig. 3), this result indicated a higher overall activation of the prefrontal cortex after training. This finding is in agreement with human fMRI studies reporting increased activation after training (Olesen et al. 2004; McNab et al. 2009). Increase of firing rate differed between task periods for FS and RS units. A significant increase in firing rate (t-test, P < 0.05) was present during the fixation period, prior to the cue appearance for RS but not FS units. Such “anticipatory” activity prior to appearance of a stimulus has been observed before in prefrontal neurons of monkeys trained to perform behavioral tasks (Qi et al. 2010). Firing rate increase was modest during the stimulus presentation period and did not reach statistical significance for either the FS or the RS units, although peak firing rate was notably higher for FS units (Fig. 4a). The greatest difference of firing rate before and after training was observed during the delay period and for RS units only (t-test, P < 0.005). The difference in delay period activity was significant even if the baseline, fixation period firing rate was subtracted from the corresponding pretraining and posttraining delay period responses (t-test, P < 0.01).
Figure 4.
Average population response. (a) Population PST histograms averaging discharges from FS neurons with significant elevated responses. Blue lines, responses prior to training (N = 25); Red lines, responses after training (N = 44). Insets above Peri-Stimulus Time Histogram indicate appearance of the first and second stimulus in the best location in the receptive field; the actual locations of the stimuli differed for each neuron. (b) Population PST histograms for RS neurons prior to (N = 290) and after training (N = 381).
Stimulus Selectivity and Discriminability
The overall increase in firing rate we observed after training was not accompanied by an increase in the selectivity for the visual stimuli, an effect commonly associated with perceptual learning. Rather, increased firing rate was observed for both preferred and nonpreferred stimuli (Fig. 5). The effect was prominent for RS units, resulting in a diminished difference between the peak and baseline rate. We obtained very similar results when we considered selectivity for stimulus location in the stimulus presentation period and in the delay period (data not shown). In essence, this effect reduced the difference between the strongest and weakest stimulus-evoked responses, lessening the apparent stimulus selectivity of a neuron. This was so, even though the overall distribution of receptive fields after training did not change appreciably; population firing rate averaged across each spatial location exhibited a similar profile and a contralateral bias (Fig. 6).
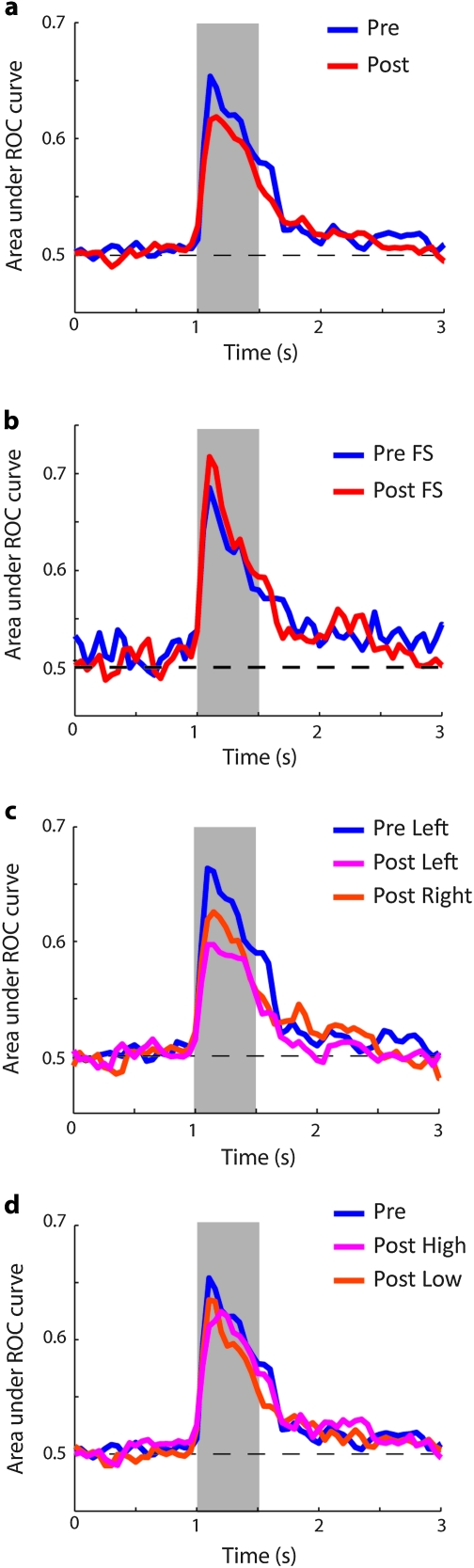
To confirm that single-unit responses failed to show increased selectivity after training, we used a ROC analysis to estimate the neuronal discriminability for the locations of the stimuli. In agreement with results shown in Figure 5, neural discrimination of stimulus locations was no greater after training, either during the stimulus presentation or delay period (Fig. 7a). In fact, the area under the ROC curve, averaged across the entire stimulus period, was significantly lower during the stimulus presentation interval (t-test, P < 0.05). This effect was accounted for by RS units alone; FS units did not exhibit a significant difference before and after training (Fig. 7b). The finding indicates that the information required for performance of the task, including encoding of stimulus attributes in the delay period, was present in neuronal firing prior to training and only declined after training.
Figure 7.
Discriminability of stimuli. (a) The average value of the area under the ROC curve is plotted comparing responses between the best spatial location and - its opposite. Data are plotted in a time-resolved fashion, using a 100-ms moving window centered on the time point plotted in the graph. Results are averaged from all neurons with significant elevated responses before training (N = 315) and after training (N = 425). Gray bar represents time of stimulus presentation. (b). ROC analysis for FS units only (N = 25 pretraining and 44 posttraining). (c) ROC analysis for data obtained prior to training from the left hemisphere of one monkey (N = 211) and after training from the left hemisphere (N = 93) and the right hemisphere (N = 170). (d) ROC analysis for data obtained before training (N = 315) and after training, from sessions divided into 2 groups, based on high (N = 212) and low (N = 213) performance.
To ensure that the differences in single-unit responses that we observed were not the result of systematic differences in the size (and by consequence, the types) of neurons isolated in the 2 phases of recording, we repeated our analysis in samples of neurons matched in terms of the SNR of their action potentials. Overall, we observed a slightly but significantly lower mean SNR ratio after training (8.0 pretraining vs. 7.2 posttraining, for neurons with significant elevated responses above baseline). The difference was significant for all 3 hemispheres with repeated recordings (t-test, P = 0.047, P = 0.027, and P < 0.0001, respectively). We therefore repeated our analysis for neurons with large SNR values, greater than 6. The mean SNR values for these pretraining and posttraining samples of neurons were not significantly different from each other(t-test, P > 0.8; means of 8.46 and 8.42, respectively). Population Peri-Stimulus Time Histograms and ROC analysis based on these matched groups of neurons appeared essentially identical to those based on the entire samples (Fig. 8). Very similar results were also obtained when we repeated our analysis based on multiunit records, which included all spikes recorded by our electrodes at each cortical site (data not shown). Finally, to ensure that the decrease in stimulus discriminability of single-unit responses was not the result of potential damage accumulated in the cortex over time, we performed ROC analysis in 2 hemispheres of one monkey; one also sampled in the pretrained phase and one sampled only after training. In both cases, discriminability was lower after training (Fig. 7c). The difference in ROC values, computed over the entire cue period, was not significant between the 2 hemispheres (t-test, P > 0.1), although the difference between the newly sampled hemisphere and the pretraining recordings was (t-test, P < 0.005). These analyses confirm that the effects we observed were the result of learning to perform the task rather than biases or systematic differences in the samples of neurons sampled.
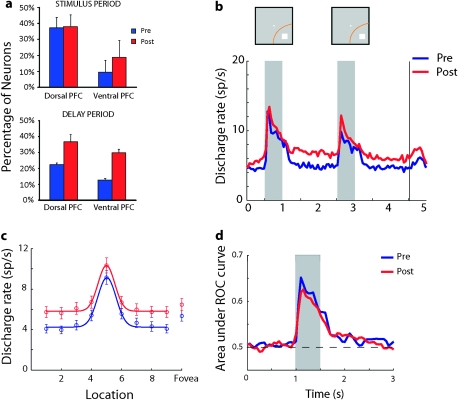
Figure 8.
Analysis of neurons matched for SNR. (a) Percentage of records with neurons matched for SNR with significantly elevated firing rate during the presentation of visual stimuli and delay period. N = 656 and 523 for dorsal PFC, pretraining, and posttraining, respectively. N = 557 and 346 for ventral PFC, pretraining, and posttraining, respectively. (b–d) Average discharge rate, selectivity, and discriminability based on responses from neurons matched for SNR with significantly elevated responses before (N = 243) and after training (N = 258). Conventions are the same as in Figures 3–5 and 7.
Behavioral Factors and Stability of Changes
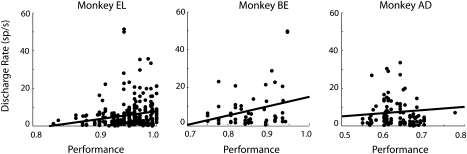
Factors related to reward rate and task difficulty have been shown to influence neuronal activity in the prefrontal cortex (Kim and Shadlen 1999), and we therefore wished to tease apart their effects from those of learning to perform the task per se. Performance in the task increased slightly during the recording phase, allowing us to determine the effects of behavioral performance in the task. We first tested whether the increased firing rate we observed after training could be the result of the increased difficulty of the task and/or the lower reward rate associated with it. If that were the case, higher firing rates would be expected in sessions with lower performance. In fact, we observed an effect in the opposite direction, of increased firing rate as a function of improved performance when the task became more familiar and presumably easier for the monkey, and the expected reward rate increased (Fig. 10). The effect was significant for 2 of the 3 monkeys (regression analysis, P < 0.005, P < 0.05, and P > 0.4, respectively). In any case, the effect of reduced discriminability was unaffected by performance levels. For each monkey, we performed the ROC analysis separately for the low performance (average 85.0%) and high performance (average 92.2%) halves of the sessions. Average ROC values showed little difference between sessions of high and low performance computed in this fashion (Fig. 7d).
Figure 10.
Effect of behavioral performance on neuronal firing rate. Each data point represents responses of a single neuron during the delay period averaged from all stimulus presentations and conditions. Data are plotted against the behavioral performance observed during the recording of the data set. Results are shown from the 3 monkeys tested in the experiment. Solid line represents linear regression.
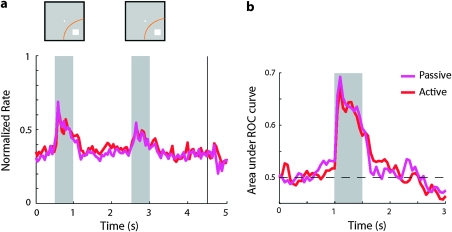
To further address whether the changes in stimulus selectivity observed after training were specifically associated with the execution of the task, we conducted additional recordings from 90 neurons of one monkey (subject EL) tested with the both spatial working memory (Fig. 1b) and passive versions of the task (Fig. 1a). Recordings were performed with the working memory task, first. We selected neurons for further testing with the passive task if responses to visual stimuli were evident during online data collection; therefore, these results are not directly comparable with the pretraining recordings, which were sampled randomly. Then trials were presented in a passive fashion, with no appearance of the choice targets. For the passive trials, the monkey was rewarded simply for maintaining fixation, as before training. In one block of trials, the stimulus always appeared at the same location (data not shown). In a subsequent block, and after the animals had already been conditioned to this passive presentation, stimuli were presented at the 9 stimulus locations, exactly as in the spatial set prior to training (Fig. 9). The results showed no significant difference in discriminability between the passive presentation and spatial working memory conditions (Fig. 9b) and no significant difference in firing rate during the fixation, stimulus presentation, or delay period (paired t-test, P > 0.5 in all cases).
Figure 9.
Passive presentation of stimuli after training. (a) Population Peri-Stimulus Time Histogram for responses collected during execution of the working memory task and during passive presentation of the same stimuli to the same neurons (N = 90). Responses are normalized to the maximum firing rate of each neuron (b) ROC analysis based on the working memory task and passively presented stimuli, after training.
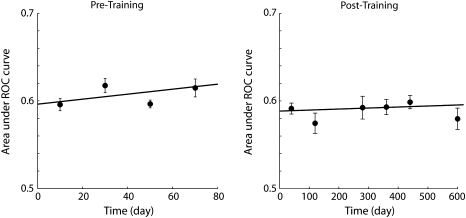
Finally, since a long time period intervened between the recordings before and after training (9, 21, and 7 months for the 3 monkeys, respectively), we also wished to determine whether the decrease in discriminability we observed was stable over time and could not have been caused by random drift of cortical properties over such a long period. We therefore computed the area under the ROC curve as a function of time (Fig. 11). Our recordings, particularly after training, spanned a long time period, allowing us to determine the stability of this measure. We found no evidence of declining discriminability in the time period prior to training that could foretell reduced discriminability after training (regression analysis, P > 0.2). After training, discriminability also remained stable as a function of time (regression analysis, P > 0.5).
Figure 11.
Discriminability as a function of time. Area under the ROC curve is plotted as a function of time (days). Each data point represents average across all neurons recorded in a bin of 20 days (before training) or 80 days long (after training). Error bars represent standard error of the mean. Solid line represents the regression line; the slope of neither curve was significantly different than zero.
Discussion
Our results shed light on how learning to perform a working memory task affects prefrontal neuronal selectivity. Effects of training on prefrontal cortex have been addressed over several decades (Sandrew et al. 1977; Kubota and Komatsu 1985; Asaad et al. 1998; Rainer and Miller 2000). The current set of experiments is the first to address neural changes in the prefrontal cortex of the same animals before any specific memory training and after they were trained to perform a working memory task. We found that learning to perform the working memory task is characterized by activation of more neurons and at higher response levels. Unexpectedly, single-unit responses were found to be less selective for stimulus properties and less able to discriminate the operant stimuli. These effects could not be accounted for by factors related to anatomical sampling or neuron selection before and after training. Our study did not address the time course of neuronal changes during training, although recent results suggest abrupt changes during the acquisition of new rules (Durstewitz et al. 2010). We also cannot rule out that learning may additionally involve transient changes in neuronal activity, when new elements of the task are introduced (Blake et al. 2002, 2005). We only examined the changes accumulated after the entire training period.
Increased Activity after Training
After learning to perform the working memory task, a greater percentage of prefrontal neurons exhibited elevated activity during the stimulus presentation and/or the subsequent delay period. Of those neurons that were active, average discharge rate also increased after training, amplifying the effect. Increased activation was particularly pronounced during the delay period, both in terms of percentage of active neurons (Fig. 3b) and in terms of firing rate (Fig. 4). This result appears expectable, as the task specifically required the subjects to maintain stimulus information in memory during the delay period, whereas no such requirement was present prior to training. We should note, however, that neurons with significantly elevated responses during the delay period and selective for the properties of the preceding stimulus have been described previously in naïve animals (Meyer et al. 2007). The increase in activation during the delay period is therefore a quantitative rather than a qualitative change over the naïve state.
Our results are consistent with human imaging studies that report increased activation after training in working memory tasks (Olesen et al. 2004; McNab et al. 2009). Although some fMRI studies report decrease in activation after training, this may be a result of increased efficiency achieved through improved strategies and requiring less engagement of the prefrontal cortex in the task (Klingberg 2010). Our paradigm did not focus on the effects of improvement in performing the task but rather compared responses prior to any understanding of the task at all and after training to execute it, allowing us to determine the effects of performing a working memory task on the activity of single neurons.
Systematic differences of training effects on firing rate were observed between FS (putative interneurons) and RS (putative pyramidal) neurons. In particular, the increase in firing rate during the delay period was accounted almost entirely by RS units. Differential influence on the 2 types of neurons has been reported previously for attention effects (Mitchell et al. 2007; Hussar and Pasternak 2009), and our results indicate that differential modulation extends to training as well.
Stimulus Selectivity
A seemingly paradoxical result of training was the decrease in stimulus selectivity of individual neurons (Fig. 4) which resulted in lower average discriminability for stimulus location (Fig. 5). This was true both during the stimulus presentation and during the delay period. This effect contrasts the results of perceptual learning, which is characterized by the emergence of neurons highly selective for relevant stimulus properties and increased stimulus selectivity (Kobatake et al. 1998; Rainer and Miller 2000; Yang and Maunsell 2004; Gilbert et al. 2009). A key distinction is that our training did not focus on improved recognition of the stimuli, which were easily discriminable and familiar to the monkeys before the pretraining recordings. Instead, we addressed the changes brought about by the incorporation of these stimuli into cognitive tasks. Therefore, it appears that the information required to discriminate and maintain the stimuli in memory was present in neuronal activity prior to training. Our results are also similar to the decreased selectivity reported in the for familiar over novel stimuli in the context of a behavioral task (Kusunoki et al. 2009).
Neuronal activity in the prefrontal cortex of subjects trained to perform working memory tasks has previously been shown to carry information both about the sensory attributes of the remembered stimuli (Constantinidis et al. 2001) and the rules that have been associated with the task (White and Wise 1999; Wallis et al. 2001). The requirement for simultaneously encoding more abstract information related to task demands after training appears to degrade the apparent selectivity for stimulus properties by decreasing the contrast between the absolute activity recorded to the best and worst stimulus.
Behavioral Effects
Although our experimental design maximized the similarity of stimulus presentations before and after training, a number of behavioral variables may have differed between the 2 conditions. These included task difficulty, attention and arousal, planning of eye movements, probability and expectation of reward. These behavioral variables influenced neuronal activity, although they could not fully account for the changes in neuronal activity, we observed after training. Overall, firing rate increased as a function of improved performance in the task (and therefore reward probability), in agreement with earlier studies (Kubota and Komatsu 1985). This effect could not explain the overall increase of firing rate after training, when overall performance and reward probability was lower than before training. The effect of reduced discriminability also proved to be stable across performance levels (Fig. 7d) and across time (Fig. 11). Eliminating the requirement for an eye movement also did not affect neuronal responses appreciably (Fig. 9).
Behavior after training ultimately differs due to learning of the task rules and due to willful execution of the task, which requires attending to the stimulus and maintaining its location in working memory. Some of the effects that we observed, such as the increase in firing rate, are consistent with the known effects of attention that have been observed in trained animals when they attend one stimulus over another (Rainer et al. 1998). The difference in firing rate during the stimulus presentation was modest, however, possibly because transient visual stimuli appearing at unpredictable locations are known to attract attention automatically (Egeth and Yantis 1997). Our study demonstrates that increased firing rate during the delay period is the most prominent effect of incorporating such transient stimuli in a working memory task. Other effects, such as the decreased stimulus selectivity and discriminability, run counter to the known effects of attention and could only be attributed to learning the task.
Funding
National Eye Institute at the National Institutes of Health (grant number R01 EY017077).
Acknowledgments
We wish to thank Fred Joelving and Bill Vaughan for their contributions to the experiments and Carl Olson, Torkel Klingberg, David Blake, Barry Stein, Ram Ramachandran, and Emilio Salinas for helpful comments on a previous version of this manuscript. Conflict of Interest: None declared.
References
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol. 2000;84:451–459. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Chafee MV, Crowe DA, Georgopoulos AP. Parallel processing of serial movements in prefrontal cortex. Proc Natl Acad Sci U S A. 2002;99:13172–13177. doi: 10.1073/pnas.162485599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci U S A. 2002;99:10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Strata F, Kempter R, Merzenich MM. Experience-dependent plasticity in S1 caused by noncoincident inputs. J Neurophysiol. 2005;94:2239–2250. doi: 10.1152/jn.00172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83:1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat Neurosci. 2001;4:311–316. doi: 10.1038/85179. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol. 2002;88:3487–3497. doi: 10.1152/jn.00188.2002. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Buchel C, Josephs O, Friston K, Dolan R. Learning-related neuronal responses in prefrontal cortex studied with functional neuroimaging. Cereb Cortex. 1999;9:168–178. doi: 10.1093/cercor/9.2.168. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Li W, Piech V. Perceptual learning and adult cortical plasticity. J Physiol. 2009;587:2743–2751. doi: 10.1113/jphysiol.2009.171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Hempel A, Giesel FL, Garcia Caraballo NM, Amann M, Meyer H, Wustenberg T, Essig M, Schroder J. Plasticity of cortical activation related to working memory during training. Am J Psychiatry. 2004;161:745–747. doi: 10.1176/appi.ajp.161.4.745. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J. Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J Neurophysiol. 2000;83:2355–2373. doi: 10.1152/jn.2000.83.4.2355. [DOI] [PubMed] [Google Scholar]
- Hussar CR, Pasternak T. Flexibility of sensory representations in prefrontal cortex depends on cell type. Neuron. 2009;64:730–743. doi: 10.1016/j.neuron.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS. Functional anatomical correlates of controlled and automatic processing. J Cogn Neurosci. 2001;13:730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- Joshua M, Elias S, Levine O, Bergman H. Quantifying the isolation quality of extracellularly recorded action potentials. J Neurosci Methods. 2007;163:267–282. doi: 10.1016/j.jneumeth.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Kobatake E, Wang G, Tanaka K. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J Neurophysiol. 1998;80:324–330. doi: 10.1152/jn.1998.80.1.324. [DOI] [PubMed] [Google Scholar]
- Kubota K, Komatsu H. Neuron activities of monkey prefrontal cortex during the learning of visual discrimination tasks with GO/NO-GO performances. Neurosci Res. 1985;3:106–129. doi: 10.1016/0168-0102(85)90025-2. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Sigala N, Gaffan D, Duncan J. Detection of fixed and variable targets in the monkey prefrontal cortex. Cereb Cortex. 2009;19:2522–2534. doi: 10.1093/cercor/bhp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Schumacher EH, Garavan H, Druzgal TJ, D'Esposito M. A functional MRI study of the influence of practice on component processes of working memory. Neuroimage. 2004;22:211–221. doi: 10.1016/j.neuroimage.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Meyer T, Constantinidis C. A software solution for the control of visual behavioral experimentation. J Neurosci Methods. 2005;142:27–34. doi: 10.1016/j.jneumeth.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Meyer T, Qi XL, Constantinidis C. Persistent discharges in the prefrontal cortex of monkeys naive to working memory tasks. Cereb Cortex. 2007;17(Suppl 1):i70–i76. doi: 10.1093/cercor/bhm063. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Moore CD, Cohen MX, Ranganath C. Neural mechanisms of expert skills in visual working memory. J Neurosci. 2006;26:11187–11196. doi: 10.1523/JNEUROSCI.1873-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Review of signal distortion through metal microelectrode recording circuits and filters. J Neurosci Methods. 2008;169:141–157. doi: 10.1016/j.jneumeth.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A, Freedman DJ, Miller EK. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, Backman L. Neural correlates of training-related memory improvement in adulthood and aging. Proc Natl Acad Sci U S A. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J Comp Neurol. 1991;310:475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Qi XL, Katsuki F, Meyer T, Rawley JB, Zhou X, Douglas KL, Constantinidis C. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci. 2010;4:12. doi: 10.3389/fnsys.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Rainer G, Miller EK. Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron. 2000;27:179–189. doi: 10.1016/s0896-6273(00)00019-2. [DOI] [PubMed] [Google Scholar]
- Sandrew BB, Stamm JS, Rosen SC. Steady potential shifts and facilitated learning of delayed response in monkeys. Exp Neurol. 1977;55:43–55. doi: 10.1016/0014-4886(77)90156-x. [DOI] [PubMed] [Google Scholar]
- Sayala S, Sala JB, Courtney SM. Increased neural efficiency with repeated performance of a working memory task is information-type dependent. Cereb Cortex. 2006;16:609–617. doi: 10.1093/cercor/bhj007. [DOI] [PubMed] [Google Scholar]
- Sigala N, Kusunoki M, Nimmo-Smith I, Gaffan D, Duncan J. Hierarchical coding for sequential task events in the monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:11969–11974. doi: 10.1073/pnas.0802569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 1983;23:775–785. doi: 10.1016/0042-6989(83)90200-6. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevenberger P, Ostensson ML, Bartfai A, Klingberg T. Computerized working memory training after stroke–A pilot study. Brain Inj. 2007;21:21–29. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry. 2000;157:1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res. 1999;126:315–335. doi: 10.1007/s002210050740. [DOI] [PubMed] [Google Scholar]
- Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]