Abstract
In root nodules of alfalfa (Medicago sativa L.), N2 is reduced to NH4+ in the bacteroid by the nitrogenase enzyme and then released into the plant cytosol. The NH4+ is then assimilated by the combined action of glutamine synthetase (EC 6.3.1.2) and NADH-dependent Glu synthase (NADH-GOGAT; EC 1.4.1.14) into glutamine and Glu. The alfalfa nodule NADH-GOGAT protein has a 101-amino acid presequence, but the subcellular location of the protein is unknown. Using immunocytochemical localization, we determined first that the NADH-GOGAT protein is found throughout the infected cell region of both 19- and 33-d-old nodules. Second, in alfalfa root nodules NADH-GOGAT is localized predominantly to the amyloplast of infected cells. This finding, together with earlier localization and fractionation studies, indicates that in alfalfa the infected cells are the main location for the initial assimilation of fixed N2.
N is an essential element for plant growth and development (Greenwood, 1982). Plants acquire N from the soil in the form of NO3− and NH4+ and from the atmosphere through symbiotic N2 fixation. Irrespective of its source, N must be reduced to NH4+ to become available for the synthesis of amino acids and other N-containing plant compounds (Lea et al., 1990; Temple et al., 1998b).
In higher plants ammonia is assimilated by the combined action of GS (EC 6.3.1.2) and GOGAT (Temple et al., 1998b). The activities of these two enzymes are interdependent, and they constitute the GS/GOGAT cycle (Lea et al., 1990). The reaction catalyzed by GS involves the ATP-dependent amination of Glu to yield Gln. GOGAT catalyzes the reductive transfer of the amido group of Gln to the α-keto position of 2-oxoglutarate, yielding two molecules of Glu (Boland and Benny, 1977; Lea et al., 1990). GOGAT, together with GS, maintains the flow of N from NH4+ into Gln and Glu. These products are then used by several aminotransferase reactions in the synthesis of amino acids (Lea et al., 1990). In higher plants GOGAT occurs as two distinct forms, using either Fd or NADH as a reductant (Suzuki and Gadal, 1984; Lea et al., 1990). In addition to reductant specificity, the enzymes differ in molecular mass, kinetics, and antigenicity (Temple et al., 1998b). Fd-GOGAT (EC 1.4.7.1) is localized in the chloroplast, where it is thought to be mainly involved in the reassimilation of ammonia derived from photorespiration (Lea et al., 1990; Temple et al., 1998b). The monomeric enzyme is suggested to be an Fe-S protein, with a molecular mass of 140 to 160 kD (Hirasawa and Tamura, 1984; Knauff et al., 1991). In contrast, NADH-GOGAT (EC 1.4.1.14) is predominantly active in nongreen tissue (Lea et al., 1990; Temple et al., 1998b). The plant NADH-GOGAT is a monomer with a molecular mass of about 200 to 240 kD (Chen and Cullimore, 1988; Anderson et al., 1989; Lea et al., 1990; Temple et al., 1998b).
In root nodules N2 is reduced to NH4+ in the bacteroids by the enzyme nitrogenase (EC 1.18.6.1) and is then released into the plant cytosol. The distribution of N assimilatory enzymes in root nodules is complex and related to the type of nitrogenous compounds that are transported from the nodules. In ureide transporters such as soybean and cowpea, cytosolic and plastid enzymes of the infected cells as well as peroxisomes and ER of uninfected cells are involved in N assimilation (Boland et al., 1982; Shelp et al., 1983; Van den Bosch and Newcomb, 1986). In amide transporters such as alfalfa and clover, immunogold localization studies indicate that AAT-2 (EC 2.6.1.1) is localized predominantly in the plastids of infected cells (Robinson et al., 1994), whereas PEP carboxylase (EC 4.1.1.31) is uniformly localized in the cytosol of both infected and uninfected cells (Robinson et al., 1996). Fractionation studies and immunogold localization indicate that GS and AS (EC 6.3.5.4) are both cytosolic enzymes (Shelp and Atkins, 1984; Brangeon et al., 1989; Forde et al., 1989; Datta et al., 1990). On the other hand, the cellular localization of NADH-GOGAT remains unclear. Although some authors suggest a cytosolic localization of the enzyme (Hecht et al., 1988), most fractionation studies suggest plastid localization (Boland et al., 1982; Shelp and Atkins, 1984; Chen and Cullimore, 1989). Alfalfa root nodule NADH-GOGAT contains a 101-amino acid presequence (Gregerson et al., 1993). The presequence analysis program P-Sort indicates that this presequence targets the protein to the ER (Nakai and Kaneshisa, 1992), but, based on the criteria of von Heijne et al. (1989), the presequence suggests mitochondrial or plastid localization.
In root nodules kinetic studies point toward the idea that NADH-GOGAT catalyzes the rate-limiting step in the primary assimilation of ammonia (Boland et al., 1980; Chen and Cullimore, 1988). This hypothesis is further supported by molecular data. In contrast to the high protein and transcript levels of enzymes involved in C and N metabolism, e.g. GS1, AAT-2, AS, and PEP carboxylase, the level of NADH-GOGAT protein and transcript are several times lower (Vance and Gantt, 1992; Vance et al., 1994). Moreover, NADH-GOGAT is the major form of GOGAT in this tissue, and enhanced expression in root nodules is restricted to effective nodulation (Gregerson et al., 1993; Vance et al., 1995). In 33-d-old nodules NADH-GOGAT transcript is localized in the distal part of the N2-fixing zone in a 5- to 15-cell-wide area similar to the distribution pattern of the nifH transcript (Trepp et al., 1999). These results indicate a close relationship between N2 fixation and NADH-GOGAT expression.
The objective of this study was to determine the subcellular localization of the NADH-GOGAT protein in alfalfa root nodule cells using both light and electron microscopy coupled to immunocytochemistry.
MATERIALS AND METHODS
Plant Material and Bacterial Strains
Alfalfa (Medicago sativa L. cv Saranac) seeds were obtained from Dr. J.F.S. Lamb (U.S. Department of Agriculture, Agricultural Research Service, St. Paul, MN). Plants were maintained in greenhouse sand benches and inoculated with effective Sinorhizobium meliloti 102F51 as described by Egli et al. (1989). For all studies the planting date was designated as d 0. At 19 and 33 d after planting and inoculation, the first two nodules from the main root were fixed and embedded for use in both the immunocytochemical and the immunogold localization.
Expression of a NADH-GOGAT Polypeptide
An 886-bp PstI-EcoRI fragment of the NADH-GOGAT cDNA (Gregerson et al., 1993) was subcloned into Bluescript pKS− (Stratagene2) and recovered as a BamHI-EcoRI fragment. This fragment was then ligated in frame to the GST in the pGEX-2T expression vector (Pharmacia Biotech) and transformed into Escherichia coli DH5α. Expression of the fusion protein was induced with isopropyl-β-thiogalactopyranoside according to the manufacturer's description. Induction times, yields, and purification progress were determined using Phast System SDS-PAGE 10% to 15% gradient gels (Pharmacia Biotech). The expressed fusion protein was insoluble; therefore, the lysed cell debris was resuspended in PBS (0.15 m NaCl, 0.01 m K2HPO4, pH 7.2) containing 1.5% sarcosyl. We were unable to purify the fusion protein using the GST-binding column. Therefore, the fusion protein was cleaved with thrombin according to the manufacturer's description. The protein was then precipitated with 30% ammonium sulfate, resuspended in PBS (pH 7.2) containing 1.5% sarcosyl, and applied to a Superose 6 column (Pharmacia Biotech). The desired column fractions were pooled and precipitated in 90% ethanol; the pellets were resuspended directly in Maizel sample buffer (Maizel, 1971) and subsequently subjected to electrophoresis and immunoblotting.
Protein Extraction and Immunoblotting
The NADH-GOGAT antibodies used were produced against purified NADH-GOGAT protein (Anderson et al., 1989). Antibodies were affinity purified, using the partly purified NADH-GOGAT polypeptide, with the elution procedure described by Smith and Fischer (1984). Immunoblot analysis was used to evaluate the specificity of the affinity-purified NADH-GOGAT antibodies. Samples of root nodules were ground in extraction buffer and prepared for immunoblotting as described by Gregerson et al. (1993).
Immunocytochemical Localization of NADH-GOGAT
For light microscopy the root nodule tissues were fixed in 4% paraformaldehyde and 0.25% glutaraldehyde in 50 mm sodium phosphate buffer (pH 7.2). The tissue was then rinsed twice in the same buffer and twice in deionized water, and dehydrated in a graded ethanol series. After the absolute ethanol was replaced with xylene, tissues were embedded in paraffin (Paraplast, Oxford Labware, St. Louis, MO). Embedded tissue was sectioned at a thickness of 7 μm and affixed to poly-l-Lys-coated slides. The paraffin was removed with xylene and the deparaffinized sections were incubated two times for 10 min in a PBS (pH 7.2) solution containing 0.1% Tween 20. Incubation with the primary antibody was performed overnight at 4°C in 500 μL of the same solution containing 15 μg of IgG. As a control, slides were incubated with the primary antibody solution containing 250 μg of partially purified NADH-GOGAT polypeptide. The sections were then washed four times for 10 min each in a PBS (pH 7.2) solution containing 0.1% Tween 20, after which an alkaline-phosphatase-conjugated secondary antibody (Bio-Rad) was applied overnight at 4°C at a 1:300 dilution. The sections were then washed four times for 10 min each in a PBS (pH 7.2) solution and transferred into 100 mm Tris-HCl (pH 9.0) containing 0.5 m NaCl and 5 mm MgCl2 in which the alkaline-phosphatase reaction was performed with nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate according to the manufacturer's description (Bio-Rad). Slides were mounted with Permount (Fisher Scientific), and the sections were viewed and photographed with a Labophot microscope (Nikon).
For immunogold labeling and electron microscopy, approximately 200-μm-thick root-nodule sections were made from 19-d-old root nodules using a hand microtome and subjected to cryofixation using a high-pressure freezer (Balzers Union, Balzers, Liechtenstein). Freeze substitution was performed in anhydrous acetone containing 2% uranyl acetate, 1% glutaraldehyde, 1% water, and 10% methanol in an freeze substitution unit 10 (Balzers Union). The substitution times were 12 h in −92°C, 8 h in −62°C, and 8 h in −32°C (Studer et al., 1992). High-pressure freezing, cryosubstitution allows the “real-to-life” preservation of tissues, and thus is superior to chemical fixation (Studer et al., 1989, 1992; Kaneko and Walther, 1995). The specimens were then embedded and polymerized in LR Gold at −20°C (London Resin, Electron Microscopy Sciences, Fort Washington, PA) (Staiger et al., 1994). Ultrathin sections of silver interference color were mounted on Formvar-coated nickel grids. Sections were blocked in PBS (pH 7.2) containing 0.1% Tween 20. Incubations with the affinity-purified primary antibodies were performed overnight at 4°C (4.5 μg of IgG in 25 μL). As a control, grids were incubated with the primary antibody solution containing 75 μg of partially purified NADH-GOGAT polypeptide. After washing in PBS (pH 7.2) containing 0.1% Tween 20, samples were incubated with the secondary antibody coupled to 18-nm gold in a dilution of 1:300 overnight at 4°C (Jackson ImmunoResearch, West Grove, PA). Poststaining was done with 2% uranyl acetate for 20 min. Sections were examined and pictured on a transmission electron microscope (Philips CM12, Eindhoven, The Netherlands). Morphometric methods were used to compare the labeling density for NADH-GOGAT in root nodules. Twenty-seven micrographs, each of randomly selected infected and uninfected cells from three individual nodules, were evaluated using the point-count method of Weibel (1979).
RESULTS
Analysis of NADH-GOGAT Antibody Specificity Using Western Blots
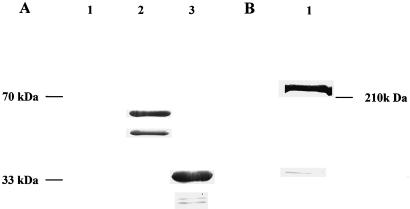
An 886-bp fragment of NADH-GOGAT cDNA was fused in frame to the GST gene in the pGEX-2T expression vector. Expression of the fusion protein in E. coli resulted in the formation of a 59-kD polypeptide, which contained GST (26 kD) and a 33.1-kD NADH-GOGAT fragment. The 59-kD fusion protein is cleavable with thrombin, resulting in a GST and a NADH-GOGAT polypeptide. An immunoblot incubated with polyclonal NADH-GOGAT antibodies is shown in Figure 1A. No signal was detected in the lane containing total protein of E. coli carrying the uninduced pGEX-GOGAT construct (Fig. 1A, lane 1). In Figure 1A, lane 2, which contains total protein of E. coli carrying the induced pGEX-GOGAT construct, antibodies reacted with two polypeptides of approximately 59 and 49 kD. In expression systems different sizes of polypeptides are not unexpected because of proteolysis and incomplete translations (Pharmacia Biotech). When the partially purified fusion protein was digested with thrombin, the expected 33.1-kD NADH-GOGAT polypeptide was released and reacted with NADH-GOGAT antibodies (Fig. 1A, lane 3). The NADH-GOGAT antibodies did not recognize any protein from pGEX-2T cells not harboring the NADH-GOGAT fragment (data not shown). When extracted root nodule protein was incubated with the affinity-purified NADH-GOGAT antiserum, a single band of approximately 220 kD was detected (Fig. 1B). This result is in agreement with previously published data (Anderson et al., 1989; Vance and Gantt, 1992; Gregerson et al., 1993).
Figure 1.
A, Immunoblot analysis of the pGEX-NADH-GOGAT fusion protein. The blot of a 15% polyacrylamide gel was probed with polyclonal NADH-GOGAT antiserum and developed with alkaline-phosphatase-conjugated secondary antibodies. Lanes 1 through 3 contain 10 μg of protein each. Lane 1 contains total protein from E. coli carrying the uninduced pGEX-NADH-GOGAT construct; lane 2 contains total E. coli protein carrying the induced pGEX-NADH-GOGAT construct; and lane 3 contains the partially purified pGEX-NADH-GOGAT construct cleaved with thrombin. B, Immunoblot of total soluble nodule protein. The blot of a 6% polyacrylamide gel was probed with affinity-purified NADH-GOGAT antibodies and developed with alkaline-phosphatase-conjugated secondary antibodies. Lane 1 contains 100 μg of soluble protein of effective nodules.
Immunocytochemical Localization of NADH-GOGAT in Alfalfa Root Nodules.
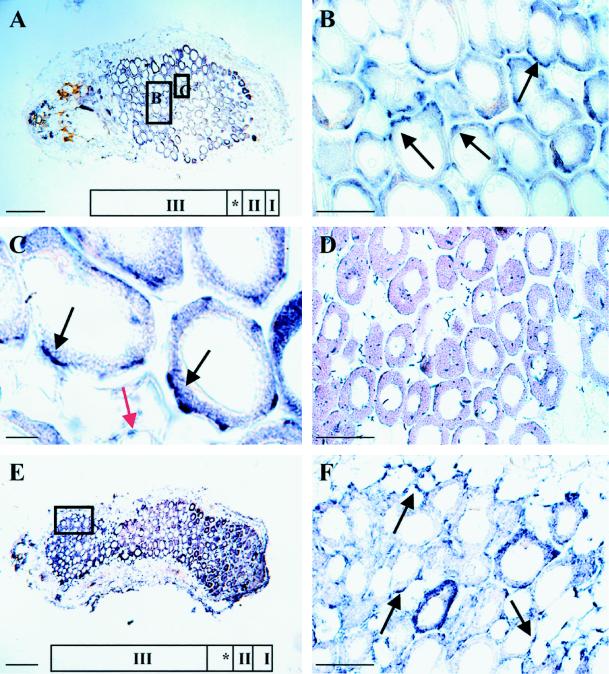
Immunocytochemical localization of NADH-GOGAT was performed on 19-d-old alfalfa root nodules. Nodule structure was classified based on the nomenclature of Vasse et al. (1990). In a longitudinal section through an alfalfa nodule (Fig. 2, A and E), five distinct zones were defined: the nodule meristem (zone I), the invasion zone (zone II), the amyloplast-rich interzone (zone II–III [*]), the N2-fixing zone and proximal inefficient zone (zone III), and the senescence zone (zone IV) (not seen in Fig. 2). The nodule parenchyma, vascular bundles, and outer cortex are seen at the periphery of the central nodule tissue. Figure 2, A through E, shows longitudinal sections through 19- and 33-d-old root nodules incubated with affinity-purified NADH-GOGAT antibodies, followed by incubation with a secondary antibody linked to alkaline phosphatase. The immunolocalization (blue spots) found after development reflects the localization of the NADH-GOGAT protein. The NADH-GOGAT protein was found throughout the interior of both the 19- and 33-d-old nodules (Fig. 2, A and E). The strongest signal was seen at the periphery of infected cells (Fig. 2B). At a higher magnification it became apparent that the protein is mainly localized in infected cells near the intercellular spaces (Fig. 2C). A weak staining was also obtained from uninfected cells (Fig. 2C).
Figure 2.
Localization of the NADH-GOGAT protein in longitudinal sections of 19-d-old (A–C) and 33-d-old (E and F) root nodules. Nodule ultrastructure was classified based on the nomenclature of Vasse et al. (1990): the meristem (zone I), the invasion zone (zone II), the interzone (*), and the N2-fixing zone (zone III). Sections A through E were probed with affinity-purified NADH-GOGAT antibodies and with alkaline-phosphatase-conjugated secondary antibodies. The signal, which reflects the localization of the NADH-GOGAT protein, is seen as blue spots. The black arrows point toward infected cells, and the red arrow points toward uninfected cells. Longitudinal section of a 19-d-old alfalfa root nodule is shown in A. Enlargement of the boxed regions in A shown in B and C includes part of the N2-fixing zone (zone III) with infected and uninfected cells. D shows control, affinity-purified NADH-GOGAT antibodies that were incubated together with the partially purified NADH-GOGAT polypeptide on a longitudinal section through a 19-d-old root nodule. E shows a longitudinal section through a 33-d-old root nodule, and F shows an enlargement of the boxed region in E that is part of the proximal region of the nodule. Bars in A and E = 270 μm; bars in B, D, and F = 70 μm; and bar in C = 15 μm.
In the accompanying paper by Trepp et al. (1999), we show that the NADH-GOGAT transcript in 33-d-old nodules is localized in a 5- to 15-cell-wide zone including the interzone and the N2-fixing zone. To address whether the NADH-GOGAT protein is abundant in the proximal part of the root nodule, longitudinal sections of 33-d-old root nodules were incubated with affinity-purified NADH-GOGAT antiserum (Fig. 2E). Our results suggest that the NADH-GOGAT protein is abundant in the proximal part of a root nodule (Fig. 2F). In addition, the strongest signal after development was observed at the periphery of infected cells, as it was in 19-d-old nodules (Fig. 2, B and C). To test the specificity of the localization, affinity-purified NADH-GOGAT antibodies were incubated together with partially purified NADH-GOGAT polypeptide. This resulted in complete loss of immunostaining and indicates that the antibodies had specific affinity for NADH-GOGAT (Fig. 2D). Two additional controls performed with the preimmune serum and background alkaline-phosphatase staining resulted in nonspecific staining (data not shown).
Immunogold Localization of NADH-GOGAT in Alfalfa Root Nodules
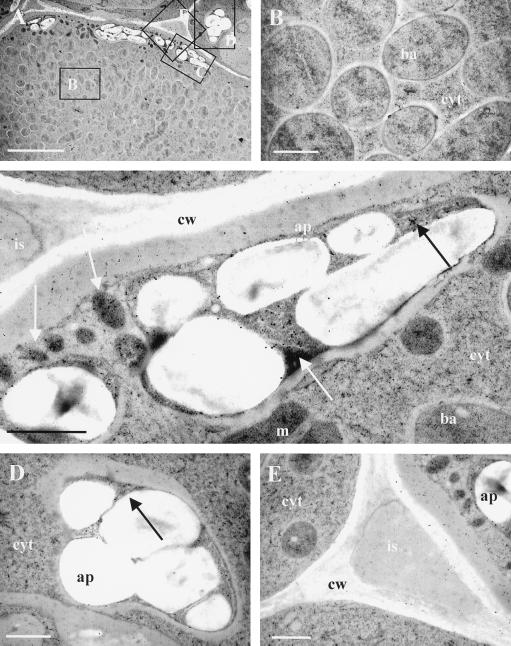
Immunogold localization of NADH-GOGAT was carried out to determine the distribution of NADH-GOGAT at the ultrastructural level. Figure 3A shows infected and uninfected root nodule cells from which all subsequent pictures were taken. Close-ups of bacteroids, amyloplasts in infected and uninfected cells, and intercellular spaces are seen in Figure 3, B to E, respectively. Evaluation of 30 individual profiles showed that gold particles mainly accumulate over the amyloplasts. Particles were counted in randomly selected areas (0.25 μm2), and the results indicate that NADH-GOGAT protein is about 3-fold more abundant in the amyloplasts of infected cells than in the amyloplasts of uninfected cells (Table I). Occasionally, some gold particles were found over intercellular spaces, cytosol, and bacteroids. However, the particle count was much lower compared with the amount of gold found over amyloplasts. As a control, the affinity-purified antibody was incubated together with the partially purified NADH-GOGAT 33.1-kD polypeptide. This resulted in a loss of specific localization of the NADH-GOGAT protein (data not shown). Two additional control experiments performed with the preimmune serum and background immunogold labeling resulted in nonspecific localization (data not shown).
Figure 3.
Immunocytochemical localization of NADH-GOGAT protein in root nodules of alfalfa. The section was probed with affinity-purified NADH-GOGAT antibodies and with secondary antibodies coupled to 18-nm gold particles. A shows the overview from which subsequent pictures were taken. Parts of an infected cell with bacteroids and cytosol are shown in B. C shows an amyloplast of an infected cell. D and E show an amyloplast of uninfected cells and intercellular space, respectively. ba, Bacteroid; cyt, cytosol; m, mitochondria; ap, amyloplast; cw, cell wall; is, intercellular space. Arrows point toward areas containing gold particles. Bar in A = 5 μm; bars in B through E = 1 μm.
Table I.
Distribution of gold particles in various alfalfa nodule cells after immunolabeling with affinity-purified NADH-GOGAT antibodies
| Cell Type | Individual Profilesa | Labeling Density |
|---|---|---|
| no. | gold particles/0.25 μm2 | |
| Cytosol | 30 | 0.13 ± 0.05 |
| Intercellular space | 30 | 0.29 ± 0.09 |
| Cell wall | 30 | 0.36 ± 0.91 |
| Mitochondria | 30 | 0.13 ± 0.05 |
| Bacteroids | 30 | 0.14 ± 0.04 |
| Amyloplasts of infected cells | 30 | 3.5 ± 0.24 |
| Amyloplasts of uninfected cells | 30 | 1.2 ± 0.92 |
All the tissues were from effective N2-fixing nodules. Labeling density values are means ± se.
Ten microscope fields from each of three individual nodules were examined.
DISCUSSION
To our knowledge, this is the first report to determine the subcellular localization of the NADH-GOGAT protein by immunocytochemistry. Our results strongly suggest that the NADH-GOGAT protein is localized in the amyloplasts of root nodules. This finding is further supported by the presence of a 101-amino acid targeting sequence on the NADH-GOGAT cDNA, which indicates compartmentalization of the NADH-GOGAT protein (Gregerson et al., 1993). P-Sort analysis indicates the presequence targets NADH-GOGAT to the ER (Nakai and Kaneshisa, 1992). Using the criteria of von Heijne et al. (1989), the presequence suggests mitochondrial or plastidic localization. Although some authors suggested a cytosolic localization of NADH-GOGAT (Hecht et al., 1988), most reported fractionation studies indicate a plastid localization for the enzyme (Shelp and Atkins, 1984; Chen and Cullimore, 1988). Plants contain two forms of GOGAT, a Fd- and a NADH-dependent form (Temple et al., 1998b). Molecular data (Sakakibara et al., 1991), immunocytochemical localization (Becker et al., 1993), biochemical studies (Wallsgrove et al., 1979), and genetic studies (Somerville and Ogren, 1980; Lea et al., 1990) provide strong evidence for the plastid localization of Fd-GOGAT. Thus, both forms of GOGAT that are found in higher plants appear to be localized in plastids.
Several studies have demonstrated that the plant NADH-GOGAT does not use NADPH as a reductant (Anderson et al., 1988; Chen and Cullimore, 1988; Lea et al., 1990). Because NADPH and not NADH is thought to be the most abundant pyridine nucleotide in plastids (Lea et al., 1990), the question arises whether amyloplasts in root nodules are able to provide sufficient NADH for NADH-GOGAT activity. The recently reported nodule-enhanced malate dehydrogenase (Miller et al., 1998) has also been localized in amyloplasts of root nodules (G.B. Trepp and C.P. Vance, unpublished data). The nodule-enhanced malate dehydrogenase also uses NADH as a reductant (Miller et al., 1998). This provides additional evidence that sufficient NADH is generated in amyloplasts of root nodules. NADH for NADH-GOGAT activity could be generated in plastids through the glycolytic degradation of starch (Plaxton, 1996). In root tissue, where Fd-GOGAT can be abundant (Matoh and Takahashi, 1982; Redinbaugh and Campbell, 1993), GOGAT activity correlates with the activity of the NADPH-generating oxidative pentose-phosphate pathway. Therefore, it has been concluded that this pathway may be involved in the generation of reductant for the reaction performed by Fd-GOGAT (Bowsher et al., 1992; Emes and Neuhaus, 1997). To become available for NADH-GOGAT, NADPH must be converted by a trans-hydrogenase into NADH (Bowsher et al., 1992). To date, this enzyme has not been characterized in plastids. Further experiments are required to determine the source of reductant for plastid-localized NADH-GOGAT.
With the localization of the NADH-GOGAT protein in alfalfa root nodules, we increase our understanding of N2 metabolism in amide-transporting legumes. In ureide transporters such as soybean, N2 assimilation occurs in both infected and uninfected cells (Boland et al., 1982; Shelp et al., 1983; VandenBosch and Newcomb, 1986). However, it appears that in the nodules of the amide transporter alfalfa, the infected cells are the main site for primary assimilation of N. Two lines of evidence support this assertion. First, NADH-GOGAT and AAT-2 proteins are predominantly present in plastids of infected cells, as shown by immunogold localization (Robinson et al., 1994) and fractionation studies (Shelp and Atkins, 1984; Chen and Cullimore, 1989). Second, several investigators have shown that GS (Shelp and Atkins, 1984; Forde et al., 1989) and AS (Shelp and Atkins, 1984) activities and protein are predominantly present in the cytosol of infected cells. Moreover, analysis of the alfalfa nodule-enhanced GS1 (Temple et al., 1995) and AS (Shi et al., 1997) cDNAs revealed that both sequences predict a cytosolic localization for these proteins. Although the plastid-localized GS2 has been detected in root nodules (Temple et al., 1998a), fractionation studies suggest that GS1 accounts for more than 90% of total nodule GS activity (Shelp and Atkins, 1984). Thus, the four enzymes involved in the primary assimilation of N in alfalfa root nodules are partitioned between two cell compartments: GS and AS in the cytosol, and NADH-GOGAT and AAT-2 in the plastids of infected cells.
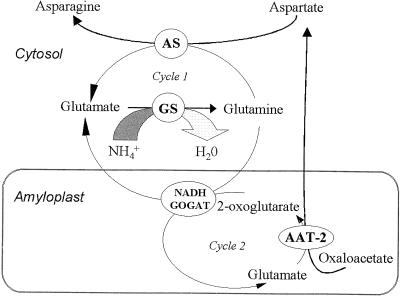
Considering the cellular location of these four enzymes, two cycles linked through NADH-GOGAT could explain how amino acids of primary N assimilation are channeled in amide-transporting legume species. A cytosolic Glu cycle (cycle 1) functioning synergistically with the amyloplast Glu cycle (cycle 2) allows metabolite exchange to occur (Fig. 4). Cycle 1 reactions are catalyzed by AS and GS, whereas AAT-2 is part of cycle 2, with NADH-GOGAT being the common link. In addition to the subcellular localization, several lines of evidence support this hypothesis. Inhibition of AAT-2 stimulates the flow of labeled carbon from Asp into Asn but inhibits labeling of Glu (Snapp and Vance, 1986), showing that Asn and Glu synthesis are linked via AAT-2. When alfalfa nodules are labeled with 14CO2 during a 20-min period, Asn, Asp, and Glu are the most abundantly labeled amino acids, showing the partitioning between the cytosolic and plastid enzyme pools (Maxwell et al., 1984). Furthermore, inhibition of NADH-GOGAT by aza-Ser results in increased incorporation of 15N2 into the amide position of both Gln and Asn (Ta et al., 1986, 1988), indicating the close linkage of Asn synthesis to NADH-GOGAT. The enhanced expression of all four genes during effective nodule development (Gregerson et al., 1993; Vance et al., 1994; Shi et al., 1997) provides further support for the possibility of these cycles occurring. If the proposed model is correct, then the link between the two cycles occurs through the NADH-GOGAT reaction and, therefore, this reaction would be the most likely regulatory point and rate-limiting step.
Figure 4.
Nitrogen assimilation in alfalfa root nodule and the cellular localization of the enzymes involved. Two linked Glu cycles are proposed to be involved in the production of Asn. Cycle 1, Cytosolic Glu cycle; cycle 2, amyloplast Glu cycle. Metabolites involved are NH4+, Gln, Glu, 2-oxoglutarate, oxaloacetate, Asp, and Asn.
To become available for the reactions catalyzed by GS and NADH-GOGAT, Gln and Glu must be transported across the plastid membrane of a root nodule cell. Chen and Cullimore (1989) have proposed a specific translocator that shuttles Gln and Glu between plastids and cytosol in Phaseolus vulgaris root nodules. An analogous system probably exists in chloroplasts of oat, where a Gln/Glu translocator has been identified (Yu and Woo, 1988). Moreover, in Arabidopsis mutants defective in chloroplast dicarboxylic acid transport, the uptake of malate, 2-oxoglutarate, Asp, and Glu into chloroplasts was severely reduced, whereas the levels of Gln were unaffected (Somerville and Ogren, 1983). These results indicate that Gln is transported by a distinct translocator into chloroplasts. It has been proposed that this Gln/Glu translocator plays an essential role during the photorespiratory nitrogen cycle (Yu and Woo, 1988), suggesting that a similar translocator may be important during root nodule N assimilation. However, in chloroplasts several translocators have been identified with overlapping substrate specificities (Fluegge and Heldt, 1991). Whether amyloplasts in alfalfa root nodules have specific amino acid transporters/translocators or several transporters/translocators with overlapping specificity, or both, has yet to be determined.
In 33-d-old alfalfa root nodules the NADH-GOGAT transcripts were localized in a 5- to 15-cell-wide zone (Trepp et al., 1999). Because the NADH-GOGAT transcript is not detectable in the proximal part of root nodules, we were curious about whether this was also the case for the NADH-GOGAT protein. Our results suggest that the NADH-GOGAT protein is present in the proximal part of root nodules. Based on the changes in bacteroid morphology from type 4 to type 5 as well as acetylene-reduction assays, Vasse et al. (1990) suggested that the proximal part of a root nodule is inefficient in N2 fixation. In 33-d-old root nodules the nifH gene, which encodes a subunit of the bacterial nitrogenase, is also expressed in a 5- to 15-cell-wide zone, and the transcript is nondetectable in the proximal part (Trepp et al., 1999). In Pisum sativum it has been estimated that the stability of the nitrogenase protein is approximately 2 d (Bisseling et al., 1980). Taken together, these results suggest that there may be little or no N2 fixation in the proximal part of a 33-d-old root nodule. This evidence leads to the question regarding the role of NADH-GOGAT in the proximal part of the organ. NADH-GOGAT, together with GS, may be involved in the remobilization of nitrogenous compounds in senescing root nodule tissue. However, the NADH-GOGAT enzyme could be inactive because of a lack of substrate or cofactors.
Several independent studies indicate an important role for GS1 during senescence (Kamachi et al., 1991, 1992; Bernhard and Matile, 1992; Feller and Fischer, 1994). However, the evidence in support of a role in senescence for NADH-GOGAT is controversial. In wheat leaves NADH-GOGAT shows a transient increase in activity during dark-induced senescence (Peeters and Van Laere, 1992). However, this does not appear to occur during natural leaf senescence in rice (Yamaya et al., 1992; Hayakawa et al., 1993, 1994). In addition, GS activity in alfalfa nodules of the early-senescing genotype in1 Saranac is comparable with that of wild type, and the activity of NADH-GOGAT in in1 Saranac nodules is dramatically reduced (Egli et al., 1989). Thus, the role of NADH-GOGAT in reassimilating N2 during root nodule senescence is unclear.
This report documents the immunogold localization of NADH-GOGAT protein to amyloplasts in alfalfa root nodules. Furthermore, the NADH-GOGAT protein is predominantly located in infected cells. We suggest, therefore, that in alfalfa, infected cells are the main site for assimilation of symbiotically fixed N2. In contrast to its transcript, the NADH-GOGAT protein is present in the proximal part of 33-d-old root nodules; the role of the enzyme in this part of the nodule remains to be determined.
ACKNOWLEDGMENTS
We thank the Integrated Microscopy Resource at the University of Wisconsin, Madison. We also thank Sue Wick and Thomas Soulen for reviewing the manuscript, and special thanks to Nikolaus Amrhein for his insightful remarks.
Abbreviations:
- AAT-2
Asp aminotransferase
- AS
Asn synthetase
- GOGAT
Glu synthase
- GS
Gln synthetase
- GST
glutathione-S-transferase
Footnotes
This work was supported in part by National Science Foundation grant no. IBN-9206890 and ETH-Zurich fellowship no. 0-28-001-91. This paper is a joint contribution from the Plant Science Research Unit, U.S. Department of Agriculture, Agricultural Research Service, and the Minnesota Agricultural Experiment Station (paper no. 98-1-13-0101, Scientific Journal Series).
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that might also be suitable.
LITERATURE CITED
- Anderson MP, Vance CP, Heichel GH, Miller SS. Purification and characterization of NADH-Glu synthase from alfalfa root nodules. Plant Physiol. 1989;90:351–358. doi: 10.1104/pp.90.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TW, Rechenmann CP, Suzuki A, Hirel B. Subcellular and immunocytochemical localization of the enzymes involved in ammonia assimilation in mesophyll and bundle-sheath cells of maize leaves. Planta. 1993;191:129–136. [Google Scholar]
- Bernhard WR, Matile P. Differential expression of glutamine synthetase genes during the senescence of Arabidopsis thaliana rosette leaves. Plant Sci. 1994;98:7–14. [Google Scholar]
- Bisseling T, Van Straten J, Houwaard F. Turnover of nitrogenase and leghemoglobin in root nodules of Pisum sativum. Biochim Biophys Acta. 1980;610:360–370. doi: 10.1016/0005-2787(80)90017-9. [DOI] [PubMed] [Google Scholar]
- Boland MJ, Benny AG. Enzymes of nitrogen metabolism in legume nodules: purification and properties of NADH-dependent Glu synthase from lupine nodules. Eur J Biochem. 1977;79:355–362. doi: 10.1111/j.1432-1033.1977.tb11816.x. [DOI] [PubMed] [Google Scholar]
- Boland MJ, Farnden KJF, Robertson JG (1980) Ammonia assimilation in nitrogen-fixing legume nodules. In WE Newton, WH Orme-Johnson, eds, Nitrogen Fixation, Vol 2. University Park Press, Baltimore, MD, pp 33–52
- Boland MJ, Hanks JF, Reynolds PHS, Blevins DG, Tolbert NE, Schubert KR. Subcellular organization of ureide biogenesis from glycolytic intermediates and ammonium in nitrogen fixing soybean nodules. Planta. 1982;155:45–51. doi: 10.1007/BF00402930. [DOI] [PubMed] [Google Scholar]
- Bowsher CG, Boulton EL, Rose J, Nayagam S, Emes MJ. Reductant for Glu synthase is generated by the oxidative pentose phosphate pathway in non-photosynthetic root plastids. Plant J. 1992;2:893–898. [Google Scholar]
- Brangeon J, Hirel B, Forchioni A. Immunogold localization of glutamine synthetase in soybean leaves, roots and nodules. Protoplasma. 1989;151:88–97. [Google Scholar]
- Chen FL, Cullimore JV. Two isoenzymes of NADH-dependent Glu synthase in root nodules of Phaseolus vulgaris L. Plant Physiol. 1988;88:1411–1417. doi: 10.1104/pp.88.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FL, Cullimore JV. Location of two isoenzymes of NADH-dependent Glu synthase in root nodules of Phaseolus vulgaris L. Planta. 1989;179:441–447. doi: 10.1007/BF00397583. [DOI] [PubMed] [Google Scholar]
- Datta DB, Xiaoyin C, Wong PP, Triplett EW. Immunocytochemical localization of glutamine synthetase in organs of Phaseolus vulgaris L. Plant Physiol. 1990;96:507–512. doi: 10.1104/pp.96.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli MA, Griffith SM, Miller SS, Anderson MA, Vance CP. Nitrogen assimilatory enzymes and enzyme protein during development and senescence of effective and plant gene-controlled ineffective alfalfa nodules. Plant Physiol. 1989;91:898–904. doi: 10.1104/pp.91.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes MJ, Neuhaus HE. Metabolism and transport in non-photosynthetic plastids. J Exp Bot. 1997;48:1995–2005. [Google Scholar]
- Feller U, Fischer A. Nitrogen metabolism in senescing leaves. Crit Rev Plant Sci. 1994;13:241–273. [Google Scholar]
- Fluegge UI, Heldt HW. Metabolite translocators of the chloroplast envelope. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:129–144. [Google Scholar]
- Forde BG, Day HM, Turton JF, Shen WJ, Cullimore JV, Oliver JE. Two glutamine synthetase genes from Phaseolus vulgaris L. display contrasting developmental and spatial patterns of expression in transgenic Lotus corniculatus plants. Plant Cell. 1989;1:391–401. doi: 10.1105/tpc.1.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DJ. Nitrogen supply and crop yield: the global scene. Plant Soil. 1982;67:45–59. [Google Scholar]
- Gregerson RG, Miller SS, Twary SN, Gantt JS, Vance CP. Molecular characterization of NADH-dependent Glu synthase from alfalfa nodules. Plant Cell. 1993;5:215–226. doi: 10.1105/tpc.5.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Nakamura T, Hattori F, Mae T, Ojima K, Yamaya T. Cellular localization of NADH-dependent Glu synthase protein in vascular bundles of unexpanded leaf blades and young grains of rice plants. Planta. 1994;193:455–460. [Google Scholar]
- Hayakawa T, Nakamura T, Mae T, Ojima K. Change in content of two Glu synthase proteins in spikelets of rice plants during ripening. Plant Physiol. 1993;101:1257–1262. doi: 10.1104/pp.101.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht U, Oelmueller R, Schmidt S, Mohr H. Action of light, nitrate and ammonium on the levels of NADH- and ferredoxin-dependent Glu synthases in the cotyledons of mustard seedlings. Planta. 1988;175:130–138. doi: 10.1007/BF00402890. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Tamura G. Flavin and iron-sulphur containing ferredoxin-linked Glu synthase from spinach leaves. J Biochem. 1984;95:983–994. doi: 10.1093/oxfordjournals.jbchem.a134725. [DOI] [PubMed] [Google Scholar]
- Kamachi K, Yamaya T, Hayakawa T, Mae T, Ojima K. Changes in cytosolic glutamine synthetase polypeptide and its mRNA in leaf blades of rice plants during natural senescence. Plant Physiol. 1992;98:1323–1329. doi: 10.1104/pp.98.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi K, Yamaya T, Mae T, Ojima K. A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol. 1991;96:411–417. doi: 10.1104/pp.96.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Walther P. Comparison of ultrastructure of germinating pea leaves prepared by high-pressure freezing-freeze substitution and conventional chemical fixation. J Electron Microsc. 1995;44:104–109. [PubMed] [Google Scholar]
- Knauff DB, Hirasawa M, Ameyibor E, Fu W, Johnson MK. Spectroscopic evidence for a [3Fe-4S] cluster in spinach Glu synthase. J Biol Chem. 1991;266:15080–15084. [PubMed] [Google Scholar]
- Lea PJ, Robinson SA, Stewart GR. The enzymology and metabolism of glutamine, Glu and asparagine. In: Miflin BJ, Lea PJ, editors. The Biochemistry of Plants: Intermediary Nitrogen Metabolism, Vol 16. San Diego, CA: Academic Press; 1990. pp. 121–159. [Google Scholar]
- Maizel JV (1971) Polyacrylamide gel electrophoresis of viral proteins. In K Maramorasch, H Koporowski, eds, Methods in Virology, Vol 5. Academic Press, New York, pp 179–246
- Matoh T, Takahashi E. Changes in the activities of ferredoxin- and NADH-Glu synthase during seedling development of peas. Planta. 1982;154:289–294. doi: 10.1007/BF00393905. [DOI] [PubMed] [Google Scholar]
- Maxwell CA, Vance CP, Heichel GH, Stade S. CO2 fixation in alfalfa and birdsfoot trefoil root nodules and partitioning of 14C to the plant. Crop Sci. 1984;24:257–264. [Google Scholar]
- Miller SS, Driscoll BT, Gregerson RG, Gantt JS, Vance CP. Alfalfa malate dehydrogenase (MDH): molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J. 1998;15:173–184. doi: 10.1046/j.1365-313x.1998.00192.x. [DOI] [PubMed] [Google Scholar]
- Nakai K, Kaneshisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters KM, Van Laere AJ. Ammonium and amino acid metabolism in excised leaves of wheat (Triticum aestivum) senescing in the dark. Physiol Plant. 1992;84:243–249. [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Redinbaugh MG, Campbell WH. Glutamine synthetase and ferredoxin-dependent Glu synthase expression in the maize (Zea mays) root primary response to nitrate. Evidence for an organ-specific response. Plant Physiol. 1993;101:1249–1255. doi: 10.1104/pp.101.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Kahn ML, Vance CP. Cellular localization of nodule-enhanced Asp aminotransferase in Medicago sativa L. Planta. 1994;207:245–250. [Google Scholar]
- Robinson DL, Pathirana SM, Gantt JS, Vance CP. Immunogold localization of nodule-enhanced phosphoenolpyruvate carboxylase in alfalfa. Plant Cell Environ. 1996;19:602–608. [Google Scholar]
- Sakakibara N, Watanabe M, Hase T, Sugiyama T. Molecular cloning and characterization of complementary DNA encoding for ferredoxin-dependent Glu synthase in maize leaf. J Biol Chem. 1991;266:2028–2035. [PubMed] [Google Scholar]
- Shelp BJ, Atkins CA. Subcellular localization of enzymes of ammonia assimilation and asparagine synthesis in root nodules of Lupinus albus L. Plant Sci Lett. 1984;36:225–230. [Google Scholar]
- Shelp BJ, Atkins CA, Storer PJ, Canvin DT. Cellular and subcellular organization of pathways of ammonia assimilation and ureide synthesis in nodules of cowpea (Vigna unguiculata L. Walp) Arch Biochem Biophys. 1983;224:429–441. doi: 10.1016/0003-9861(83)90229-1. [DOI] [PubMed] [Google Scholar]
- Shi L, Twary SN, Yoshioka H, Gregerson RG, Miller SS, Samac DA, Gantt JS, Unkefer PJ, Vance CP. Nitrogen assimilation in alfalfa: isolation and characterization of an asparagine synthetase gene showing enhanced expression in root nodules and dark-adapted leaves. Plant Cell. 1997;9:1330–1356. doi: 10.1105/tpc.9.8.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Fischer PA. Identification, developmental regulation and response to heat shock of two antigenically related forms of major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp SS, Vance CP. Asparagine biosynthesis in alfalfa (Medicago sativa L.) root nodules. Plant Physiol. 1986;82:390–395. doi: 10.1104/pp.82.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL. Inhibition of photosynthesis in Arabidopsis mutants lacking in leaf Glu synthase activity. Nature. 1980;286:257–259. [Google Scholar]
- Somerville SC, Ogren WL. An Arabidopsis thaliana mutant defective in chloroplast dicarboxylate transport. Proc Natl Acad Sci USA. 1983;80:1290–1294. doi: 10.1073/pnas.80.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger D, Kappeler S, Muller M, Apel K. The proteins encoded by two tapetum-specific transcripts, Satap35 and Satap44, from Sinapis alba L. are localized in the exine cell wall layer of developing microspores. Planta. 1994;192:221–231. [PubMed] [Google Scholar]
- Studer D, Hennecke H, Mueller M. High-pressure freezing of soybean nodules leads to an improved preservation of ultrastructure. Planta. 1992;188:155–163. doi: 10.1007/BF00216809. [DOI] [PubMed] [Google Scholar]
- Studer D, Michel M, Mueller M. High-pressure freezing comes of age. Scanning Microsc Suppl. 1989;3:253–269. [PubMed] [Google Scholar]
- Suzuki A, Gadal P. Glutamate synthase: physiochemical and functional properties of different forms in higher plants and in other organisms. Physiol Veg. 1984;22:471–486. [Google Scholar]
- Ta TC, Faris MA, MacDowall FDH. Pathways of nitrogen metabolism in nodules of alfalfa (Medicago sativa L.) Plant Physiol. 1986;80:1002–1005. doi: 10.1104/pp.80.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta TC, MacDowall FDH, Faris MA. Assimilation and partitioning of labeled nitrogen from 15N2 and 15NO3− by alfalfa (Medicago sativa L.) J Plant Physiol. 1988;132:239–244. [Google Scholar]
- Temple SJ, Bagga S, Sengupta-Gopalan C. Down-regulation of specific members of the glutamine synthetase gene family in alfalfa by antisense RNA technology. Plant Mol Biol. 1998a;37:535–547. doi: 10.1023/a:1006099512706. [DOI] [PubMed] [Google Scholar]
- Temple SJ, Heard J, Ganter G, Dunn K, Sengupta-Gopalan C. Characterization of a nodule-enhanced glutamine synthetase from alfalfa: nucleotide sequence, in situ localization, and transcript analysis. Mol Plant Microbe Interact. 1995;8:218–227. doi: 10.1094/mpmi-8-0218. [DOI] [PubMed] [Google Scholar]
- Temple SJ, Vance CP, Gantt JS. Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 1998b;3:51–56. [Google Scholar]
- Trepp GB, van de Mortel M, Yoshioka H, Miller SS, Samac DA, Gantt JS, Vance CP. NADH-Glu synthase in alfalfa root nodules. Genetic regulation and cellular expression. Plant Physiol. 1999;119:817–828. doi: 10.1104/pp.119.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBosch KA, Newcomb EH. Immunogold localization of nodule-specific uricase in developing soybean nodules. Planta. 1986;167:198–201. doi: 10.1007/BF00391217. [DOI] [PubMed] [Google Scholar]
- Vance CP, Gantt JS. Control of nitrogen and carbon metabolism in root nodules. Physiol Plant. 1992;85:266–274. [Google Scholar]
- Vance CP, Gregerson RG, Robinson DL, Miller SS, Gantt JS. Primary assimilation of nitrogen in alfalfa nodules: molecular features of the enzymes involved. Plant Sci. 1994;101:51–64. [Google Scholar]
- Vance CP, Miller SS, Gregerson RG, Samac DA, Robinson DL, Gantt JS. Alfalfa NADH-dependent Glu synthase: structure of the gene and importance in symbiotic N2 fixation. Plant J. 1995;8:345–358. doi: 10.1046/j.1365-313x.1995.08030345.x. [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G. Correlation between ultrastructure, differentiation of bacteroids and nitrogen fixation. J Bacteriol. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Stepphun J, Herrmann RG. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- Wallsgrove RM, Lea PJ, Miflin BJ. Distribution of the enzymes of nitrogen assimilation within the pea leaf cell. Plant Physiol. 1979;63:232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER (1979) Stereological Methods, Vol 1. Academic Press, New York
- Yamaya T, Hayakawa T, Tanasawa K, Kamachi K, Mae T, Ojima K. Tissue distribution of Glu synthase and glutamine synthetase in rice leaves. Occurrence of NADH-dependent Glu synthase protein and activity in the unexpanded non-green leaf blades. Plant Physiol. 1992;100:1427–1432. doi: 10.1104/pp.100.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Woo KC. Glutamine transport and the role of glutamine translocator in chloroplasts. Plant Physiol. 1988;88:1048–1054. doi: 10.1104/pp.88.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]