Abstract
Background. Data on the natural history of human papillomavirus (HPV)–related genital warts (GWs) in men are sparse. We described the distribution of HPV types in incident GWs and estimated GW incidence and time from type-specific incident HPV infections to GW detection in a multinational cohort of men aged 18–70 years.
Methods. Participants included 2487 men examined for GWs and tested for HPV every 6 months and followed up for a median of 17.9 months. Samples were taken from 112 men with incident GWs to test for HPV DNA by polymerase chain reaction.
Results. Incidence of GWs was 2.35 cases per 1000 person-years, with highest incidence among men aged 18–30 years (3.43 cases per 1000 person-years). HPV 6 (43.8%), HPV 11 (10.7%), and HPV 16 (9.8%) were the genotypes most commonly detected in GWs. The 24-month cumulative incidence of GWs among men with incident HPV 6/11 infections was 14.6% (95% confidence interval [CI], 7.5%–21.1%). Median time to GW detection was 17.1 months (95% CI, 12.4–19.3 months), with shortest time to detection among men with incident infections with HPV 6/11 only (6.2 months; 95% CI, 5.6–24.2 months).
Conclusions. HPV 6/11 plays an important role in GW development, with the highest incidence and shortest time to detection among men with incident HPV 6/11 infection.
Anogenital human papillomavirus (HPV) is the most common sexually transmitted infection in the United States [1]. More than 100 HPV types have been identified, and approximately 40 of these infect the anogenital region. Genital warts (GWs) are a common HPV-related disease associated with HPV types 6 and 11 [2]. In the United States, 5.6% of sexually active adults aged 18–59 years have self-reported ever receiving a diagnosis of GWs [3], and 1% of US adults aged 18–45 years are estimated to have GWs at any given time [4]. Although GWs are benign and not associated with mortality, they are a source of psychosocial distress [5] and can cause physical discomfort, including pain, bleeding, and itching [6]. GWs are highly infectious; 65% of persons who have sex with a partner with GWs will develop GWs [7]. A high rate of recurrence makes treatment difficult and costly [8]. Approximately $200 million is spent annually in the United States for GW treatment [9].
HPV vaccination may be an effective approach for primary prevention of GWs [10]. However, incidence rates for GWs and estimates of time from HPV infection to GW detection are necessary parameters for modeling the effectiveness of GW prevention through vaccination. Few published studies have reported the HPV type distribution in GWs, the incidence of GWs, and the time from HPV infection to GWs in men. Most published incidence rates of GWs among US men are based on data from private insurance claims [11, 12, 13]. These data likely underestimate true incidence because they exclude individuals who do not seek treatment or who are not privately insured. Likewise, little is known regarding the median time from HPV infection to GW detection, with only 1 published study to date conducted among young university students positive for HPV 6/11 [14].
The purpose of this study was to describe the prevalence of HPV types detected in newly acquired GWs and estimate GW incidence and time from type-specific incident HPV infection to GW detection in a multinational cohort of men aged 18–70 years.
METHODS
Study Population
The HPV in Men (HIM) Study is a multinational prospective study of men aged 18–70 years that examines the natural history of HPV infection in men. Participants were enrolled in the HIM Study from July 2005 through September 2009 and met the following inclusion criteria: (1) aged 18–70 years; (2) resided in southern Florida; Sao Paulo, Brazil; or the state of Morelos, Mexico; (3) reported no previous diagnosis of penile or anal cancer; (4) reported no prior diagnosis of genital or anal warts; (5) had not participated in an HPV vaccine clinical trial; (6) reported no prior diagnosis of human immunodeficiency virus (HIV) infection or AIDS; (7) were not currently receiving treatment for a sexually transmitted infection; (8) had not been imprisoned, homeless, or in drug treatment in the previous 6 months; and (9) were willing to complete 10 scheduled visits every 6 months over 4 years.
In the United States, men were recruited from a large university and the general population in Tampa, Florida, through flyers, brochures, and advertisements in local and university newspapers. In Brazil, men were recruited from the general population of the metropolitan area of São Paulo through several advertisements and from a large urogenital care clinic. Participants in Brazil also included the partners of healthy women who had participated in an HPV natural history study in Sao Paulo. In Cuernavaca, Mexico, men were recruited through a state health plan, from local factories, and from the military. All participants provided written informed consent, and study protocols were approved by institutional review boards at each study site. A more detailed description of the study design and population is reported elsewhere [15, 16, 17]. The present study includes 2487 men who enrolled in the HIM Study before 1 January 2009, did not have GWs detected at enrollment, and completed at least one 6-month follow-up visit.
GW Identification
GWs were identified by visual inspection of the external genitalia by a trained clinician at each clinic visit and included both verrucous and flat lesions. All GWs were sampled with a saline prewetted Dacron swab for the presence of HPV DNA. If multiple GWs were detected, a separate specimen was obtained from each lesion. Specimens were also obtained from healthy genital skin on the coronal sulcus/glans penis, penile shaft, and scrotum for HPV DNA testing. GWs were sampled before healthy genital skin to avoid interspecimen contamination. Lesions that appeared to be related to herpes simplex virus or a benign condition, such as skin tags or cysts, were not sampled for HPV DNA.
HPV DNA Testing
DNA was extracted from samples using the QIAamp Mini kit (Qiagen), according to the manufacturer’s instructions. The polymerase chain reaction (PCR) consensus primer system PGMY 09/11 was used to amplify a fragment of the HPV L1 gene. Every PCR plate included a negative (H2O) and a positive (CaSki cell DNA) control to test for possible contamination. The Linear Array HPV Genotyping Test (Roche Diagnostics) was used to test for the presence of 37 HPV types, including 13 oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66) and 24 nononcogenic types (6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 67–73, 81–84, IS39, and CP6108). A sample was considered HPV positive if HPV DNA was detected by PCR or if it tested positive for at least 1 of the 37 HPV genotypes. Samples that amplified HPV DNA by PCR but did not test positive for a specific HPV genotype were considered unclassified infections. β-Globin was detected in 93% (112 of 120) of GW samples. The 8 men with β-globin–negative GW samples (all negative for HPV DNA) were excluded from all analyses.
Statistical Analysis
GW incidence rates were calculated by dividing the number of incident cases by the number of person-months of follow-up. Person-months were measured as the number of months from the date of enrollment until the date that the incident GW was detected or until the date of the last clinic visit for men who did not develop GWs. Incidence rates were calculated for individual HPV types and groups of HPV types (nononcogenic or oncogenic HPV types) detected on the surface of the GW. The 95% confidence intervals (CIs) for incidence rates were calculated based on the Poisson distribution [18]. All incidence rates were reported per 1000 person-years.
The Kaplan–Meier method was used to estimate the 12- and 24-month cumulative risk of developing GWs (regardless of the HPV type detected on the wart) after specific types of incident genital HPV infections. Incident HPV infections were infections detected at a follow-up visit after a man tested negative for the same HPV type at enrollment. Men who did not develop GWs were censored at the date of their last study visit. The log-rank test was used to test for differences in risk of GWs by age group (18–30 years, 31–44 years, and 45–70 years) and by type of incident HPV infection (HPV 6/11 only, HPV 6/11 and other types, and HPV types other than 6/11). Among the 112 men who developed GWs, the median time from a type-specific incident HPV infection to GW detection was calculated as time in months from the date an incident HPV infection was detected until the date that the GW was detected. The time from HPV infection to GW detection was calculated for the development of any GW, regardless of whether the wart had the same HPV type present in the incident infection.
RESULTS
Study participants were followed up for a median of 17.9 months (range, 4.5–46.9 months; interquartile range 7.0–29.6 months). A total of 112 men developed incident GWs during follow-up. Multiple warts were detected in 13 of the 112 men (12 men had 2 warts, and 1 man had 3 warts). The mean age (SD) of participants was 32.6 (11.4) years (range, 18–70 years), with 49% of men aged 18–30 years. Forty-five percent of men self-reported white race, and 45.2% self-identified as Hispanic. At baseline, 64.8% of men tested positive for HPV DNA on the normal genital skin, and 5.0% tested positive for HPV types 6 or 11.
Table 1 presents GW incidence rates and the distribution of HPV type groups (oncogenic vs nononcogenic) detected on the surface of GWs. The overall incidence rate for a newly acquired GW was 2.35 cases per 1000 person-years. HPV DNA was detected in 80.4% of GWs, and 45.5% of GWs tested positive for multiple HPV types. Unclassified infections that tested positive for HPV DNA by PCR but did not hybridize a specific HPV type occurred in 5.4% of GWs. Forty-two percent of GWs had nononcogenic HPV types only, 5.4% had oncogenic HPV types only, and 27.7% tested positive for a mix of oncogenic and nononcogenic HPV types. HPV 6 and/or 11 was detected in 53.6% of the 112 incident GWs.
Table 1.
Incidence Rates of Grouped Human Papillomavirus Types Detected on Genital Warts
| HPV type detected in genital warts | No. (%)a | Incidence, cases per 1000 person-years (95% CI) |
| Incidence of any GW | 112 (100.0) | 2.35 (1.94–2.83) |
| Any HPVb | 90 (80.4) | 1.89 (1.52–2.33) |
| Positive for multiple HPV types | 51 (45.5) | 1.05 (.78–1.39) |
| Unclassified infectionsc | 6 (5.4) | 0.13 (.05–.27) |
| Nononcogenic HPV types only | 47 (42.0) | 0.99 (.73–1.31) |
| Oncogenic HPV types only | 6 (5.4) | 0.13 (.05–.27) |
| Both nononcogenic and oncogenic HPV types | 31 (27.7) | 0.65 (.44–.93) |
| HPV 6/11 | 60 (53.6) | 1.26 (.96–1.62) |
| HPV 16/18 | 14 (12.5) | 0.29 (.16–.49) |
Abbreviations: CI, confidence interval; GW, genital wart; HPV, human papillomavirus.
Denominator is the 112 men who developed incident GWs.
Includes unclassified HPV infections.
Infections that tested positive for HPV DNA by polymerase chain reaction but did not test positive for any of the 37 HPV types.
Table 2 presents HPV type–specific incidence rates and the proportion of GWs that tested positive for specific HPV types. Nononcogenic types HPV 6 (43.8%) and HPV 11 (10.7%) were the most common types detected and had the highest incidence rates (1.03 cases per 1000 person-years and 0.25 cases per 1000 person years, respectively). All other HPV types were found in ≤10% of GWs and had incidence rates of <1.0 cases per 1000 person-years. Other common nononcogenic HPV types detected were 62 (9.8%; 0.23 cases per 1000 person-years) and 84 (8.9%; 0.21 cases per 1000 person-years). The most common oncogenic HPV types detected were 16 (9.8%; 0.21 cases per 1000 person-years) and 52 (6.2%; 0.15 cases per 1000 person-years). A total of 58.9% of men who developed GWs had an HPV infection prior to GW development with ≥1 of the HPV types detected on the surface of the GW; 65.3% of men with GWs positive for HPV 6 and 58.3% of men with GWs positive for HPV 11 had a preceding HPV infection with types 6 and 11, respectively (data not shown).
Table 2.
Incidence Rates by Individual Human Papillomavirus Types Detected on Genital Warts
| HPV type | No. of men who developed GW with HPV type (%)a | Incidence of GW, cases per 1000 person-years (95% CI) |
| Nononcogenic HPV types | ||
| 6 | 49 (43.8) | 1.03 (.76–1.36) |
| 11 | 12 (10.7) | 0.25 (.13–.44) |
| 26 | 0 (0.0) | … |
| 40 | 7 (6.2) | 0.15 (.06–.30) |
| 42 | 6 (5.4) | 0.13 (.05–.27) |
| 53 | 8 (7.1) | 0.17 (.07–.33) |
| 54 | 5 (4.5) | 0.11 (.03–.25) |
| 55 | 7 (6.2) | 0.15 (.06–.30) |
| 61 | 2 (1.8) | 0.04 (.01–.15) |
| 62 | 11 (9.8) | 0.23 (.12–.41) |
| 64 | 0 (0.0) | … |
| 67 | 1 (0.9) | 0.02 (.00–.12) |
| 68 | 3 (2.7) | 0.06 (.01–.18) |
| 69 | 0 (0.0) | … |
| 70 | 0 (0.0) | … |
| 71 | 2 (1.8) | 0.04 (.01–.15) |
| 72 | 2 (1.8) | 0.04 (.01–.15) |
| 73 | 1 (0.9) | 0.02 (.00–.12) |
| 81 | 1 (0.9) | 0.02 (.00–.12) |
| 82 | 1 (0.9) | 0.02 (.00–.12) |
| 83 | 2 (1.8) | 0.04 (.01–.15) |
| 84 | 10 (8.9) | 0.21 (.10–.39) |
| IS39 | 0 (0.0) | … |
| CP6108 | 7 (6.2) | 0.15 (.06–.3) |
| Oncogenic HPV types | ||
| 16 | 11 (9.8) | 0.21 (.10–.39) |
| 18 | 4 (3.6) | 0.08 (.02–.22) |
| 31 | 1 (0.9) | 0.02 (.00–.12) |
| 33 | 0 (0.0) | … |
| 35 | 0 (0.0) | … |
| 39 | 5 (4.5) | 0.11 (.03–.25) |
| 45 | 1 (0.9) | 0.02 (.00–.12) |
| 51 | 6 (5.4) | 0.13 (.05–.27) |
| 52 | 7 (6.2) | 0.15 (.06–.30) |
| 56 | 1 (0.9) | 0.02 (.00–.12) |
| 58 | 4 (3.6) | 0.08 (.02–.22) |
| 59 | 5 (4.5) | 0.11 (.03–.25) |
| 66 | 6 (5.4) | 0.13 (.05–.27) |
Abbreviations: CI, confidence interval; GW, genital wart; HPV, human papillomavirus.
Denominator is the 112 men who developed incident GWs.
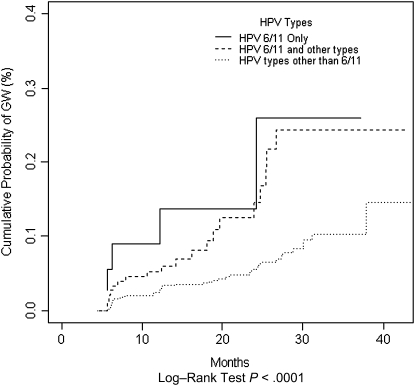
The cumulative risk of developing GWs was 1.7% (95% CI, 1.2%–2.3%) at 12 months and 5.2% (95% CI, 4.1%–6.4%) at 24 months, and the median time to detection of any GW was 17.1 months (95% CI, 12.4–19.3 months) (Table 3). Among men with an incident HPV infection with any type, cumulative incidence of GWs was 2.4% (95% CI, 1.6%–3.3%) at 12 months and 6.8% (95% CI, 5.0%–8.6%) at 24 months. Although not statistically significant, cumulative incidence at 12 months was higher among men with incident HPV infections with nononcogenic types only (4.1%; 95% CI, 2.2%–6.0%), compared with men with incident infections with oncogenic types only (1.8%; 95% CI, .0%–3.5%) or a mix of oncogenic and nononcogenic types (1.5%; 95% CI, .5%–2.4%; P = .39). Men with an incident HPV 6/11 infection had the highest probability of developing GWs (Figure 1). Twelve months after an incident HPV infection, 8.9% (95% CI, .0%–18.1%) of men with HPV 6/11 only, 5.2% (95% CI, 1.8%–8.5%) of men with HPV 6/11 and other types, and 2.5% (95% CI, 1.5%–3.4%) of men with HPV types other than 6/11 developed GWs. The time from an incident HPV infection to GW detection was significantly shorter among men with an infection with HPV 6/11 only (6.2 months; 95% CI, 5.6–24.2 months), compared with men with HPV 6/11 and other types (13.3 months; 95% CI, 6.3–19.6 months) or men with an infection with HPV types other than 6/11 (18.2 months; 95% CI, 12.4–23.6 months; P < .0001) (Table 3).
Table 3.
Cumulative Incidence of Genital Warts at 12 and 24 Months and Median Time From Incident Human Papillomavirus Infection to Genital Wart Detection
| Incident genital HPV infectiona | 12-month cumulative incidence, % (95% CI) | 24-month cumulative incidence, % (95% CI) | Median time from incident genital HPV infection to GW detection, months (95% CI) |
| Any GW | 1.7 (1.2–2.3)b | 5.2 (4.1–6.4)b | 17.1 (12.4–19.3)c |
| Any HPV type | 2.4 (1.6–3.3) | 6.8 (5.0.-8.6) | 12.6 (12.1–18.4) |
| Nononcogenic HPV types only | 4.1 (2.2–6.0) | 6.7 (3.9–9.4) | 7.6 (6.2–12.2) |
| Oncogenic HPV types only | 1.8 (.0–3.5) | 5.5 (.9–9.9) | 19.4 (6.2–23.5) |
| Both nononcogenic and oncogenic HPV types | 1.5 (.5–2.4) | 6.8 (4.3–9.3) | 18.8 (12.7–23.9) |
| HPV 6/11d | 5.8 (2.6–9.0) | 14.6 (7.5–21.1) | 12.2 (6.2–18.9) |
| HPV 6/11 only | 8.9 (.0–18.1) | 13.7 (.0–25.8) | 6.2 (5.6–24.2) |
| HPV 6/11 and other HPV types | 5.2 (1.8–8.5) | 14.5 (6.7–21.7) | 13.3 (6.3–19.6) |
| HPV types other than 6/11 | 2.5 (1.5–3.4) | 5.5 (3.8–7.3) | 18.2 (12.4–23.6) |
Abbreviations: CI, confidence interval; GW, genital wart; HPV, human papillomavirus.
Incident genital HPV infection that occurred before development of GWs.
Cumulative incidence of GWs among all 2487 men regardless of HPV infection status.
Median time from enrollment visit to GW detection.
Includes the men in the “HPV 6/11 only” and “HPV 6/11 and other HPV types” categories.
Figure 1.
Cumulative incidence of genital warts among men with incident human papillomavirus (HPV) infection due to HPV 6/11 only, HPV 6/11 and other HPV types, and HPV types other than 6/11.
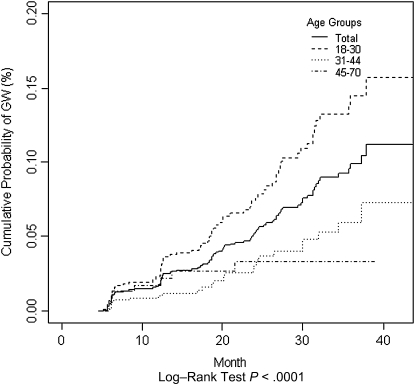
Incidence of GWs significantly varied across age groups (P < .0001; Figure 2). Although the 24-month cumulative incidence of GWs was highest among younger men (7.4%; 95% CI, 5.4%–9.4%), middle-aged adults (3.1%; 95% CI, 1.6%–4.6%) and older men (3.3%; 95% CI, 0.9%–5.6%) remained at risk for GWs (Table 4). There were no statistically significant differences in GW incidence across countries in each age group (data not shown).
Figure 2.
Cumulative incidence of genital warts, by age group.
Table 4.
The 12- and 24-Month Cumulative Incidence and Median Time to Genital Wart Incidence by Age Group
| Age group (years) | Incidence, cases per 1000 person-years (95% CI) | 12-month cumulative incidence,% (95% CI) | 24-month cumulative incidence,% (95% CI) | Median time to GW detection, months (95% CI) |
| 18–30 | 3.43 (2.72–4.27) | 2.3 (1.4–3.2) | 7.4 (5.4–9.4) | 17.2 (12.3–19.5) |
| 31–44 | 1.37 (.89–2.02) | 1.0 (.3–1.7) | 3.1 (1.6–4.6) | 18.9 (11.9–24.2) |
| 45–70 | 1.27 (.55–2.51) | 1.7 (.2–3.2) | 3.3 (.9–5.6) | 7.6 (5.8–13.6) |
Abbreviations: CI, confidence interval; GW, genital wart; HPV, human papillomavirus.
DISCUSSION
In this multinational cohort of men aged 18–70 years, we estimated GW incidence and time from HPV infection to GW detection and described the distribution of HPV types detected on the surface of incident GWs. Men with incident HPV 6/11 infection had the highest incidence of GWs and shortest time from HPV infection to GW detection. HPV 6 (43.8%) and HPV 11 (10.7%) were the most common types detected on GWs, but there was also a high prevalence of oncogenic types, including HPV 16 (9.8%).
The incidence rate of GWs among all men in our study was 2.35 cases per 1000 person-years, with the highest incidence of 3.43 cases per 1000 person-years observed among men aged 18–30 years. Our findings are similar to incidence estimates from studies using private health insurance claims that reported GW incidence rates of 1.70 [11], 1.62 [12], and 1.10 [13] cases per 1000 person-years. Those studies also observed the highest GW incidence among younger men. Two studies observed peak GW incidence among men aged 25–29 years (5.01 cases per 1000 person-years [13] and 2.7 cases per 1000 person-years [11]), and one study observed the highest incidence among men aged 20–29 years (3.1 cases per 1000 person-years [12]). Higher GW incidence rates have been observed among individuals in the placebo arms of HPV vaccine clinical trials: 8.7 cases per 1000 person-years among female patients aged 15–26 years [19] and 15.8 cases per 1000 person-years among male patients aged 16–26 years [10]. An enrollment criterion for the clinical trials was having ≤4 lifetime sexual partners. The median number of lifetime sexual partners in the current study was 6; thus, the lower GW incidence observed in the current study, compared with the clinical trials, is unexpected. One possible explanation is that young individuals just beginning to be sexually active and, therefore, exposed to HPV for the first time have not developed the immune response to quickly clear an infection. Time to clearance of an HPV infection is significantly longer among younger men [17], who may consequently have a greater likelihood of developing a lesion.
Approximately 15% of men in the current study developed GWs within 24 months after an incident HPV 6/11 infection. This is lower than the percentage in a cohort of university students, in which 58% of men [14] and approximately 60% of women [20] developed GWs within 24 months after an incident HPV 6/11 infection. The age distribution of participants in each study may partially account for the difference. The student cohort only included individuals 18–21 years, whereas our study included men aged 18–70 years. However, the 24-month cumulative incidence of GWs after an incident HPV 6/11 infection was only 22.5% among men aged 18–21 years in our study. Differences in time intervals between clinic visits may also contribute to our lower incidence. Men in our study had a slightly longer time of 6 months between visits, compared with the cohort of students who were examined every 4 months. Because the median time to clearance of GWs was 5.9 months in the female students [20], it is possible that there were men in our study who developed and cleared a new GW between the 6-month clinic visits.
Among men in our study with an incident HPV 6/11 infection, the median time to GW detection was 12.2 months, similar to the median time of 11.0 months reported among male university students with an incident HPV 6/11 infection [14]. Women tend to have a shorter time from HPV 6/11 infection to GW detection; the placebo arm of a vaccine trial in women reported median times of 5.0 months [19], and the study of female university students reported a median time of 2.9 months [20]. It is not known why GWs develop more slowly in men, but this observation is consistent with findings of peak GW incidence occurring at a slightly older age in men than in women [11, 13].
The prevalence of HPV 6/11 in GWs in our study was 54%. Previous studies reported the HPV 6/11 prevalence in GWs to be 86% among young women in the placebo arm of an HPV vaccine trial [19], 89% in men from Hong Kong [21], 90% among French men aged 18–72 years [22], and >95% in 2 small US studies that included <50 men [23, 24]. We also observed a high proportion of oncogenic HPV types in GWs (33.1%), with HPV 16 (9.8%) being the most common type detected after HPV 6 (43.8%) and HPV 11 (10.7%). This finding is consistent with other studies that also reported a high prevalence of oncogenic HPV types in GWs [19, 21, 22, 24].
The major limitation of this study was reliance on visual inspection to identify GWs. Without histologic confirmation that lesions were GWs, it is possible that other conditions, such as penile intraepithelial neoplasia, were incorrectly classified as GWs. Misclassification could partially explain why men with genital HPV infections with only oncogenic types had the longest time from infection to lesion development. Because our GW incidence rates are similar to those found in previous studies [11, 12, 13], it is unlikely that there was extensive misclassification. The generalizability of our findings may also be limited, because men who agree to participate in a 4-year study are likely to not be representative of the underlying population at each study site. However, our findings are likely more generalizable to a broader population than are findings from clinical trials, which have a more select group of individuals because of more stringent selection criteria. Also, HPV detection in the current study was based on samples obtained by sampling the surface of the GWs, and therefore, the types detected may not represent the types present in the lesions.
The major strength of the current study was the longitudinal study design and long duration of follow-up. Repeated measures of HPV status over follow-up enabled the examination of how time to GW development differed after incident HPV infections with specific types. We also included men from a broader age range than most previous studies, which allowed us to examine how incidence of GWs differed with age.
This study is one of the first to examine progression from HPV infection to GW development including men from across the lifespan. Although younger men had the highest incidence of GWs, middle-aged adult and older men still remained at risk of acquiring GWs. HPV 6/11 appears to play an important role in GW development, with the highest incidence and shortest time to GW development observed among men with incident HPV 6/11 infection. Future studies should confirm these incidence estimates among histologically confirmed GWs.
Notes
Disclaimer.
The publication and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support.
This work was supported by a National Institutes of Health grant (R01-CA098803-05 to A. R. G.).
Potential conflicts of interest.
A. R. G. receives support from Merck, is a member of the Merck Young Women’s Advisory Board, and serves on the speakers’ bureau for Merck. L. L. V. is a consultant for Merck, Sharp, and Dohme. All other authors: no conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cates W., Jr. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association Panel. Sex Transm Dis. 1999;26:S2–7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(Suppl 3):S3/35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, National Health and Nutrition Examination Survey, 1999–2004. Sex Transm Dis. 2008;35:357–60. doi: 10.1097/OLQ.0b013e3181632d61. [DOI] [PubMed] [Google Scholar]
- 4.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 5.Jeynes C, Chung MC, Challenor R. ‘Shame on you'—the psychosocial impact of genital warts. Int J STD AIDS. 2009;20:557–60. doi: 10.1258/ijsa.2008.008412. [DOI] [PubMed] [Google Scholar]
- 6.Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35:S210–24. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]
- 7.Oriel JD. Natural history of genital warts. Br J Vener Dis. 1971;47:1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey CJ. Therapy for genital human papillomavirus-related disease. J Clin Virol. 2005;32(Suppl 1):S82–90. doi: 10.1016/j.jcv.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107–22. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis. 2003;36:1397–403. doi: 10.1086/375074. [DOI] [PubMed] [Google Scholar]
- 12.Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748–52. doi: 10.1097/01.olq.0000145851.76025.ad. [DOI] [PubMed] [Google Scholar]
- 13.Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25:2343–51. doi: 10.1185/03007990903136378. [DOI] [PubMed] [Google Scholar]
- 14.Arima Y, Winer RL, Feng Q, et al. Development of genital warts after incident detection of human papillomavirus infection in young men. J Infect Dis. 2010;202:1181–4. doi: 10.1086/656368. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–7. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1990;131:373–5. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 19.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 20.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–8. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 21.Chan PK, Luk AC, Luk TN, et al. Distribution of human papillomavirus types in anogenital warts of men. J Clin Virol. 2009;44:111–4. doi: 10.1016/j.jcv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Aubin F, Pretet JL, Jacquard AC, et al. Human papillomavirus genotype distribution in external acuminata condylomata: a large French national study (EDiTH IV) Clin Infect Dis. 2008;47:610–5. doi: 10.1086/590560. [DOI] [PubMed] [Google Scholar]
- 23.Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995;33:2058–63. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–22. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]