(See the article by Mugwanya et al, on pages 1912–7.)
In this issue of the Journal, Mugwanya [1] and colleagues report a crossover randomized controlled trial (RCT) comparing the effect of high-dose valacyclovir (1.5 g twice daily) and standard-dose acyclovir (400 mg twice daily) on plasma human immunodeficiency type 1 (HIV-1) RNA levels. In this proof-of-concept trial, 32 dually HIV-1/herpes simplex virus type 2 (HSV-2) seropositive individuals not receiving antiretroviral therapy (ART) took each regimen for 12 weeks with a 2-week washout period. Plasma HIV-1 RNA was significantly lower during high-dose valacyclovir treatment (mean, 0.62 log10 copies/mL). Compared with levels while not receiving treatment, plasma HIV levels were >1 log10 copies/mL lower when individuals were taking high-dose valacyclovir. High-dose valacyclovir was reported to be well tolerated and safe.
These results provide an impetus to refocus on the potential role of high-dose acyclovir or valacyclovir for preventing HIV transmission and slowing HIV disease progression. In this editorial, we consider whether such a strategy is likely to be effective.
EPIDEMIOLOGICAL EVIDENCE FOR THE ROLE OF HSV SUPPRESSIVE THERAPY ON HIV TRANSMISSION
Biological and observational epidemiological evidence for the synergy between HSV-2 and HIV infections led to active research on the potential role of HSV suppression for HIV prevention in the late 1990s/early 2000s [2]. Several proof-of-concept RCTs evaluated the impact of standard-dose HSV suppressive therapy (acyclovir 400 mg or 800 mg twice daily, and valacyclovir 500 mg twice daily) on plasma and genital HIV levels in dually HSV-2/HIV seropositive individuals not yet eligible for ART [3–9]. The trials of valacyclovir and higher dose acyclovir [3, 6, 7, 9] found modest reductions in plasma HIV RNA levels (PVLs) (0.25–0.53 log10 copies/mL) over periods of up to 3 months. Similar effects were seen on genital HIV RNA levels. Smaller or no reductions were seen in trials of standard-dose acyclovir (400 mg twice daily) [4, 5, 8], although adherence was also lower in 2 of these trials, so this differential effect may not be entirely attributable to the different regimen.
The Partners in Prevention HSV/HIV Transmission Study took this research a step further, examining whether standard-dose acyclovir taken by an HIV-positive individual would reduce HIV transmission to their HIV-negative partner [10] and decrease HIV progression [11]. A total of 3408 HIV-discordant couples in 14 sites in Africa were randomized to either acyclovir or placebo and followed up for ≤12 months [10]. A 73% reduction in the occurrence of HSV-2 genital ulcers, and a 0.25 log10 reduction in HIV PVL levels were seen (similar to that seen in the previous studies), but there was no impact of acyclovir on HIV transmission (hazard ratio, 0.92 [95% confidence interval {CI}, .61–1.41]). This result, together with results from 2 RCTs showing no effect of standard-dose acyclovir on HIV acquisition [12, 13], essentially halted research on HSV suppression for HIV prevention.
One explanation for the disappointing findings was that this acyclovir dose provided ineffective HSV-2 suppression to reduce HIV transmission. This theory is supported by data presented at the recent International Society for Sexually Transmitted Diseases Research conference, which showed that pharmacokinetic differences result in acyclovir exposure being lower in black African than non-African participants [14]. Higher doses of suppressive therapy may, thus, be needed to reduce HIV transmission in black African populations. Moreover, in vitro studies have shown that acyclovir has a direct effect on HIV-1 reverse transcriptase [15, 16]. HIV-1 reverse transcriptase is less sensitive to acyclovir than is HSV2; therefore, a higher dose of acyclovir or valacyclovir may increase the direct inhibitory effect on HIV [17]. Results of a study comparing standard-dose acyclovir with valacyclovir 1000 mg daily showed larger reductions in HIV PVL on high-dose HSV suppression, but no difference in HSV suppression [18]. The findings by Mugwanya et al provide additional evidence that high-dose valacyclovir may be more effective than standard dose acyclovir in preventing HIV transmission.
POPULATION-LEVEL EFFECT OF HSV-2 INFECTION
On the basis of relative risks from observational studies, Freeman et al estimated that 38%–60% of new HIV infections in women and 8%–49% of new HIV infections in men may be attributable to HSV-2 in sub-Saharan Africa [19]. Mathematical modeling studies have shown that HSV-2 amplified HIV transmission at the population level and its role in the HIV epidemic increased over time [20–22]. HSV-2 has a unique role in the HIV epidemic, compared with other sexually transmitted infections (STIs). Unlike other STIs, which can be sustained only in populations with high rates of partner change, the lifelong nature of HSV-2 infection means that infection can be sustained even in populations with low rates of partner change [22]. The high prevalence of HSV-2, and its role in increasing acquisition and transmission of HIV, have greatly increased the proportion of the population who can sustain HIV [22]. Accordingly, HSV-2 may have played a leading role in fuelling HIV transmission among low-risk populations [22].
IMPACT OF HSV TREATMENT ON HIV TRANSMISSION
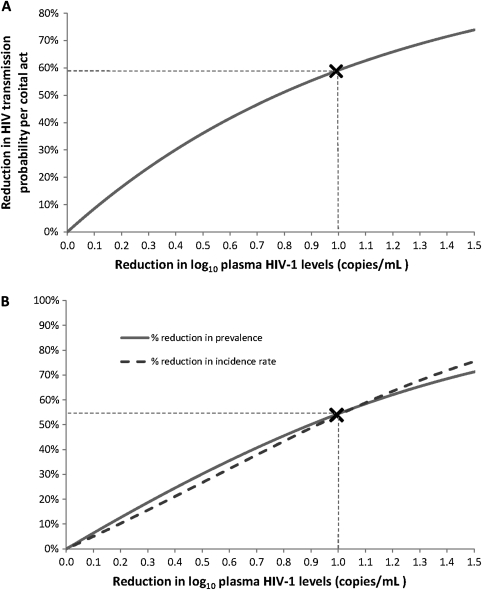
HIV transmission is closely linked with HIV-1 RNA PVL [23], and provision of ART to reduce PVL is a highly effective method of HIV control for both vertical and sexual transmission [24, 25]. The recent HPTN052 RCT of 1763 HIV-discordant couples from 13 sites in Africa, Asia, and the Americas found that provision of ART at a CD4 count of 350–500 cells/mm3 provided almost complete protection against HIV transmission [24]. The impact of high-dose valacyclovir on HIV PVL is more modest, but substantial reductions in HIV infectiousness can be expected. Using the relationship between HIV PVL and transmission probability per coital act, as measured in the Rakai study [23], a reduction of 1 log10 copies/mL in viral load, similar to that found in Mugwanya et al, implies a reduction of 59% in HIV infectiousness (Figure 1A) [22, 26]. Similarly based on the Partners in Prevention cohort, this decrease may result in a 50%–67% reduction in HIV transmission risk [27].
Figure 1.
Impact of high-dose valacylovir treatment on human immunodeficiency virus (HIV) transmission and the HIV epidemic. A, The reduction in HIV transmission probability per coital act expected with a reduction in HIV plasma viral load. The X marker signifies the reduction in infectiousness expected with a reduction of 1 log10 copies/mL in HIV plasma viral load, similar to the viremia reduction observed by Mugwanya et al. B, Impact of intervention aimed at reducing community-level HIV plasma viral load on HIV prevalence and incidence rate at endemic equilibrium. The X marker signifies the impact on prevalence and incidence if the community-level viral load is reduced at the population level by similar magnitude to that observed in Mugwanya et al.
IMPACT OF HSV TREATMENT ON THE HIV EPIDEMIC
The impact of standard-dose HSV suppressive therapy at the population level was estimated by Baggaley et al [28], who found that the reduction in plasma HIV-1 levels seen in 2 trials [5, 7], was likely to result in a very moderate impact, as decreases in infectiousness are counterbalanced by increases in the duration of infectiousness. Mathematical modeling should now explore whether the greater reductions in HIV PVL in the study by Mugwanya and colleagues may lead to a greater population impact overall. Extending earlier modeling work [26, 29], we estimate that such a reduction in HIV PVL may have substantial population level benefits (Figure 1B). A reduction of 1 log10 copies/mL in community HIV viral load, as observed by Mugwanya et al, may reduce HIV prevalence and incidence in the long term by as much as 55%. Achieving such large reductions in PVL at a population level poses challenges but should be considered, particularly where ART provision at CD4 count ≥350 cells/mm3 is not viable.
EFFECT OF HSV-2 INFECTION AND TREATMENT ON HIV DISEASE PROGRESSION
Despite the lack of impact on HIV transmission, the Partners in Prevention trial reported a modest effect of HSV suppressive therapy on HIV disease progression [11]. The primary endpoint was either CD4 count <200 cells/mL3, ART initiation, or non–trauma-related death. Standard-dose acyclovir reduced the risk of HIV disease progression by 16% (95% CI, 2%–29%) and delayed the risk of CD4 counts falling below 350 cells/mm3 by 19% (95% CI, 7%–29%). A systematic review of observational studies found that with a 1 log10 increase in viral load, risk of HIV progression increased 2-fold [30], suggesting that the larger reduction in HIV PVL observed in the study by Mugwanya et al has the potential for a greater impact on disease progression. In HPTN052, early ART was associated with a 41% reduction in HIV-related clinical events [24]. However, with many countries unable to provide early ART, an intervention that slows disease progression and delays time to ART may be of clinical benefit, in addition to reducing HIV transmission risk.
CONCLUSIONS
Mugwanya and colleagues demonstrate that high-dose valacyclovir has a substantial effect on HIV plasma levels, surpassed only by ART. HSV suppressive therapy is simpler to implement than ART, with no laboratory monitoring required. Standard-dose suppressive therapy has been used for many years and has a well-established safety profile. Despite years of use, acyclovir resistance is stable, although more frequent in immune-compromised individuals [31]. No resistant strains were found in genital samples from both HIV-negative and HIV-positive participants of recent trials with >12 months follow-up [32]. However, it would be important to monitor for resistant strains. Providing high-dose HSV suppressive therapy to individuals not eligible for ART may have added benefits for HIV patients, including increasing timely ART provision by improving retention in HIV services, as has been suggested for cotrimoxazole prophylaxis in South Africa [33].
There are a number of caveats, however, including whether the effect observed over 12 weeks in this highly controlled trial will translate into an effective real-life, affordable intervention to reduce HIV transmission and progression. Given the direct effect of acyclovir on HIV, there would need to be monitoring for emergent HIV-1 resistant strains as have been reported in vitro [16, 34], although samples analyzed from other HSV suppressive therapy trials do not report emergence of resistant strains [35, 36]. Yet, under current World Health Organization guidelines to start ART at a CD4 count of 350 cells/mm3, these results suggest a potential role for high-dose HSV suppressive therapy to reduce HIV transmission in dually HIV-1/HSV-2 seropositive individuals not yet eligible for ART or who want to delay starting ART.
Notes
Financial support.
This work was supported by a Wellcome Trust Value in People award to C. T., the Qatar National Research Fund (NPRP 08-068-3-024 to L. J. A.), and MRC grant G0700837 to H. A. W.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Ngure K, Celum C. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared to standard dose acyclovir among HIV-1/HSV-2 co-infected persons: a randomized, cross-over trial. J Infect Dis. 2011 doi: 10.1093/infdis/jir649. 204:1912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Herpes simplex virus type 2: programmatic and research priorities in developing countries (Report of a WHO/UNAIDS/LSHTM Workshop; London 14–16 February 2001) Geneva: WHO/UNAIDS: 2001. http://www.who.int/hiv/pub/sti/en/hiv_aids_2001.05.pdfAccessed 30 September 2011. [Google Scholar]
- 3.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan FM, Pascoe SJ, Barlow KL, et al. A randomised placebo-controlled trial to explore the effect of suppressive therapy with acyclovir on genital shedding of HIV-1 and herpes simplex virus type 2 among Zimbabwean sex workers. Sex Transm Infect. 2008;84:548–53. doi: 10.1136/sti.2008.031153. [DOI] [PubMed] [Google Scholar]
- 5.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–9. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49:77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 7.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 8.Tanton C, Weiss HA, Rusizoka M, et al. Long-term impact of acyclovir suppressive therapy on genital and plasma HIV RNA in Tanzanian women: a randomized controlled trial. J Infect Dis. 2010;201:1285–97. doi: 10.1086/651696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 10.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–9. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Hendrix C, Celum C, et al. Acyclovir achieves lower concentration in African HIV–, HSV2+ women compared to non-African populations, possibly explaining lower herpes suppression. Sex Transm Infect 2011; 87(Suppl_1): A79. [Google Scholar]
- 15.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–93. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisco A, Vanpouille C, Margolis L. A missed point in deciphering the viral synergy between herpes simplex virus and HIV. Lancet Infect Dis. 2009;9:522–3. doi: 10.1016/S1473-3099(09)70209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perti T, Baeten JM, Johnston C, et al. High-dose valacyclovir decreases plasma HIV-1 levels more than standard dose acyclovir in HIV-1, HSV-2 positive persons: a randomised, crossover trial. Sex Transm Infect. 2011;87(Suppl 1):A350. [Google Scholar]
- 19.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 20.Orroth KK, White RG, Korenromp EL, et al. Empirical observations underestimate the proportion of human immunodeficiency virus infections attributable to sexually transmitted diseases in the Mwanza and Rakai sexually transmitted disease treatment trials: simulation results. Sex Transm Dis. 2006;33:536–44. doi: 10.1097/01.olq.0000204667.11192.71. [DOI] [PubMed] [Google Scholar]
- 21.Freeman EE, Orroth KK, White RG, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83:i17–24. doi: 10.1136/sti.2006.023549. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One. 2008;3:e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999;353:781–5. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–6. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 27.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baggaley RF, Griffin JT, Chapman R, et al. Estimating the public health impact of the effect of herpes simplex virus suppressive therapy on plasma HIV-1 viral load. AIDS. 2009;23:1005–13. doi: 10.1097/QAD.0b013e32832aadf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Raddad LJ, Longini IM., Jr No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008;22:1055–61. doi: 10.1097/QAD.0b013e3282f8af84. [DOI] [PubMed] [Google Scholar]
- 30.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–85. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–72. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson-Jones D, Wald A, Celum C, et al. Use of acyclovir for suppression of human immunodeficiency virus infection is not associated with genotypic evidence of herpes simplex virus type 2 resistance to acyclovir: analysis of specimens from three phase III trials. J Clin Microbiol. 2010;48:3496–503. doi: 10.1128/JCM.01263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohler PK, Chung MH, McGrath CJ, Benki-Nugent SF, Thiga JW, John-Stewart GC. Implementation of free cotrimoxazole prophylaxis improves clinic retention among antiretroviral therapy-ineligible clients in Kenya. AIDS. 2011;25:1657–1. doi: 10.1097/QAD.0b013e32834957fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tchesnokov EP, Obikhod A, Massud I, et al. Mechanisms associated with HIV-1 resistance to acyclovir by the V75I mutation in reverse transcriptase. J Biol Chem. 2009;284:21496–504. doi: 10.1074/jbc.M109.024026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeten JM, Lingappa J, Beck I, et al. Herpes simplex virus type 2 suppressive therapy with acyclovir or valacyclovir does not select for specific HIV-1 resistance in HIV-1/HSV-2 dually infected persons. J Infect Dis. 2011;203:117–21. doi: 10.1093/infdis/jiq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeGoff J, Tanton C, Delaugerre C, et al. No selection of nucleoside reverse transcriptase inhibitor resistance associated mutations by acyclovir suppressive therapy in herpes simplex virus-2/HIV-1 dually infected persons. AIDS. 2010;24:2595–6. doi: 10.1097/QAD.0b013e32833e5176. [DOI] [PubMed] [Google Scholar]