Abstract
Background. Despite suppression of plasma human immunodeficiency virus type 1 (HIV-1) RNA by antiretroviral therapy to levels below clinical assay detection, infection and immune activation may persist within the central nervous system and possibly lead to continued brain injury. We hypothesized that intensifying therapy would decrease cerebrospinal fluid (CSF) infection and immune activation.
Methods. This was a 12-week, randomized, open-label pilot study comparing addition of the integrase inhibitor raltegravir to no treatment augmentation, with an option for rollover to raltegravir. CSF and plasma were analyzed for HIV-1 RNA using a single-copy assay. CSF and blood immune activation was assessed by neopterin concentrations and CD4+ and CD8+ T-cell surface antigen expression.
Results. Primary analysis compared 14 intensified (including rollovers) to 9 nonintensified subject experiences. Median HIV-1 RNA levels in all samples were lower in CSF (<.3 copies/mL) than in plasma (<.9 copies/mL; P < .0001), and raltegravir did not reduce HIV-1 RNA, CSF neopterin, or CD4+ and CD8+ T-cell activation.
Conclusions. Raltegravir intensification did not reduce intrathecal immunoactivation or alter CSF HIV-1 RNA levels in subjects with baseline viral suppression. With and without raltegravir intensification, HIV RNA levels in CSF were very low in the enrolled subjects.
Clinical Trials Registration. NCT00672932.
Human immunodeficiency virus type 1 (HIV-1) establishes infection in the central nervous system (CNS) during primary infection [1, 2] and remains detectable thereafter in the cerebrospinal fluid (CSF) of nearly all untreated individuals [3–5]. Although the HIV-1 RNA concentration in CSF in untreated patients varies, on average it is approximately 10-fold lower than in plasma [6]. During primary infection, HIV-1 populations in CSF and plasma are often genetically homogenous [7], but later virus in CSF exhibits increasing genetic compartmentalization, particularly in patients with HIV-associated dementia (HAD), indicating autonomous HIV-1 replication within the CNS [8, 9].
Untreated HIV-1 infection is nearly always accompanied by intrathecal immune activation reflected in elevations of CSF biomarkers, perhaps most notably by increased CSF neopterin [10, 11]. Although clinically asymptomatic in most patients, this infection and immune activation may lead to gradual brain injury [12–14] and in some untreated patients evolves to a more severe HIV-1 encephalitis that manifests with the cognitive and motor dysfunction characteristic of the AIDS dementia complex [15], now commonly referred to as HAD [12]. Although the pathogenesis of brain injury in HAD is not precisely understood, it likely involves host inflammatory mediators that serve as important neuropathogenic signals and toxins and, hence, in a broad sense can be considered immunopathological [16].
HIV-1 CNS infection generally responds well to combination antiretroviral therapy (ART), so that when HIV-1 RNA levels in plasma become undetectable by standard assays (<50 copies/mL) the same is true of CSF [17, 18], although notable exceptions have been observed [10, 19]. Whereas ART largely prevents HAD [20, 21], less severe neurological impairment remains common in treated populations, and CNS injury may possibly continue despite ART [14, 22, 23]. Although the pathogenesis of this type of chronic injury is even less well understood than that of HAD, continued immune activation, despite suppressive therapy that reduces both plasma and CSF to levels below detection by standard assays, may be important [24].
One explanation tying these observations together is that CNS is a sanctuary site where HIV-1 replication continues at low levels that, despite being undetected by conventional assays, are sufficient to drive CNS immune activation and continued brain injury. To test this theory and assess an additional avenue of therapy, we undertook a randomized, open-label pilot clinical trial of treatment intensification adding the integrase inhibitor raltegravir to suppressive regimens [25, 26]. At the time the study began, raltegravir was a new agent to which the study participants would not have been previously exposed. It is a potent antiretroviral with a novel mode of action and generally favorable entry into the CNS [27–29]. The primary outcome measure was reduction in CSF neopterin, a pteridine metabolite produced largely by activated macrophages [30]. Its CSF concentration reflects CNS macrophage activation, increases with disease severity, is especially elevated in HAD [11], and generally responds well to ART, although not always returning to normal levels [24, 31]. After the study began, we added measurement of HIV-1 RNA levels in CSF by a single-copy assay (SCA). Because of its sensitivity and precision, SCA has become the standard for studying the effect of treatment intensification on persistent plasma HIV-1 [32–34], but it had not been applied to CSF samples. We additionally measured cell surface markers of T-cell activation in CSF and blood [35].
METHODS
Study Design
This was a 1:1 randomized, open-label pilot study in which treated, virally suppressed subjects either added raltegravir 400 mg twice daily to their current regimens or received no additional treatment for 12 weeks. Those randomized to no intensification had the option to roll over to receive raltegravir for 12 weeks after the initial period of no added therapy. The study was approved by the University of California, San Francisco Committee on Human Research and was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Entry criteria included HIV-1 infection treated with ART for >2 years, at least 3 antiretroviral drugs (not including ritonavir to boost drug concentrations), plasma suppression to <50 copies/mL for >1 year, and plasma and CSF HIV-1 RNA <50 copies/mL at screening. Additional criteria included the following: >18 years of age; predicted medication adherence; no contraindications to lumbar puncture (LP); no active opportunistic infection or neurological disease confounding evaluations; no previous exposure or contraindications to raltegravir; and not taking immunomodulating drugs.
After providing consent, subjects underwent a screening evaluation that included LP and blood sampling, and standardized neurological assessments as previously described [17, 36, 37]. Those meeting entry criteria were randomized to either starting open-label raltegravir 400 mg twice daily for 12 weeks or no additional drug. At baseline and 4 and 12 weeks, subjects underwent full evaluation, including LP. A visit at 8 weeks included only blood sampling and brief clinical assessment for toxicity or clinical change. For subjects who rolled over, the 12-week visit was used as the baseline for subsequent raltegravir effect, and they underwent a similar schedule of evaluations at the subsequent 4, 8, and 12 weeks. For the main analysis, the subjects randomized to receive no raltegravir were compared with the combined group of those initially randomized to raltegravir and those rolling over to raltegravir. Treatment adherence was assessed by direct questioning and pill count. Theoretical CNS drug activity in the absence of raltegravir was examined using the CNS Penetration Effectiveness (CPE) score as recently updated [23].
CSF and Plasma HIV-1 RNA
For study entry, CSF and plasma HIV-1 RNA was measured using the Abbott RealTime HIV-1 Assay with a lower quantitative limit of 40 copies/mL. Although not initially planned, after the study was under way we added assessment of batch CSF (and plasma) HIV-1 RNA levels using a sensitive SCA method [38]. In brief, up to 8 mL of CSF or plasma, with a known amount of RCAS (an avian retrovirus) added as an internal standard, was centrifuged at 100 000g, and the pellet was extracted and subjected to complementary DNA synthesis followed by real-time polymerase chain reaction amplification of a 79-base pair region of HIV-1 Gag or a portion of the RCAS genome. HIV-1 RNA levels were determined using a standard curve constructed with HIV-1 of known RNA copy number. To ensure that the extraction process was successful, the level of RCAS was measured using a separate standard curve constructed with RCAS of known RNA copy number. HIV-1 RNA results by SCA each represent the median of triplicate determinations.
CSF Soluble Immunological and Other Outcome Measurements
Neopterin was measured in cell-free CSF and plasma by enzyme-linked immunoassay according to the manufacturer’s instructions (BRAHMS Aktiengesellschaft). CSF white blood cell (WBC) counts and differential, blood CD4+ and CD8+ T-cell counts, CSF and blood albumin used to compute the CSF-blood albumin ratio [39], CSF total protein, and blood metabolic profile were all performed in the San Francisco General Hospital Clinical Laboratories using standard methods.
CSF and Blood T-Cell Activation by Multiparameter Flow Cytometry
CSF and blood CD4+ and CD8+ T-cell activation were assessed by the percentage of cells in fresh specimens coexpressing surface CD38 and human leukocyte antigen (HLA)–DR or CCR5 as described previously [35, 40]. Flow cytometry data were compensated and analyzed with FlowJo software version 8.8 (Tree Star).
Neurological Evaluations
All subjects underwent a standardized bedside medical and neurological evaluation. Performance was monitored using 4 brief quantitative tests (timed gait, grooved pegboard, finger tapping, and digit symbol) to obtain a simple aggregate quantitative neurological performance score (QNPZ-4) [41, 42].
Statistics
Changes from baseline to follow-up test intervals at 12 weeks for each of the outcome variables were compared independently by unpaired parametric or nonparametric t tests. Proportions were compared using Fisher exact test. All P values were 2-sided with values <.05 considered significant. Descriptive and comparative statistical analyses were performed using GraphPad Prism 5 software (GraphPad).
RESULTS
Subjects
Of 37 subjects screened, 18 met criteria and were randomized. One subject in the raltegravir group was censored when a pharmacological study showed no drug in either plasma or CSF [27]. Six subjects randomized to no drug later rolled over to receive raltegravir. Primary analysis treated the rollover subjects as independent and compared 14 intensified to 9 nonintensified subject experiences. The baseline characteristics of the subjects are shown in Table 1, grouped as all subjects at entry, those not receiving raltegravir after initial randomization, and the combined subjects receiving raltegravir both initially and after rollover. There is thus overlap among these groups.
Table 1.
Baseline Subject Characteristicsa
| Characteristic | All at entry (n = 17) | No RAL (n = 9) | Combined RAL (n = 14) |
| Age (years) | 52.7 (6.4) | 54.3 (5.3) | 53.7 (6.8) |
| Male/Female (no.) | 16/1 | 8/1 | 13/1 |
| Time since HIV diagnosis (years) | 16.6 (6.7) | 16.5 (7.0) | 17.8 (5.8) |
| Time on treatment (years) | 6.5 (4.2) | 5.5 (3.3) | 6.8 (4.4) |
| QNPZ-4 | −0.3 (1.0) | −0.5 (1.0) | −0.2 (0.8) |
| Blood T cells (cells/μL) | |||
| CD4+ | 513.1 (185.5) | 469.8 (185.5) | 544.2 (259.7) |
| CD8+ | 910.0 (440.1) | 976.6 (430.3) | 945.4 (417.9) |
| HIV-1 RNA (copies/mL) | |||
| Plasma | <50 | <50 | <50 |
| CSF | <50 | <50 | <50 |
| CSF WBCs (per μL) | 2.1 (1.9) | 2.2 (2.0) | 2.2 (1.6) |
| Neopterin (nmol/L) | |||
| Plasma | 9.4 (4.9) | 11.3 (5.6) | 8.5 (4.1) |
| CSF | 5.6 (2.0) | 6.6 (2.4) | 6.0 (3.6) |
| CSF-blood albumin ratio | 5.65 (2.43) | 5.87 (2.86) | 5.65 (2.20) |
| Cell activation (flow) | |||
| CSF CD8+ (CD38+/HLA-DR+) | 28.5 (12.8) | 32.0 (15.0) | 29.2 (12.9) |
| Blood CD8+ (CD38+/HLA-DR+) | 16.3 (6.7) | 18.5 (8.2) | 15.8 (7.3) |
| CSF CD8+ (CCR5+) | 82.3 (7.8) | 83.2 (10.2) | 82.5 (6.2) |
| Blood CD8+ (CCR5+) | 55.6 (12.9) | 58.0 (11.9) | 54.2 (14.6) |
| CSF CD4+ (CD38+/HLA-DR+) | 10.3 (6.2) | 11.8 (6.7) | 9.2 (6.0) |
| Blood CD4+ (CD38+/HLA-DR+) | 5.5 (2.6) | 6.4 (3.1) | 5.3 (2.2) |
| CSF CD4+ (CCR5+) | 53.2 (13.3) | 55.5 (17.2) | 52.6 (11.0) |
| Blood CD4+ (CCR5+) | 27.9 (13.0) | 31.8 (15.2) | 29.2 (11.3) |
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; HLA-DR, human leukocyte antigen–DR; QNPZ, quantitative neurological performance score; RAL, raltegravir; WBC, white blood cell.
All data are presented as mean (SD) unless otherwise specified.
The subjects were predominantly male, with a long history of infection (mean time from diagnosis, 17 years) and treatment (mean, 7 years). They were on a variety of ART combinations with a mean number of drugs of 3.6 (standard deviation [SD] 0.7) for the overall group and a mean CPE score of 1.8 (SD 1.0). There was no significant difference in this score between those initially randomized to either arm or between the final comparison groups.
As defined by entry, all plasma and CSF HIV-1 RNA levels were <50 copies/mL. Blood CD4+ T-cell counts were relatively preserved (mean, 513 cells/μL). CSF WBCs and neopterin levels were also low, and CSF-blood albumin ratios were normal, indicating preservation of the blood-brain barrier [39]. CSF CD4+ and CD8+ T-cell activation measured by the percentage of cells coexpressing CD38 and HLA-DR and expressing CCR5 was higher in CSF than in blood and comparable to previous observations of treated suppressed patients [35, 43].
Effects of Intensification on CSF HIV-1 Assessed by SCA
Because SCA was originally developed for plasma samples, we conducted brief preliminary experiments to ensure that this method could also measure low levels of HIV-1 RNA in the CSF. First, we spiked 10 mL of CSF from an uninfected patient with 5 μL of a plasma sample from an HIV-1–infected individual with a known level of HIV-1 RNA. In the spiked CSF sample, we measured a median of 432 copies per well compared with 334 copies per well (SD 84) usually found in the positive control. To ensure efficient quantification at even lower levels, we spiked 2 uninfected CSF samples with 1 μL of the positive control and measured a median of 44 copies per well compared with 27 copies when the control was added to water (P = .10).
Because use of the SCA method with its optimization using a relatively large volume of CSF or plasma was not part of the original study plan, some of the samples were insufficient to detect the planned sensitivity of .3 copies/mL. Some of the assays also failed for technical reasons. Table 2 shows the results of the SCA assessment for all the intervals successfully tested. The differences in the limits of detection relate to the amount of fluid available for each assay. Thus, if there were <.3 copies detected, this implies that 7 mL of fluid was available. To evaluate this sample volume effect on the different samples, we examined the distribution of the available sample volumes, whether different between CSF and plasma or among the different treatment groups. The median volume for CSF was 6.5 mL and for plasma was 6 mL (P = .13, Mann–Whitney U test). The median for CSF samples without and with treatment intensification was 6.5 mL (P = .37); likewise, the medians for plasma samples obtained without and with treatment intensification were 6 mL (P = .73). Thus, although there were some differences in the available sample volumes, this did not appear to influence the overall results.
Table 2.
Single-Copy Assay HIV RNA Results
| Baseline |
Week 4 No RAL |
Week 12 No RAL |
Week 4 RAL |
Week 12 RAL |
||||||
| CSF | Plasma | CSF | Plasma | CSF | Plasma | CSF | Plasma | CSF | Plasma | |
| Subject | (RNA copies/mL)a | |||||||||
| 1 | <0.2 | 0.2 | <0.3 | <0.3 | … | <0.3 | ||||
| 2 | <0.3 | <0.3 | <0.5 | <0.5 | … | … | ||||
| 3 | <0.3 | 2.1 | <0.3 | 3.3 | … | 2.9 | ||||
| 4 | <0.5 | 0.8 | <0.5 | <2 | <0.4 | 2.8 | <0.5 | 1.4 | <0.4 | 0.7 |
| 5 | <0.3 | 2.3 | <0.3 | 0.4 | <0.3 | 0.2 | 0.3 | 1.8 | 0.3 | <0.3 |
| 6 | 0.5 | 0.9 | 0.9 | … | <0.6 | … | 2.8 | 3.6 | <0.3 | <0.5 |
| 7 | <0.3 | 1.0 | <0.3 | … | <0.3 | … | <0.3 | … | … | 1.4 |
| 8 | … | 4.1 | 0.1 | … | <0.5 | 12 | <0.5 | 10.0 | 0.1 | <0.9 |
| 9 | <0.5 | 2.1 | <0.9 | … | <0.9 | … | <0.5 | … | <0.5 | <2.0 |
| 10 | <0.4 | <0.3 | <0.3 | <0.5 | <0.3 | <0.4 | ||||
| 11 | <0.3 | 2.1 | <0.3 | 4.6 | 0.2 | 2.6 | ||||
| 12 | <0.3 | 0.7 | <0.3 | <0.3 | <0.3 | <0.3 | ||||
| 13 | <0.3 | 1.0 | 0.4 | <0.3 | <0.3 | … | ||||
| 14 | <0.3 | <0.3 | <0.3 | 5.6 | … | 53 | ||||
| 15 | <0.3 | 1.4 | <0.3 | 0.7 | <0.3 | 0.6 | ||||
| 16 | <0.5 | 0.4 | … | … | <0.3 | <0.3 | ||||
| 17 | <0.3 | <0.3 | <0.3 | 1.3 | … | … | ||||
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; RAL, raltegravir.
Samples with detectable HIV-1 RNA are emphasized by bold font.
As shown in Table 2, the amount of HIV-1 RNA in CSF was low in all of the CSF samples. At baseline, only 1 of the 16 CSF samples assessed was positive (with .5 copies/mL). This compared with 13 of 17 plasma samples with similar levels of detection. This low detection rate in CSF was noted in follow-up at weeks 4 (2 of 9) and 12 (0 of 6) in the nonintensified group and in weeks 4 (3 of 13) and 12 (2 of 11) in the raltegravir-treated groups, without difference in the frequency in the groups (P = .69, by Fisher exact test applied to the 4-week and 12-week results of the 2 groups).
In addition to the lack of an effect on the detection rate in CSF, substituting a value .1 copy below the limit of detection in the individual assay for quantitative approximation, there was no difference in the median values of CSF HIV-1 RNA concentrations at 4 and 12 weeks between the unintensified and the raltegravir-treated groups (P = .97, Mann–Whitney U test). In both groups, this median was below the .3 copies detection limit.
Comparison of CSF to plasma underscored the low levels of HIV RNA in CSF. Thus, taking into account all samples, the frequency of viral detection in CSF was 8 of 56 (14%) compared with 32 of 50 (64%) in plasma (P < .001, Fisher exact test). Using the rules for estimating the concentrations in CSF and plasma described above, the median for all CSF samples was .2 copies (interquartile range [IQR], 0.2–0.4), whereas for all plasma samples it was .9 copies (IQR, 0.3–2.2; P < .0001, Mann–Whitney U test).
Effects of Intensification on Nonvirological Outcomes
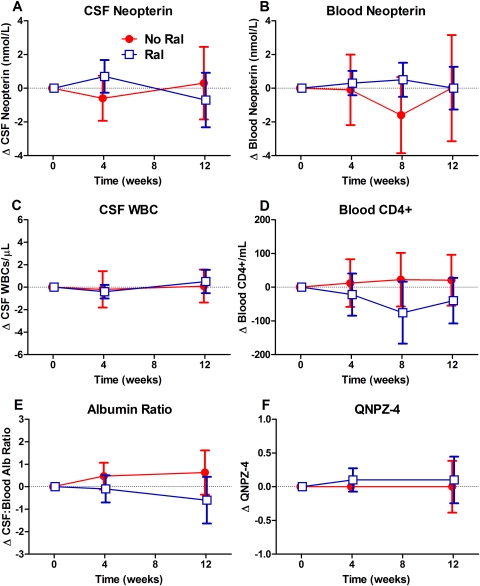
Figure 1 shows the changes from baseline in CSF neopterin and some of the secondary outcome measures of the 2 comparison groups, whereas Table 2 compares the changes at 12 weeks. The CSF neopterin (Figure 1A) varied little over the course of treatment without significant difference in the changes between the groups. Likewise, changes in the blood neopterin (Figure 1B), CSF WBC counts (Figure 1C), blood CD4+ T-cell (Figure 1D) and CD8+ T-cell counts (data not shown), CSF-blood albumin ratios (Figure 1E), and QNPZ-4 scores did not differ between the comparison group (t test).
Figure 1.
Changes in the main and some of the secondary outcome variables with raltegravir intensification. The mean changes in the no raltegravir (No Ral) group are indicated by closed red circles and red lines, whereas the raltegravir (Ral)-treated subjects are shown with open blue circles and blue lines; error bars depict 95% confidence intervals. A, Cerebrospinal fluid (CSF) concentrations. B, Blood neopterin concentrations. C, CSF white blood cell count. D, Blood CD4+ T-cell counts. E, CSF-blood albumin ratio. F, Quantitative neurological performance score (QNPZ-4) performance. None of these changes were different between the 2 groups.
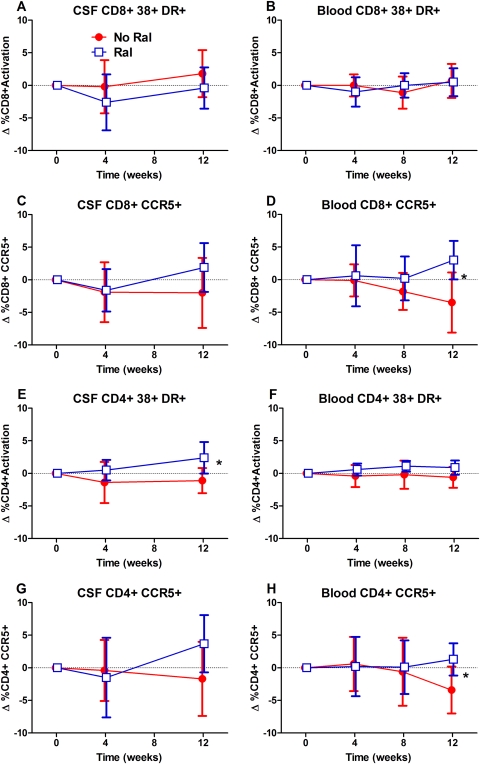
Differences between the 2 groups among the T-cell activation marker changes (Figure 2, Table 3) were noted in the blood CD8+ and CD 4+ CCR5 expression (Figure 2D and H) and in CSF CD4+ T-cell coexpression of CD38 and HLA-DR (2E). In each of these cases, the activation was higher in the raltegravir intensification arm. Changes in the other cell activation measurements between groups were not significant.
Figure 2.
Cerebrospinal fluid (CSF) and blood T-cell activation changes with raltegravir intensification. Symbols are the same as in Figure 1. Panels indicate changes in CSF and blood CD8+ T-cell activation as assessed by coexpression of CD38 and human leukocyte antigen (HLA)-DR on CD3+ and CD8+ lymphocytes (A and B); CSF and blood CD8+ expression of CCR5 (C and D); CSF and blood CD4+ T-cell activation (E and F); and CSF and blood CD4+ CCR5 expression (G and H). *P < .05 as described in text.
Table 3.
Comparison of Week 12 Changesa
| No RAL (n = 9) | RAL (n = 14) | P value (t test) | |
| Blood T cells (cells/μL) | |||
| CD4+ | 20.7 ± 34.7 | −40.0 ± 32.4 | .23 |
| CD8+ | −15.7 ± 66.9 | −37.7 ± 35.1 | .75 |
| CSF WBCs (per μL) | 0.1 ± 0.7 | 0.5 ± 0.5 | .64 |
| Neopterin (nmol/L) | |||
| Plasma | 0.0 ± 1.4 | 0.0 ± 0.6 | .90 |
| CSF | 0.3 ± 1.1 | 0.1 ± 0.3 | .45 |
| QNPZ-4 | −0.00 ± 0.18 | 0.08 ± 0.16 | .73 |
| CSF-blood albumin ratio | 0.634 ± 0.454 | −0.621 ± 0.494 | .09 |
| Cell activation (flow) | |||
| Blood CD4+ (%CD38+/HLA-DR+) | −0.61 ± 0.74 | 0.91 ± 0.54 | .10 |
| CSF CD4+ (%CD38+/HLA-DR+) | −1.11 ± 0.89 | 2.36 ± 1.16 | .04 |
| Blood CD4+ (%CCR5+) | −3.44 ± 1.66 | 1.30 ± 1.19 | .03 |
| CSF CD4+ (%CCR5+) | −1.66 ± 2.63 | 3.72 ± 2.21 | .13 |
| Blood CD8+ (%CD38+/HLA-DR+) | 0.66 ± 1.20 | 0.51 ± 1.03 | .93 |
| CSF CD8+ (%CD38+/HLA-DR+) | 1.80 ± 1.67 | −0.39 ± 1.52 | .36 |
| Blood CD8+ (%CCR5+) | −3.48 ± 2.11 | 3.00 ± 1.40 | .01 |
| CSF CD8+ (%CCR5+) | −2.01 ± 2.47 | 1.91 ± 1.79 | .20 |
| Blood monocyte (%CD14+CD16+) | 0.63 ± 1.35 | −0.51 ± 1.81 | .65 |
| CSF monocyte (%CD14+CD16+) | −1.23 ± 5.99 | 8.70 ± 2.30 | .13 |
| Blood monocyte (%CD14+CCR5+) | −1.02 ± 1.30 | 0.75 ± 0.86 | .25 |
| CSF monocyte (%CD14+CCR5+) | −0.75 ± 3.00 | 10.82 ± 5.66 | .22 |
P values <.05 designated in boldface are not corrected for multiple comparisons.
Abbreviations: CSF, cerebrospinal fluid; HLA-DR, human leukocyte antigen–DR; QNPZ, quantitative neurological performance score; RAL, raltegravir; SEM, standard error of the mean; WBC, white blood cell.
All data are presented as change mean ± SEM unless otherwise specified.
DISCUSSION
This study explored whether treatment intensification with an additional antiretroviral drug having a different mechanism of action from the drugs already being taken—in this case the integrase inhibitor raltegravir—would reduce CSF biomarkers of immune activation and CSF HIV-1 RNA. Our underlying mechanistic hypotheses centered on the capacity of raltegravir to further inhibit low levels of viral replication in the CNS despite clinically measured suppressive therapy and thereby reduce the residual intrathecal immune activation. Our main outcomes were CSF neopterin and CSF HIV-1 RNA measured by SCA, but we also measured CSF T-cell activation, WBC count, CSF-blood albumin ratio, and the QNPZ-4 index of neurological performance. None of these measures showed improvement with raltegravir augmentation in this well-treated group of subjects with low baseline CSF viral burden and immunoactivation. This was similar to the absence of systemic effects on the HIV-1 viral load in plasma and blood immune activation markers in this study and in larger studies [25, 33, 44–46], which also did not detect an effect of intensification, with the exception of one that found an increase in 2–long terminal repeat circles, suggesting possible inhibition of low-level replication in a subset of patients [44]. The only measured changes in T-cell activation showed higher rather than lower levels in the intensified group, and in each case the level of difference was small and might be explained by the multiple comparisons.
There are at least 3 possible explanations for the lack of observed effect of intensification in this small study. First, raltegravir might not appreciably inhibit CNS infection. Although there is little direct study documenting the CNS efficacy of raltegravir in isolation, pharmacokinetic studies, including one involving some of the subjects included in this study [27], have shown that the drug commonly achieves CSF levels in the low therapeutic range, albeit well below those in plasma [28, 29]. In addition, depending on interpretation of therapeutically effective concentrations, drug exposure was likely sufficient to inhibit CNS replication, although the period of treatment may possibly have been too short to detect an effect.
Second, the underlying hypothesis might not be sound: there may be little or no continued HIV-1 replication within the CNS, and local viral replication may not cause the persistent immunoactivation in the CSF. This explanation actually has 2 parts related to (1) residual ongoing viral replication in the CNS and (2) the mechanism of continued intrathecal immunoactivation. With respect to continued infection in treated patients, pathological studies do not generally detect active infection at autopsy in treated patients [47]. However, this is the first study to use a very sensitive SCA method to examine residual CSF HIV-1 RNA in very well-treated subjects, and it shows that indeed even at baseline there was very little CSF virus despite detectible residual virus in many of the plasma samples, similar to that in other studies [25, 33, 34, 45, 46]. Thus, overall, HIV-1 RNA was detected in only 8 of 56 CSF samples (14%) compared with 32 of 50 (64%) plasma samples. Using the rough estimation method described earlier of subtracting .1 copies from the limits of detection for each sample below these limits, the median CSF copy number was .2 copies/mL while that of the plasma was near .9 copies/mL, a CSF-plasma relationship not much different from the usual 1:10 ratio found in untreated patients, although higher than in patients failing therapy in which the ratio is closer to 1:100 [6, 17, 35]. Overall, the very low levels of CSF HIV-1 RNA and the lack of further reduction by intensification suggest that there was indeed little active CNS viral replication in these subjects, with the general caveat that even our SCA applied to CSF may be insensitive to low-level infection within the brain parenchyma.
In untreated infection, virus detected in CSF may originate either from the bloodstream (so-called transitory or noncompartmentalized infection) or from local sources in the CNS (autonomous or compartmentalized infection) [9, 48]. To further elucidate the origins of the very low-level infection in treated subjects, it will be crucial to similarly explore the origins of CSF HIV found in these patients. While technically formidable, this will be essential to understanding the meaning of detecting CSF HIV in treated patients, particularly patients with higher amounts of virus than noted here. Is it spillover from the residual plasma virus or from long-lived memory CD4+ T cells, or is it released from local resident cells, macrophage-microglia, or even astrocytes? This issue may take on greater importance in relation to therapeutic viral eradication.
Stimulation of CSF neopterin by local infection is supported by the association of this pteridine with CNS infection and disease [11, 30] and with previous observations that an increase in these low levels in CSF correlates with viral escape [10]. Thus, it is likely that local infection can “drive” CSF neopterin concentrations. However, the low residual levels seen in the broader experience of treated patients [11, 18] may also reflect other processes, including a continued immunological abnormality in the absence of local infection [49].
Third, the underlying hypothesis might not have actually been addressed because of the particular makeup of the subject group with minimal CNS infection and immunoactivation that left little room to discern a therapeutic effect. In addition to the very low levels of virus in CSF, CSF neopterin concentrations were also quite low, similar to our previous control experience (mean 5.3 nmol/L, SD 2.2), although higher than a larger series of HIV-uninfected individuals without neurological disease (4.2 nmol/L, SD 0.8) [11]. Whether the unanticipated low levels observed in our subjects related to their duration of treatment, their drug combinations (some of which included more than 3 drugs), treatment adherence, self-selection for a CNS intensification study, or other factors, they may have comprised an atypical or extreme group. This issue needs to be examined by further application of SCA to a larger cohort.
Nonetheless, our findings are consonant with a previous study of treatment intensification using either enfurvitide, maraviroc, or lopinavir/ritonavir over a shorter period of time (8 weeks) [50]. Although the proportion of CSF samples in which HIV-1 could be detected and the levels of HIV-1 detected were higher in that study using a different method for HIV-1 RNA detection, still no change in the CSF neopterin was detected.
In summary, despite the favorable characteristics of raltegravir for CNS treatment, we found no evidence that intensification reduced either intrathecal immunoactivation or CSF HIV-1 RNA in our subjects. This is not only consistent with the previous CSF report but also with systemic effects of treatment intensification [25, 33, 44–46]. A remarkable facet of our findings was the low amount of CSF viral RNA and neopterin detected in the subject group. This suggests that treatment can indeed be effective in reducing the CNS viral burden and intrathecal immune activation. It remains to be seen whether this is common or whether our subjects were indeed an unusual group.
Notes
Financial support.
This work was supported by the National Institutes of Health (NIH) (R21MH083520), Merck & Co (IISP ID: 33054), Swedish Doctors against AIDS Foundation, Foundation for AIDS Research, the Wallenberg Foundation, and the National Center for Research Resources via the University of California, San Francisco Clinical and Translational Sciences Institute (UL1 RR024131). The contents are solely the responsibility of the authors and do not represent the official views of the NIH or other funding agencies.
Potential conflicts of interest.
R. W. P. has received funding from Merck to support this investigator-initiated research study and an honorarium from Abbott for a conference presentation. All other authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–9. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 2.Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–45. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–88. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 4.McArthur JC, McClernon DR, Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–98. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 5.Spudich SS, Huang W, Nilsson AC, et al. HIV-1 chemokine coreceptor utilization in paired cerebrospinal fluid and plasma samples: a survey of subjects with viremia. J Infect Dis. 2005;191:890–8. doi: 10.1086/428095. [DOI] [PubMed] [Google Scholar]
- 6.Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197(Suppl 3):S294–306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84:2395–407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington PR, Schnell G, Letendre SL, et al. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23:907–15. doi: 10.1097/QAD.0b013e3283299129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5:e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eden A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–25. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 14.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–35. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 17.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–96. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 18.Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69:1536–41. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]
- 19.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 20.d’Arminio Monforte A, Cinque P, Mocroft A, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–8. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 21.Lescure F-X, Omland LH, Engsig FN, et al. Incidence and impact on mortality of severe neuro-cognitive disorders in persons with and without HIV: a Danish nationwide cohort study. Clin Infect Dis. 2010;52:235–43. doi: 10.1093/cid/ciq041. [DOI] [PubMed] [Google Scholar]
- 22.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 23.Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–65. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–73. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:1–10. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archin NM, Cheema M, Parker D, et al. Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell infection. PLoS One. 2010;5:e9390. doi: 10.1371/journal.pone.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz A, Gisslen M, Spudich S, et al. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One. 2009;4:e6877. doi: 10.1371/journal.pone.0006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croteau D, Letendre S, Best BM, et al. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother. 2010;54:5156–60. doi: 10.1128/AAC.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calcagno A, Bonora S, Bertucci R, Lucchini A, D’Avolio A, Di Perri G. Raltegravir penetration in the cerebrospinal fluid of HIV-positive patients. AIDS. 2010;24:931–2. doi: 10.1097/QAD.0b013e3283319954. [DOI] [PubMed] [Google Scholar]
- 30.Wirleitner B, Schroecksnadel K, Winkler C, Fuchs D. Neopterin in HIV-1 infection. Mol Immunol. 2005;42:183–94. doi: 10.1016/j.molimm.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–83. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 32.Josefsson L, Dahl V, Palmer S. Can HIV infection be eradicated through use of potent antiviral agents? Curr Opin Infect Dis. 2010;23:628–32. doi: 10.1097/QCO.0b013e32833ff1d0. [DOI] [PubMed] [Google Scholar]
- 33.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–9. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinclair E, Ronquillo R, Lollo N, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47:544–52. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Probasco JC, Spudich SS, Critchfield J, et al. Failure of atorvastatin to modulate CSF HIV-1 infection: results of a pilot study. Neurology. 2008;71:521–4. doi: 10.1212/01.wnl.0000325006.84658.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho EL, Spudich SS, Lee E, Fuchs D, Sinclair E, Price RW. Minocycline fails to modulate cerebrospinal fluid HIV infection or immune activation in chronic untreated HIV-1 infection: results of a pilot study. AIDS Res Ther. 2011;8:17. doi: 10.1186/1742-6405-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–90. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 40.Neuenburg JK, Cho TA, Nilsson A, et al. T-cell activation and memory phenotypes in cerebrospinal fluid during HIV infection. J Acquir Immune Defic Syndr. 2005;39:16–22. doi: 10.1097/01.qai.0000155036.03004.a0. [DOI] [PubMed] [Google Scholar]
- 41.Price RW, Yiannoutsos CT, Clifford DB, et al. Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. AIDS. 1999;13:1677–85. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- 42.Wright EJ, Grund B, Robertson K, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75:864–73. doi: 10.1212/WNL.0b013e3181f11bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shacklett BL, Cox CA, Wilkens DT, et al. Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J Infect Dis. 2004;189:2202–12. doi: 10.1086/421244. [DOI] [PubMed] [Google Scholar]
- 44.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 45.Dinoso JB, Kim SY, Wiegand AM, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203:960–8. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Everall I, Vaida F, Khanlou N, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–70. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrington PR, Haas DW, Ritola K, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. J Virol. 2005;79:7959–66. doi: 10.1128/JVI.79.13.7959-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yilmaz A, Verhofstede C, D’Avolio A, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–96. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]