Abstract
A genome-wide association study (GWAS) identified single-nucleotide polymorphisms (SNPs) at 1p11.2 and 14q24.1 (RAD51L1) as breast cancer susceptibility loci. The initial GWAS suggested stronger effects for both loci for estrogen receptor (ER)-positive tumors. Using data from the Breast Cancer Association Consortium (BCAC), we sought to determine whether risks differ by ER, progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), grade, node status, tumor size, and ductal or lobular morphology. We genotyped rs11249433 at 1p.11.2, and two highly correlated SNPs rs999737 and rs10483813 (r2= 0.98) at 14q24.1 (RAD51L1), for up to 46 036 invasive breast cancer cases and 46 930 controls from 39 studies. Analyses by tumor characteristics focused on subjects reporting to be white women of European ancestry and were based on 25 458 cases, of which 87% had ER data. The SNP at 1p11.2 showed significantly stronger associations with ER-positive tumors [per-allele odds ratio (OR) for ER-positive tumors was 1.13, 95% CI = 1.10–1.16 and, for ER-negative tumors, OR was 1.03, 95% CI = 0.98–1.07, case-only P-heterogeneity = 7.6 × 10−5]. The association with ER-positive tumors was stronger for tumors of lower grade (case-only P= 6.7 × 10−3) and lobular histology (case-only P= 0.01). SNPs at 14q24.1 were associated with risk for most tumor subtypes evaluated, including triple-negative breast cancers, which has not been described previously. Our results underscore the need for large pooling efforts with tumor pathology data to help refine risk estimates for SNP associations with susceptibility to different subtypes of breast cancer.
INTRODUCTION
Genome-wide association studies (GWASs) have successfully identified common single-nucleotide polymorphisms (SNPs) associated with breast cancer risk (1–9). The relative risks associated with these SNPs are small (per allele OR < 1.3), and large samples sizes are necessary to obtain more precise estimates of risk particularly for tumor subtypes. Evaluating the associations between susceptibility loci and tumor subtypes could allow for improved risk assessment; and predicting the risk for specific tumor subtypes may lead to targeted early detection or prevention strategies. A recent multi-stage Cancer Genetic Markers of Susceptibility (CGEMS) GWAS, which included 1145 cases of invasive breast cancer and 1142 controls in the first stage, and 8625 cases and 9657 controls in a replication stage, identified SNPs on 1p11.2 and 14q24.1 to be associated with breast cancer risk (4). Data suggested associations for both SNPs were stronger for estrogen receptor (ER)-positive tumors than for ER-negative tumors, especially for 1p11.2. However, sample sizes in the initial report were limited in being able to detect differences by tumor subtype (4).
The Breast Cancer Association Consortium (BCAC) is an international consortium of breast cancer studies formed to identify and validate genetic risk factors associated with breast cancer (1,9–18). The aim of this study was to more accurately estimate breast cancer risk associated with the 1p.11.2 rs11249433 SNP and two 14q24.1 (RAD51L1) highly correlated SNPs (rs999737, rs10483813, r2= 0.98), and to investigate whether these breast cancer susceptibility SNPs are associated with specific tumor types. Analyses were based on data from a maximum of 39 case–control or cohort studies in BCAC that included 46 036 invasive breast cancer cases and 46 930 unaffected controls.
RESULTS
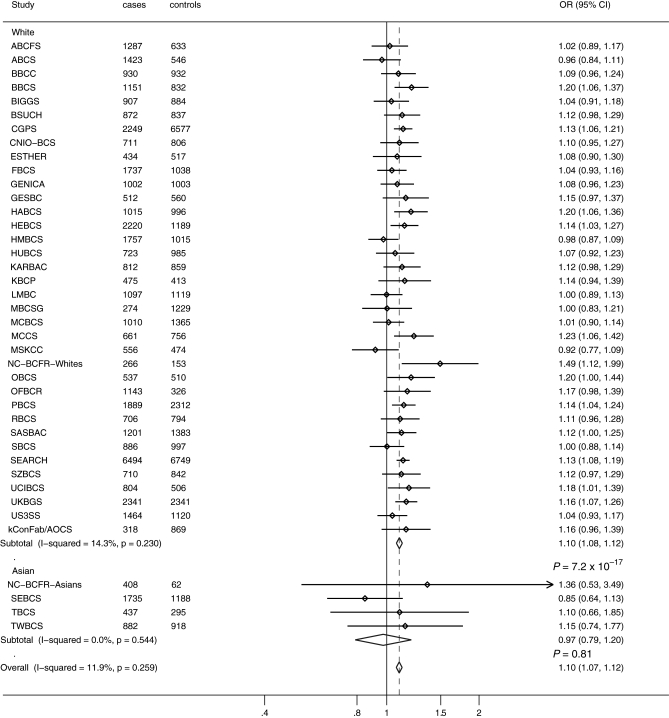
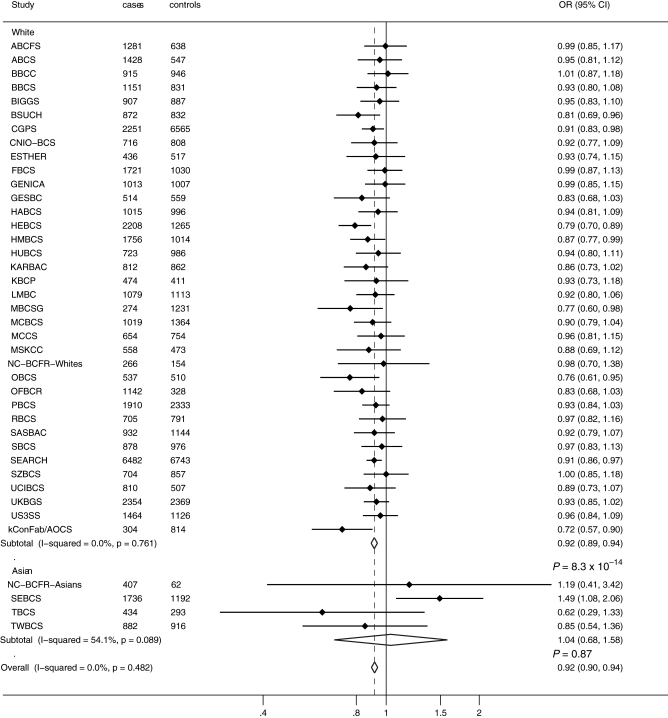
Study acronyms are defined in Supplementary Material, Table S1, and estimated allele frequencies for each study and P for departure from Hardy–Weinberg equilibrium for the controls are reported in Supplementary Material, Table S2. The frequency of the C-allele for rs11249433 at 1p11.2 ranged between 16 and 26% among white women of European ancestry control groups, and was substantially lower for women of Asian ancestry (2% for Asians versus 23% for Europeans). The frequency of the A-allele for rs10483813 or T-allele for rs999737 at 14q24.1 ranged between 32 and 44% across European ancestry control groups, and was also substantially lower for women of Asian ancestry (3% for Asians versus 40% for Europeans). We estimated per-allele odds ratios (ORs) and 95% confidence intervals (CI) for invasive breast cancer, considering European and Asian women separately, for SNPs at the 1p11.2 and 14q24.1 (RAD51L1) using data from 39 studies (Figs 1 and 2).
Figure 1.
Per-allele OR estimates and 95% CIs for 1p11.2 rs11249433 and breast cancer risk by study. Analysis was based on 46 036 invasive breast cancer cases and 46 930 controls from 39 studies. Differences in total numbers are due to missing genotype data. Study acronyms are defined in Supplementary Material, Table S1.
Figure 2.
Per-allele OR estimates and 95% CIs for 14q24.1 (RAD51L1) rs10483813 or rs999737 and breast cancer risk by study. Analysis was based on 46 036 invasive breast cancer cases and 46 930 controls from 39 studies. Differences in total numbers are due to missing genotype data. Study acronyms are defined in Supplementary Material, Tables S1.
Analyses of 1p11.2 SNP rs11249433 and breast cancer risk
Based on the analysis of subjects reporting to be of European ancestry (42 574 invasive cases and 44 467 controls) from 36 studies, the estimated OR per C-allele for rs11249433 was 1.10 (95% CI = 1.08–1.12; P =7.2 × 10−17, study heterogeneity I2= 14.3 P = 0.23; Fig. 1). Based on four studies with subjects reporting to be of Asian ancestry (3462 cases and 2463 controls), the estimated per-allele OR was 0.97 (95% CI = 0.79–1.20; P = 0.81; study heterogeneity I2= 0.0 P = 0.54; Fig. 1). Since the minor alleles for the SNPs analyzed were substantially rarer in Asian populations, we did not observe any significant risk associations in this group, and we had significantly fewer subjects of Asian ancestry, so subsequent analyses were restricted to subjects reporting to be of European ancestry. The estimated ORs for heterozygotes and homozygotes in subjects of European ancestry were: heterozygote OR 1.09 (95% CI = 1.05–1.13; P =2.9 × 10−5); homozygote OR 1.22 (95% CI = 1.17–1.27; P =1.3 × 10−19); Supplementary Material, Figure S1.
Using logistic regression models adjusting for study, and data from 1395 DCIS cases and 26 662 controls, there was no evidence for an association between rs11249433 and risk of ductal carcinoma in situ (DCIS): OR 0.98 (95% CI = 0.90–1.06; P= 0.57). There was no evidence of differences in OR by age [1.04 (95% CI = 0.97–1.11), 1.10 (95% CI = 1.04–1.15), 1.11 (95% CI = 1.06–1.16), and 1.10 (95% CI = 1.061.14) for age categories <40, 40–49, 50–59 and ≥60 years, respectively; P= 0.70 for heterogeneity]. Analysis excluding cases selected for family history gave similar estimates to analyses of all invasive cases: per-allele OR 1.11 (95% CI = 1.08–1.15). There was also no evidence of differences in the per-allele ORs when case groups were defined by the presence or absence of a first-degree family member with breast cancer (P = 0.56 for heterogeneity).
Analyses of 14q24.1 (RAD51L1) rs10483813/rs999737 SNPs and breast cancer risk
Based on the analysis of subjects reporting to be of European ancestry from 36 studies, the estimated OR per A-allele for the rs10483813 or T-allele for the rs999737 14q24.1 (RAD51L1) SNPs was 0.92 (95% CI = 0.89–0.94; P =8.3 × 10−14, study heterogeneity I2= 0, P= 0.76; Fig. 2). The estimated per-allele OR for subjects of Asian ancestry (3459 cases and 2463 controls) from four studies was 1.04 (95% CI = 0.68–1.58; P = 0.87) with some evidence of heterogeneity in OR across studies (I2= 54.1, P= 0.09; Fig. 2). Since the minor alleles for the SNPs analyzed were substantially rarer in Asian populations, we did not observe any significant risk associations, and we had significantly fewer subjects of Asian ancestry, so subsequent analyses were restricted to subjects reporting to be of European ancestry. The estimated ORs for rs10483813/rs999737 in European women were: heterozygote OR 0.93 (95% CI = 0.90–0.95; P =3.54 × 10−7); homozygote OR 0.82 (95% CI = 0.77–0.88; P =6.0 × 10−9); Supplementary Material, Figure S2.
Using data from 1397 DCIS cases and 26 455 controls, the estimated per-allele logistic regression models adjusted for study the OR for DCIS was 0.92 (95% CI = 0.83–1.01; P= 0.08), similar to that for invasive disease. Analysis by age groups did not provide evidence of differences in the OR by age [0.97 (95% CI = 0.90–1.06), 0.90 (95% CI = 0.85–0.95), 0.88 (95% CI = 0.840.92) and 0.96 (95% CI = 0.921.00) for age categories <40, 40–49, 50–59, and ≥60 years, respectively; P= 0.17 for heterogeneity]. Analysis excluding invasive cases selected for family history gave similar estimates to those for all studies: per-allele OR 0.92 (95% CI = 0.88–0.95). There was also no evidence of a difference in the per-allele OR when case groups were defined by first-degree family history of breast cancer (P = 0.24 for heterogeneity).
Analyses of 1p11.2 SNP rs11249433 and 14q24.1 (RAD51L1) rs10483813/rs999737 SNPs by ER, PR and HER2 status of tumors
The majority of studies (26 of 36 studies with women reporting to be of European ancestry) contributed information on the pathology of the breast tumor, and analyses were based on up to 35 209 controls and 25 458 cases. The 1p11.2–rs11249433 SNP exhibited a stronger association with ER-positive tumors than that with ER-negative tumors (Table 1). Per-allele ORs for ER-positive and ER-negative tumors were 1.13 (95% CI = 1.10–1.16) and 1.03 (95% CI = 0.981.07), respectively (case-only P-heterogeneity = 7.6 × 10−5). In contrast, for rs10483813/rs999737, there was an association with both ER-positive and ER-negative disease, with per-allele OR of 0.90 (95% CI = 0.87–0.93) for ER-positive and 0.93 (95% CI = 0.88–0.98) for ER-negative tumors (case-only P-heterogeneity = 0.42). Analyses by PR status for the 1p11.2 and 14q24.1 (RAD51L1) SNPs showed similar results to those observed by ER status (Table 1). The estimated OR for rs11249433 was slightly higher for HER2-negative than that for HER2-positive disease (case-only P-heterogeneity = 0.23, Table 1), but no difference by HER2 status was observed when ER/PR-positive and ER/PR-negative cases were considered separately (case-only P-heterogeneity = 0.80 and 0.49, respectively; Table 2). There was a slight suggestion of stronger effects for the rs10483813/rs999737 SNP and HER2-positive tumors with per-allele OR of 0.91 (95% CI = 0.86–0.95) for HER2-negative and 0.85 (95% CI = 0.78–0.92) for HER2-positive tumors (case-only P-heterogeneity = 0.08, Table 1). There was still some suggestion of a difference by HER2 status among ER/PR-positive tumors (case-only P-heterogeneity = 0.02, Table 2); however, there was no suggestion of differences among ER/PR-negative cases by HER2 expression (case-only P-heterogeneity = 0.56, Table 2).
Table 1.
Per-allele OR and 95% CIs for the association of SNPs at 1p11.2 rs11249433 and 14q24.1 (RAD51L1) rs10483813 or rs999737 and breast cancer risk by ER, PR and HER2 tumor expression for cases and controls reporting European Caucasian ancestry
| Locus | SNP | Case–control |
P | Case-only P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI |
P | n | OR | 95% CI |
||||||
| ER+ tumors versus controls | ER− tumors versus controls | |||||||||||
| 1p11.2 | rs11249433 | 16 874 | 1.13 | 1.10 | 1.16 | 3.71E–18 | 5099 | 1.03 | 0.98 | 1.07 | 0.21 | 7.6 E–05 |
| 14q24.1 | rs10483813 or rs999737 | 16 693 | 0.90 | 0.87 | 0.93 | 1.32E–09 | 5060 | 0.93 | 0.88 | 0.98 | 0.004 | 0.42 |
| PR+ tumors versus controls | PR− tumors versus controls | |||||||||||
| 1p11.2 | rs11249433 | 12 708 | 1.13 | 1.10 | 1.17 | 7.55E–16 | 6624 | 1.07 | 1.03 | 1.11 | 0.001 | 0.007 |
| 14q24.1 | rs10483813 or rs999737 | 12 545 | 0.91 | 0.88 | 0.95 | 7.28E–07 | 6582 | 0.90 | 0.86 | 0.94 | 0.00001 | 0.42 |
| HER2− tumors versus controls | HER2+ tumors versus controls | |||||||||||
| 1p11.2 | rs11249433 | 7138 | 1.11 | 1.06 | 1.15 | 8.36E–07 | 1964 | 1.06 | 0.99 | 1.13 | 0.09 | 0.231 |
| 14q24.1 | rs10483813 or rs999737 | 7137 | 0.91 | 0.86 | 0.95 | 5.84E–05 | 1956 | 0.85 | 0.78 | 0.92 | 1.04E–04 | 0.077 |
Analysis included a maximum of 35 209 controls and 22 116 cases with genotypes and ER status (cases-only); 35 210 controls and 19 471 cases for PR analysis; 28 194 controls and 9 178 cases for HER2. Differences in total number are due to missing genotype data. ORs are adjusted by study and are for European Caucasians only. Case-only P-value was used to test for heterogeneity, and was estimated using a polytomous logistic regression model with receptor status as the outcome adjusted by study.
Table 2.
Per-allele ORs and 95% CIs for the association of SNPs at 1p11.2–rs11249433 and 14q24.1 (RAD51L1) rs10483813 or rs999737 and breast cancer risk by ER. PR and HER2 expression in tumors for cases and controls reporting European Caucasian ancestry
| Locus | SNP | Case–control |
Case-only P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI |
P | n | OR | 95% CI |
P | |||||
| ER+/PR+ and HER2− | ER+/PR+ and HER2+ | |||||||||||
| 1p11.2 | rs11249433 | 5834 | 1.10 | 1.06 | 1.15 | 4.58E–06 | 1296 | 1.09 | 1.00 | 1.18 | 0.037 | 0.80 |
| 14q24.1 | rs10483813 or rs999737 | 5828 | 0.92 | 0.88 | 0.97 | 0.002 | 1296 | 0.82 | 0.74 | 0.90 | 0.0001 | 0.02 |
| Triple-negative tumors | ER−, PR− and HER2+ | |||||||||||
| 1p11.2 | rs11249433 | 1155 | 1.07 | 0.98 | 1.17 | 0.11 | 635 | 1.02 | 0.91 | 1.14 | 0.71 | 0.49 |
| 14q24.1 | rs10483813 or rs999737 | 1160 | 0.89 | 0.80 | 0.98 | 0.02 | 627 | 0.93 | 0.82 | 1.07 | 0.30 | 0.56 |
Analysis included a maximum of 28 194 controls and 8997 cases. ORs are adjusted by study and are for European Caucasians only. Case-only P-value was used to test for heterogeneity, and was estimated using a polytomous logistic regression model comparing ER+/PR+ and HER2+ versus ER+/PR+ and HER2–tumors and triple-negative versus ER−/PR− and HER2+ tumors, respectively. Differences in total number are due to missing genotype data.
Analyses of 1p11.2 SNP rs11249433 and 14q24.1 (RAD51L1) rs10483813/rs999737 SNPs by other tumor characteristics
The 1p11.2 rs11249433 SNP showed a stronger association with tumors of lower grade (P= 7 × 10−6; Table 3). There was some indication of a higher risk for low-grade rather than higher grade ER-positive tumors (adjusted case-only P= 6.7 × 10−3; Table 3), and no association with ER-negative tumors of any grade (adjusted case-only P= 0.99; Table 3). There was no difference in risk by grade for the rs10483813/rs999737 14q24.1 (RAD51L1) SNPs (Table 3). There was evidence of a higher risk for ER-positive tumors of lobular compared with ductal tumors for rs11249433 (1p11.2) (P= 0.01; Table 3), but no evidence for such differences in risk for rs10483813/rs999737 (P= 0.81; Table 3). We found no evidence of heterogeneity for risk associated with 1p11.2 SNP rs11249433 and 14q24.1 (RAD51L1) rs10483813/rs999737 SNPs by node status or tumor size (Tables 4 and 5).
Table 3.
Per-allele ORs and 95% CI for the association of SNPs at 1p11.2–rs112494331p11.2 rs11249433 and 14q24.1 (RAD51L1) rs10483813 or rs999737 and breast cancer risk by tumor grade and histology stratified by ER tumor expression
| Locus | SNP | Cases (grade) |
Grade 1 |
Grade 2 |
Grade 3 |
Case-only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | OR | 95% CI | OR | 95% CI | OR | 95% CI | P | |||||
| All tumors | ||||||||||||||
| 1p11.2 | rs11249433 | 5222 | 10952 | 7471 | 1.18 | 1.13 | 1.23 | 1.12 | 1.09 | 1.16 | 1.05 | 1.01 | 1.09 | 7.01E–06 |
| 14q24.1 | rs10483813 or rs999737 | 5193 | 10851 | 7301 | 0.88 | 0.84 | 0.93 | 0.93 | 0.9 | 0.97 | 0.91 | 0.87 | 0.95 | 0.51 |
| ER-positive tumors only | ||||||||||||||
| 1p11.2 | rs11249433 | 3697 | 7307 | 3204 | 1.18 | 1.13 | 1.25 | 1.13 | 1.09 | 1.17 | 1.08 | 1.02 | 1.14 | 6.67E–03 |
| 14q24.1 | rs10483813 or rs999737 | 3680 | 7244 | 3111 | 0.87 | 0.82 | 0.92 | 0.92 | 0.88 | 0.96 | 0.89 | 0.83 | 0.95 | 0.50 |
| ER-negative tumors only | ||||||||||||||
| 1p11.2 | rs11249433 | 286 | 1120 | 2618 | 1.01 | 0.85 | 1.2 | 1.03 | 0.94 | 1.12 | 1.01 | 0.95 | 1.07 | 0.99 |
| 14q24.1 | rs10483813 or rs999737 | 285 | 1116 | 2588 | 1.06 | 0.87 | 1.29 | 0.92 | 0.83 | 1.02 | 0.89 | 0.83 | 0.96 | 0.19 |
| Cases (histology) | Ductal | Lobular | Other | Ductal/lobular | ||||||||||
| Locus | SNP | Ductal | Lobular | Other | OR | 95% CI | OR | 95% CI | OR | 95% CI | P | |||
| All tumors | ||||||||||||||
| 1p11.2 | rs11249433 | 19 197 | 3742 | 2381 | 1.10 | 1.07 | 1.13 | 1.21 | 1.16 | 1.28 | 1.11 | 1.04 | 1.18 | 0.0001 |
| 14q24.1 | rs10483813 or rs999737 | 18 940 | 3709 | 2361 | 0.91 | 0.88 | 0.94 | 0.91 | 0.85 | 0.96 | 0.92 | 0.86 | 0.99 | 0.81 |
| ER-positive tumors only | ||||||||||||||
| 1p11.2 | rs11249433 | 10 558 | 2460 | 972 | 1.12 | 1.09 | 1.16 | 1.22 | 1.15 | 1.29 | 1.09 | 1.00 | 1.20 | 0.01 |
| 14q24.1 | rs10483813 or rs999737 | 10 398 | 2443 | 962 | 0.91 | 0.88 | 0.95 | 0.89 | 0.83 | 0.95 | 0.90 | 0.81 | 1.01 | 0.56 |
| ER-negative tumors only | ||||||||||||||
| 1p11.2 | rs11249433 | 3544 | 296 | 399 | 1.01 | 0.96 | 1.07 | 1.16 | 0.99 | 1.37 | 1.04 | 0.90 | 1.20 | 0.15 |
| 14q24.1 | rs10483813 or rs999737 | 3516 | 292 | 395 | 0.93 | 0.88 | 0.99 | 0.85 | 0.70 | 1.04 | 0.93 | 0.78 | 1.10 | 0.27 |
Analysis included a maximum of 35 082 controls and max. 23 800 cases with genotypes, and grade status (cases only); and 33 535 controls and max. 25 458 cases with genotypes, and histopathology information (cases only). ORs are adjusted by study and are for European Caucasians only. Case-only P-value was used to test for heterogeneity, and was estimated using a polytomous logistic regression model with ductal histology as the referent.
Table 4.
Per-allele ORs and 95% CIs for the association of SNPs at 1p11.2 rs11249433 and 14q24.1 (RAD51L1) rs10483813 or rs999737 and breast cancer risk by node status
| Locus | SNP | Case–control |
Case-only P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Node-positive tumors versus controls |
Node-negative tumors versus controls |
|||||||||
| Node + cases | OR | 95% CI | P | Node− cases | OR | 95% CI | P | |||
| 1p11.2 | rs11249433 | 8868 | 1.11 | 1.05–1.16 | 1.05E−04 | 13,747 | 1.11 | 1.07–1.16 | 1.8 E−07 | 0.80 |
| 14q24.1 | rs10483813 or rs999737 | 8798 | 0.90 | 0.87–0.93 | 1.32E−09 | 13,520 | 0.94 | 0.89–0.98 | 0.01 | 0.53 |
Analysis included a maximum of 33 284 controls and max 22 755 cases with genotypes, and node information (cases-only). ORs are adjusted by study and are for European Caucasians only. Case-only P-value was used to test for heterogeneity, and was estimated using a polytomous logistic regression model with node-positive status as the referent.
Table 5.
Per-allele ORs and 95% CIs for the association of SNPs at 1p11.2 rs11249433 and 14q24.1 (RAD51L1) rs10483813 or rs999737 and breast cancer risk by tumor size
| Locus | SNP | Cases (tumor size) |
Size ≤1 cm |
Size >1–2 cm |
Size >2 cm |
Case-only P | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤1cm | >1–2 cm | >2 cm | OR | 95% CI |
P | OR | 95% CI |
P | OR | 95% CI |
P | ||||||
| 1p11.2 | rs11249433 | 3910 | 8716 | 7448 | 1.14 | 1.09 | 1.20 | 1.16E−07 | 1.09 | 1.05 | 1.13 | 2.81E−06 | 1.12 | 1.07 | 1.16 | 1.02E−08 | 0.72 |
| 14q24.1 | rs10483813 or rs999737 | 3807 | 8581 | 7375 | 0.95 | 0.90 | 1.01 | 0.08 | 0.90 | 0.86 | 0.94 | 1.33E−06 | 0.91 | 0.87 | 0.95 | 3.18E−05 | 0.49 |
Analysis included max. 30 771 controls and max. 20 193 cases with genotypes, and tumor size information (cases-only). ORs are adjusted by study and are for European Caucasians only. Case-only P-value was used to test for heterogeneity, and was estimated using a polytomous logistic regression models constraining the effect size to increase linearly across levels.
DISCUSSION
Our large study has confirmed the associations with breast cancer risk for both rs11249433 SNP at 1p11.2 and rs10483813/rs999737 at 14q24.1 and refined the risk estimates by clinically important tumor characteristics. The estimated ORs for rs11249433 for women of European ancestry were lower than reported by Thomas et al. (4) (Thomas et al. reported heterozygote OR = 1.16 versus BCAC OR = 1.09; and homozygote OR = 1.30 versus BCAC OR = 1.22). The estimated homozygote OR for rs10483813/rs999737 was also attenuated toward null in this study (Thomas et al. reported heterozygote OR = 0.94 versus BCAC OR = 0.93; and homozygote OR = 0.70 versus BCAC OR = 0.82). This attenuation may reflect an overestimation in the initial GWAS reports due to ‘winner's curse’.
In addition to the estimates of association for European women, we also estimated risks for Asian women based on 3462 cases and 2463 controls from four studies. Neither locus showed evidence for an association in this group, but the estimated per-allele ORs for Asians were both consistent with that reported for Europeans. The wide confidence intervals in Asians were due to the smaller sample size but also the low minor allele frequencies in (both MAF < 3%). Future studies involving larger numbers of subjects of other race/ethnicities will be necessary to clarify the issue of consistency of findings across racial/ethnic groups.
For the 1p11.2 rs11249433 SNP, we found evidence for a greater OR for ER-positive versus ER-negative disease, consistent with the initial report (4). Thomas et al. reported a P value of 0.001 for heterogeneity from case-only analysis for this same SNP. This observation was based on 6586 cases, 1314 of which were ER-negative in the initial GWAS report. We investigated the association of these SNPs and ER expression based on 22 116 cases; of which, 5099 were ER-negative and found little evidence of any association with ER-negative disease. Our data showed that the 1p11.2 locus was most strongly associated with ER-positive tumors that are of low grade and lobular histology, which are more likely to be screen-detected and tend to have good prognosis. In contrast, rs10483813/rs999737 was associated with multiple tumor types, and showed little evidence for a difference in OR by tumor characteristics except for potentially HER2 expression. In particular, the SNP showed clear evidence for an association with both ER-positive and ER-negative disease and refutes the initial finding reported by Thomas et al. Our results by ER status are also consistent with parallel findings assessing modification of risk in BRCA1/2 carriers by The Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA), which show that rs11249433 modifies risk of BRCA2 carriers but rs10483813/rs999737 has no apparent association with risk on the background of familial risk conferred by BRCA1 or BRCA2 mutations (19). Further, our estimates for risk of DCIS suggested similar effects to invasive disease for the14q24.1 region, which we did not observe for the 1p11.2 region. Together, these data do not support the previous report that the 14q24.1 rs10483813/rs999737 SNP associations are stronger for ER-positive breast cancer (4), and rather our data indicate that this locus confers susceptibility to various subtypes of breast cancer.
The rs11249433 SNP locus is located in a relatively large non-genic region of high linkage disequilibrium (LD) very close to the centromere of chromosome 1, a region notoriously difficult to map. The closest neighboring genes to this SNP are genes in the low-affinity Fc gamma receptor family, FCGR1B, and the transmembrane protein coding gene NOTCH2. SNPs in this region have recently been associated with type 2 diabetes (20). Recent pooled analysis have shown diabetes and related conditions to increase risk of death for breast cancer (21); however, epidemiological studies of type 2 diabetes and breast cancer risk have given mixed results (22–24). A recent study found some evidence of increased NOTCH2 expression in breast tumors in carriers of the C allele of rs11249433, suggesting that the breast cancer susceptibility at this locus may be mediated through variation in NOTCH2 expression (25).
Both rs999737 and rs10483813 lie within an LD block in intron 10 of RAD51L1 (also known as RAD51B). RAD51L1 is a member of the Rad51-like proteins that are involved in double-strand break (DSB) repair and homologous recombination (26). Rare mutations in other genes in this pathway (notably BRCA1 and BRCA2) predispose to high risks of breast cancer, and most recently common susceptibility variants in another DSB repair genes (near MERIT40 on chromosome 19p13) have been shown to modify risk for BRCA1 mutation carriers (27). Assuming that the risk association is mediated through an effect on RAD51L1 expression/function, the identification and confirmation of the 14q24.1 (RAD51L1) locus increases the number of genes within the repair pathway that may be important for susceptibility to breast and other cancers.
The analyses presented here have resulted in refined relative risk estimates on the largest sample size to date for overall breast cancer risk and risk for specific tumor subtypes, a very important consideration for low-risk alleles of modest effect that will, in the future, be used together in risk models to assess the likelihood that women will be predisposed to breast cancer. Our analyses of these two loci highlight the notion that some susceptibility factors are more strongly associated with specific subtypes (e.g. 1p11.2 SNPs are more strongly associated with ER-positive tumors of low grade and lobular histology), while other loci are associated across different subtypes of breast cancer (e.g. 14q24.1). These findings demonstrate the importance of conducting large studies with tumor pathology data in order to refine risk estimates for all risk-associated SNPs identified by GWAS and other studies, to provide the most robust SNP risk models possible for assessing predisposition to different types of breast cancer.
Key strengths of our study are its large sample size, and data on tumor characteristics. Our study had >80% power at P< 0.05 to detect an OR of 1.1 for ER and PR subtype analysis and 70% power for the rarer HER2+ breast cancers. A limitation is the use of non-standardized data on tumor markers since data were derived from studies using different tissue collection and processing protocols, immunohistochemical assays, and criteria for pathology review. Nevertheless, we observed consistent associations across studies, indicating that our findings are robust and highlight that breast tumors are etiologically distinct. Further genetic mapping and functional analyses will be required to determine the genetic variants underlying both these susceptibility loci signals, and to delineate the biological pathways involved in susceptibility to different subtypes of breast cancer.
MATERIALS AND METHODS
Study samples
Thirty-nine breast cancer studies participating in BCAC contributed data for cases and controls for the 1p11.2 SNP rs11249433, and at least one of the two highly correlated SNPs rs10483813 or rs999737 at14q24.1 (RAD51L1) (see Supplementary Material, Table S1 for a list of studies and abbreviations, and a more detailed description of participating studies). After excluding subjects that did not report to be of European or Asian ancestry, the number of subjects available for analysis was 46 036 invasive breast cancer cases, and 46 930 controls from case–control or prospective cohort studies. Data on age and race/ethnicity of participants was provided by each study. Primary analysis estimated per-allele OR for Europeans and Asian separately. Thirty-six studies from Europe, North America and Australia included predominantly women of white European ancestry. Except for the NC-BCFR study, women whose reported race/ethnicity was non-European were excluded from analyses. The NC-BCFR study had >100 subjects reporting European or Asian ancestry, and was separated into two groups for analysis: NC-BCFR whites and NC-BCFR Asians. Analyses of Asian women included four studies, one each from the USA (NC-BCFR Asians), Korea (SEBCS), Taiwan (TWBCS) and Thailand (TBCS). We also had data on 1397 cases with ductal carcinoma in situ (DCIS) from 24 studies from women of European descent.
Pathology and tumor markers
The final numbers available for analysis were 46 036 invasive breast cancer cases and 46 930 controls from 39 studies and pathology data included in each analysis are shown in Tables 1–5. Of the 36 studies that reported women of European ancestry, the majority provided information on histopathologic subtype (24 studies: 76% ductal, 15% lobular, 9% other histologies), grade of differentiation (25 studies; 22% grade 1, 46% grade 2 and 32% grade 3 or higher), tumor size (21 studies: 19% with the size of 1 cm or less, 43% with the size of >1–2 cm and 37% with the size of >2 cm) and nodal involvement (26 studies: 60% node positive). Twenty-six studies provided data on ER and PR status and 18 on HER2 status.
Genotyping
Genotyping for three SNPs (rs11249433, rs10483813 and rs999737) was performed in the framework of BCAC as described previously (10–13,15). Most studies carried out genotyping using Taqman nuclease assay (Taqman®), with reagents designed by Applied Biosystems (http://www.appliedbiosystems.com/) as Assays-by-Design™ and genotyping performed using the ABI PRISM 7900HT, 7700 or 7500 Sequence Detection Systems according to manufacturer's instructions. A few studies (GENICA, HEBCS, kConFab/AOCS, LMBC, MBCSG and SASBAC) used the Sequenom iPLEX MassARRAYTM system (Sequenom, San Diego, CA, USA) with oligonucleotide design performed using MassARRAY Assay Design software (version 3.1). Genotyping platform used by each study are indicated in Supplementary Material, Table S2.
Out of 40 studies that performed the genotyping, data from only one study were excluded due to not meeting the BCAC quality control (QC) guidelines: (i) individual samples were excluded based on the number of SNPs that were typed in this phase of genotyping by each study, which were three SNPs (rs11249433, rs10483813/rs999737 and rs2046210). Any given sample was excluded if it failed genotyping for two of the three SNPs. (ii) All samples on any one plate were excluded if the plate had a SNP call rate <90%; (iii) all genotype data for any SNP were excluded if the overall call rate was <95%; or data for any SNP where duplicate concordance was <98%. For any SNP for which the P-value for departure from Hardy-Weinberg equilibrium for controls was <0.005, clustering of the intensity plots was reviewed manually by a single person and clustering was judged to be fine. In addition, all genotyping centers assayed an identical plate of 94 control CEPH DNA samples referred to as the Coriell plate (HAPMAPPT01, Coriell Institute for Medical Research, Cambden, NJ, USA); which also included five internal duplicates. Studies had to achieve a call rate >90% and concordance >98% in order for their data to be included. After applying these QC guidelines, data were available for a total of 39 studies (see Supplementary Material, Table S1). For the 14q24.1 SNP data, 33 studies genotyped rs10483813, and five studies (GENICA, HEBCS, LMBC, SASBAC and KCONFAB-AOCS) genotyped rs999737. One study from Italy (MBCSG) genotyped both SNPs (r2= 0.98, based on 1217 control samples, Supplementary Material, Table S3). For the MBCSG study, data for rs10483813 were used in the analysis.
Statistical analysis
Departure from Hardy–Weinberg equilibrium was tested for controls from each center using Pearson's χ2-test with 1df. We presented the association of each SNP with breast cancer risk assessed by meta-analysis using genotype frequencies in cases and controls. We also performed multiple logistic regression adjusted for study which gave similar results to meta-analysis (data not shown). For each SNP, we performed one analysis estimating the separate odds ratios (ORs) and 95% confidence intervals (CIs) for heterozygotes and homozygote variants relative to the common-allele homozygotes, and another analysis assuming a log-additive model to estimate the OR per variant allele, assuming a log-additive model. Between-study heterogeneity in OR was expressed using the I2 statistic. Polytomous logistic regression was used to estimate the OR for each breast cancer subtype (comparing case subtypes with all controls). OR and 95% CI were estimated assuming a log-additive model for the association with genotype, adjusted by study. Heterogeneity between genotype OR for different tumor subtypes was assessed using logistic regression analyses restricted to cases (case-only analyses) with the tumor characteristic as the outcome variable. For tumor subtypes with more than two levels (i.e. grade and size), we used a polytomous logistic regression model constraining the strength of association to increase linearly across levels (e.g. the parameter for grade 3 versus grade 1 was constrained to be twice that for grade 2 versus grade 1). All statistical tests were two-sided. To test if the per-allele ORs differed by age or family history, a likelihood ratio test was used from fitting logistic regression models with and without interaction terms. All analyses were carried out using Stata: Release 9 (College Station, TX, USA).
SUPPLEMENTARY MATERIAL
FUNDING AND ACKNOWLEDGEMENTS
The Breast Cancer Association Consortium (BCAC) was supported by Cancer Research UK grant C1287/A12014. D.F.E. is a Principal Research Fellow of Cancer Research UK.
The ABCFS was supported by the National Health and Medical Research Council of Australia (NHMRC) [145604], the NIH [CA102740-01A2], and by the United States National Cancer Institute, NIH [CA-95-011] through cooperative agreements with members of the Breast Cancer Family Registry and principal investigators Cancer Care Ontario [CA69467], Columbia University [CA69398], Fox Chase Cancer Center [CA69631], Huntsman Cancer Institute [CA69446], Northern California Cancer Center [CA69417] and University of Melbourne [CA69638]. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of collaborating centers in the Breast CFR, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government or the Breast CFR. The ABCFS was initially supported by the NHMRC, the New South Wales Cancer Council and the Victorian Health Promotion Foundation. J.L.H. is an Australia Fellow of the NHMRC and Victorian Breast Cancer Research Consortium Group Leader. M.C.S. is a Senior Research Fellow of the NHMRC and Victorian Breast Cancer Research Consortium Group Leader. This research was supported by the Victorian Government through Victorian Cancer Agency funding of the Victorian Breast Cancer Research Consortium.
The ABCS study was supported by the Dutch Cancer Society [grants NKI 2001–2423; 2007–3839] and the Dutch National Genomics Initiative. ABCS acknowledges the Family Cancer Clinic at the NKI-AVL and Richard van Hien and Sten Cornelissen for DNA plating.
The BBCC study was partly funded by the ELAN Funding of the University of Erlangen.
The BBCS is funded by Cancer Research UK and Breakthrough Breast Cancer and acknowledges NHS funding to the NIHR Biomedical Research Centre, and the National Cancer Research Network (NCRN).
The BSUCH study was supported by the Dietmar-Hopp Foundation and the Helmholtz Society.
The CGPS Funding: The CGPS was supported by the Chief Physician Johan Boserup and Lise Boserup Fund, the Danish Medical Research Council and Herlev Hospital. Acknowledgements: We thank all the individuals who took part in these studies and all the researchers, clinicians, technicians and administrative staff who have enabled this work to be carried out.
The CNIO-BCS was supported by the Genome Spain Foundation, the Red Temática de Investigación Cooperativa en Cáncer and grants from the Asociación Española Contra Cáncer and the Fondo de Investigación Sanitario [PI081120 to J.B., PI081583 to R.L.M.]. We thank Charo Alonso, Tais Moreno, Guillermo Pita, Primitiva Menendez and Anna González-Neira.
FBCS Cancer Research UK (C8620/A83); US Military Acquisition (ACQ) Activity, Era of Hope Award (W81XWH-05–1–0204) The samples were collected and screened for BRCA mutations through funding from Cancer Research UK; US Military Acquisition (ACQ) Activity, Era of Hope Award (W81XWH-05–1–0204) and the Institute of Cancer Research (UK). This study makes use of data generated by the Wellcome Trust Case Control Consortium (WTCCC) 2. A full list of the investigators who contributed to the generation of the data is available from the WTCCC website. C.T. is funded by a Medical Research Council (UK) Clinical Research Fellowship.
The GENICA study was supported by the German Human Genome Project and funded by the Federal Ministry of Education and Research (BMBF) Germany grants 01KW9975/5, 01KW9976/8, 01KW9977/0 and 01KW0114. Genotyping analyses were supported by Robert Bosch Foundation of Medical Research, Stuttgart, Germany. The GENICA network would also like to acknowledge 1. Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, and University of Tübingen, Germany; [C.J., H.B.]; Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany [Yon-Dschun Ko, Christian Baisch]; Institute of Pathology, University of Bonn, Bonn, Germany [Hand-Peter Fischer]. Molecular Genetics of Breast Cancer, Deutsches Krebsforschungszentrum (DKFZ), Heidelberg, Germany [Ute Hamann]; Institute for Prevention and Occupational Medicine of the German Social Accident Insurance (IPA), Bochum, Germany [TB, Beate Pesch, Sylvia Rabstein, Volker Harth].
The GESBC study was supported by the Deutsche Krebshilfe e. V. [70492] and GESBC genotyping in part by the state of Baden-Württemberg through the Medical Faculty of the University of Ulm [P.685].
The HABCS study was supported by an intramural grant from Hannover Medical School and by a grant from the German Research Foundation [DFG, Do761/2–1].
The HEBCS study has been financially supported by the Helsinki University Central Hospital Research Fund, Academy of Finland (132473), the Finnish Cancer Society, and the Sigrid Juselius Foundation.
HUBCS was supported by a grant from the German Federal Ministry of Research and Education (RUS08/017).
The KARBAC study was supported by The Swedish Cancer Society, The Stockholm Cancer Society, The Gustav V Jubilee Foundation and The Bert von Kantzow Foundation.
KConFab/AOCS: kConFab (the Kathleen Cuningham Consortium for Research into Familial Breast Cancer) thanks Heather Thorne, Eveline Niedermayr, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the Clinical Follow-Up Study (funded by NHMRC grants 145684, 288704 and 454508) for their contributions to this resource, and the many families who contribute to kConFab. kConFab is supported by grants from the National Breast Cancer Foundation, the National Health and Medical Research Council (NHMRC) and by the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. The Australian Ovarian Cancer Study (AOCS) Management Group (D. Bowtell, G. Chenevix-Trench, A. deFazio, D. Gertig, A. Green and P.M. Webb) gratefully acknowledges the contribution of all the clinical and scientific collaborators (see http://www.aocstudy.org/). The Australian Cancer Study Management Group (A. Green, P. Parsons, N. Hayward, P.M.Webb, and D. Whiteman) thank all of the project staff, collaborating institutions and study participants. A.B.S. is an NHMRC Senior Research Fellow, and G.C.T. is an NHMRC Senior Principal Research Fellow.
The KBCP was supported by Grants from the Finnish Cancer Society; the Academy of Finland (grant number 127220); EVO Research Fund (grant number 5654113 and 5501); EVO research funding of Vaasa Hospital District (grant number 100449); and the strategic funding of the University of Eastern Finland. We thank Mrs. Helena Kemiläinen, Mrs Aija Parkkinen and Mrs. Eija Myöhänen for their skillful technical assistance.
The LMBC Leuven Multidisciplinary Breast Center (LMBC) is supported by the ‘Stichting tegen Kanker’ (232–2008). B.T.Y is funded by FWO. We acknowledge Gilian Peuteman, Dominiek Smeets, Thomas Van Brussel for technical support.
MBCSG is supported by grants from Ministero della Salute (Extraordinary National Cancer Program 2006 ‘Alleanza contro il Cancro’, and ‘Progetto Tumori Femminili’ to P.R.), Ministero dell'Universita’ e Ricerca (RBLAO3-BETH to P.R.), Fondazione Italiana per la Ricerca sul Cancro (Special Project ‘Hereditary tumors’), Associazione Italiana per la Ricerca sul Cancro (4017 and by funds from Italian citizens who allocated the 5 × 1000 share of their tax payment in support of the Fondazione IRCCS Istituto Nazionale Tumori, according to Italian laws (INT-Institutional strategic projects ‘‘5 × 1000’).
The MCBCS was supported by National Institutes of Health grant, R01 CA122340, and an NCI Specialized Program of Research Excellence (SPORE) in breast cancer, P50 CA116201.
The MCCS study was supported by Cancer Council Victoria and by NHMRC grants 209057, 251533, 396414, 504711 and 504715. The MEC study was supported by National Institutes of Health grants R01-CA63464 and R37-CA54281.
The MSKCC study was supported by the Breast Cancer Research Foundation, the Normal and Carol Stone Genetics Research Fund, and the Robert, the Robert and Kate Niehaus Clinical Genetics Initiative and the Lymphoma Foundation.
The NC-BCFR and OFBCR are funded by the National Cancer Institute, National Institutes of Health under RFA-CA-06-503 and through cooperative agreements with members of the Breast Cancer Family Registry (BCFR) and Principal Investigators, including Cancer Care Ontario (U01 CA69467), the Cancer Prevention Institute of California (formerly the Northern California Cancer Center, U01 CA69417) and the University of Melbourne (U01 CA69638). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the BCFR, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government or the BCFR.
OBCS was supported by research grants from the Finnish Cancer Foundation, the Sigrid Juselius Foundation, the Academy of Finland, the University of Oulu, and the Oulu University Hospital.
The OFBCR was supported by the National Cancer Institute, National Institutes of Health under RFA # CA- 06-503 and through cooperative agreements with members of the Breast Cancer Family Registry (BCFR) and Principal Investigators, including Cancer Care Ontario (U01 CA69467), Northern California Cancer Center (U01 CA69417) and University of Melbourne (U01 CA69638) and by Cancer Care Ontario. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the BCFR, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government or the BCFR.
The PBCS was supported by Intramural Research Funds of the National Cancer Institute, Department of Health and Human Services, USA.
The RBCS study was supported by Dutch Cancer Society: DDHK 2004-3124. RBCS would like to acknowledge Petra Bos, Jannet Blom, Ellen Crepin, Elisabeth Huijskens and Annette Heemskerk for their contribution in data management.
The SASBAC study was supported by funding from the Agency for Science, Technology and Research of Singapore (A*STAR), the US National Institute of Health (NIH) and the Susan G. Komen Breast Cancer Foundation.
The SBCS study Funding: Breast Cancer Campaign and Yorkshire Cancer Research. SBCS would like to acknowledge Helen Cramp, Sue Higham, Dan Connley and Saba Balasubramanian.
SEARCH study was supported by Cancer Research UK grants: C490/A1102, C8197/A10123, C490/A10119, C490/A11020, C1287/A10118 and A.M.D. was funded by CR-UK grant C8197/A10865. The pathology work in Cambridge was supported by the NIHR Cambridge Biomedical Research Centre and by the Cambridge Experimental Cancer Medicine Centre.
The SZBCS was supported by Grant PBZ_KBN_122/P05/2004. K.J. is a fellow of International PhD program, Postgraduate School of Molecular Medicine, Warsaw Medical University, supported by the Polish Foundation of Science.
The TBCS was funded by The National Cancer Institute Thailand.
The TWBCS study was supported by the Institute of Biomedical Sciences, Academia Sinica, National Sciences Council and Taiwan Biobank.
The UCIBCS study was supported by the National Institutes of Health, National Cancer Institute grants CA-58860 and the Lon V Smith Foundation grant LVS-39420.
The US3SS study was supported by Massachusetts (K.M.E., R01CA47305), Wisconsin (P.A.N., R01 CA47147) and New Hampshire (L.T.-E., R01CA69664) centers, and Intramural Research Funds of the National Cancer Institute, Department of Health and Human Services, USA.
Supplementary Material
APPENDIX
The list of study abbreviations.
ABCFS: Australian Breast Cancer Family Study; ABCS: Amsterdam Breast Cancer Study; BBCC: Bavarian Breast Cancer Cases and Controls; BBCS: British Breast Cancer Study; BIGGS: Breast Cancer in Galway Genetic Study; BSUCH: Breast Cancer Study of the University of Heidelberg; CGPS: Copenhagen General Population Study; CNIO-BCS: Spanish National Cancer Centre Breast Cancer Study; ESTHER: ESTHER Breast Cancer Study; FBCS: ICR Familial Breast Cancer Study; GENICA: Gene Environment Interaction and Breast Cancer in Germany; GESBC: Genetic Epidemiology Study of Breast Cancer by Age 50; HABCS: Hannover Breast Cancer Study; HEBCS: Helsinki Breast Cancer Study; HMBCS: Hannover-Minsk Breast Cancer Study; HUBCS: Hannover-Ufa Breast Cancer Study; KARBAC: Karolinska Breast Cancer Study; KConFab-AOCS: Kathleen Cuningham Foundation Consortium for research into Familial Breast Cancer/Australian Ovarian Cancer Study; KBCP: Kuopio Breast Cancer Project; LMBC: Leuven Multidisciplinary Breast Centre; MBCSG: Milan Breast Cancer Study Group; MCBCS: Mayo Clinic Breast Cancer Study; MCCS: Melbourne Collaborative Cohort Study; NC-BCFR: Northern California Breast Cancer Family Registry; OBCS: Oulu Breast Cancer Study; OFBCR: Ontario Familial Breast Cancer Registry; PBCS: NCI Polish Breast Cancer Study; RBCS: Rotterdam Breast Cancer Study; SASBAC: Singapore and Sweden Breast Cancer Study; SBCS: Sheffield Breast Cancer Study; SEARCH: Study of Epidemiology and Risk factors in Cancer Heredity; SEBCS: Seoul Breast Cancer Study; SZBCS: IHCC-Szczecin Breast Cancer Study; TBCS: IARC-Thai Breast Cancer Study; TWBCS: Taiwanese Breast Cancer Study; UCIBCS: UCI Breast Cancer Study; UKBGS: UK Breakthrough Generations Study; US3SS: US Three State Study.
REFERENCES
- 1.Easton D.F., Pooley K.A., Dunning A.M., Pharoah P.D., Thompson D., Ballinger D.G., Struewing J.P., Morrison J., Field H., Luben R., et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold B., Kirchhoff T., Stefanov S., Lautenberger J., Viale A., Garber J., Friedman E., Narod S., Olshen A.B., Gregersen P., et al. Genome-wide association study provides evidence for a breast cancer risk locus at 6q22.33. Proc. Natl Acad. Sci. USA. 2008;105:4340–4345. doi: 10.1073/pnas.0800441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter D.J., Kraft P., Jacobs K.B., Cox D.G., Yeager M., Hankinson S.E., Wacholder S., Wang Z., Welch R., Hutchinson A., et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat. Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas G., Jacobs K.B., Kraft P., Yeager M., Wacholder S., Cox D.G., Hankinson S.E., Hutchinson A., Wang Z., Yu K., et al. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1) Nat. Genet. 2009;41:579–584. doi: 10.1038/ng.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull C., Ahmed S., Morrison J., Pernet D., Renwick A., Maranian M., Seal S., Ghoussaini M., Hines S., Healey C.S., et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat. Genet. 2010;42:504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng W., Long J., Gao Y.T., Li C., Zheng Y., Xiang Y.B., Wen W., Levy S., Deming S.L., Haines J.L., et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat. Genet. 2009;41:324–328. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stacey S.N., Manolescu A., Sulem P., Rafnar T., Gudmundsson J., Gudjonsson S.A., Masson G., Jakobsdottir M., Thorlacius S., Helgason A., et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat. Genet. 2007;39:865–869. doi: 10.1038/ng2064. [DOI] [PubMed] [Google Scholar]
- 8.Stacey S.N., Manolescu A., Sulem P., Thorlacius S., Gudjonsson S.A., Jonsson G.F., Jakobsdottir M., Bergthorsson J.T., Gudmundsson J., Aben K.K., et al. Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat. Genet. 2008;40:703–706. doi: 10.1038/ng.131. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed S., Thomas G., Ghoussaini M., Healey C.S., Humphreys M.K., Platte R., Morrison J., Maranian M., Pooley K.A., Luben R., et al. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat. Genet. 2009;41:585–590. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the Breast Cancer Association Consortium. J. Natl. Cancer Inst. 2006;98:1382–1396. doi: 10.1093/jnci/djj374. [DOI] [PubMed] [Google Scholar]
- 11.Cox A., Dunning A.M., Garcia-Closas M., Balasubramanian S., Reed M.W., Pooley K.A., Scollen S., Baynes C., Ponder B.A., Chanock S., et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat. Genet. 2007;39:352–358. doi: 10.1038/ng1981. [DOI] [PubMed] [Google Scholar]
- 12.Dunning A.M., Healey C.S., Baynes C., Maia A.T., Scollen S., Vega A., Rodriguez R., Barbosa-Morais N.L., Ponder B.A., Low Y.L., et al. Association of ESR1 gene tagging SNPs with breast cancer risk. Hum. Mol. Genet. 2009;18:1131–1139. doi: 10.1093/hmg/ddn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnatty S.E., Couch F.J., Fredericksen Z., Tarrell R., Spurdle A.B., Beesley J., Chen X., Gschwantler-Kaulich D., Singer C.F., Fuerhauser C., et al. No evidence that GATA3 rs570613 SNP modifies breast cancer risk. Breast Cancer Res. Treat. 2009;117:371–379. doi: 10.1007/s10549-008-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt M.K., Reincke S., Broeks A., Braaf L.M., Hogervorst F.B., Tollenaar R.A., Johnson N., Fletcher O., Peto J., Tommiska J., et al. Do MDM2 SNP309 and TP53 R72P interact in breast cancer susceptibility? A large pooled series from the Breast Cancer Association Consortium. Cancer Res. 2007;67:9584–9590. doi: 10.1158/0008-5472.CAN-07-0738. [DOI] [PubMed] [Google Scholar]
- 15.Milne R.L., Benitez J., Nevanlinna H., Heikkinen T., Aittomaki K., Blomqvist C., Arias J.I., Zamora M.P., Burwinkel B., Bartram C.R., et al. Risk of estrogen receptor-positive and -negative breast cancer and single-nucleotide polymorphism 2q35-rs13387042. J. Natl. Cancer Inst. 2009;101:1012–1018. doi: 10.1093/jnci/djp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Closas M., Hall P., Nevanlinna H., Pooley K., Morrison J., Richesson D.A., Bojesen S.E., Nordestgaard B.G., Axelsson C.K., Arias J.I., et al. Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet. 2008;4:e1000054. doi: 10.1371/journal.pgen.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudet M.M., Milne R.L., Cox A., Camp N.J., Goode E.L., Humphreys M.K., Dunning A.M., Morrison J., Giles G.G., Severi G., et al. Five polymorphisms and breast cancer risk: results from the Breast Cancer Association Consortium. Cancer Epidemiol. Biomarkers Prev. 2009;18:1610–1616. doi: 10.1158/1055-9965.EPI-08-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank B., Wiestler M., Kropp S., Hemminki K., Spurdle A.B., Sutter C., Wappenschmidt B., Chen X., Beesley J., Hopper J.L., et al. Association of a common AKAP9 variant with breast cancer risk: a collaborative analysis. J. Natl. Cancer Inst. 2008;100:437–442. doi: 10.1093/jnci/djn037. [DOI] [PubMed] [Google Scholar]
- 19.Antoniou A.C., Kartsonaki C., Sinilnikova O.M., Soucy P., McGuffog L., Healey S., Lee A., Peterlongo P., Manoukian S., Peissel B., et al. Common alleles at 6q25.1 and 1p11.2 are associated with breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum. Mol. Genet. 2011;20:3304–3321. doi: 10.1093/hmg/ddr226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeggini E., Scott L.J., Saxena R., Voight B.F., Marchini J.L., Hu T., de Bakker P.I., Abecasis G.R., Almgren P., Andersen G., et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seshasai S.R., Kaptoge S., Thompson A., Di Angelantonio E., Gao P., Sarwar N., Whincup P.H., Mukamal K.J., Gillum R.F., Holme I., et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabat G.C., Kim M., Caan B.J., Chlebowski R.T., Gunter M.J., Ho G.Y., Rodriguez B.L., Shikany J.M., Strickler H.D., Vitolins M.Z., et al. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int. J. Cancer, 2009;125:2704–2710. doi: 10.1002/ijc.24609. [DOI] [PubMed] [Google Scholar]
- 23.Barclay A.W., Petocz P., McMillan-Price J., Flood V.M., Prvan T., Mitchell P., Brand-Miller J.C. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am. J. Clin. Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 24.Xue F., Michels K.B. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am. J. Clin. Nutr. 2007;86:s823–835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 25.Fu Y.P., Edvardsen H., Kaushiva A., Arhancet J.P., Howe T.M., Kohaar I., Porter-Gill P., Shah A., Landmark-Hoyvik H., Fossa S.D., et al. NOTCH2 in breast cancer: association of SNP rs11249433 with gene expression in ER-positive breast tumors without TP53 mutations. Mol. Cancer. 2010;9:113. doi: 10.1186/1476-4598-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lio Y.C., Mazin A.V., Kowalczykowski S.C., Chen D.J. Complex formation by the human Rad51B and Rad51C DNA repair proteins and their activities in vitro. J. Biol. Chem. 2003;278:2469–2478. doi: 10.1074/jbc.M211038200. [DOI] [PubMed] [Google Scholar]
- 27.Antoniou A.C., Wang X., Fredericksen Z.S., McGuffog L., Tarrell R., Sinilnikova O.M., Healey S., Morrison J., Kartsonaki C., Lesnick T., et al. A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat. Genet. 2010;42:885–892. doi: 10.1038/ng.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.