Abstract
Increasing evidence suggests that the accumulation of amyloid beta (Aβ) in synapses and synaptic mitochondria causes synaptic mitochondrial failure and synaptic degeneration in Alzheimer's disease (AD). The purpose of this study was to better understand the effects of Aβ in mitochondrial activity and synaptic alterations in neurons from a mouse model of AD. Using primary neurons from a well-characterized Aβ precursor protein transgenic (AβPP) mouse model (Tg2576 mouse line), for the first time, we studied mitochondrial activity, including axonal transport of mitochondria, mitochondrial dynamics, morphology and function. Further, we also studied the nature of Aβ-induced synaptic alterations, and cell death in primary neurons from Tg2576 mice, and we sought to determine whether the mitochondria-targeted antioxidant SS31 could mitigate the effects of oligomeric Aβ. We found significantly decreased anterograde mitochondrial movement, increased mitochondrial fission and decreased fusion, abnormal mitochondrial and synaptic proteins and defective mitochondrial function in primary neurons from AβPP mice compared with wild-type (WT) neurons. Transmission electron microscopy revealed a large number of small mitochondria and structurally damaged mitochondria, with broken cristae in AβPP primary neurons. We also found an increased accumulation of oligomeric Aβ and increased apoptotic neuronal death in the primary neurons from the AβPP mice relative to the WT neurons. Our results revealed an accumulation of intraneuronal oligomeric Aβ, leading to mitochondrial and synaptic deficiencies, and ultimately causing neurodegeneration in AβPP cultures. However, we found that the mitochondria-targeted antioxidant SS31 restored mitochondrial transport and synaptic viability, and decreased the percentage of defective mitochondria, indicating that SS31 protects mitochondria and synapses from Aβ toxicity.
INTRODUCTION
Alzheimer's disease (AD) is the most common neurodegenerative disorder in the aged population. It is characterized by the progressive decline of memory and cognition, as well as changes in behavior and personality (1). The pathological hallmarks of AD are amyloid plaques and neurofibrillary tangles (1,2). Both plaques and tangles occur late in disease progression, leaving the role as causative agents in neurodegeneration questionable. AD is also associated with inflammatory responses, synaptic damage and mitochondrial structural and functional abnormalities (3–10). The best correlate to loss of memory and cognitive decline is synaptic loss (11–13). However, the molecular events leading to synaptic loss in AD are unknown. Mutations that cause hereditary AD in humans and in animal models of AD indicate that the initial pathogenic event for AD involves abnormal processing of the amyloid beta (Aβ) precursor protein (AβPP) leading to Aβ formation (14).
Aβ is a 38–43 amino acid protein that is produced by the sequential cleavage of AβPP by β-secretase and γ-secretase (2). Aβ exists in multiple forms, forms aggregates and accumulates in different subcellular organelles of neurons in AD patients (15,16). The most prevalent forms of Aβ are Aβ40 and Aβ42; Aβ42 has been shown to be more toxic. Aβ42 is also known to self-aggregate into several sizes and conformations, the smallest of which are oligomers. Aβ42 also appears as multimeric, diffusible, soluble aggregations ranging from dimers to species less than about 100 kDa. Protofibrils are soluble intermediates of Aβ that are >100 kDa, and fibrils represent the earliest forms of insoluble Aβ, which comprises the plaques (17).
It is widely thought that various forms of oligomeric Aβ may be the key initiators of cell death in AD (14,18). Amyloid plaque burden is known to correlate poorly with memory deficits in AD patients; however, synaptic loss is a strong predictor of the clinical symptoms of AD. Oligomeric Aβ is known to be toxic to neurons and can induce synaptic degeneration and loss (19–21). The mechanism by which oligomeric Aβ leads to synaptic degeneration and loss is not clear, but there is strong evidence that mitochondria contribute significantly to the process (21–25).
Highly interrelated changes in mitochondria and synaptic function appear early in AD neurodegeneration (16,26). Monomeric and oligomeric Aβ are known to associate with mitochondrial membranes (27–32) and to interact with the mitochondrial proteins Drp1 (21,27), ABAD (33) and cyclophilin D (34). Oligomeric Aβ has been found to induce free radical production, alter mitochondrial function and decrease ATP synthesis (35). Further, the mitochondrial processes of fission and fusion and of transport are known to be disrupted by oligomeric Aβ (21–25,36–39). These oligomeric, Aβ-induced impairments in mitochondrial function are likely to precede and contribute to synaptic degeneration (16,40).
However, most studies of Aβ that so far used cell-culture models of AD acutely expose primary neurons and/or other mammalian cells to high concentrations of pre-aggregated Aβ (Aβ40, Aβ42 and Aβ25–35) (9,22–25). Although this method is advantageous in that this exposure to specific species of Aβ is controlled, prolonged exposure to low doses of secreted Aβ more directly models the disease. However, there are no published reports that investigate the toxic effects of Aβ in axonal transport of mitochondria, structural and functional alterations of mitochondria in primary neurons derived from AβPP mice. Further, it is still unclear how dysfunctional mitochondria alter synaptic activities in neurons from AβPP mice. For the first time, we studied mitochondrial transport, dynamics and function and synaptic alterations using primary neurons from AβPP mice.
In the current study, we aimed to determine whether mitochondrial dysfunction exists in primary neurons from AβPP mice. We found that AβPP neurons exhibit mitochondrial deficiencies similar to those seen in AD. These deficiencies correlate temporally with an accumulation of Aβ oligomer and increased apoptotic neuronal death in the transgenic cultures. Our results indicate that intraneuronal accumulation of oligomeric Aβ leads to mitochondrial dysfunction, to synaptic deficiencies and, ultimately, to neurodegeneration in primary neurons from AβPP mice. Additionally, we determined whether the mitochondria-targeted antioxidant SS31 could mitigate the effects of oligomeric Aβ. We found that after treatment, mitochondrial transport was largely restored, along with a restoration of synaptic viability.
RESULTS
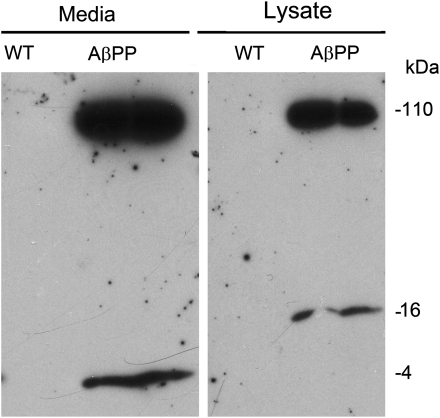
AβPP cultures secrete Aβ into the medium and accumulate a 16 kDa oligomer in neurons
Cell cultures were grown to 16 DIV, at which point both medium and cells were harvested for western blot analysis. Media and cell lysates were immunoblotted with the 6E10 antibody for Aβ. Wild-type (WT) cultures did not show 6E10 staining in either medium or cell lysates. Media from AβPP cultures contained significant amounts of the 4 kDa Aβ monomer, as well as 100 kDa secreted AβPP (Fig. 1). In the cell lysate, we observed 110 kDa full-length AβPP as well as a band at 16 kDa, which corresponded to oligomeric Aβ (Fig. 1).
Figure 1.
Four-kiloDalton Aβ is secreted into the media of AβPP-derived neurons. Twenty-five micrograms of the cell lysate or 10 µl of conditioned cell media from WT and AβPP cultures (16 DIV) was resolved on a 10–20% tricine gel, and AβPP and Aβ proteins were detected using AβPP- and Aβ-specific antibody (6E10, which recognizes Aβ residues 1–16) after following standard immunoblotting procedure. Left panel, media from the AβPP and WT cultures show an accumulation of 4 kDa Aβ monomer and 100 kDa-secreted AβPP. Right panel, cell lysates from AβPP and WT cultures show an accumulation of 110 kDa full-length AβPP and 16 kDa Aβ oligomer.
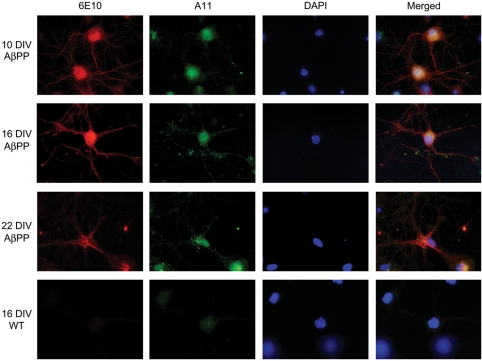
AβPP cultures contain oligomeric Aβ in neurites and cell bodies
At all time points, the cells stained with 6E10 and the A11 antibody revealed a relatively consistent 6E10 immunoreactivity (Fig. 2). However, A11 immunoreactivity increased over time in the cultures. At 10 DIV, no A11 immunoreactivity was seen in AβPP cultures. By 16 DIV, A11 was stained in the neurites, and by 22 DIV, A11 staining was observed in the cell body and neurites. WT cultures did not stain specifically with the A11 antibody.
Figure 2.
Aβ oligomer accumulates in cell neurites and soma over time. Double-immunocytochemistry with the A11 (anti-oligomer: green) and 6E10 (red) antibodies revealed 6E10 immunoreactivity present at all time points examined (10, 16, 22 DIV), indicated by increased staining in the cell body by 22 DIV. A11 immunoreactivity was not seen in appreciable amounts at 10 DIV, but was found extensively throughout neurites at 16 DIV. By 22 DIV, A11 staining was found within the cell body. WT neurons did not exhibit specific staining with the 6E10 or A11 antibodies. Images were all collected with a 100× objective.
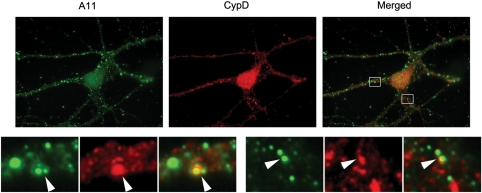
Oligomeric Aβ localizes in mitochondria
With the A11 anti-oligomer antibody, the Aβ oligomers colocalized the mitochondrial matrix marker cyclophilin D (Fig. 3). Cells harvested at 16 DIV showed pronounced A11 staining in the neuronal processes. Although the distribution of puncta was not highly correlated with cyclophilin D, significant co-labeling was found, suggesting that oligomeric Aβ targets mitochondria.
Figure 3.
A11 immunoreactivity colocalizes with cyclophilin D staining. Double-labeling with A11 (green) and cyclophilin D (red) showed that Aβ oligomers colocalize with a mitochondrial matrix marker (cyclophilin D). Lower panels are enlargements of insets on the original images. Images were taken with a 100× objective. Arrowheads indicate colocalization.
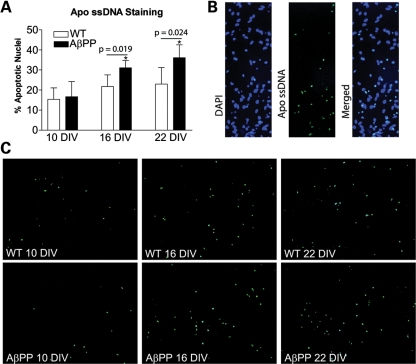
AβPP cultures exhibit increased apoptotic cell death
Apoptotic nuclei were quantified in both WT and AβPP cultures at 10, 16 and 22 DIV via staining for single-stranded DNA. We found that aged primary neurons that overexpress AβPP exhibited increased apoptosis at 16 DIV (Fig. 4A). At 10 DIV, WT and AβPP cultures showed no difference in apoptosis frequency, with 15.4 ± 5.7 and 16.7 ± 7.5% apoptotic nuclei, respectively. At 16 DIV, the AβPP cultures contained an increased number of apoptotic nuclei (WT, 21.8 ± 5.8%; AβPP, 31.1 ± 3.7%; P = 0.019), and the increase remained at 22 DIV (WT, 23.0 ± 8.2%; AβPP, 36.1 ± 6.5%; P = 0.024). Most of the nuclei labeled for single-stranded DNA also appeared to be pyknotic in the DAPI channel (Fig. 4B), which further validates the specificity of Apo-ssDNA staining for apoptotic nuclei.
Figure 4.
Primary neurons from AβPP mice undergo premature apoptotic cell death compared with WT neurons. (A) Cells stained with the Apo-ssDNA antibody for apoptosis were counted and normalized to DAPI-stained nuclei in order to assess the percentage of apoptotic nuclei (n = 5 cultures from independent pups per group). (B) Apo-ssDNA-stained cells colocalized with pyknotic nuclei from DAPI stain. (C) Representative images of Apo-ssDNA-stained cultures from WT and AβPP-derived cultures harvested at 10, 16 and 22 DIV.
AβPP cultures exhibit decreased synaptic gene expression as well as mitochondrial dynamic gene expression, indicating increased fission
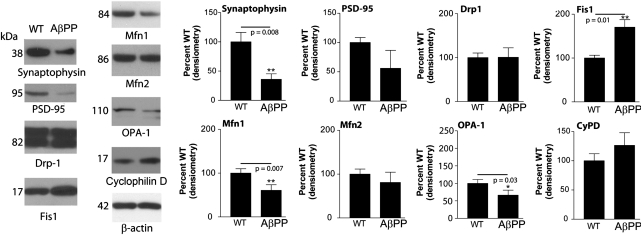
Quantitative real-time RT–PCR was used to measure the mRNA expression levels of genes involved in synapse formation, antioxidant defense and mitochondrial dynamics (Table 1), in the 16 DIV WT and AβPP cultures. Overall, we found that synaptic genes and peroxiredoxins were less in the AβPP cultures than in the WT cultures. Also, genes involved in mitochondrial dynamics were regulated in line with increased mitochondrial fission in AβPP cultures. Nine synaptic genes were examined, seven of which showed a statistically significant reduction in the AβPP neurons, compared with WT. The largest fold-change reduction was found in synaptophysin (−2.0-fold). The mRNA expression for PSD-95, synapsin 1, synapsin 2, synaptobrevin 1, synatpobrevin 2 and GAP43 were also reduced. Peroxiredoxins tended to be reduced in five of the six mRNAs that were measured. However, none of these reductions reached statistical significance.
Table 1.
mRNA fold change of synaptic, mitochondrial fission/fusion and peroxiredoxin genes in primary neurons from AβPP cultures relative to WT neurons
| Synaptic |
Peroxyredoxins |
Mitochondrial dynamics/matrix |
|||
|---|---|---|---|---|---|
| PSD-95 | −1.5* | Prx1 | −1.3 | Fis1 | 1.5* |
| Synaptophysin | −2.0* | Prx2 | −1.1 | Drp1 | 1.1 |
| Synapsin1 | −1.5** | Prx3 | −1.3 | Mfn1 | −1.6* |
| Synapsin2 | −1.4** | Prx4 | −1.2 | Mfn2 | −1.1 |
| Synaptobrevin1 | −1.3* | Prx5 | −1.1 | OPA-1 | −1.3 |
| Synaptobrevin2 | −1.3* | Prx6 | 1.0 | CypD | 1.6** |
| GAP43 | −1.2** | ||||
| Neurogranin | 1.0 | ||||
| Synaptopodin | −1.1 | ||||
mRNA fold changes for synaptic genes, mitochondrial genes and peroxiredoxins were calculated. Gene expression was normalized to β-actin. n = 3 cultures per group.
*P ≤ 0.05, **P ≤ 0.01 compared with WT.
Notably, the genes involved in mitochondrial dynamics were regulated in a way that would predict increased mitochondrial fragmentation in the AβPP cultures. Fis1, which mediates mitochondrial fission, was significantly increased in AβPP cultures, whereas Mfn1 (fusion) was significantly decreased. Drp1 also tended to increase in the AβPP cultures, and Mfn2 and Opa1 tended to decrease.
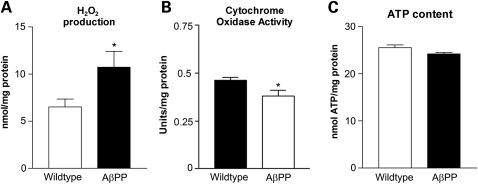
Hydrogen peroxide production is increased in AβPP cultures whereas cytochrome oxidase activity and ATP are decreased
Hydrogen peroxide levels were significantly increased in AβPP cultures than in the WT culture, at 16 DIV (Fig. 5A). In the WT cultures, hydrogen peroxide levels were 6.52 ± 0.84 nmol/mg protein (mean ± SEM), whereas levels in the AβPP cultures were 10.73 ± 1.68 nmol/mg protein (P = 0.033). Conversely, cytochrome c oxidase activity was decreased in AβPP cultures compared with WT (Fig. 5B). The activity levels of WT cytochrome c oxidase were 0.4633 ± 0.062 U/mg protein (mean ± SEM), whereas AβPP cultures were 0.3802 ± 0.03125 U/mg protein (P = 0.011). ATP levels in the AβPP cultures were slightly less compared with ATP levels in the WT culture (Fig. 5C); however, this difference did not reach statistical significance. In the WT culture, ATP was 25.5 ± 0.6 nmol ATP/mg protein (mean ± SEM), whereas the AβPP cultures were 24.2 ± 0.3 nmol ATP/mg protein (P = 0.062).
Figure 5.
Mitochondrial function is impaired in AβPP cultures. (A) The content of hydrogen peroxide increased in AβPP compared with that in WT neurons. n = 4 independent cultures per group. (B) Cytochrome c oxidase activity decreased in AβPP neurons compared with WT. n = 6 independent cultures per group. (C) ATP levels were slightly decreased in the AβPP cultures compared with the ATP levels in the WT cultures. n = 4 independent cultures per group.
Synaptic proteins are decreased, and mitochondrial dynamics proteins are shifted toward fission in AβPP cultures
Protein changes for synaptic markers, synaptophysin, and PSD95 were performed with western blot analysis (Fig. 6). Total protein levels of synaptophysin and PSD95 were significantly reduced in AβPP cultures, compared with the levels in WT cultures, indicating a reduction of synapses in the transgenic cultures (Fig. 6A and B). Protein changes in mitochondrial proteins (Drp1, Fis1, Mfn1, Mfn2 and Opa1) were also probed with western blot analysis (Fig. 6). Fis1 protein was higher in AβPP transgenic cells than in WT cells. The level of Drp1 did not change in the AβPP cultures. When the fusion protein Mfn1 was probed, it was less in the AβPP cultures than in the WT culture.
Figure 6.
Western blot analysis of AβPP cultures shows reduced expression of proteins involved in synaptic formation and mitochondrial fusion, but increased expression of the mitochondrial fission protein Drp1. Protein samples were probed for synaptophysin, PSD-95, Drp1, Fis1, Mfn1, Mfn2, OPA-1, cyclophilin D and β-actin. Representative blots are shown with quantification. n = 3 cultures per group. *P ≤ 0.05, **P ≤ 0.01 compared with WT.
Mitochondria in the soma of AβPP primary neurons are fragmented
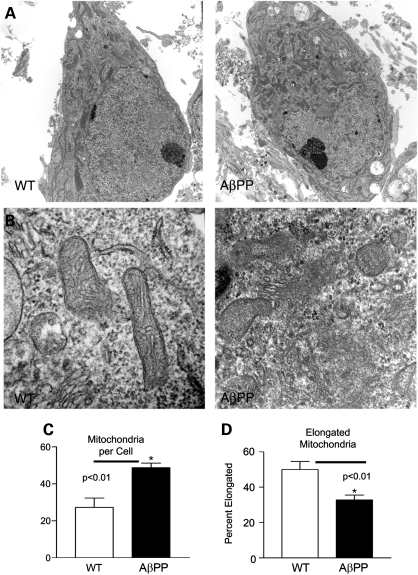
We used TEM to determine the number and morphology of mitochondria in the cell bodies of primary neurons (Fig. 7). WT and AβPP neuronal cultures were collected for EM imaging (Fig. 7A). AβPP transgenic neurons had more mitochondria per cell body than did the WT neurons (48.8 ± 7.6 AβPP and 27.2 ± 16.1 WT, respectively; Fig. 4B). Elongated and round mitochondria were identified and quantified in each image, and the percent of elongated mitochondria was calculated (Fig. 7C). AβPP transgenic neurons had a lower percentage of elongated mitochondria compared with the WT neurons (32.8 ± 8.3% AβPP and 49.9 ± 14.5% WT, respectively). Closer examination revealed AβPP transgenic neurons with many fragmented mitochondria, the membranes of which were disrupted and were without clear cristae (Fig. 7, upper right panel). These fragmented mitochondria were rarely observed in the WT neurons.
Figure 7.
Transmission electron microscopy analysis of cell body mitochondria shows increased numbers of rounded mitochondria in AβPP neurons. (A) Representative images of cell bodies are shown from AβPP-derived and WT primary neuronal cultures at 16 DIV. (B) Close examination reveals that many mitochondria in AβPP neurons lack clear membrane structure and appear fragmented. (C) Mitochondria per cell body were counted. n ≥ 10 images per group. (D) Elongated mitochondria were counted and normalized to total mitochondria to calculate the percent of elongated mitochondria. n ≥ 10 images per group.
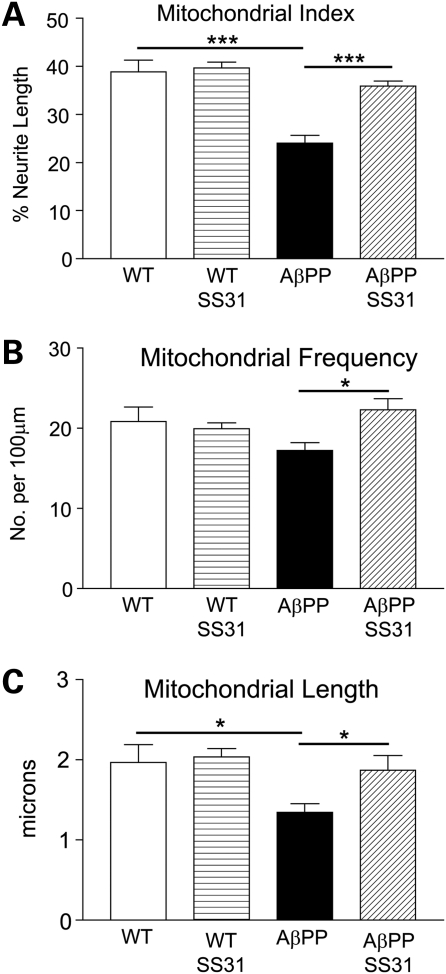
Mitochondria in the neurites of AβPP primary neurons are fewer in number and shorter in length
Cultures of DsRed-mito-transfected AβPP primary neurons and WT primary neurons were treated with SS31 or a vehicle for 48 h. Then the cultures were harvested at 16 DIV to analyze the mitochondrial content. The mitochondrial index was calculated as the percentage of neurites occupied by mitochondria. The AβPP cells had a lower mitochondrial index than did the WT cells (AβPP: 24.1 ± 1.6%; WT: 38.8 ± 2.4%; P = 0.001). The AβPP cells that were treated with SS31 had a significantly higher mitochondrial index than did the WT cells that were untreated; indeed, the index of the SS31-treated AβPP cells returned to near-WT levels (AβPP-SS31: 35.9 ± 0.9%, P = 0.0001 compared with AβPP untreated). SS31 treatment did not alter the mitochondrial index in the WT neurons compared with untreated (WT-SS31: 39.7 ± 3.5%).
Since the mitochondrial index depends on both the length of mitochondria and the number of mitochondria per neurite (mitochondrial frequency), these two parameters were also calculated. Both mitochondrial length and mitochondrial frequency were reduced in the AβPP cultures compared with the WT cultures (AβPP length: 1.35 ± 0.31 μm; WT length: 1.97 ± 0.54 μm; P = 0.038). However, regarding mitochondrial frequency, the reduction did not reach statistical significance (WT: 20.8 ± 4.5 mitochondria/100 μm; AβPP: 17.2 ± 2.7 mitochondria/100 μm; P = 0.122). Both frequency and the length of the mitochondria were significantly increased in the SS31-treated AβPP cultures, compared with the untreated (mitochondrial frequency: 22.3 ± 3.4 mitochondria/100 μm, P = 0.014, compared with AβPP untreated, mitochondrial length: 1.87 ± 0.45 μm, P = 0.038 compared with AβPP untreated). Neither mitochondrial frequency nor the mitochondrial length was affected greatly in the WT neurons that were treated with SS31 (mitochondrial frequency: 19.9 ± 2.1 mitochondria/100 μm, mitochondrial length: 2.04 ± 0.29 μm).
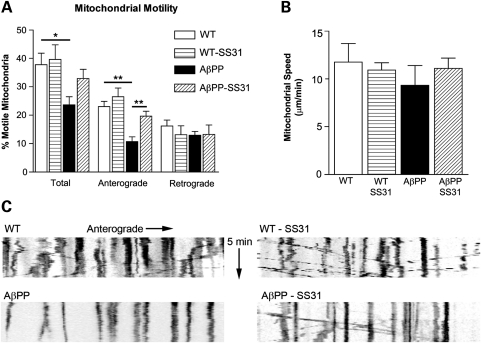
Mitochondrial anterograde transport in AβPP neurons is impaired, but is rescued by SS31
Mitochondrial motility in axonal projections was measured in AβPP and WT neurons at 16 DIV (Fig. 8). The total proportion of recorded moving mitochondria in WT neurons was 37.83 ± 3.97%, whereas the AβPP neurons had 23.69 ± 2.82% moving mitochondria (P = 0.031). When neuronal cultures were treated with SS31, the motion increased only slightly in the WT cultures (39.65 ± 5.10%), but the motion in the AβPP neurons returned to pre-treatment levels (32.94 ± 3.26%, P = 0.077 compared with AβPP untreated), indicating that SS31 rescued mitochondrial index. When the anterograde and retrograde mitochondrial movements were evaluated, retrograde motion was found to be largely unaffected; most of the effects were due to altered anterograde movements. The proportion of mitochondria that moved retrograde after SS31 treatment was as follows: AβPP: 12.96 ± 1.34%; AβPP-SS31: 13.28 ± 3.27%; WT: 16.18 ± 2.09%; and WT-SS31: 13.14 ± 3.07%. No groups exhibited differences that reached statistical significance. On the other hand, anterograde motion was severely diminished in the AβPP cultures compared with the WT cultures (AβPP: 10.72 ± 1.63%; WT: 23.02 ± 1.86%; P = 0.003). SS31 treatment rescued anterograde motion in AβPP neurons and also slightly enhanced anterograde motion in the WT cultures (AβPP-SS31: 19.66 ± 1.74%; WT-SS31: 26.51 ± 3.04%; P = 0.010 compared with AβPP untreated).
Figure 8.
Primary neurons from AβPP mice show reduced anterograde axonal transport of mitochondria but are rescued by mitochondria-targeted antioxidant, SS31. (A) Percent of motile mitochondria in neuronal cultures of AβPP, AβPP+SS31, WT, and WT+SS31. (B) Mitochondrial speed in all four neuronal cultures. (C) Anterograde movements of mitochondria in neuronal cultures of AβPP, AβPP+SS31, WT and WT+SS31. *P < 0.05; **P < 0.005.
The speed of motion was not significantly altered in AβPP SS31-treated or -untreated cultures. In the WT neurons, the mitochondria moved with an average speed of 11.76 ± 1.95 mm/min, and in the AβPP neurons, they moved at 9.33 ± 2.07 μm/min (P = 0.424). In the SS31-treated WT cells, the speed was unaffected compared with untreated neurons (WT-SS31: 10.92 ± 0.77 μm/min), whereas SS31-treated AβPP cells moved at 11.09 ± 1.08 μm/min (P = 0.485 compared with AβPP untreated).
DISCUSSION
We characterized the mitochondrial and synaptic viability of primary neurons derived from the Tg2576 mouse. In AβPP neurons, measurement of mRNA and protein levels showed that synaptic constituents were decreased compared with the WT cells, and mitochondrial dynamics shifted toward increased fission. Anterograde movement in the AβPP neurons was also impaired. These changes temporally corresponded to Aβ oligomer accumulation in synapses and mitochondria, as well as increased apoptotic cell death within the AβPP cultures. Taken together, our observations suggest that intraneuronal Aβ oligomer accumulation leads to mitochondrial and synaptic dysfunction and subsequent neurotoxicity. The addition of the mitochondria-targeted antioxidant, SS31, to growth media was sufficient to rescue deficits in mitochondrial motility, mitochondrial dynamics and synaptic protein expression.
In the AβPP neurons, we first characterized the formation of Aβ and Aβ-related aggregates. Perhaps the most common use of the AβPP primary neuronal cultures has been to evaluate AβPP processing and Aβ secretion (41–43). Characterization of the Aβ aggregation states within these cultures has been relatively understudied. Previous reports have shown that Aβ oligomers accumulate intracellularly in AβPP cultures (44). Furthermore, conditioned media from these cultures are known to contain toxic components that lead to neuritic degeneration when applied to WT cells (45). We observed two predominant bands in the medium from the AβPP neurons, one at ∼100 kDa and the other a 4 kDa form of Aβ. This observation is in agreement with previous reports (41) and indicates that the main species of Aβ in culture media is monomeric. Furthermore, the intraneuronal oligomeric Aβ accumulation found in our study is in agreement with Takahashi et al. (44). In our study, we saw that oligomeric Aβ accumulates over time, with increased immunocytochemical staining at 16 and 22 DIV. At 16 DIV, we found that a portion of A11 immunopositive oligomeric Aβ colocalized with mitochondria, confirming previous reports (21,27,28,34). Accumulation of the Aβ oligomer corresponded to increased apoptotic cell death within the cultures.
We next studied synaptic proteins in WT and AβPP neuronal cultures and found significantly decreased mRNA and protein levels in AβPP neurons relative to WT neurons. These findings agree with earlier reports that Aβ causes synaptic deficiencies in AβPP neurons. We also studied peroxiredoxins (endogenous cytoprotective antioxidant enzymes) and found slightly decreased mRNA levels of peroxiredoxins (1–5) in primary AβPP neuronal cultures relative to WT cultures. We speculate that Aβ increased free radicals production in neuronal cultures that express Aβ, and endogenous cytoprotective peroxiredoxins may not be able to scavenge completely free radicals induced by Aβ. Further, increased levels of Aβ in AβPP cultures may suppress endogenous antioxidant enzymes including peroxiredoxins. Therefore, decreased levels of peroxiredoxins are the result of Aβ toxicity in AβPP cultures.
We report, for the first time, that, in AβPP transgenic primary neurons, the mitochondria exhibit increased fission and decreased fusion in terms of mRNA and protein levels. As predicted from these results, the mitochondria were also more numerous and less elongated within the cell bodies of AβPP neurons than in WT neurons. Moreover, close examination of mitochondria using TEM revealed extensive, fragmented morphology in the AβPP neurons, but not in the WT neurons. This fragmented morphology was characterized by mitochondria that were small and rounded, and had an indistinct matrix. Additionally, cytochrome c oxidase activity was decreased in the AβPP neurons, indicating impaired mitochondrial function. It has previously been suggested that an imbalance in mitochondrial fission and fusion may be an early event in AD progression (23,35,46). Increases in mitochondrial fission proteins with corresponding decreases in fusion proteins have been observed in brain tissues from AD patients (21,23). Recently, this idea has been supported with studies of M17 cells that were found to overexpress mutant AβPP (22,23). In these cells, mitochondrial morphology and distribution were abnormal: fragmented mitochondria accumulated in the perinuclear area. Our laboratory has previously seen fragmentation of mitochondria in a neuronal cell line that we had treated with exogenous Aβ (25), and others have also found that exogenous Aβ oligomers induce mitochondrial fragmentation in primary hippocampal neurons (23,47).
The tight coupling between oxidative metabolism and neuronal activity is well known and has led to the use of cytochrome oxidase activity levels as a surrogate marker for neural activity that is highly localized and responds to sustained alterations in neuronal function (48). Also, an association between metabolic activity, as measured by cytochrome oxidase activity, and mitochondrial size has been described in neurons, with larger mitochondria in active dendrites staining robustly for cytochrome oxidase activity. The larger mitochondrial size allows for increased matrix content, where oxidative phosphorylation occurs (48). In our experiments reported here, we found smaller mitochondria in the AβPP neurons, whereas the number of mitochondria was greater in the cell body. The reduction in mitochondrial size would predict reduced mitochondrial matrix content and the corresponding decrease in cytochrome oxidase activity that we observed. Based on the tight association between neural activity and cytochrome oxidase activity, our observations would suggest that the neuronal activity in AβPP neurons may also be reduced.
Drp1, a multifunctional protein, is not only responsible for mitochondrial fission in the cell body (46) but also is localized to neurites and synapses where Drp1 is responsible for regulating neurite outgrowth and synaptic development and function (49–53). We measured Drp1 mRNA and protein levels in AβPP and WT cells and did not find alterations in Drp1 mRNA or protein levels in AβPP neurons; however, using immunocytochemistry, we did find a difference in the cellular distribution of Drp1. In the WT cells, we saw that Drp1 localized largely throughout the cytoplasm. There were also areas of intense staining, corresponding to neurite outgrowth. In the AβPP cultures, we saw a reduction in the staining associated with neurite outgrowth, but we found similar levels in the cytoplasm. This finding was similar to our earlier observations after primary neurons were treated with Aβ25–35, Drp1 staining colocalized frequently with mitochondria, indicating an increase in mitochondrial fragmentation (38). Mitochondrial fragmentation was also evident in the redistribution of mitochondria along the neurites. Both the number and length of mitochondria decreased in the AβPP neurons, leading to a drastic reduction in the mitochondrial mass within the neurite (Fig. 9). This effect was largely rescued by treatment with SS31.
Figure 9.
Mitochondrial index, frequency and length were reduced in primary neurons from AβPP mice. (A) Mitochondrial index, (B) mitochondrial frequency and (C) mitochondrial length in neuronal cultures of AβPP, AβPP+SS31, WT, and WT+SS31. *P < 0.05; **P < 0.005.
Our observation that anterograde mitochondrial transport is decreased in AβPP neurons provides a probable explanation for the observed redistribution of mitochondria in neurites. Previous studies showed that mitochondrial transport is impaired after the addition of exogenous Aβ (24,36–38). However, this is the first report that overexpression of mutant AβPP in neurons can lead to mitochondrial transport deficiencies. Furthermore, previous reports have found that exogenous Aβ causes impaired organelle transport that can be rescued by a reduction in tau (54) or pharmacologically with NMDA receptor antagonists or GSK3β antagonists (55).
We also found that the mitochondria-targeted antioxidant SS31 can rescue Aβ-induced deficiencies in mitochondrial transport. Reversal of transport deficiencies can account for the observed rescue of mitochondrial distribution. Further, the observed SS31 rescue of mitochondrial transport suggests that mitochondrial viability is a key determinant of anterograde transport. This observation is supported by an earlier finding that the direction of mitochondrial transport correlates highly with mitochondrial membrane potential (56), with depolarized mitochondria moving retrograde, and with polarized mitochondria moving anterograde. Mechanistically, the SS31 peptide targets the inner mitochondrial membrane, due to the electrostatic attraction between these cationic peptides (positive charge) and the highly anionic cardiolipin molecules (negative charge) of the inner mitochondrial membrane. Further, SS31 has a dimethyltyrosine residue, allowing SS31 to scavenge oxyradicals and inhibit linoleic acid and low-density lipoprotein oxidation. By reducing mitochondrial ROS, SS31 was able to prevent the opening of the mitochondrial permeability transition pore, prevent mitochondrial swelling and reduce cytochrome c release in response to a high Ca2+ overload. These findings suggest that SS31 rescues Aβ-induced mitochondrial toxicity in AβPP neurons, thus warranting the study of SS31 as a potential drug to treat patients with AD.
Evidence for the involvement of synaptic mitochondria in AD degeneration is emerging. Recently, several groups demonstrated a direct association between Aβ and mitochondria (21,27,28,34). Based on this initial observation, Du et al. (34) used an AD mouse model to determine that the accumulation of Aβ in mitochondria occurs early in AD progression, preferentially in synaptic mitochondria rather than in whole tissue lysates. This accumulation is coincident with impairments in mitochondrial respiratory control ratio as well as increased levels of oxidative stress.
Overall, our data support a model where the disruption of the normal mitochondrial life cycle may be a key component of neurodegeneration in AD. Under normal conditions, mitochondrial biogenesis is thought to occur mainly in the cell soma, near the nucleus, which codes for the vast majority of mitochondrial constituents. This concept was demonstrated with PC-12 cells (57); however, there is another published report that demonstrates mitochondrial biogenesis within axons of intact neurons (58). One explanation for this is that the newly synthesized mitochondria from the soma may be transported into axons and dendrites, in order to fuel the high ATP demands of neuronal transmission. As the mitochondria age within neurites, they may lose polarization, which could signal that they will next move retrograde toward the soma where they will encounter more viable mitochondria with which they can fuse and refresh their constituents. The recycled mitochondria would then be available to redistribute to other areas of the cell. The balance of this recycling, coupled with autophagy and biogenesis, is thought to maintain the overall health of mitochondria in the cell (59). Under disease conditions, impairment of mitochondria would interrupt their normal lifecycle, disallowing effective mitochondrial recycling and subsequent replenishment of healthy functioning mitochondria in neurites. This problem would be exacerbated by an accumulation of toxin in the synaptic mitochondria. In such a case, lifespan of synaptic mitochondria would decrease, and the recycling of these dysfunctional organelles would possibly introduce accumulated Aβ into the pool of functional mitochondria within the cell body.
Since synaptic deficiencies are the best correlate to dementia (13), it is essential that an AD model exhibit synaptic degeneration to verify its relevance to the disease. Synaptic deficiencies and oligomeric Aβ accumulation within AβPP primary neurons were first characterized by Tampellini and Gouras (13). They found that by 19 DIV, the neurons showed decreased levels of synaptophysin, GluR1 and the PSD-95 protein, which correlated with decreased synaptic function in AβPP cultures compared with synaptic function in WT cultures (60). Further, these neurons showed oligomeric Aβ accumulation in the cell processes that were localized to endosomal-like vesicles, evidenced by immunostaining with the oligomer-specific M16 antibody. This observation also extended to aged Tg2576 mouse tissue, where oligomers were observed in the degenerating processes and not the healthy neuropil, by immunogold staining (44). In support of the observed synaptic deficiencies, Wu et al. (61) recently found that AβPP primary neurons exhibited profound spinal loss, dendritic simplification and neuritic dystrophies, but WT neurons did not. These deficiencies were attributed to an accumulation of Aβ within the media, evidenced by induced neurodegeneration in the WT cultures after they were incubated with a conditioned medium from the AβPP cells. In the present work, we used western blotting and immunocytochemsitry to confirm that AβPP primary neurons exhibited reductions in synaptic proteins and mRNA for synaptophysin and PSD-95. Additionally, we found that AβPP neurons showed reduced mRNA transcript levels of other synaptic constituents, including synapsin, synaptobrevin and GAP43.
Although our data clearly show a temporal correlation between mitochondrial deficiency, synaptic deficiency and cell death, the mechanistic links are still not fully elucidated. It has been proposed that deficiencies in mitochondrial function near active synapses can trigger synaptic degeneration by decreasing available ATP. Since synapses are sites of high ATP consumption, any disruption in the supply of ATP might be detrimental to the ability of cells to regulate ion gradients and buffer intracellular Ca2+. Although no one has directly measured local concentrations of ATP in synapses to test this attractive hypothesis, other evidence has emerged to connect mitochondrial function to synaptic degeneration. A recent report suggests that in AβPP mice, mitochondria-mediated caspase-3 activation, specifically localized to postsynaptic fractions, led to a decrease in hippocampal dendritic spine density and a deficiency in the long-term depression, via Aβ-induced AMPA-receptor dysregulation (62). Therefore, synaptic degeneration may be a highly regulated process, initiated by mitochondria, that is dysfunctional in AD. Previous reports have shown that AMPA-receptor surface expression was decreased in cultures, leading to spinal loss and decreased AMPA responsiveness after the exposure of neurons to Aβ (63,64). Further, reductions in the GluR1 subunit of the AMPA receptor were reported in AβPP primary neurons (60). Mitochondrial involvement in this process was demonstrated in a study, in which primary hippocampal neurons exposed to Aβ oligomers underwent a rapid reduction (30 min) in GluR1-positive spines. The probability of an individual spine showing GluR1 immunoreactivity associated with the probability that a mitochondrion exists in close proximity to the spine (65). Taken together, there is evidence that the mechanism inducing AMPA endocytosis and spine and synapsis degeneration involves mitochondria-mediated caspase activation (62,66).
In this study, we found that primary cultures of AβPP neurons are subjected to progressive accumulation of Aβ oligomers and correspondingly develop a phenotype that is characterized by increased oxidative stress, increased mitochondrial fission, decreased mitochondrial transport in axons, decreased synapse maintenance and increased apoptotic cell death. These findings further elucidate the response of primary neurons to low levels of secreted and internalized Aβ oligomers. These observations also support the hypothesis that mitochondrial dysfunction is an early event in the development of AD. Our data also illustrate the multifaceted nature of mitochondrial dysfunction in AD and suggest that targeting mitochondrial viability may provide a valuable clinical option for the prevention of AD. Findings from this study will advance AD and mitochondrial fields in terms of (i) improving basic understanding of Aβ, Aβ-induced mitochondrial defects and synaptic alterations and (ii) may help developing drugs/agents that target Aβ and mitochondria as therapeutic approaches.
MATERIALS AND METHODS
AβPP mice and primary neuronal cultures
To understand the toxic effects of Aβ in mitochondrial and synaptic dynamics, using primary neurons from AβPP mice (Tg2576 line) (67) and non-transgenic, WT littermates, we investigated the nature (monomers and oligomers) and localization of Aβ and its toxic effects to mitochondria and synaptic proteins. The mice were housed at the Oregon National Primate Research Center of Oregon Health & Science University (OHSU). The OHSU Institutional Animal Care and Use Committee approved all procedures for animal care according to guidelines set forth by the National Institutes of Health.
Primary neurons were prepared for the Aβ, mitochondrial and synaptic studies, as previously described (25,38,39). Briefly, WT and AβPP mice (Tg2576 mouse line) were decapitated, and the brains were removed and maintained in room-temperature HABG (Hibernate A medium, Brain Bits, Springfield, IL, USA) supplemented with B-27 (Invitrogen, Carlsbad, CA, USA) and 0.5 mm GlutaMAX (Invitrogen). The cortex or hippocampus was dissected and reserved for culture, and the cerebellum was used for genotyping. Tissue was minced and then transferred to a solution of 2 mg/ml papain (Worthington Biochemical Corp., Lakewood, NJ, USA) dissolved in Hibernate A without calcium (Brain Bits) and supplemented with 0.5 mm GlutaMAX. Tissue was digested for 30 min at 30°C in a shaking water bath and then removed to 2 ml HABG. Digested tissue was triturated 10 times with a fire-polished, siliconized, 9 in. glass pipette. Non-dissociated tissue was allowed to settle for 2 min, and then the supernatant was removed to a fresh tube. An additional 2 ml of HABG was added, and the process was repeated until 6 ml of dissociated cells were collected. Cells were centrifuged at 200g for 2 min and then washed with 2 ml of HABG. The pellets were resuspended in Neurobasal (Invitrogen) supplemented with B-27 and 0.5 mm GlutaMAX. Live cells were counted, using the trypan blue exclusion method, and were plated at 500 cells/mm2 on poly-d-lysine (PDL)-coated plates or cover slips. Cells were moved to a 37°C, 5% CO2 incubator and allowed to adhere for 1 h. The medium was then changed to Neurobasal, supplemented with B-27 minus antioxidants and 0.5 mm GlutaMAX. One-half of the growth medium was changed every 3 days.
Real time RT–PCR
To determine the mRNA expression levels of mitochondrial and synaptic genes in AβPP-expressing neurons and non-transgenic WT neurons, using quantitative Real time RT–PCR, we measured mRNA levels of Drp1, Fis1, Mfn1, Mfn1, Opa1, CypD (mitochondrial genes), peroxiredoxins 1–6 (cytoprotective genes) and synaptophysin, synapsin 1 and 2, synaptobrevin 1 and 2, GAP43, neurogranin, synaptopodin and PSD95 (synaptic genes), as described in Manczak et al. (25) and Gutala and Reddy (68). Using the Primer Express software (Applied Biosystems), we designed the oligonucleotide primers for the housekeeping genes, β-actin, GAPDH, synaptic and mitochondrial genes and oligonucleotide sequences as follows: Drp1 forward primer 5′ GCGCTGATCCCGCGTCAT and reverse primer 5′ CCGCACCCACTGTGTTGA 3′; Fis1 forward primer 5′ GCCCCTGCTACTGGACCAT and reverse primer 5′ CCCTGAAAGCCTCACACTAAGG 3′; Mfn1 forward primer 5′ TCTCCAAGCCCAACATCTTCA 3′ and reverse primer 5′ ACTCCGGCTCCGAAGCA 3′; Mfn2 forward primer 5′ ACAGCCTCAGCCGACAGCAT 3′ and reverse primer 5′ TGCCGAAGGAGCAGACCTT 3′; Opa1 forward primer 5′ TGGGCTGCAGAGGATGGT and reverse primer 5′ CCTGATGTCACGGTGTTGATG 3′; CypD forward primer 5′ CAGCCAAGCCCTCCAACTC 3′ and reverse primer 5′ GCCGATGTCCACGTCAAAG 3′; Prx1 forward primer 5′ TGGCTCGACCCTGCTGATAG 3′ and reverse primer 5′ GGAGCAGGATACCCAATTTTTG 3′; Prx2 forward primer 5′ CCCCTGAATATCCCTCTGCTT 3′ and reverse primer 5′ CAGGGCAGGCTAAGGGAAAG 3′; Prx3 forward primer 5′ GGCCCCATTTCTTGGAT 3′ and reverse primer 5′ CAGGGCAGGCTAAGGGAAAG 3′; Prx4 forward primer 5′ CCTGTTGCGGACCGAATCT 3′ and reverse primer 5′ GGGTCCGGAACCGTTCAT 3′; Prx5 forward primer 5′ CCCGATCAAGGTGGGAGAT 3′ and reverse primer 5′ CCCGGTTCCCCTTCAAATA 3′; Prx6 forward primer 5′ TCTGGCAAAAAATACCTCCGTTA 3′ and reverse primer 5′ GCCCCAATTTCCGCAAAG 3′; β-actin forward primer 5′ ACGGCCAGGTCATCACTATTC and reverse primer 5′ AGGAAGGCTGGAAAAGAGCC 3′; GAPDH forward primer 5′ TTCCCGTTCAGCTCTGGG 3′ and reverse primer 5′ CCCTGCATCCACTGGTGC 5′; synaptophysin forward primer 5′ CATTCAGGCTGCACCAAGTG 3 and reverse primer 5′ TGGTAGTGCCCCCTTTAACG 3′; PSD95 forward primer 5′ GGACATTCAGGCGCACAAG and reverse primer 5′ TCCCGTAGAGGTGGCTGTTG 3′; synapsin 1 forward primer 5′ TGAGGACATCAGTGTCGGGTAA 3′ and reverse primer 5′ GGCAATCTGCTCAAGCATAGC 3′; synapsin 2 forward primer 5′ TCCCACTCATTGAGCAGACATACT 3′ and reverse primer 5′ GGGAACGTAGGAAGCGTAAGC 3′; synaptobrevin 1 forward primer 5′ TGCTGCCAAGCTAAAAAGGAA 3′ and reverse primer 5′ CAGATAGCTCCCAGCATGATCA 3′; synaptobrevin 2 forward primer 5′ CGGAAGAGTCAGTCTCCATTGG 3′ and reverse primer 5′ CACCTGCAGATAATGTCGTGCTA 3′; GAP43 forward primer 5′ CTGAGGAGGAGAAAGACGCTGTA 3′ and reverse primer 5′ TCCTGTCGGGCACTTTCC 3′; neurogranin forward primer 5′ CCTCAACACCGGCAATGG 3′ and reverse primer 5′ AATATCGTCGTCTGGCTTGGA 3′; synaptopodin forward primer 5′ TCCTGCGCCCTGAACCTA 3′ and reverse primer 5′ GACGGGCGACAGAGCATAGA 3′.
Briefly, total RNA was isolated from neurons representing three independent cultures, in six-well plates, using TRIzol. Reverse transcription was performed with 2 μg of total RNA from each sample, using the Superscript III First Strand Synthesis System for RT–PCR (Invitrogen). RNA was combined with oligo-dT20, 1μl of oligo (dt) and 1 μl of dNTPs (10 mm each) in a total volume of 12 μl; and then heated to 65°C for 5 min. The mixture was chilled on ice, and then 4 μl of 5× first-strand buffer, 2 μl of 0.1 m DTT and 1μl of RNAse out were added. Samples were incubated at 42°C for 2 min, and then 1μl of Superscript III (40 U/ml) was added. After a 50 min incubation at 42°C, the reaction was inactivated by heating at 70°C for 15 min.
Real-time quantitative PCR was performed using an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA) in a 25 μl volume. The reaction mixture for each primer comprised 1× PCR buffer, 2 mm MgCl2, 250 μm dNTPs, 0.3× SYBR Green, 3.12% DMSO, 0.015 U/μl GoldTaq, 50 ng of cDNA, 200 nm. Both β-actin and GAPDH were measured as housekeeping genes with which to normalize gene expression data. However, we chose β-actin for normalization because this non-mitochondrial gene generated a less variable CT value between samples. CT values for gene products were normalized to β-actin CT values, and comparisons were made between experimental groups, using the ΔCT method. Briefly, the ΔCT value was calculated for each sample (CT gene of interest minus CT β-actin). Then the calibrator value was the average ΔCT value for the control samples. The calibrator value was subtracted from ΔCT for each control and from the experimental sample to derive ΔΔCT. Fold change was calculated as 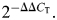 Average fold change was calculated for each experimental group.
Average fold change was calculated for each experimental group.
Western blot analysis
To determine whether endogenously produced Aβ alters mitochondrial and synaptic proteins, we used western blotting to analyze protein levels of Drp1, Fis1, Mfn1, Mfn2, OPA-1, cyclophilin D (mitochondrial), synaptophysin, PSD-95 (synaptic) proteins in neurons from AβPP mice and WT mice. We also measured the AβPP/Aβ species using Aβ antibody, 6E10 in cells and the conditioned medium at 16 DIV. Protein lysates were made by harvesting individual wells of a six-well plate in a Brij buffer (10 mm Tris, pH 7.7, 2 mm EDTA, 150 mm NaCl, 0.875% Brij 97 Sigma, 0.125% NP40) and 1× Sigma protease inhibitor cocktail. Cells were scraped and collected, and then the cell membranes were disrupted by sonication (three rounds of 20 s on ice). Protein concentrations were determined with the BCA protein assay (Pierce/Thermo Scientific). Polyacrylamide gels (10%) were loaded with 25 μg of protein per well and were run to completion. AβPP/Aβ was probed, using 10–20% tricine gels, on which 25 μg of cell lysate or 10 μl of conditioned cell media were loaded. Proteins were transferred in a transfer buffer (25 mm Tris, 190 mm glycine, 20% methanol) overnight at 15 V to PVDF membranes (Perkin Elmer), and then washed with TBST (50 mm Tris, 150 mm NaCl, 0.1% Tween-20, pH 7.4). Membranes were then incubated for 1h at room temperature in a blocking buffer (5% dry milk dissolved in TBST). Blots were probed overnight with primary antibodies for AβPP/Aβ (6E10, 1:400 mouse monoclonal; Covance), Drp1 (1:200 rabbit polyclonal; Novus Biologicals, Inc.), Fis1 (1:1500 rabbit polyclonal; Protein Tech Group), Mfn1 (1:200 rabbit polyclonal; Santa Cruz Biotech), Mfn2 (1:200 rabbit polyclonal; Santa Cruz Biotech), OPA-1 (1:400 mouse monoclonal; BD Transduction Laboratories), cyclophilin D (1:400 mouse monoclonal; Calbiochem), synaptophysin (1:1000 mouse monoclonal; Millipore/Chemicon) or PSD-95 (1:200 rabbit polyclonal; AbCam) diluted in a blocking buffer. After the addition of the primary antibodies, membranes were washed three times with TBST for 5 min and incubated with secondary antibodies (1:5000 sheep-anti-mouse-HRP or donkey-anti-rabbit-HRP as appropriate; GE Healthcare) for 2 h at room temperature. The membranes were again washed three times with TBST. Then the proteins were detected with the Supersignal West Pico chemiluminescent reagent (Thermo Scientific). Scanned images of the exposed X-ray film were analyzed with ImageJ to determine relative band intensity. Quantification was performed on samples from three independent cultures for each condition.
Immunocytochemistry
To determine the localization of Aβ, oligomeric Aβ and mitochondrial proteins in neurons that express Aβ, we performed immunocytochemical analysis of 6E10 (for Aβ), A11 (for Aβ oligomers) and cyclophilin D in neurons from AβPP mice and WT mice using the method described in Calkins and Reddy (38). Briefly, we plated cells on 13 mm round cover slips coated with PDL contained within a 24-well plate. After 16 DIV, the media were removed, and the cells were fixed with 4% paraformaldehyde in PBS for 10–15 min at room temperature. Cover slips were washed with PBS, cell membranes were permeablized with 0.1% Triton X-100 in PBS for 5 min and then a blocking solution was applied (2% normal goat serum, 1% BSA in PBS). All incubations were carried out in a humidified environment. Samples were blocked for 2 h at room temperature and then incubated with primary antibodies diluted in blocking solutions overnight at 4°C. 6E10 (1:500 mouse monoclonal), A11 (1:50 rabbit polyclonal) and cyclophilin D (1:200, mouse monoclonal) were probed. After the primary antibodies were incubated, the cells were washed three times with PBS and then incubated with either goat-anti-rabbit-Alexa488 or goat-anti-mouse-Alexa568 (both 1:500, Invitrogen/Molecular Probes) for 2 h at room temperature. Cells were washed three times in PBS, and then the cover slips were mounted on slides with a ProLong Gold antifade mounting reagent (Invitrogen). Cells were imaged using a Zeiss Axioskop 40 FL microscope.
Apo-ssDNA
To determine whether Aβ is involved in causing cell death in neurons expressing Aβ, we measured apoptotic nuclei in neurons from AβPP mice and WT mice, using a Apo-ssDNA kit (Cell Technology, Mountain View, CA, USA), with minor modifications from the manufacturer's instructions. Primary neurons grown on glass PDL-coated cover slips were harvested into an ice-cold fixative and allowed to fixate at −20°C overnight. Then the cover slips were transferred to a denaturation buffer and heated to 70°C in a humid environment for 10 min. Once the samples were cooled to room temperature, the slips were washed with 1× wash buffer and then incubated with 0.1% Triton X-100 for 10 min at room temperature. Cells were then incubated in a 1% blocking solution for 1 h at room temperature, after which time the primary antibodies (diluted 1:10 in blocking buffer) were added for 1 h at room temperature. After being washed, cells were incubated with secondary antibodies (1:100 in blocking buffer) for 1 h at room temperature and then washed three times with a wash buffer. DAPI (600 nm in PBS) was added to the cells for 5 min, and then the samples were washed three times in PBS before they were mounted on slides. Images were obtained with a Zeiss Axioplan inverted microscope, and images were analyzed with the ImageJ software. The number of Apo ssDNA-stained apoptotic nuclei was normalized to the total number of DAPI-stained nuclei. Three images were analyzed from random locations on a cover slip, and the average value was calculated as the percent of apoptotic nuclei. Five WT and five AβPP cultures from independent pups were evaluated at each time point.
Hydrogen peroxide production
To determine the effect of Aβ in free radical production, in neurons that express Aβ, we measured hydrogen peroxide levels in whole cell lysates of primary neurons from AβPP mice and WT mice, using the method described in Manczak et al. (25). Briefly, hydrogen peroxide was measured with the Amplex Red Hydrogen Peroxide/Peroxidase Kit (Invitrogen), according to manufacturer's instructions. Independent experiments were performed on WT and AβPP cultures (n = 4), as previously described (25,27). Briefly, hydrogen peroxide was measured in the mitochondria from WT and AβPP primary neurons. The reaction mixture contained a sample protein, Amplex Red reagent (50 μm), horseradish peroxidase (0.1 U/ml) and reaction buffer (1×). The reaction was incubated at room temperature for 30 min and read on a fluorescence spectrophotometer (530 nm excitation, 590 nm emission). The concentration of the protein was estimated using the BCA method (Pierce Biotechnology). Results were expressed as nmol/mg protein.
Cytochrome c oxidase activity
To determine the effect of Aβ in cytochrome oxidase activity in neurons that express Aβ, we measured cytochrome oxidase activity in whole cell lysates of primary neurons from AβPP mice and WT mice, using the method described in Manczak et al. (25). Briefly, cells were scraped from six-well plates into a homogenization buffer containing 280 mm sucrose, 10 mm Tris, pH 7.4, and 1 mm EDTA. Then the whole lysate was centrifuged at 200g for 10 min at 4°C. The cells were resuspended in 1 ml of a homogenization buffer and transferred to a dounce homogenizer. After 10 strokes, the cell lysate was spun at 600g for 10 min at 4°C to remove debris and extremely large organelles. The supernatant was then centrifuged at 16 000g for 10 min at 4°C. The resulting pellet (mitochondrial fraction) was then assayed with the Cytochrome c Oxidase Assay Kit (Sigma), as described in Manczak et al. (2,7). The mitochondrial fraction was resuspended in 50 μl of an enzyme dilution buffer with 1 mm n-dodecyl-β-d-maltoside. The concentration of protein was determined using the BCA method. Mitochondrial protein (2 μg) was added to 1.1 ml of the reaction solution, which contained 50 µl of 0.22 mm ferricytochrome c fully reduced by dithiothreitol, Tris–HCl (pH 7.0) and 120 mm potassium chloride. The rate that the absorbance decreased at 550 nm was recorded for 90 s at 10 s intervals, using a Beckman DU640B spectrophotometer. Cytochrome c oxidase activity was measured as U/mg of mitochondrial protein. Results were derived from six independent cultures per condition.
ATP content
To determine the effect of Aβ on cellular ATP in neurons that express Aβ, we measured ATP levels in whole cell lysates of primary neurons from AβPP mice and WT mice, with the ATP determination kit (Invitrogen/Molecular Probes). Cells were lysed with a Brij buffer and immediately assayed for ATP content. Briefly, a standard curve was made from 5–50 μm ATP in a Brij buffer. Equal volumes of the sample lysate or the standard (10 μl) were added to individual wells of a 96-well plate. The reaction mixture (90 μl) was injected into the well, using the automatic injection feature of a Synergy HT microplate reader (Bio-Tek). The well was incubated for 10 s to allow the reaction to proceed, and luminescence was measured for 2 s. Protein concentration was determined by the BCA assay, and values were calculated as nmol ATP/mg cellular protein. Results were derived from four independent cultures per condition.
Transmission electron microscopy
To determine whether mitochondrial morphology is altered in neurons that express Aβ, we performed transmission electron microscopy in primary neurons from AβPP mice and WT mice (25). Cells were fixed in 100 mm sodium cacodylate (pH 7.2), 2.5% glutaraldehyde, 1.6% paraformaldehyde, 0.064% piric acid and 0.1% ruthenium red. They were gently washed and post-fixed for 1 h in 1% osmium tetroxide plus 8% potassium ferricyanide, in 100 mm sodium cacodylate, pH 7.2. The cells were washed in water, dehydrated and infiltrated overnight in 50% acetone and 50% Epon 812. Then they were infiltrated with 100% Epon 812 resin and embedded into the resin. After polymerization, 60–80 nm sections were cut on a Reicher ultramicrotome and stained in lead citrate for 5 min. They were then rinsed and post-stained in uranyl acetate for 30 min, and then rinsed again and dried. TEM was performed at 60 kV on a Philips Morgagne TEM, equipped with a CCD, and images were collected at original magnifications of 1000–37 000×. The number of mitochondria per cell body was counted from at least 10 cells in all groups. The proportion of ‘elongated’ mitochondria was estimated as the number of mitochondria appearing with a length that was at least twice the width, divided by the total number of mitochondria counted.
Mitochondrial motility
To determine whether endogenously produced Aβ impair mitochondrial transport, we assessed mitochondrial motility, in primary neurons from AβPP mice and WT mice, as previously described (38). Briefly, mitochondria were labeled by transfecting pDsRed2-mito (Clontech) into the hippocampal neurons at day 2 (DIV) with Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. DsRed-labeled neuronal mitochondria were observed in cultures up to 21 days after transfection (data not shown). Axonal processes were determined by morphological characteristics. Axons were identified as processes stemming from the soma that are two to three times longer than other processes (69,70). Recordings were made on axonal segments, ∼20–100 µm from the soma. A series of time-lapse images was captured every 5 s, using a Leica SP5 AOBS confocal microscope with a heated (37°C), 5% CO2-controlled stage, for 5 min. Z-stacks at each time point were collapsed to maximum projections, and the time series were archived as avi files. The ImageJ software, with a multiple kymograph plug-in, was used to analyze the avi files. Mitochondrial movements (direction and speed) were determined from the kymographic images. Mitochondria were considered stationary if they did not move more than 2 μm during the entire recording period. Each series of images was recorded for at least three randomly selected Ds-Red-mito-labeled cells per culture and four cultures of independent pups per condition.
Mitochondrial content in neurites
To determine the mitochondrial distribution from AβPP mice, we assessed the mitochondria in neurites in the neuronal cultures from AβPP mice and WT mice. Neuronal cultures were fixed with 4% paraformaldehyde for 5 min at room temperature and then washed with PBS. Images of cell bodies with neurites extending at least 100 mm were collected using a Leica SP5 AOBS confocal microscope with a 63× objective. GFP and Ds-Red were analyzed with measurement tools in ImageJ to determine the neurite mitochondrial index, the average mitochondrial length and the number of mitochondria per neurite length. Data were collected from at least four cells per culture and six cultures of independent pups per condition.
Statistical analysis
All data were analyzed using the Prism3 software from GraphPad Software (San Diego, CA, USA). Results are expressed as mean ± SEM. The data were analyzed by unpaired Student's t-test. Data with more than two groups were analyzed by one-way ANOVA with Tukey's post hoc comparison. Statistical significance was attained at P ≤ 0.05.
Conflict of Interest statement. None declared.
FUNDING
This research was supported by NIH grants AG028072, RR00163 and P30-NS061800 (Aicher, PI), and Alzheimer Association grant IIRG-09-92429.
REFERENCES
- 1.Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 3.Keller J.N., Pang Z., Geddes J.W., Begley J.G., Germeyer A., Waeg G., Mattson M.P. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J. Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow R.H., Khan S.M.A. Mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med. Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer's disease mitochondrial cascade hypothesis. J. Alzheimers Dis. 2010;20((Suppl. 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R.L., Atwood C.S., Johnson A.B., Kress Y., Vinters H.V., Tabaton M., et al. Mitochondrial abnormalities in Alzheimer's disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 8.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 9.Barsoum M.J., Yuan H., Gerencser A.A., Liot G., Kushnareva Y., Gräber S., Kovacs I., Lee W.D., Waggoner J., Cui J., et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greig N.H., Utsuki T., Ingram D.K., Wang Y., Pepeu G., Scali C., Yu Q.S., Mamczarz J., Holloway H.W., Giordano T., et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl Acad. Sci. USA. 2005;102:17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoni-Freddari C., Fattoretti P., Casoli T., Meier-Ruge W., Ulrich J. Morphological adaptive response of the synaptic junctional zones in the human dentate gyrus during aging and Alzheimer's disease. Brain Res. 1990;517:69–75. doi: 10.1016/0006-8993(90)91009-6. [DOI] [PubMed] [Google Scholar]
- 12.DeKosky S.T., Scheff S.W., Styren S.D. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- 13.Tampellini D., Gouras G.K. Synapses, synaptic activity and intraneuronal abeta in Alzheimer's disease. Front. Aging. Neurosci. 2010;2:13. doi: 10.3389/fnagi.2010.00013. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 15.Broersen K., Rousseau F., Schymkowitz J. The culprit behind amyloid beta peptide related neurotoxicity in Alzheimer's disease: oligomer size or conformation? Alzheimers Res. Ther. 2010;2:12. doi: 10.1186/alzrt36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy P.H., Manczak M., Mao P., Calkins M.J., Reddy A.P., Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer's disease: implications for synaptic damage and cognitive decline. J. Alzheimers Dis. 2010;20(Suppl. 2)):S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh D.M., Selkoe D.J. A beta oligomers - a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein W.L., Krafft G.A., Finch C.E. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends. Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 19.Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacor P.N., Buniel M.C., Chang L., Fernandez S.J., Gong Y., Viola K.L., Lambert M.P., Velasco P.T., Bigio E.H., Finch C.E., Krafft G.A., Klein W.L. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J. Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manczak M., Calkins M.J., Reddy P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Su B., Siedlak S.L., Moreira P.I., Fujioka H., Wang Y., Casadesus G., Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl Acad. Sci. USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Su B., Lee H.G., Li X., Perry G., Smith M.A., Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J. Neurosci. 29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du H., Guo L., Yan S., Sosunov A.A., McKhann G.M., Yan S.S. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc. Natl Acad. Sci. USA. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manczak M., Mao P., Calkins M.J., Cornea A., Reddy A.P., Murphy M.P., Szeto H.H., Park B., Reddy P.H. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J. Alzheimers Dis. 2010;20(Suppl. 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy P.H., Beal M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends. Mol. Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manczak M., Anekonda T.S., Henson E., Park B.S., Quinn J., Reddy P.H. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 28.Devi L., Prabhu B.M., Galati D.F., Avadhani N.G., Anandatheerthavarada H.K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J. Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crouch P.J., Blake R., Duce J.A., Ciccotosto G.D., Li Q.X., Barnham K.J., Curtain C.C., Cherny R.A., Cappai R., Dyrks T., Masters C.L., Trounce I.A. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1–42. J. Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caspersen C., Wang N., Yao J., Sosunov A., Chen X., Lustbader J.W., Xu H.W., Stern D., McKhann G., Yan S.D. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 31.Hansson Petersen C.A., Alikhani N., Behbahani H., Wiehager B., Pavlov P.F., Alafuzoff I., Leinonen V., Ito A., Winblad B., Glaser E., Ankarcrona M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl Acad. Sci. USA. 2009;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao J., Irwin R.W., Zhao L., Nilsen J., Hamilton R.T., Brinton R.D. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lustbader J.W., Cirilli M., Lin C., Xu H.W., Takuma K., Wang N., Caspersen C., Chen X., Pollak S., Chaney M., et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 34.Du H., Guo L., Fang F., Chen D., Sosunov A.A., McKhann G.M., Yan Y., Wang C., Zhang H., Molkentin J.D., et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy P.H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Perry G., Smith M.A., Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener. Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rui Y., Tiwari P., Xie Z., Zheng J.Q. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J. Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calkins M.J., Reddy P.H. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim. Biophys. Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calkins M.J., Reddy P.H. Assessment of newly synthesized mitochondrial DNA using BrdU labeling in primary neurons from Alzheimer's disease mice: implications for impaired mitochondrial biogenesis and synaptic damage. Biochim. Biophys. Acta. 2011;1812:1182–1189. doi: 10.1016/j.bbadis.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy P.H., Beal M.F. Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res. Brain Res. Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Qiu Z., Naten D.L., Liston J.C., Yess J., Rebeck G.W. A novel approach for studying endogenous abeta processing using cultured primary neurons isolated from APP transgenic mice. Exp. Neurol. 2001;170:186–194. doi: 10.1006/exnr.2001.7703. [DOI] [PubMed] [Google Scholar]
- 42.Bürger S., Yafai Y., Bigl M., Wiedemann P., Schliebs R. Effect of VEGF and its receptor antagonist SU-5416, an inhibitor of angiogenesis, on processing of the β-amyloid precursor protein in primary neuronal cells derived from brain tissue of Tg2576 mice. Int. J. Dev. Neurosci. 2010;28:597–604. doi: 10.1016/j.ijdevneu.2010.07.231. [DOI] [PubMed] [Google Scholar]
- 43.Gong B., Chen F., Pan Y., Arrieta-Cruz I., Yoshida Y., Haroutunian V., Pasinetti G.M. SCFFbx2-E3-ligase-mediated degradation of BACE1 attenuates Alzheimer's disease amyloidosis and improves synaptic function. Aging Cell. 2010;9:1018–10131. doi: 10.1111/j.1474-9726.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi R.H., Almeida C.G., Kearney P.F., Yu F., Lin M.T., Milner T.A., Gouras G.K. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J. Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M.H., Kuo C.Y. Application of high throughput perfusion micro 3-D cell culture platform for the precise study of cellular responses to extracellular conditions - effect of serum concentrations on the physiology of articular chondrocytes. Biomed. Microdevices. 2011;13:131–141. doi: 10.1007/s10544-010-9478-2. [DOI] [PubMed] [Google Scholar]
- 46.Reddy P.H., Reddy T.P., Manczak M., Calkins M.J., Shirendeb U., Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain. Res. Rev. 2011;67:103–118. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paula-Lima A.C., Adasme T., SanMartín C., Sebollela A., Hetz C., Carrasco M.A., Ferreira S.T., Hidalgo C. Amyloid β-peptide oligomers stimulate RyR-mediated Ca2+ release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid. Redox. Signal. 2011;14:1209–1223. doi: 10.1089/ars.2010.3287. [DOI] [PubMed] [Google Scholar]
- 48.Wong-Riley M.T. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- 49.Ishihara N., Nomura M., Jofuku A., Kato H., Suzuki S.O., Masuda K., Otera H., Nakanishi Y., Nonaka I., Goto Y., et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 50.Wakabayashi J., Zhang Z., Wakabayashi N., Tamura Y., Fukaya M., Kensler T.W., Iijima M., Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H., Chen Y., Jones A.F., Sanger R.H., Collis L.P., Flannery R., McNay E.C., Yu T., Schwarzenbacher R., Bossy B., et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc. Natl Acad. Sci. USA. 2008;105:2169–2174. doi: 10.1073/pnas.0711647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verstreken P., Ly C.V., Venken K.J., Koh T.W., Zhou Y., Bellen H.J. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Li Z., Okamoto K., Hayashi Y., Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Vossel K.A., Zhang K., Brodbeck J., Daub A.C., Sharma P., Finkbeiner S., Cui B., Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decker H., Lo K.Y., Unger S.M., Ferreira S.T., Silverman M.A. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J. Neurosci. 2010;30:9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller K.E., Sheetz M.P. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 57.Davis A.F., Clayton D.A. In situ localization of mitochondrial DNA replication in intact mammalian cells. J. Cell Biol. 1996;135:883–893. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amiri M., Hollenbeck P.J. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev. Neurobiol. 2008;68:1348–1361. doi: 10.1002/dneu.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Twig G., Shirihai O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Almeida C.G., Tampellini D., Takahashi R.H., Greengard P., Lin M.T., Snyder E.M., Gouras G.K. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Wu H.Y., Hudry E., Hashimoto T., Kuchibhotla K., Rozkalne A., Fan Z., Spires-Jones T., Xie H., Arbel-Ornath M., Grosskreutz C.L., Bacskai B.J., Hyman B.T. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J. Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Amelio M., Cavallucci V., Middei S., Marchetti C., Pacioni S., Ferri A., Diamantini A., De Zio D., Carrara P., Battistini L., et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat. Neurosci. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ting J.T., Kelley B.G., Lambert T.J., Cook D.G., Sullivan J.M. Amyloid precursor protein overexpression depresses excitatory transmission through both presynaptic and postsynaptic mechanisms. Proc. Natl Acad. Sci. USA. 2007;104:353–358. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rui Y., Gu J., Yu K., Hartzell H.C., Zheng J.Q. Inhibition of AMPA receptor trafficking at hippocampal synapses by beta-amyloid oligomers: the mitochondrial contribution. Mol. Brain. 2010;3:10. doi: 10.1186/1756-6606-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan S.L., Griffin W.S., Mattson M.P. Evidence for caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer's disease. J. Neurosci. Res. 1999;57:315–323. doi: 10.1002/(SICI)1097-4547(19990801)57:3<315::AID-JNR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 67.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 68.Gutala R.V., Reddy P.H. The use of real-time PCR analysis in a gene expression study of Alzheimer's disease post-mortem brains. J. Neurosci. Methods. 2004;132:101–107. doi: 10.1016/j.jneumeth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Martínez A., Lübke J., Del Río J.A., Soriano E., Frotscher M. Regional variability and postsynaptic targets of chandelier cells in the hippocampal formation of the rat. J. Comp. Neurol. 1996;376:28–44. doi: 10.1002/(SICI)1096-9861(19961202)376:1<28::AID-CNE2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 70.Brown M.R., Sullivan P.G., Geddes J.W. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J. Biol. Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]