Abstract
Epileptic seizures, particularly infantile spasms, are often seen in infants with tuberous sclerosis complex (TSC) soon after birth. It is feared that there are long-term developmental and cognitive consequences from ongoing, frequent epilepsy. In addition, the hallmark brain pathology of TSC, cortical tubers and giant cells are fully developed at late gestational ages. These observations have led us to examine the benefit of prenatal rapamycin in a new fetal brain model of TSC. In this Tsc1cc Nes-cre+ mouse model, recombination and loss of Tsc1 in neural progenitor cells leads to brain enlargement, hyperactivation of mTOR, and neonatal death on P0 due to reduced pup–maternal interaction. A single dose of prenatal rapamycin given to pregnant dams (1 mg/kg, subcutaneous) rescued the lethality of mutant mice. This one dose of prenatal rapamycin treatment reduced hyperactivation of the mTOR pathway in the mutant brain without causing apparent pregnancy loss. Continued postnatal rapamycin beginning at day 8 extended the survival of these mice to a median of 12 days with complete suppression of hyperactive mTOR. However, the rapamycin-treated mutants developed enlarged brains with an increased number of brain cells, displaying marked runting and developmental delay. These observations demonstrate the therapeutic benefit and limitations of prenatal rapamycin in a prenatal-onset brain model of TSC. Our data also suggest the possibility and limitations of this approach for TSC infants and mothers.
INTRODUCTION
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder characterized by benign tumors in multiple organ systems, including the brain, kidney, lung, skin and heart (1,2). Multiple brain manifestations of TSC, such as epileptic seizures, autism spectrum disorder, global developmental delay, sleep disorders and other mental health disorders, are seen in most TSC patients, which raise major issues for the patients and their families (3). Neurological symptoms including infantile spasms are often seen within a few months of birth, and can have a major influence on development and are associated with subsequent cognitive disabilities (4,5). A hallmark feature of TSC neuropathology is the occurrence of cortical tubers and subependymal nodules. Since tuber-like lesions have been identified in the developing neocortex of TSC patients as early as 20 weeks of gestation (6,7), the current model is that these lesions develop during corticogenesis from neural progenitor cells through one or more genetic mechanisms (8,9).
TSC is caused by inactivating mutations in either TSC1 or TSC2, which encode the TSC1/hamartin and TSC2/tuberin proteins. These proteins form a heterodimeric complex which negatively regulates levels of GTP-bound Rheb. Rheb-GTP activates mTOR serine/threonine kinase complex 1 (mTORC1), and enhances multiple growth and metabolic pathways, including enhancement of protein translation through phosphorylation of ribosomal protein S6 at Ser 235/6 and 240/4 sites (10). The mTOR kinase is also a key component of mTOR complex 2 (mTORC2) (2), which activates Akt (11,12). Loss of either TSC1 or TSC2 leads to elevated Rheb-GTP levels, and robust activation of mTORC1 and cell growth (10,13,14).
mTORC1 inhibitors including rapamycin and its analogues have shown benefit in several mouse brain models of TSC (15–17). In addition, both rapamycin and everolimus, a rapamycin analogue, have shown major benefit in the treatment of TSC patients with giant cell astrocytomas (SEGAs) (18,19). However, treatment with rapamycin during infancy in TSC has not been studied in any detail (20–22). Here, we report the therapeutic value of prenatal rapamycin treatment in a new, severe, prenatal-onset brain model of TSC.
RESULTS
A new early onset TSC brain model; rescue from lethality at birth by rapamycin
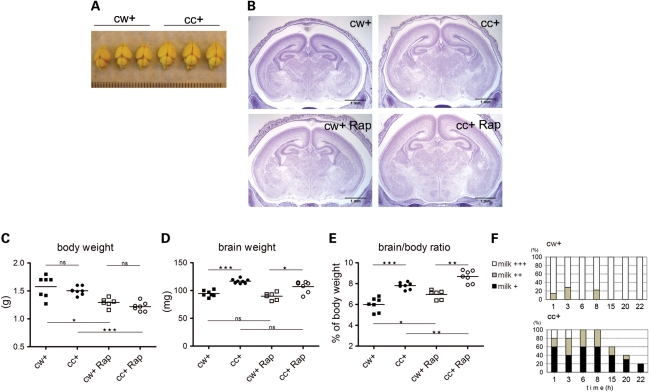
To assess the potential benefit of prenatal rapamycin administration, we developed a new brain-specific Tsc1 knockout, using the conditional Tsc1c allele and a nestin promoter-driven cre allele (Nes-cre). Nestin is expressed in neuroprogenitor cells during early brain development (23), and Tsc1cc Nes-cre+ mutant brains were expected to mimic the abnormal brain development, including ectopic and enlarged cells, seen in TSC embryos and infants (1,6). We found Tsc1cc Nes-cre+ mice were born in normal Mendelian ratios, but died within 24h after birth (Fig. 1). The mutant mice showed normal body weight (Fig. 2C) and normal organ development (data not shown) but had enlarged brains (increased in weight by 23% on average) (Fig. 2A, D and E). The mutant brains showed grossly normal brain architecture, but the entire brain and especially the cerebral cortex were enlarged (Fig. 2B). During the first 24h, we observed that the mutant pups had less milk in their stomachs than littermate controls (Fig. 2F), and were often separated from the rest of the litter in the maternal nest. As a result of their reduced milk consumption, they had lower blood glucose levels than littermate controls [controls 48.8 ± 3.0 mg/dl (n = 17), mutants 32.2 ± 3.0 mg/dl (n = 18), P= 0.0006]. Therefore, mutant lethality on the day of birth (postnatal day 0, P0) was likely due to impaired maternal pup interaction with resulting malnutrition, hypoglycemia and hypothermia.
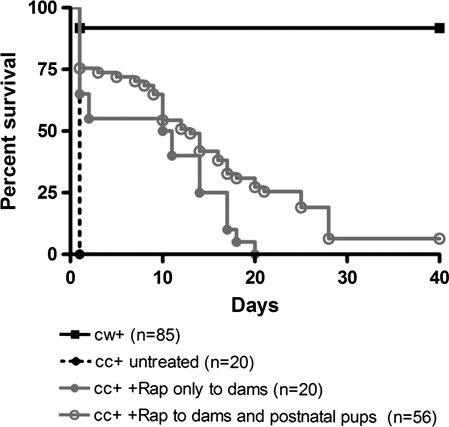
Figure 1.
Survival of Tsc1cc Nes-cre+ mice; response to rapamycin treatment. Survival of Tsc1cc Nes-cre+ (cc+) mice either untreated, treated with a single dose of prenatal rapamycin +/− a single dose to the mother at P3 (cc+ Rap only to dams, 1 mg/kg, 1 dose between E15 and 17), or with a combination of prenatal and postnatal rapamycin treatment (cc+ Rap to dams and postnatal pups; P8–19, 1 mg/kg IP every other day; P21–death, 3 mg/kg, IP every other day); in comparison to littermate controls (cw+).
Figure 2.
Phenotype of Tsc1cc Nes-cre+ mice; response to rapamycin treatment. (A) P0 brains of mutant and littermate control mice. (B) Brain histology at P0. Scale = 1 mm. (C–E) Body weight (C), brain weight (D) and brain–body weight ratio (E) of P0 mutant and control mice, with and without prenatal rapamycin treatment. (F) Reduced milk ingestion in mutant mice. The amount of milk in the stomach was scored as: +++, full; ++, less than half full; or +, barely seen or none. x-axis is hours after birth. cw+, n = 9; cc+, n = 11, from 3 litters. *P < 0.05, **P < 0.01 and ***P< 0.001.
Next, we examined whether prenatal administration of rapamycin could improve the survival of Tsc1cc Nes-cre+ mice. A single dose of rapamycin (1 mg/kg) was given subcutaneously to seven pregnant wild-type (Tsc1cc) dams bearing Tsc1cc Nes-cre+ embryos and control littermates (Tsc1cwNes-cre+) between embryonic days 15–17 (E15–17) (Fig. 1). We found that this one prenatal injection of rapamycin significantly extended the survival of the mutant mice, with one surviving as long as P20 (Fig. 1, P= 0.0245, log-rank test). All pregnant dams successfully gave birth, and there was no obvious loss of pups at birth due to rapamycin. However, prenatal rapamycin treatment was associated with a significant reduction in body weight in both mutants (19%) and controls (18%) at P0 (Fig. 2C). It had no effect on brain weight in either mutants or controls (Fig. 2D), so that the brain:body weight ratio was also significantly increased in both mutants and controls in response to rapamycin (Fig. 2E). Therefore, we examined the effects of rapamycin on mTOR signaling and neuropathology in these mice.
Restored mTOR pathway and increased neural cell density by a single dose of prenatal rapamycin treatment
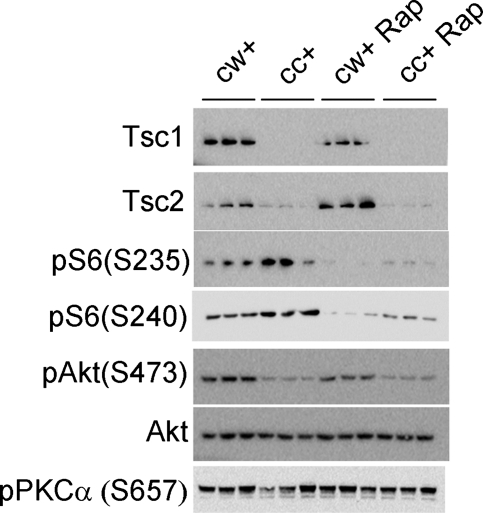
Brain lysates from P0 Tsc1cc Nes-cre+ mice showed nearly undetectable levels of Tsc1 protein, as well as reduced levels of Tsc2 protein and hyperactivation of mTORC1, indicated by the higher phosphorylation of ribosomal protein S6 (Fig. 3). The single prenatal rapamycin injection given at E16 strongly suppressed mTORC1 and lowered the levels of pS6(Ser235) and pS6(Ser240) at P0 in both control and mutant brains. This finding is consistent with our previous pharmacokinetic studies, indicating that brain levels of rapamycin persist in mice for at least 48h after a single intraperitoneal (IP) injection (17). Together, these data point to the effective mTORC1 blockade and therapeutic effect of rapamycin, consistent with the improvement in survival of the mutant mice.
Figure 3.
mTOR signaling in P0 Tsc1cc Nes-cre+ mice at birth, with or without prenatal rapamycin treatment. Immunoblot analysis of brain lysates from P0 mutant mice (cc+) and control littermates (cw+) with/without prenatal rapamycin treatment at E16 shows rapamycin-reduced hyper-phosphorylation of S6 due to complete loss of Tsc1 in mutant brains.
However, prenatal rapamycin treatment did not enhance activation of Akt, as assessed by phosphorylation at Ser473 in either control or mutant mice, such that pAkt(Ser473) levels remained low in rapamycin-treated mutant mice. In addition, mTORC2 activity as assessed by phosphorylation levels of PKCα(Ser657) (12) was similar among all four groups at P0. These data indicate that although mTORC1 activity was curtailed, the down-regulation of Akt due to loss of Tsc1 was not improved by this single dose of prenatal rapamycin treatment.
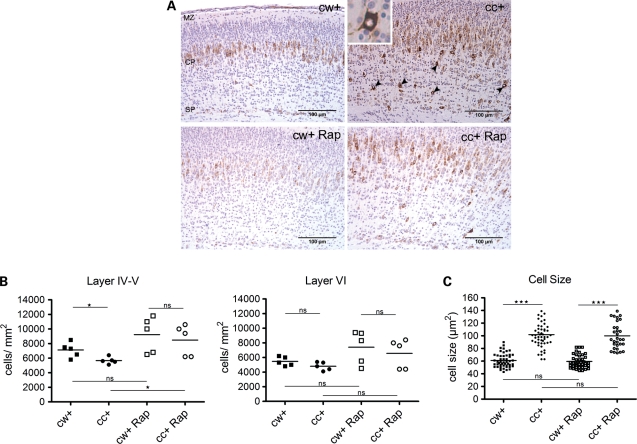
Corresponding to the immunoblot findings, we found that the cortex of mutant mice contained larger cells with aberrant pS6(Ser235) positivity (Fig. 4A). Most notable in the mutant brains was the occurrence of strongly pS6(Ser235)+ individual cells in the lower cortical plate (layers IV–VI) (Fig. 4A, inset). However, mutant brains showed normal layer formation by both marker studies (Cux1 and CTIP2 staining) and BrdU birth date analysis (data not shown). Prenatal rapamycin treatment decreased the levels of aberrant pS6(Ser235) staining seen in the enlarged cells in mutant mice (Fig. 4A). Thus, we conclude that the loss of Tsc1 causes a major increase in mTORC1 activity in most brain cells, but does not affect cell migration or cortical layer formation, and this increased mTORC1 activity is reversed by a single prenatal dose of rapamycin.
Figure 4.
Histology, cell density and size in P0 Tsc1cc Nes-cre+ mice at birth, with or without prenatal rapamycin treatment. (A) IHC with phospho-S6(Ser235) antibody in primary motor cortex of P0 mutant (cc+) and control (cw+) mice with/without prenatal rapamycin treatment at E16. Scale = 100 μm. Marginal zone, MZ; cortical plate, CP; subplate, SP. Neural cells showing hyperactivation of mTOR with heavy pS6 staining were seen in the deeper cortical plate of untreated mutant brain (arrowheads and inset). (B) Cell density measurement in layers IV–V (left) and layer VI (right) at P0. (C) Cell size measurement in layer V neurons in motor cortex at P0 indicates marked cell enlargement (n > 30 cells, three mice). *P < 0.05, **P < 0.01 and ***P< 0.001.
The mutant brains showed a significantly lower neural cell density, especially in the lower cortical plate (developing layer IV and V) (Fig. 4B, left). In addition, cell size in the cortical plate of mutants was significantly increased in comparison to littermate controls (Fig. 4C). With the single prenatal rapamycin treatment, we did not observe any change in cell size (Fig. 4C) or brain weight (Fig. 2D) in either mutants or controls. However, there was a significant increase in layer IV–V cell density in treated mutants, such that rapamycin-treated control and mutant mice had similar cell densities (Fig. 4B). We did not observe any difference in cell proliferation at P0, by PCNA staining or BrdU incorporation (data not shown), in comparison of Tsc1cc Nes-cre+ and littermate controls. Therefore, in aggregate, these data indicate that blockade of mTORC1 in the fetal brain by this limited treatment regimen with rapamycin results in increased neural cell numbers without affecting neural cell migration in this model.
Longer term benefit of rapamycin in Tsc1cc Nes-cre+ mice
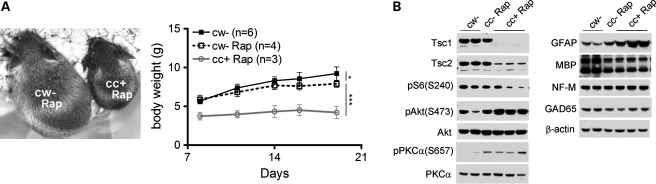
To attempt to improve the survival and functional outcomes of Tsc1cc Nes-cre+ mice beyond that achieved by a single prenatal rapamycin treatment, we treated juvenile controls and mutants with rapamycin by IP injection starting at postnatal day 8 (P8), and continued this therapy at gradual increasing doses (P8–P19, 1 mg/kg, every 3–4 days; >P21, 3 mg/kg, 3 times/week). We did not attempt additional prenatal treatments due to concern that we would see fetal wastage and worsening of the weight loss seen with a single prenatal dose (Fig. 2C). Addition of postnatal rapamycin treatment extended the survival of mutant mice, with the longest survivor making it out to 40 days (Fig. 1). However, all of the treated mutants showed significant developmental delay and severe neurological symptoms, with Straub tail, tremor and delayed eye opening beyond age 3 weeks. Rapamycin-treated mutants also had very poor weight gain (Fig. 5A). Rapamycin-treated littermate controls also showed a reduction in weight gain, in comparison to untreated littermate controls, but this was minor in contrast to the near complete lack of weight gain in rapamycin-treated mutant mice (Fig. 5A). Rapamycin-treated controls also showed no neurologic symptoms.
Figure 5.
Growth and brain development of Tsc1cc Nes-cre+ mice with pre- and postnatal rapamycin treatment. (A) Growth retardation in rapamycin-treated mutant mice (cc+). Representative images (left, at P28) and weight gain (right) of control and mutant mice with pre- and postnatal rapamycin treatment. (B) Immunoblotting using brain lysates of P21 control and mutant mice with prenatal and postnatal rapamycin treatment. Brains were collected 48h after the final rapamycin treatment. Note the marked reduction in pS6(S240) and MBP, and increase in pAkt(S473), pPKCα(S657) and GFAP in rapamycin-treated mutant mice.
mTORC1 activity in brain lysates of mutants that continued on rapamycin was nearly completely suppressed, with levels of pS6(Ser240) below those of untreated controls (Fig. 5B). Similarly, there was an increase in levels of pAkt(Ser473), consistent with chronic mTORC1 suppression, and evidence of increased mTORC2 activity as shown by the increase in pPKCα(Ser657). Therefore, these data suggest that prolonged rapamycin treatment in vivo may cause a shift toward increased mTORC2 activity concomitant with the reduction in mTORC1 activity. Rapamycin-treated mutant brains showed no change in the levels of neurofilament or GAD67 expression, suggesting that neuronal and inhibitory neuron development was normal (Fig. 5B). In contrast, expression of GFAP was markedly increased in the mutants consistent with progressive astrogliosis. In addition, myelin basic protein (MBP) expression was reduced in both treated controls and treated mutant brains (Fig. 5B), indicative of hypomyelination. Hypomyelination may be due to inhibition of mTORC1 activity in oligodendrocytes (24,25). These observations indicate that while rapamycin is quite effective in blocking mTORC1 activation in the Tsc1cc Nes-cre+ brain, there is hyperactivation of Akt, which may possibly contribute to the poor development and survival of these mice.
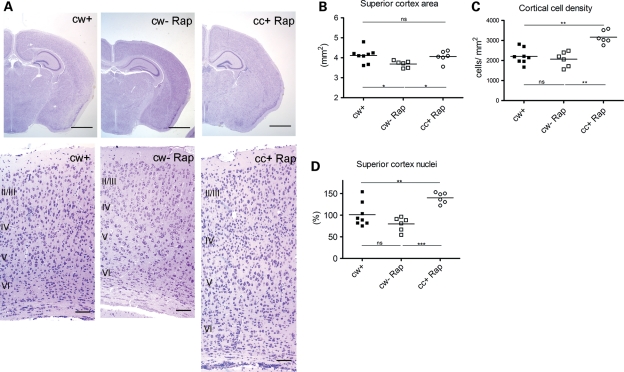
Rapamycin-treated mutants had enlarged brains with enlarged cerebral cortex in comparison to rapamycin-treated controls (Fig. 6A and B). In addition, we found that rapamycin-treated mutant brains had increased neural cell density in the cerebral cortex in comparison to both untreated and treated controls (Fig. 6C). Combining the increased density of neural cells and enlarged brain size, the total cell number in the rapamycin-treated mutant brains were increased by 40 and 75% in comparison to untreated control and rapamycin-treated control brains, respectively (Fig. 6D). Neuronal differentiation is impaired in neuroprogenitor cells lacking Tsc2 (26), and it is likely that it was not fully normalized on the rapamycin treatment regimen given here. Therefore, we suspect that either persistent mTORC1 activation during the interval from P0 to P8, or strong inhibition of mTORC1 and distortion in the regulation of mTORC1 vs. mTORC2 as well as enhanced Akt activity, may have led to reduction in neural pruning via apoptosis, to increase overall neural number in the mutant mice.
Figure 6.
Brain histology of Tsc1cc Nes-cre+ mice with pre- and postnatal rapamycin treatment. (A) Brain histology at P21. Scale = 1 mm (top), 50 mm (bottom). Note the expansion of cell cortex and increased numbers of neural cells in the cc+ Rap mouse section. (B–D) Superior cortex area (B), cortical cell density (C) and estimated total brain cells (D) in P21 mutant and control mice with pre- and postnatal rapamycin treatment. *P < 0.05, **P < 0.01 and ***P< 0.001.
DISCUSSION
TSC is often a devastating neurologic disorder that often causes considerable morbidity in affected infants (2,4,5). Epilepsy occurs in the vast majority (∼90%) of individuals with TSC, with 70% experiencing epilepsy during the first year of life (2). About a third of TSC infants develop infantile spasms, which are considered a catastrophic epilepsy syndrome, and are often associated with subsequent profound neurocognitive impairment (3). Although the therapeutic armamentarium for this seizure disorder is improving, there is still a critical need for more effective therapies. In all, >50% of TSC individuals have some degree of cognitive impairment, which can be profound with speech never attained (2). Due to these common and severe consequences of this disorder, there has been interest in the clinical utility of mTORC1 inhibitors for a variety of TSC manifestations, stimulated by the knowledge that unregulated activation of mTORC1 occurs in all cells lacking either TSC1 or TSC2. In recent years, there has been strong clinical evidence that both rapamycin and everolimus (a rapamycin derivative) are effective in the control of subependymal giant cell astrocytomas in TSC (18,19); and for treatment of renal angiomyolipomas and pulmonary lymphangioleiomyomatosis in both TSC and non-TSC patients (27,28). In addition, there is preliminary evidence that rapamycin may be beneficial for seizure control and behavioral issues in young children with TSC (18). Thus, it is logical to consider the possibility that rapamycin or related drugs might be beneficial at earlier stages during human development, especially considering that a major portion of brain development has occurred at the time of birth.
Rapamycin and everolimus have both been shown to be effective in the postnatal treatment of a wide variety of TSC mouse models (15–17,29,30). They are particularly effective in the treatment of mouse brain models of TSC, including those in which neurons (15,17), radial glial cells (30) or other brain cell subsets (15,16) have been targeted for recombination and loss of either Tsc1 or Tsc2. However, this is the first study in which prenatal treatment with rapamycin has been explored.
We report the therapeutic value of prenatal treatment with the mTORC1 inhibitor, rapamycin, in a new mouse brain model of TSC. After pilot studies established that low-dose rapamycin [1 mg/kg subcutaneous (SC)] was well tolerated by pregnant dams at E14–E18 without pregnancy loss, we found that a single rapamycin dose in the E15–17 interval lead to a dramatic improvement in survival in a new mouse TSC brain model (Tsc1cc Nes-cre+) in which there is recombination and loss of Tsc1 in neuroprogenitor cells beginning during early embryogenesis. Death of Tsc1cc Nes-cre+ pups occurred uniformly on P0, due to apparent hypoglycemia and neurobehavioral effects of recombination and loss of Tsc1, manifest as poor mother–pup interaction, with limited milk suckling and loss from the maternal nest.
A single dose of rapamycin rescued this early death phenotype to a major extent with a median survival of 10 days and maximum of 20. However, this treatment caused an 18–19% reduction in weight of the newborn pups. Despite the marked improvement in survival, there were also continuing severe manifestations in the Tsc1cc Nes-cre+ mice, including very poor weight gain, developmental delay and neurological symptoms. Rapamycin may have contributed to these clinical features to some extent; however, only a minor weight gain delay without other phenotype was seen in control mice treated on this same regimen. It is also likely that the absence of treatment during the P0–P8 interval contributed to clinical features in these mice. However, given the small size of these pups and the requirement for neonatal care by the mother, we felt that it was unwise to administer rapamycin during that period. A third, and in our view most likely possibility, is that rapamycin did not completely reverse the brain effects of loss of Tsc1. We were guided in our choice of dose by previous experience and detailed pharmacokinetic studies, in which we demonstrated that rapamycin at 6 mg/kg given IP led to supra-therapeutic levels, though substantial benefit, in a neuronal model of TSC (17). Thus, we lowered the dose to 1 mg/kg starting on P8. Our analyses at 3 weeks of age demonstrated that this dose was very effective at suppressing mTORC1 activity, and enhancing Akt activity. It is notable that this same rapamycin treatment regimen had less effect in blocking mTORC1 activity in control compared with mutant mice (Fig. 5B), which we suspect is due to delay in blood brain barrier development in the mutant mice compared with control mice, leading to markedly higher brain rapamycin levels in the mutants. Further dose titration might yield somewhat greater benefit, although monitoring and assessment of levels is quite difficult in these small mice. In addition, in pilot studies we tried two prenatal doses of rapamycin at E11–12 and E15–16, and observed a poorer outcome for mutant pups.
Although mTORC1 was effectively inhibited on this treatment regimen, we did observe significant pathologic features in the treated mice, including reduced myelination, increased GFAP and a 50% increase in the total number of neural cells in cerebral cortex. Neuronal differentiation is impaired in neuroprogenitor cells lacking Tsc2 (26), and a reduced number of post-mitotic neurons was noted in a radial glial model of TSC (30). With treatment in this model, however, we achieved both effective blockade of mTORC1 and activation of Akt. Akt has several downstream signaling partners in addition to Tsc2, which regulate both growth and apoptosis, and it is possible that activation of Akt may have led to enhanced survival of immature neurons/neural progenitors in postnatal brains, increasing the total number of neural cells in cerebral cortex. Reduced myelination, due to both neuronal and oligodendrocyte effects in this model, as well as astrogliosis may also have contributed to the poor development and neurologic symptoms seen in these mice.
The dramatic therapeutic response to rapamycin in this new model prompts consideration of trials of this and related compounds in both pregnant mothers bearing offspring affected with TSC, as well as TSC infants with severe neurologic manifestations. Currently, the U.S. National Transplantation Pregnancy Registry (NTPR) reports that pregnancy while on immunosuppressants after transplantation, including rapamycin, should be considered high risk (31). However, there are several reported cases of successful delivery without apparent maternal or fetal complications by mothers who were taking rapamycin during pregnancy for immunosuppression of transplanted organs (21,22). Nonetheless, with our observations of both benefit from prenatal rapamycin, but also toxicity in the form of reduced birth weight and reduced neonatal weight gain, lead to a recommendation for caution in consideration of use of prenatal rapamycin during pregnancy.
MATERIAL AND METHODS
Mouse procedures
Mice bearing the Nestin-cre allele (B6.Cg-Tg(Nes-cre)1Kln, here denoted Nes-cre) were obtained from Jackson Laboratories. Generation and genotyping of Tsc1c and homozygous transgene alleles in mice were described previously (17). We use c to denote a conditional, floxed allele of Tsc1 that is converted to a null allele by exposure to the cre recombinase; and w to denote a wild-type allele of Tsc1. Mice were mainly generated from breedings between Tsc1cc females and Tsc1cw Nes-cre+ or Tsc1cw Nes-cre++ males. Mice with either Tsc1cc (cc− for short), Tsc1cw (cw−) or Nes-cre+Tsc1cw (cw+) genotypes showed no major difference in development or phenotype, and were all used as control mice. Tsc1cc mice are maintained in a congenic strain in our lab (mixture of BALB/c, C57BL and 129S4/SvJae), reducing genetic variation within this colony. Timed matings were performed using the vaginal plug to assess fertilization.
Rapamycin (LC Laboratories) was prepared as described previously (17) with dilution in 0.25% Tween 80, 0.25% PEG 400 for injection. One dose SC rapamycin injection was given to pregnant dams between E15 and 17, and in some cases an additional dose was given to the mothers when they were nursing P3 pups. Pups were treated with rapamycin by IP injection every 3–4 days starting at P8 (1 mg/kg, IP) and changed to 3 times per week after P21 (3 mg/kg, IP). All procedures were carried out in accordance with the Guide for the Humane Use and Care of Laboratory Animals, and the study was approved by the Animal Care and Use Committee of Children's Hospital Boston.
Materials
Antibodies were obtained from the following sources: Tsc1(#4906), phospho-Ser235/236-S6(#2211), phospho-Ser240/244-S6(#2215), PKCα(#2056) and IRS1(#2382) antibodies from Cell Signaling Technology; Tsc2 (SC-893), Akt(SC-1618) antibodies, as well as HRP-labeled anti-mouse, anti-goat, anti-rabbit secondary antibodies from Santa Cruz Biotechnology; phospho-Ser473-Akt antibodies(M3628) from Dako; phosphor-Ser657-PKCα antibody(#06–822) from Millipore; β-actin antibody (A4700) from Sigma; MBP(AB980), and GAD67 (MAB351) antibodies from Chemicon; GFAP (SMI-22) and Pan-neurofilament (SMI311) antibodies from Covance.
Histology and immunohistochemistry
Histology sections of newborn mouse brains were prepared after decapitation and 2–4 days fixation with Bouin's solution (Sigma). Adult mice were euthanized in a CO2 chamber and fixed with Bouin's. Following paraffin embedding, 5 μm sections were stained with either cresyl violet (Nissl) or H&E, or used for immunohistochemistry (IHC). IHC was performed after deparaffinization and rehydration steps and antigen retrieval in Citrate buffer (pH8), using the Envision system (DAKO). The primary motor cortex in comparable coronal brain sections containing the anterior hippocampus was analyzed for cell density and size measurements. Cell density of layers IV–VI in developing cortical plate in P0 brain was measured by counting Nissl-positive cells in 20 000 μm2 (n = 5). Layer VI was defined as the layer adjacent to the subplate, and layers IV–V were defined as the layers containing the largest cortical neurons. For cell size measurement, the area of the three largest layer IV–V cells in every 65 000 μm2 was measured by ImageJ software (n > 30 cells, n = 3 mice). All data in graphs are presented with means (bars). Statistical comparisons were made using the Mann–Whitney t-test.
Western blotting
Animals were euthanized and brains were rapidly removed, dissected and flash frozen in liquid N2. Total tissue lysates for western blotting were prepared and analyzed as described previously (17).
Suckling behavior analysis
Newborn pups born to Tsc1cc dams were monitored every 2h after birth. The amount of milk in the stomach of these pups was scored as: (3) full; (2) less than half or (1) barely seen.
FUNDING
This work was supported by National Institutes of Health (1P01NS24279-16, 2R37NS031535-14) and the Tuberous Sclerosis Alliance (postdoctoral fellowship award to J.G.).
ACKNOWLEDGEMENTS
We thank Mustafa Sahin for helpful comments and discussions.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Jozwiak J., Jozwiak S., Wlodarski P. Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol. 2008;9:73–79. doi: 10.1016/S1470-2045(07)70411-4. [DOI] [PubMed] [Google Scholar]
- 2.Kwiatkowski D.J., Thiele E.A., Whittemore V.H., editors. Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Wiley-Blackwell, Weinheim, Germany; 2010. [Google Scholar]
- 3.Holmes G.L., Stafstrom C.E. Tuberous Sclerosis Study Group. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 4.Doherty C., Goh S., Poussaint T.Y., Erdag N., Thiele E.A. Prognostic significance of tuber count and location in tuberous sclerosis complex. J. Child Neurol. 2005;20:837–841. doi: 10.1177/08830738050200101301. [DOI] [PubMed] [Google Scholar]
- 5.Thiele E.A. Managing epilepsy in tuberous sclerosis complex. J. Child Neurol. 2004;19:680–686. doi: 10.1177/08830738040190090801. [DOI] [PubMed] [Google Scholar]
- 6.Park S.H., Pepkowitz S.H., Kerfoot C., DeRosa M.J., Poukens V., Wienecke R., DeClue J.E., Vinters H.V. Tuberous sclerosis in a 20-week gestation fetus: immunohistochemical study. Acta Neuropathol. 1997;94:180–186. doi: 10.1007/s004010050691. [DOI] [PubMed] [Google Scholar]
- 7.Bordarier C., Lellouchtubiana A., Robain O. Cardiac rhabdomyoma and tuberous sclerosis in 3 fetuses—a neuropathological study. Brain Dev. 1994;16:467–471. doi: 10.1016/0387-7604(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 8.Qin W., Kozlowski P., Taillon B.E., Bouffard P., Holmes A.J., Janne P., Camposano S., Thiele E., Franz D., Kwiatkowski D.J. Ultra deep sequencing detects a low rate of mosaic mutations in tuberous sclerosis complex. Hum. Genet. 2010;127:573–582. doi: 10.1007/s00439-010-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crino P.B., Aronica E., Baltuch G., Nathanson K.L. Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology. 2010;74:1716–1723. doi: 10.1212/WNL.0b013e3181e04325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabatini D.M. mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 11.Guertin D.A., Stevens D.M., Thoreen C.C., Burds A.A., Kalaany N.Y., Moffat J., Brown M., Fitzgerald K.J., Sabatini D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKC alpha but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Huang J.X., Wu S.L., Wu C.L., Manning B.D. Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Res. 2009;69:6107–6114. doi: 10.1158/0008-5472.CAN-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fingar D.C., Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 14.Laplante M., Sabatini D.M. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 2009;19:1046–1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehninger D., Han S., Shilyansky C., Zhou Y., Li W.D., Kwiatkowski D.J., Ramesh V., Silva A.J. Reversal of learning deficits in a Tsc2(+/−) mouse model of tuberous sclerosis. Nat. Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng L.H., Xu L., Gutmann D.H., Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meikle L., Pollizzi K., Egnor A., Kramvis I., Lane H., Sahin M., Kwiatkowski D.J. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krueger D.A., Care M.M., Holland K., Agricola K., Tudor C., Mangeshkar P., Wilson K.A., Byars A., Sahmoud T., Franz D.N. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 19.Franz D.N., Leonard J., Tudor C., Chuck G., Care M., Sethuraman G., Dinopoulos A., Thomas G., Crone K.R. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 20.McKay D.B., Josephson M.A. Pregnancy after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2008;3:117–125. doi: 10.2215/CJN.02980707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sifontis N.M., Coscia L.A., Constantinescu S., Lavelanet A.F., Moritz M.J., Armenti V.T. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698–1702. doi: 10.1097/01.tp.0000252683.74584.29. [DOI] [PubMed] [Google Scholar]
- 22.Chu S.H., Liu K.L., Chiang Y.J., Wang H.H., Lai P.C. Sirolimus used during pregnancy in a living related renal transplant recipient: a case report. Transplant. Proc. 2008;40:2446–2448. doi: 10.1016/j.transproceed.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P.C., Bock R., Klein R., Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan S.P., Flores A.I., Wang F., Macklin W.B. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyler W.A., Gangoli N., Gokina P., Kim H.A., Covey M., Levison S.W., Wood T.L. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J. Neurosci. 2009;29:6367–6378. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onda H., Crino P.B., Zhang H.B., Murphey R.D., Rastelli L., Gould Rothberg B.E., Kwiatkowski D.J. Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol. Cel. Neurosci. 2002;21:561–574. doi: 10.1006/mcne.2002.1184. [DOI] [PubMed] [Google Scholar]
- 27.Bissler J.J., McCormack F.X., Young L.R., Elwing J.M., Chuck G., Leonard J.M., Schmithorst V.J., Laor T., Brody A.S., Bean J., et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormack F.X., Inoue Y., Moss J., Singer L.G., Strange C., Nakata K., Barker A.F., Chapman J.T., Brantly M.L., Stocks J.M., et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollizzi K., Malinowska-Kolodziej I., Stumm M., Lane H., Kwiatkowski D.J. Equivalent benefit of mTORC1 blockade and combined PI3K-mTOR blockade in a mouse model of tuberous sclerosis. Mol. Cancer. 2009;8:38. doi: 10.1186/1476-4598-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Way S.W., McKenna J., Mietzsch U., Reith R.M., Wu H.C., Gambello M.J. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum. Mol. Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coscia L.A., Armenti V.T. Transplantation: pregnancy outcomes in kidney recipients: more data are needed. Nat. Rev. Nephrol. 2010;6:131–132. doi: 10.1038/nrneph.2009.232. [DOI] [PubMed] [Google Scholar]